Microbially Mediated Arsenic-Nitrogen Biogeochemical Coupling Across Vertical Distribution in Coastal Wetlands
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area and Sampling Sites
2.2. Sample Processing
2.3. Physico-Chemical Analysis
2.4. Bacterial DNA Extraction and 16s rDNA Sequencing
2.5. Statistical Analysis
3. Results and Discussion
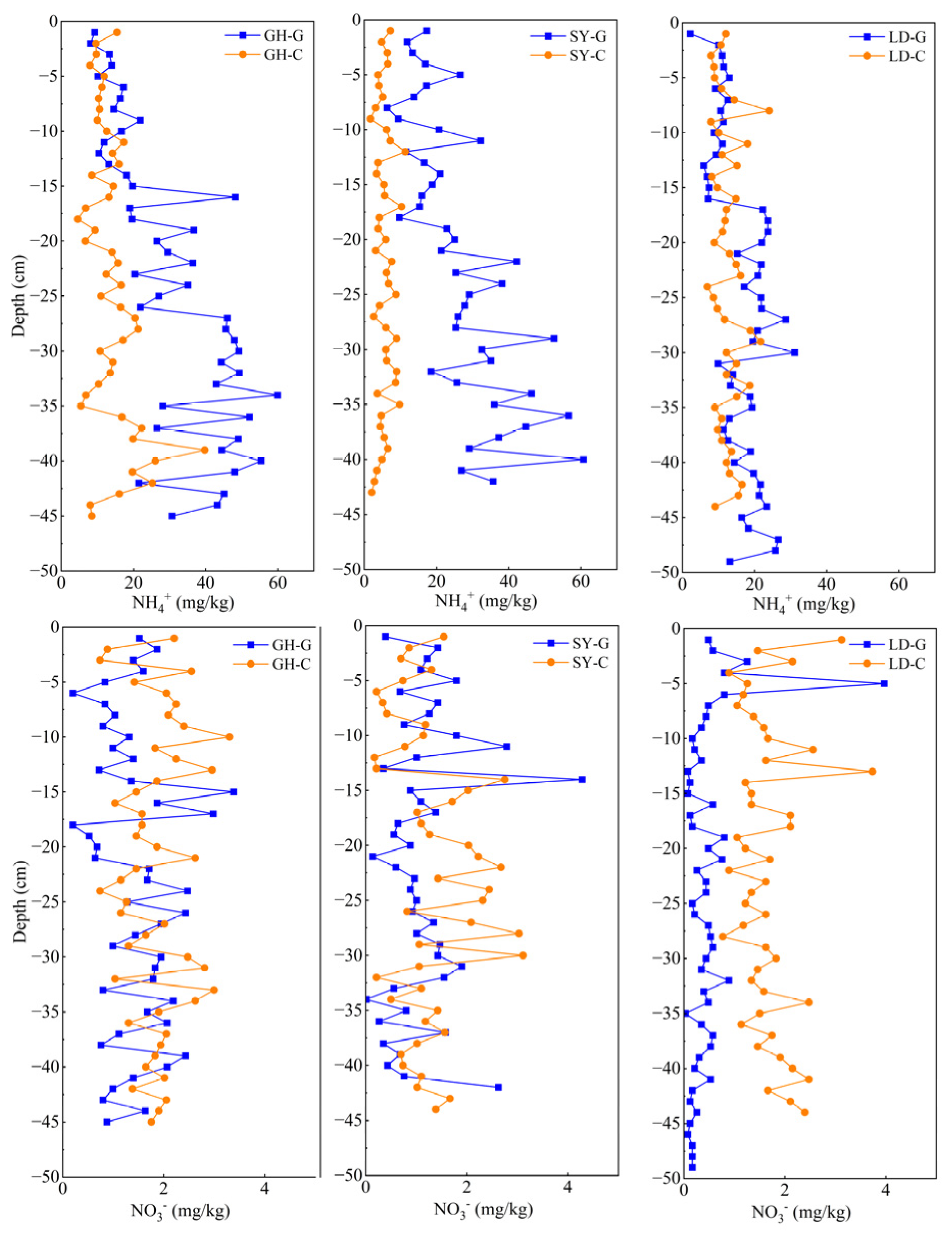
3.1. Arsenic and Nitrogen Vertical Distribution in Pore Water
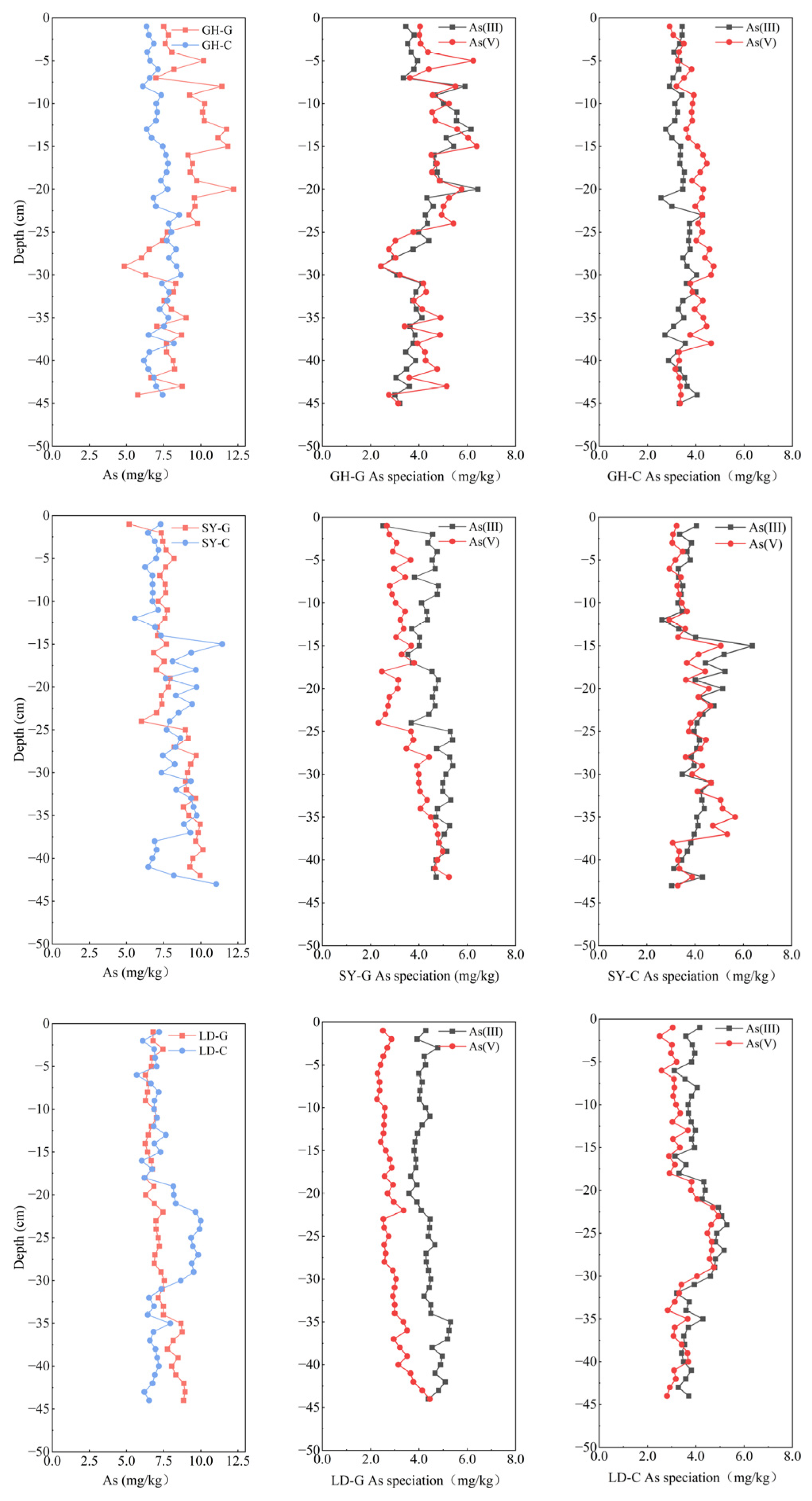
3.2. Total Arsenic and Arsenic Species in Sediment
3.3. Vertical Distribution of Nitrogen Species in the Sediments
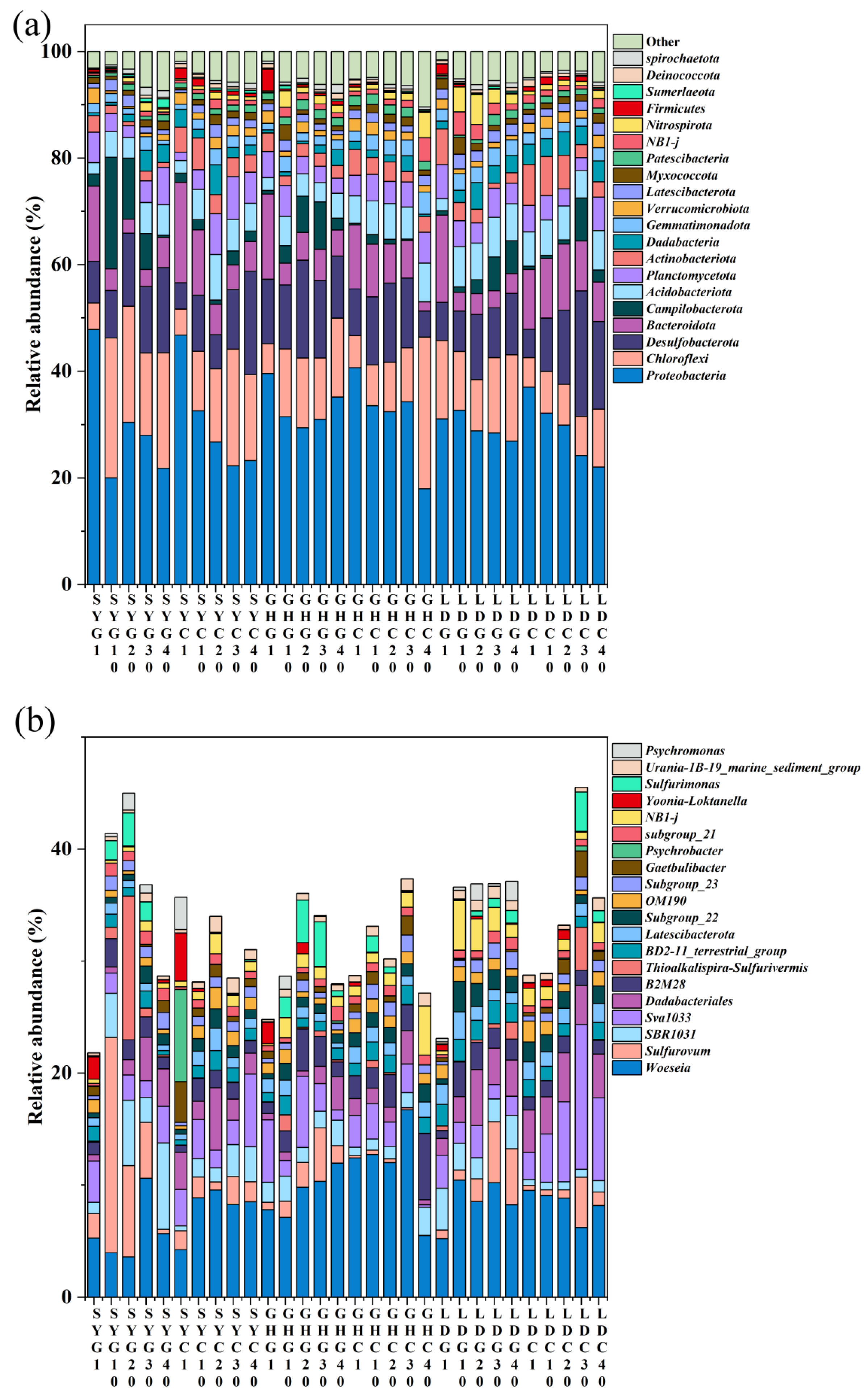
3.4. Microbial Analysis in Sediment
3.4.1. Microbial Diversity
3.4.2. Microbial Community Composition and Potential Arsenic-Nitrogen Coupling Functional Microorganisms
3.5. Interactions of Microorganisms with Arsenic and Nitrogen
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Chakraborty, S.; Bhar, K.; Saha, S.; Chakrabarti, R.; Pal, A.; Siddhanta, A. Novel arsenic nanoparticles are more effective and less toxic than As (III) to inhibit extracellular and intracellular proliferation of Leishmania donovani. J. Parasitol. Res. 2014, 2014, 187640. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Xiong, H.Y.; Zhang, J.C.; Wang, W.X. Transfer and bioavailability of inorganic and organic arsenic in sediment-water-biota microcosm. Aquat. Toxicol. 2021, 232, 105763. [Google Scholar] [CrossRef] [PubMed]
- Alsaffar, Z.; Cúrdia, J.; Borja, A.; Irigoien, X.; Carvalho, S. Consistent variability in beta-diversity patterns contrasts with changes in alpha-diversity along an onshore to offshore environmental gradient: The case of Red Sea soft-bottom macrobenthos. Mar. Biodivers. 2019, 49, 247–262. [Google Scholar] [CrossRef]
- Zhu, Y.G.; Yoshinaga, M.; Zhao, F.J.; Rosen, B.P. Earth abides arsenic biotransformations. Annu. Rev. Earth Planet. Sci. 2014, 42, 443–467. [Google Scholar] [CrossRef]
- Couture, R.M.; Gobeil, C.; Tessier, A. Arsenic, iron and sulfur co-diagenesis in lake sediments. Geochim. Cosmochim. Acta 2010, 74, 1238–1255. [Google Scholar] [CrossRef]
- Zan, F.Y.; Huo, S.L.; Zhang, J.T.; Zhang, L.; Xi, B.D.; Zhang, L.Y. Arsenic fractionation and contamination assessment in sediments of thirteen lakes from the East Plain and Yungui Plateau Ecoregions, China. J. Environ. Sci. 2014, 26, 1977–1984. [Google Scholar] [CrossRef]
- Wu, J.W.; Liang, J.L.; Björn, L.O.; Li, J.T.; Shu, W.S.; Wang, Y.T. Phosphorus-arsenic interaction in the ‘soil-plant-microbe’ system and its influence on arsenic pollution. Sci. Total Environ. 2022, 802, 149796. [Google Scholar] [CrossRef]
- Planer-Friedrich, B. Sulfur being an overlooked promoter of groundwater arsenic contamination. Nat. Water 2023, 1, 134–135. [Google Scholar] [CrossRef]
- Xiu, W.; Gai, R.X.; Chen, S.Z.; Ren, C.; Lloyd, J.R.; Bassil, N.M. Ammonium-enhanced arsenic mobilization from aquifer sediments. Environ. Sci. Technol. 2024, 58, 3449–3461. [Google Scholar] [CrossRef]
- Feng, M.; Du, Y.H.; Li, X.M.; Li, F.B.; Qiao, J.T.; Chen, G. Insight into universality and characteristics of nitrate reduction coupled with arsenic oxidation in different paddy soils. Sci. Total Environ. 2023, 866, 161342. [Google Scholar] [CrossRef]
- Weng, T.N.; Liu, C.W.; Kao, Y.H.; Hsiao, S.S. Isotopic evidence of nitrogen sources and nitrogen transformation in arsenic-contaminated groundwater. Sci. Total Environ. 2017, 578, 167–185. [Google Scholar] [CrossRef] [PubMed]
- Li, X.M.; Qiao, J.T.; Li, S.; Häggblom, M.M.; Li, F.B. Bacterial communities and functional genes stimulated during anaerobic arsenite oxidation and nitrate reduction in a paddy soil. Environ. Sci. Technol. 2020, 54, 2172–2181. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Chai, C.W.; ThomasArrigo, L.K.; Zhao, S.C.; Kretzschmar, R.; Zhao, F.J. Nitrite accumulation is required for microbial anaerobic iron oxidation, but not for arsenite oxidation, in two heterotrophic denitrifiers. Environ. Sci. Technol. 2020, 54, 4036–4045. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.H. Impact of microorganisms on arsenic biogeochemistry: A review. Water Air Soil. Pollut. 2014, 225, 1–25. [Google Scholar] [CrossRef]
- Zhang, J.; Zhou, W.X.; Liu, B.B.; He, J.; Shen, Q.; Zhao, F.J. Anaerobic arsenite oxidation by an autotrophic arsenite-oxidizing bacterium from an arsenic-contaminated paddy soil. Environ. Sci. Technol. 2015, 49, 5956–5964. [Google Scholar] [CrossRef]
- Zhang, J.; Zhao, S.C.; Xu, Y.; Zhou, W.X.; Huang, K.; Tang, Z. Nitrate stimulates anaerobic microbial arsenite oxidation in paddy soils. Environ. Sci. Technol. 2017, 51, 4377–4386. [Google Scholar] [CrossRef]
- Sun, W.; Sierra, R.; Field, J.A. Anoxic oxidation of arsenite linked to denitrification in sludges and sediments. Water Res. 2008, 42, 4569–4577. [Google Scholar] [CrossRef]
- Jamieson, J.; Prommer, H.; Kaksonen, A.H.; Sun, J.; Siade, A.J.; Yusov, A. Identifying and quantifying the intermediate processes during nitrate-dependent iron(II) oxidation. Environ. Sci. Technol. 2018, 52, 5771–5781. [Google Scholar] [CrossRef]
- Liu, T.X.; Chen, D.D.; Li, X.M.; Li, F.B. Microbially mediated coupling of nitrate reduction and Fe(II) oxidation under anoxic conditions. FEMS Microbiol. Ecol. 2019, 95, fiz030. [Google Scholar] [CrossRef]
- Liu, J.B.; Zhang, H.B.; Wang, H.F.; He, B.H.; Wang, H.X. Remediation of arsenic- and nitrate-contaminated groundwater through iron-dependent autotrophic denitrifying culture. Environ. Res. 2024, 257, 119239. [Google Scholar] [CrossRef]
- Zhang, M.M.; Kolton, M.; Häggblom, M.M.; Sun, X.Y.; Yu, K.; He, B. Anaerobic ammonium oxidation coupled to arsenate reduction, a novel biogeochemical process observed in arsenic-contaminated paddy soil. Geochim. Cosmochim. Acta 2022, 335, 11–22. [Google Scholar] [CrossRef]
- Duan, L.Q.; Song, J.M.; Zhang, Y.T.; Yin, M.L.; Yuan, H.M.; Li, X.G. Unraveling seasonal shifts in microbial and geochemical mediated arsenic mobilization at the estuarine sediment-water interface under redox changes. Sci. Total Environ. 2024, 912, 168939. [Google Scholar] [CrossRef] [PubMed]
- Li, W.H.; Siddique, M.S.; Liu, M.J.; Graham, N.; Yu, W.Z. The migration and microbiological degradation of dissolved organic matter in riparian soils. Water Res. 2022, 224, 119080. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Velez, J.D.; Harvey, J.W.; Cardenas, M.B.; Kiel, B. Denitrification in the Mississippi River network controlled by flow through river bedforms. Nat. Geosci. 2015, 8, 941–945. [Google Scholar] [CrossRef]
- Hou, L.J.; Zheng, Y.L.; Liu, M.; Gong, J.; Zhang, X.L.; Yin, G.Y. Anaerobic ammonium oxidation (anammox) bacterial diversity, abundance, and activity in marsh sediments of the Yangtze Estuary. J. Geophys. Res. Biogeosci. 2013, 118, 1237–1246. [Google Scholar] [CrossRef]
- Gao, D.Z.; Liu, C.; Li, X.F.; Zheng, Y.L.; Dong, H.P.; Liang, X. High importance of coupled nitrification-denitrification for nitrogen removal in a large periodically low-oxygen estuary. Sci. Total Environ. 2022, 846, 157516. [Google Scholar] [CrossRef]
- Liu, Z.Y.; Pan, S.M.; Sun, Z.Y.; Ma, R.F.; Chen, L.H.; Wang, Y. Heavy metal spatial variability and historical changes in the Yangtze River estuary and North Jiangsu tidal flat. Mar. Pollut. Bull. 2015, 98, 115–129. [Google Scholar] [CrossRef]
- Liu, F.D.; Mo, X.; Kong, W.J.; Song, Y. Soil bacterial diversity, structure, and function of Suaeda salsa in rhizosphere and non-rhizosphere soils in various habitats in the Yellow River Delta, China. Sci. Total Environ. 2020, 740, 140144. [Google Scholar] [CrossRef]
- Zhang, Q.Q.; Yan, Z.Z.; Li, X.Z. Iron plaque formation and rhizosphere iron bacteria in Spartina alterniflora and Phragmites australis on the redoxcline of tidal flat in the Yangtze River Estuary. Geoderma 2021, 392, 115000. [Google Scholar] [CrossRef]
- Sanz, E.; Muñoz-Olivas, R.; Cámara, C.; Kumar Sengupta, M.; Ahamed, S. Arsenic speciation in rice, straw, soil, hair and nails samples from the arsenic-affected areas of Middle and Lower Ganga plain. J. Environ. Sci. Health Part A 2007, 42, 1695–1705. [Google Scholar] [CrossRef]
- Lestari, L.; Harmesa, H.; Kaysupi, M.T.; Kampono, I.; Prayitno, H.B.; Budiyanto, F. Determination of trace metal content in certified reference marine sediment by three acid digestion and flame atomic absorption Spectrometry. AIP Conf. Proc. 2022, 2493, 030015. [Google Scholar] [CrossRef]
- Zhang, Z.N.; Yin, N.Y.; Du, H.L.; Cai, X.L.; Cui, Y.S. The fate of arsenic adsorbed on iron oxides in the presence of arsenite-oxidizing bacteria. Chemosphere 2016, 151, 108–115. [Google Scholar] [CrossRef] [PubMed]
- Farmaki, E.G.; Thomaidis, N.S.; Pasias, I.N.; Baulard, C.; Papaharisis, L.; Efstathiou, C.E. Environmental impact of intensive aquaculture: Investigation on the accumulation of metals and nutrients in marine sediments of Greece. Sci. Total Environ. 2014, 485, 554–562. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Liu, R.; Jian, Y.T.; Ma, T. Spatial distribution and factors influencing the various forms of iron in alluvial–lacustrine clayey aquitard. Water 2023, 15, 3934. [Google Scholar] [CrossRef]
- Xiao, C.C.; Chen, Y.Z.; Ma, T.; Xiong, W. Impact of pressure on arsenic released from pore water in clayey sediment. Toxics 2022, 10, 738. [Google Scholar] [CrossRef]
- Zhang, S.Y.; Zhang, J.J.; Niu, L.L.; Chen, Q.; Zhou, Q.; Xiao, N. Escalating arsenic contamination throughout Chinese soils. Nat. Sustain. 2024, 7, 766–775. [Google Scholar] [CrossRef]
- Bowen, J.L.; Giblin, A.E.; Murphy, A.E.; Bulseco, A.N.; Deegan, L.A.; Johnson, D.S. Not all nitrogen is created equal: Differential effects of nitrate and ammonium enrichment in coastal wetlands. BioScience 2020, 70, 1108–1119. [Google Scholar] [CrossRef]
- Bai, J.H.; Ouyang, H.; Deng, W.; Zhu, Y.M.; Zhang, X.L.; Wang, Q.G. Spatial distribution characteristics of organic matter and total nitrogen of marsh soils in river marginal wetlands. Geoderma 2005, 124, 181–192. [Google Scholar] [CrossRef]
- Lin, Z.J.; Wang, X.; Wu, X.; Liu, D.H.; Yin, Y.L.; Zhang, Y.; Xing, B. Nitrate reduced arsenic redox transformation and transfer in flooded paddy soil-rice system. Environ. Pollut. 2018, 243, 1015–1025. [Google Scholar] [CrossRef]
- Nguyen, V.K.; Tran, H.T.; Park, Y.; Yu, J.; Lee, T. Microbial arsenite oxidation with oxygen, nitrate, or an electrode as the sole electron acceptor. J. Ind. Microbiol. Biotechnol. 2017, 44, 857–868. [Google Scholar] [CrossRef]
- Zhu, X.B.; Zeng, X.C.; Chen, X.M.; Wu, W.W.; Wang, Y.X. Inhibitory effect of nitrate/nitrite on the microbial reductive dissolution of arsenic and iron from soils into pore water. Ecotoxicology 2019, 28, 528–538. [Google Scholar] [CrossRef] [PubMed]
- Phillips, D.J.H. Arsenic in aquatic organisms: A review, emphasizing chemical speciation. Aquat. Toxicol. 1990, 16, 151–186. [Google Scholar] [CrossRef]
- Fattorini, D.; Notti, A.; Halt, M.N.; Gambi, M.C.; Regoli, F. Levels and chemical speciation of arsenic in polychaetes: A review. Mar. Ecol. 2005, 26, 255–264. [Google Scholar] [CrossRef]
- He, Z.F.; Li, F.L.; Dominech, S.; Wen, X.H.; Yang, S.Y. Heavy metals of surface sediments in the Changjiang (Yangtze River) Estuary: Distribution, speciation and environmental risks. J. Geochem. Explor. 2019, 198, 18–28. [Google Scholar] [CrossRef]
- Shu, Q.; Ma, Y.Y.; Liu, Q.; Zhang, S.J.; Hu, Z. Levels and ecological risk of heavy metals in the surface sediments of tidal flats along the North Jiangsu coast, China. Mar. Pollut. Bull. 2021, 170, 112663. [Google Scholar] [CrossRef]
- Zhang, N.; Dai, Z.; Wang, F.; Yang, S.; Cao, W. Geographical factor dominates spatial patterns of potential nitrate reduction rates in coastal wetland sediments in Fujian Province, China. Front. Earth Sci. 2024, 12, 1399200. [Google Scholar] [CrossRef]
- Guo, T.; Li, L.G.; Zhai, W.W.; Xu, B.L.; Yin, X.L.; He, Y. Distribution of arsenic and its biotransformation genes in sediments from the East China Sea. Environ. Pollut. 2019, 253, 949–958. [Google Scholar] [CrossRef]
- Mamindy-Pajany, Y.; Bataillard, P.; Séby, F.; Crouzet, C.; Moulin, A.; Guezennec, A.-G. Arsenic in marina sediments from the Mediterranean coast: Speciation in the solid phase and occurrence of thioarsenates. Soil. Sediment. Contam. Int. J. 2013, 22, 984–1002. [Google Scholar] [CrossRef]
- Huang, B.; Yuan, Z.J.; Li, D.Q.; Zheng, M.H.; Nie, X.D.; Liao, Y.H. Effects of soil particle size on the adsorption, distribution, and migration behaviors of heavy metal(loid)s in soil: A review. Environ. Sci. Process. Impacts 2020, 22, 1596–1615. [Google Scholar] [CrossRef]
- Xiang, L.H.; Wang, Y.F.; Jiang, C.D.; Liu, Q.; Li, W.X. Heavy metal contamination in surface sediments of intertidal zone in central Jiangsu Province: Distribution, source, and assessment. Mar. Geol. Front. 2024, 40, 1–11. [Google Scholar] [CrossRef]
- Lan, T.; Han, Y.; Roelcke, M.; Nieder, R.; Cai, Z.C. Temperature dependence of gross N transformation rates in two Chinese paddy soils under aerobic condition. Biol. Fertil. Soils 2014, 50, 949–959. [Google Scholar] [CrossRef]
- Lin, X.B.; Li, X.F.; Gao, D.Z.; Liu, M.; Cheng, L. Ammonium production and removal in the sediments of Shanghai river networks: Spatiotemporal variations, controlling factors, and environmental implications. J. Geophys. Res. Biogeosci. 2017, 122, 2461–2478. [Google Scholar] [CrossRef]
- Wang, F.Z.; Lu, Z.Y.; Tobias, C.R.; Wang, Y.; Xiao, K.; Yu, Q.B. Salt marsh expansion into estuarine mangrove mudflats reduces nitrogen removal capacity. Catena 2023, 232, 107459. [Google Scholar] [CrossRef]
- Gao, X.P.; Bi, Y.X.; Su, L.; Lei, Y.; Gong, L.; Dong, X.H. Unveiling the nitrogen and phosphorus removal potential: Comparative analysis of three coastal wetland plant species in lab-scale constructed wetlands. J. Environ. Manag. 2024, 351, 119864. [Google Scholar] [CrossRef]
- Zhang, Z.; Furman, A. Soil redox dynamics under dynamic hydrologic regimes—A review. Sci. Total Environ. 2021, 763, 143026. [Google Scholar] [CrossRef]
- Zhang, X.; Yang, Y.Q.; Fu, Q.L.; Hu, H.Q.; Zhu, J.; Liu, M.X. Comparing effects of ammonium and nitrate nitrogen on arsenic accumulation in brown rice and its dynamics in soil-plant system. J. Soils Sediments 2021, 21, 2650–2658. [Google Scholar] [CrossRef]
- Canfield, D.E.; Glazer, A.N.; Falkowski, P.G. The evolution and future of Earth’s nitrogen cycle. Science 2010, 330, 192–196. [Google Scholar] [CrossRef]
- Huang, J.X.; Xu, X.; Wang, M. Responses of soil nitrogen fixation to Spartina alterniflora invasion and nitrogen addition in a Chinese salt marsh. Sci. Rep. 2016, 6, 20384. [Google Scholar] [CrossRef]
- Wang, X.D.; Feng, J.G.; Ao, G.; Qin, W.K.; Han, M.G.; Shen, Y.W. Globally nitrogen addition alters soil microbial community structure, but has minor effects on soil microbial diversity and richness. Soil. Biol. Biochem. 2023, 179, 108982. [Google Scholar] [CrossRef]
- Chaudhary, D.R.; Kim, J.; Kang, H. Influences of different halophyte vegetation on soil microbial community at temperate salt marsh. Microb. Ecol. 2018, 75, 729–738. [Google Scholar] [CrossRef]
- Gu, C.; Shi, J.Y.; Rui, J.L.; Yu, Y.M.; Huang, W.B.; Lu, Z.N. Halophyte vegetation influences soil microbial community of coastal salt marsh. J. Ocean. Univ. China 2022, 21, 1549–1556. [Google Scholar] [CrossRef]
- An, J.X.; Liu, C.; Wang, Q.; Yao, M.J.; Rui, J.P.; Zhang, S.H. Soil bacterial community structure in Chinese wetlands. Geoderma 2019, 337, 290–299. [Google Scholar] [CrossRef]
- Yang, C.; Zhang, H.; Feng, Y.Y.; Hu, Y.S.; Chen, S.K.; Guo, S.S. Effect of microbial communities on nitrogen and phosphorus metabolism in rivers with different heavy metal pollution. Environ. Sci. Pollut. Res. Int. 2023, 30, 87398–87411. [Google Scholar] [CrossRef] [PubMed]
- Luo, T.; Sun, Y.; Liang, W.H.; Zheng, Q.N.; Kong, S.; Xue, L. The alternation of flood and ebb tide induced arsenic release and migration from coastal tidal flat sediments in Yellow Sea wetlands: An ex-situ study. J. Clean. Prod. 2024, 448, 141730. [Google Scholar] [CrossRef]
- Zhang, M.M.; Tan, Y.F.; Fan, Y.J.; Wu, J.; Yu, L.T. Insights into nitrite accumulation and microbial structure in partial denitrification (PD) process by the combining regulation of C/N ratio and nitrate concentration. J. Environ. Chem. Eng. 2023, 11, 109891. [Google Scholar] [CrossRef]
- Guo, J.Y.; Li, Q.H.; Gao, Q.F.; Shen, F.; Yang, Y.; Zhang, X.B. Comparative study on the treatment of swine wastewater by VFCW-MFC and VFCW: Pollutants removal, electricity generation, microorganism community. J. Environ. Manag. 2023, 342, 118299. [Google Scholar] [CrossRef]
- Li, Y.Y.; Liu, L.; Wang, H.J. Mixotrophic denitrification for enhancing nitrogen removal of municipal tailwater: Contribution of heterotrophic/sulfur autotrophic denitrification and bacterial community. Sci. Total Environ. 2022, 814, 151940. [Google Scholar] [CrossRef]
- Zhang, D.C.; Li, X.D.; Wu, Y.H.; Xu, X.W.; Liu, Y.X.; Shi, B.Z. Microbe-driven elemental cycling enables microbial adaptation to deep-sea ferromanganese nodule sediment fields. Microbiome 2023, 11, 160. [Google Scholar] [CrossRef]
- D’Angelo, T.; Goordial, J.; Lindsay, M.R.; McGonigle, J.; Booker, A.; Moser, D. Replicated life-history patterns and subsurface origins of the bacterial sister phyla Nitrospirota and Nitrospinota. ISME J. 2023, 17, 891–902. [Google Scholar] [CrossRef]
- Castelle, C.J.; Hug, L.A.; Wrighton, K.C.; Thomas, B.C.; Williams, K.H.; Wu, D. Extraordinary phylogenetic diversity and metabolic versatility in aquifer sediment. Nat. Commun. 2013, 4, 2120. [Google Scholar] [CrossRef]
- Mußmann, M.; Pjevac, P.; Krüger, K.; Dyksma, S. Genomic repertoire of the Woeseiaceae/JTB255, cosmopolitan and abundant core members of microbial communities in marine sediments. ISME J. 2017, 11, 1276–1281. [Google Scholar] [CrossRef] [PubMed]
- Yu, T.T.; Li, M.; Niu, M.Y.; Fan, X.B.; Liang, W.Y.; Wang, F.P. Difference of nitrogen-cycling microbes between shallow bay and deep-sea sediments in the South China Sea. Appl. Microbiol. Biotechnol. 2018, 102, 447–459. [Google Scholar] [CrossRef] [PubMed]
- Giovannelli, D.; Chung, M.; Staley, J.; Starovoytov, V.; Le Bris, N.; Vetriani, C. Sulfurovum riftiae sp. nov., a mesophilic, thiosulfate-oxidizing, nitrate-reducing chemolithoautotrophic epsilonproteobacterium isolated from the tube of the deep-sea hydrothermal vent polychaete Riftia pachyptila. Int. J. Syst. Evol. Microbiol. 2016, 66, 2697–2701. [Google Scholar] [CrossRef] [PubMed]
- Luo, T.; Qin, W.; Wang, Y.Z.; Sun, Y.; Kong, S. Arsenic mobility and microbial community composition in the sediments of coastal wetlands driven by tidal action. J. Environ. Sci. 2024, 153, 99–108. [Google Scholar] [CrossRef] [PubMed]
- Baloza, M.; Henkel, S.; Kasten, S.; Holtappels, M.; Molari, M. The impact of sea ice cover on microbial communities in Antarctic shelf sediments. Microorganisms 2023, 11, 1572. [Google Scholar] [CrossRef]
- Bao, P.; Su, J.Q.; Hu, Z.Y.; Häggblom, M.M.; Zhu, Y.G. Genome sequence of the anaerobic bacterium Bacillus sp. strain ZYK, a selenite and nitrate reducer from paddy soil. Stand. Genom. Sci. 2014, 9, 646–654. [Google Scholar] [CrossRef]
- Wu, S.J.; Wang, L.R.; Gan, R.; Tong, T.; Bian, H.; Li, Z.Q. Signature arsenic detoxification pathways in Halomonas sp. strain GFAJ-1. Mbio 2018, 9, e00515-18. [Google Scholar] [CrossRef]
- Zecchin, S.; Colombo, M.; Cavalca, L. Exposure to different arsenic species drives the establishment of iron- and sulfur-oxidizing bacteria on rice root iron plaques. World J. Microbiol. Biotechnol. 2019, 35, 117. [Google Scholar] [CrossRef]
- Coskun, Ö.K.; Özen, V.; Wankel, S.D.; Orsi, W.D. Quantifying population-specific growth in benthic bacterial communities under low oxygen using H218O. ISME J. 2019, 13, 1546–1559. [Google Scholar] [CrossRef]
- Guo, C.; Zhang, X.; Luan, S.; Zhou, H.; Liu, L.; Qu, Y. Diversity and structure of soil bacterial community in intertidal zone of Daliao River estuary, Northeast China. Mar. Pollut. Bull. 2021, 163, 111965. [Google Scholar] [CrossRef]
- Xie, Y.; Tian, X.; He, Y.; Dong, S.; Zhao, K. Nitrogen removal capability and mechanism of a novel heterotrophic nitrification–aerobic denitrification bacterium Halomonas sp. DN3. Bioresour. Technol. 2023, 387, 129569. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Song, X.; Cheng, X.; Huang, Z.; Dong, D.; Li, X. Enhanced denitrification of biodegradable polymers using Bacillus pumilus in aerobic denitrification bioreactors: Performance and mechanism. Bioresour. Technol. 2024, 394, 130240. [Google Scholar] [CrossRef] [PubMed]
- D’Aquino, A.; Kalinainen, N.; Auvinen, H.; Andreottola, G.; Puhakka, J.A.; Palmroth, M.R.T. Effects of inorganic ions on autotrophic denitrification by Thiobacillus denitrificans and on heterotrophic denitrification by an enrichment culture. Sci. Total Environ. 2023, 901, 165940. [Google Scholar] [CrossRef]
- Zhang, M.; Zhang, T.; Shao, M.F.; Fang, H.H.P. Autotrophic denitrification in nitrate-induced marine sediment remediation and Sulfurimonas denitrificans-like bacteria. Chemosphere 2009, 76, 677–682. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.; Zhang, D.; Cao, H.; Zhang, Y.; Zhao, D.; Zeng, W.; Lei, H. Identification of aerobic-denitrifying Psychrobacter cryohalolentis strain F5-6 and its nitrate removal at low temperature. Int. Biodeterior. Biodegrad. 2022, 172, 105426. [Google Scholar] [CrossRef]
- Zhang, Z.; Yin, N.; Cai, X.; Wang, Z.; Cui, Y. Arsenic redox transformation by Pseudomonas sp. HN-2 isolated from arsenic-contaminated soil in Hunan, China. J. Environ. Sci. 2016, 47, 165–173. [Google Scholar] [CrossRef]
- Bagade, A.; Nandre, V.; Paul, D.; Patil, Y.; Sharma, N.; Giri, A.; Kodam, K. Characterisation of hyper tolerant Bacillus firmus L-148 for arsenic oxidation. Environ. Pollut. 2020, 261, 114124. [Google Scholar] [CrossRef]
- Li, Y.; Guo, L.; Yang, R.; Yang, Z.; Zhang, H.; Li, Q.; Cao, Z.G.; Zhang, X. Thiobacillus spp. and Anaeromyxobacter spp. mediate arsenite oxidation-dependent biological nitrogen fixation in two contrasting types of arsenic-contaminated soils. J. Hazard. Mater. 2023, 443, 130220. [Google Scholar] [CrossRef]
- Liao, V.H.-C.; Chu, Y.-J.; Su, Y.-C.; Hsiao, S.-Y.; Wei, C.-C.; Liu, C.-W.; Liao, C.-M.; Shen, W.-C. Arsenite-oxidizing and arsenate-reducing bacteria associated with arsenic-rich groundwater in Taiwan. J. Contam. Hydrol. 2011, 123, 20–29. [Google Scholar] [CrossRef]
- Luo, T.; Zheng, Q.; Yu, J.; Liang, W.; Sun, Y.; Quan, G.; Zhou, F. Roles of nanoparticles in arsenic mobility and microbial community composition in arsenic-enriched soils. J. Environ. Sci. 2024, 138, 301–311. [Google Scholar] [CrossRef]
- Xin, X.; Li, B.; Liu, X.; Yang, W.; Liu, Q. Starting-up performances and microbial community shifts in the coupling process (SAPD-A) with sulfide autotrophic partial denitrification (SAPD) and anammox treating nitrate and ammonium contained wastewater. J. Environ. Manag. 2023, 331, 117298. [Google Scholar] [CrossRef]
- Wan, K.; Yu, Y.; Hu, J.; Liu, X.; Deng, X.; Yu, J.; Chi, R. Recovery of anammox process performance after substrate inhibition: Reactor performance, sludge morphology, and microbial community. Bioresour. Technol. 2022, 357, 127351. [Google Scholar] [CrossRef]




| NH4+ | pH | EC | ACE | Shannon | Obs | ||
|---|---|---|---|---|---|---|---|
| NH4+ | Correlation coefficient | 1 | 0.422 * | 0.481 ** | 0.055 | 0.136 | 0.045 |
| Significance | 0.023 | 0.008 | 0.776 | 0.483 | 0.815 | ||
| pH | Correlation coefficient | 0.422 * | 1 | 0.619 ** | 0.189 | 0.271 | 0.271 |
| Significance | 0.023 | <0.001 | 0.326 | 0.155 | 0.155 | ||
| EC | Correlation coefficient | 0.481 ** | 0.619 ** | 1 | 0.471 ** | −0.529 ** | −0.509 ** |
| Significance | 0.008 | <0.001 | 0.01 | 0.003 | 0.005 | ||
| ACE | Correlation coefficient | 0.055 | 0.189 | 0.471 ** | 1 | 0.899 ** | 0.966 ** |
| Significance | 0.776 | 0.326 | 0.01 | <0.001 | <0.001 | ||
| Shannon | Correlation coefficient | 0.136 | 0.271 | 0.529 ** | 0.899 ** | 1 | 0.913 ** |
| Significance | 0.483 | 0.155 | 0.003 | <0.001 | <0.001 | ||
| Obs | Correlation coefficient | 0.045 | 0.271 | 0.509 ** | 0.966 ** | 0.913 ** | 1 |
| Significance | 0.815 | 0.155 | 0.005 | <0.001 | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zou, Y.; Xue, L.; Luo, T.; Kong, S.; Zhao, Z.; Ding, L.; Liu, K.; Gao, H.; Wu, H. Microbially Mediated Arsenic-Nitrogen Biogeochemical Coupling Across Vertical Distribution in Coastal Wetlands. Water 2025, 17, 1255. https://doi.org/10.3390/w17091255
Zou Y, Xue L, Luo T, Kong S, Zhao Z, Ding L, Liu K, Gao H, Wu H. Microbially Mediated Arsenic-Nitrogen Biogeochemical Coupling Across Vertical Distribution in Coastal Wetlands. Water. 2025; 17(9):1255. https://doi.org/10.3390/w17091255
Chicago/Turabian StyleZou, Yang, Lili Xue, Ting Luo, Sheng Kong, Zirui Zhao, Liang Ding, Kexin Liu, Huaxin Gao, and Hao Wu. 2025. "Microbially Mediated Arsenic-Nitrogen Biogeochemical Coupling Across Vertical Distribution in Coastal Wetlands" Water 17, no. 9: 1255. https://doi.org/10.3390/w17091255
APA StyleZou, Y., Xue, L., Luo, T., Kong, S., Zhao, Z., Ding, L., Liu, K., Gao, H., & Wu, H. (2025). Microbially Mediated Arsenic-Nitrogen Biogeochemical Coupling Across Vertical Distribution in Coastal Wetlands. Water, 17(9), 1255. https://doi.org/10.3390/w17091255






