Preparation of Triiodide Resin Using Potassium Iodide and Peracetic Acid: Application to Wastewater Treatment
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Apparatus
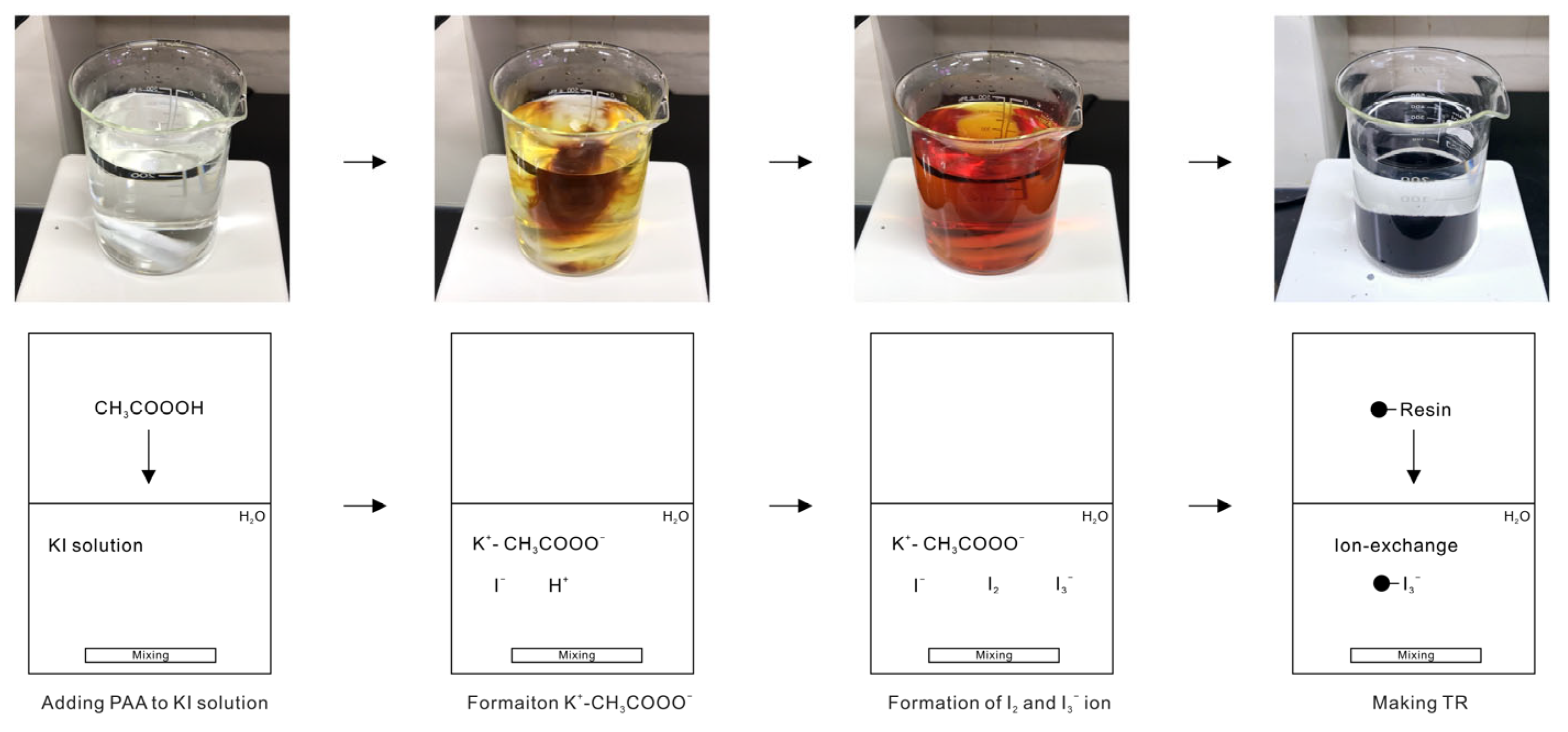
2.2. Formation of I2 and I3− Ion in KI Solution by Reaction with PAA
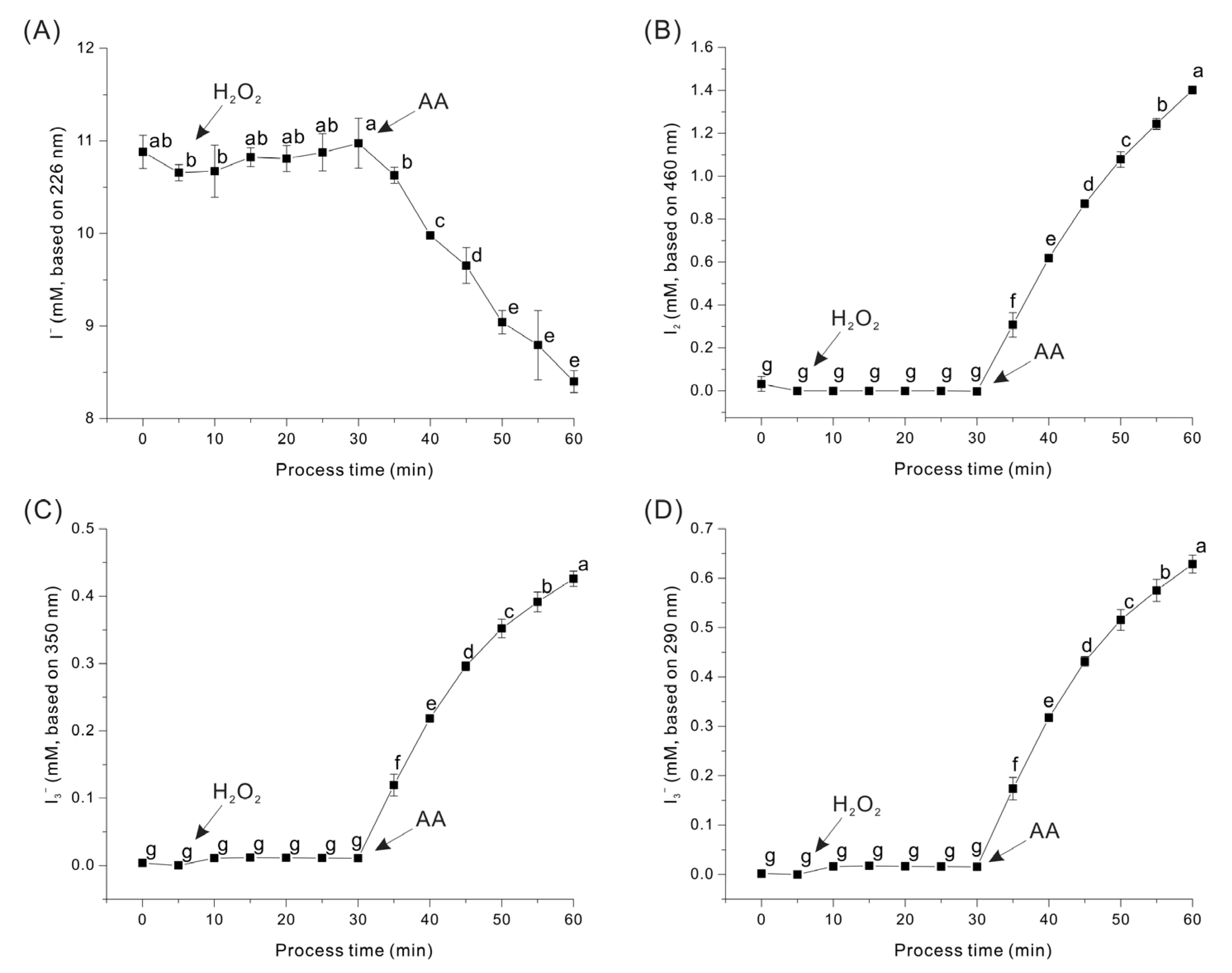
2.3. Reaction of AA and H2O2 with KI
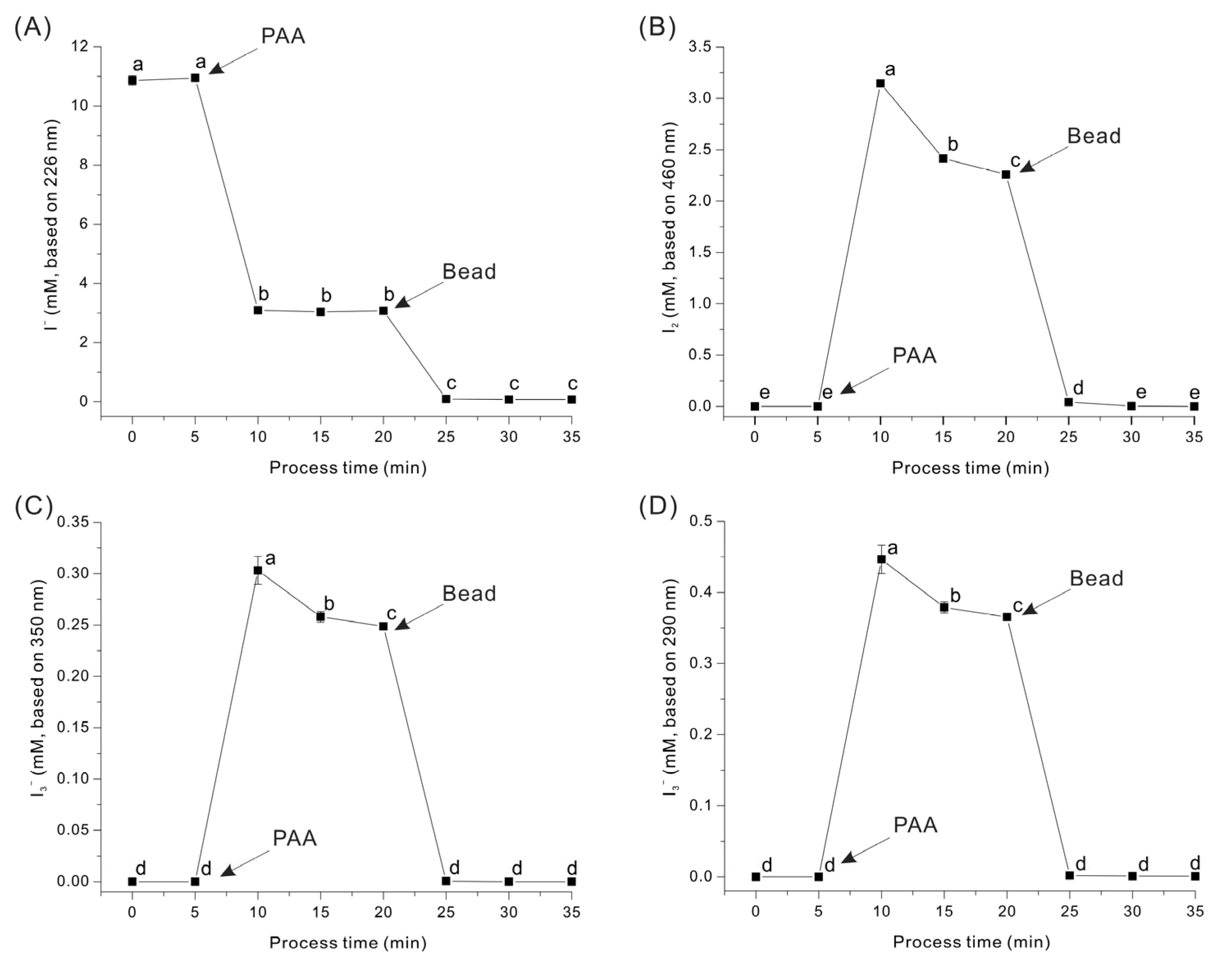
2.4. Manufacture of TR
2.5. Treatment of Anaerobically Treated Livestock Wastewater by TR
2.6. Statistical Analysis
3. Results and Discussion
3.1. Formation of I2 and I3− Ion by Chemical Reaction of KI and PAA
3.2. Triiodide Resin (TR) Manufacture
3.3. Antibacterial Effect of TR
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Tang, W.; Pei, Y.; Zheng, H.; Zhao, Y.; Shu, L.; Zhang, H. Twenty years of China’s water pollution control: Experiences and challenges. Chemosphere 2022, 295, 133875. [Google Scholar] [CrossRef] [PubMed]
- Gitis, V.; Hankins, N. Water treatment chemicals: Trends and challenges. J. Water Process Eng. 2018, 25, 34–38. [Google Scholar] [CrossRef]
- Ellis, K. Water disinfection: A review with some consideration of the requirements of the third world. Crit. Rev. Environ. Sci. Technol. 1991, 20, 341–407. [Google Scholar] [CrossRef]
- Ghernaout, D.; Ghernaout, B. From chemical disinfection to electrodisinfection: The obligatory itinerary? Desalin. Water Treat. 2010, 16, 156–175. [Google Scholar] [CrossRef]
- Hove, P.R.; Mobley, D.; Magunda, F.; Call, D.R. Deploying elemental iodine in a vapor form to disinfect water and to clear biofilms. Int. J. Environ. Res. Public Health 2020, 17, 3489. [Google Scholar] [CrossRef]
- Backer, H.; Hollowell, J. Use of iodine for water disinfection: Iodine toxicity and maximum recommended dose. Environ. Health Perspect. 2000, 108, 679–684. [Google Scholar] [CrossRef]
- Black, A.; Kinman, R.; Thomas Jr, W.; Freund, G.; Bird, E. Use of iodine for disinfection. J. Am. Water Works Assn. 1965, 57, 1401–1421. [Google Scholar] [CrossRef]
- Chang, S.L.; Morris, J.C. Elemental iodine as a disinfectant for drinking water. Ind. Eng. Chem. 1953, 45, 1009–1012. [Google Scholar] [CrossRef]
- Sen, A.; Sharma, S.; Dutta, S.; Shirolkar, M.M.; Dam, G.K.; Let, S.; Ghosh, S.K. Functionalized ionic porous organic polymers exhibiting high iodine uptake from both the vapor and aqueous medium. ACS Appl. Mater. Interfaces 2021, 13, 34188–34196. [Google Scholar] [CrossRef]
- Küpper, F.C.; Kroneck, P.M. Iodine bioinorganic chemistry: Physiology, structures, and mechanisms. Iodine Chem. Appl. 2014, 555–589. [Google Scholar] [CrossRef]
- Jung, S.-H.; Yeon, J.-W.; Hong, S.Y.; Kang, Y.; Song, K. The oxidation behavior of iodide ion under gamma irradiation conditions. Nucl. Sci. Eng. 2015, 181, 191–203. [Google Scholar] [CrossRef]
- Some, S.; Sohn, J.S.; Kim, J.; Lee, S.-H.; Lee, S.C.; Lee, J.; Shackery, I.; Kim, S.K.; Kim, S.H.; Choi, N. Graphene-iodine nanocomposites: Highly potent bacterial inhibitors that are bio-compatible with human cells. Sci. Rep. 2016, 6, 20015. [Google Scholar] [CrossRef] [PubMed]
- Farooq, W.; Mishra, S.K.; Moon, M.; Suh, W.I.; Shrivastav, A.; Kumar, K.; Kwon, J.H.; Park, M.S.; Yang, J.-W. Energy efficient process for microalgae cell disruption for oil recovery using triiodide resin. Algal Res. 2016, 13, 102–108. [Google Scholar] [CrossRef]
- Barton, D.N.; Robshaw, T.J.; Okusanya, O.; Kim, D.; Pepper, S.E.; Sharrad, C.A.; Lee, T.S.; Ogden, M.D. Remediation of radioiodine using polyamine anion exchange resins. J. Ind. Eng. Chem. 2019, 78, 210–221. [Google Scholar] [CrossRef]
- Reynier, N.; Lastra, R.; Laviolette, C.; Fiset, J.-F.; Bouzoubaâ, N.; Chapman, M. Comparison of uranium recovery by ion exchange from sulfuric acid liquor in iodide and chloride media. Solvent Extr. Ion Exch. 2016, 34, 188–200. [Google Scholar] [CrossRef]
- Ashley, K.R.; Whitener, G.D.; Schroeder, N.C.; Ball, J.R.; Radzinski, S.D. Sorption behavior of pertechnetate ion on reillextm-hpq anion exchange resin from hanford and melton valley tank waste simulants and sodium hydroxide/sodium nitrate solutions. Solvent Extr. Ion Exch. 1998, 16, 843–859. [Google Scholar] [CrossRef]
- Shkinev, V.; Spivakov, B.Y.; Geckeler, K.; Bayer, E. Anion exchange extraction and enrichment from aqueous solutions by quarternary ammonium reagents. Solvent Extr. Ion Exch. 1989, 7, 499–510. [Google Scholar] [CrossRef]
- Ashley, K.R.; Ball, J.R.; Pinkerton, A.B.; Abney, K.D.; Norman, C. Sorption behavior of pertechnetate on reillex (tm)-HPQ anion exchange resin from nitric acid solution. Solvent Extr. Ion Exch. 1994, 12, 239–259. [Google Scholar] [CrossRef]
- Stevens, K.A.; Jaykus, L.-A. Bacterial separation and concentration from complex sample matrices: A review. Crit. Rev. Microbiol. 2004, 30, 7–24. [Google Scholar] [CrossRef]
- Liu, H.F.; Ma, J.; Winter, C.; Bayer, R. Recovery and purification process development for monoclonal antibody production. MAbs 2010, 2, 480–499. [Google Scholar] [CrossRef]
- Vijay Rakesh Reddy, S.; Sudhakar Rao, D.; Sharma, R.; Preethi, P.; Pandiselvam, R. Role of ozone in post-harvest disinfection and processing of horticultural crops: A review. Ozone Sci. Eng. 2022, 44, 127–146. [Google Scholar] [CrossRef]
- Fina, L.R.; Hassouna, N.; Horacek, G.L.; Lambert, J.P.; Lambert, J.L. Viricidal capability of resin-triiodide demand-type disinfectant. Appl. Environ. Microbiol. 1982, 44, 1370–1373. [Google Scholar] [CrossRef] [PubMed]
- Nam, K.; Shin, W.-S.; Jeong, B.-r.; Park, M.S.; Yang, J.-W.; Kwon, J.-H. Use of a triiodide resin for isolation of axenic cultures of microalgal Nannochloropsis gaditana. Bioresour. Technol. 2015, 191, 391–394. [Google Scholar] [CrossRef]
- Taylor, S.; Fina, L.; Lambert, J. New water disinfectant: An insoluble quaternary ammonium resin-triiodide combination that releases bactericide on demand. Appl. Microbiol. 1970, 20, 720–722. [Google Scholar] [CrossRef]
- Yan, L.; Zhang, S.; Kang, Q.; Meng, X.; Li, Z.; Liu, T.; Ma, T.; Lin, Z. Iodine conversion chemistry in aqueous batteries: Challenges, strategies, and perspectives. Energy Storage Mater. 2023, 54, 339–365. [Google Scholar] [CrossRef]
- Valizadeh, B.; Nguyen, T.N.; Smit, B.; Stylianou, K.C. Porous Metal–Organic Framework@ Polymer Beads for Iodine Capture and Recovery Using a Gas-Sparged Column. Adv. Funct. Mater. 2018, 28, 1801596. [Google Scholar] [CrossRef]
- Gazda, D.B.; Lipert, R.J.; Fritz, J.S.; Porter, M.D. Investigation of the iodine–poly (vinylpyrrolidone) interaction employed in the determination of biocidal iodine by colorimetric solid-phase extraction. Anal. Chim. Acta 2004, 510, 241–247. [Google Scholar] [CrossRef]
- Bayer, G.; Shayganpour, A.; Bayer, I.S. Efficacy of a New Alcohol-Free Organic Acid-Based Hand Sanitizer against Foodborne Pathogens. Toxics 2023, 11, 938. [Google Scholar] [CrossRef]
- Kazantseva, N.; Ernepesova, A.; Khodjamamedov, A.; Geldyev, O.; Krumgalz, B. Spectrophotometric analysis of iodide oxidation by chlorine in highly mineralized solutions. Anal. Chim. Acta 2002, 456, 105–119. [Google Scholar] [CrossRef]
- Cason, D.; Neumann, H. Stability of the Chloro-complexes of Iodine in Aqueous Solution1. J. Am. Chem. Soc. 1961, 83, 1822–1828. [Google Scholar] [CrossRef]
- Kim, G.-H.; Jung, J.-Y.; Lim, H.-J.; Jung, S.M.; Kwon, J.-H. Photometric determination of peracetic acid by reaction with potassium iodide solution. Anal. Sci. 2023, 39, 383–388. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Singh, J.; Vasishth, A.; Kumar, A.; Pattnaik, S.S. Emerging Materials in Advanced Oxidation Processes for Micropollutant Treatment Process. In Advanced Oxidation Processes for Micropollutant Remediation; CRC Press: Boca Raton, FL, USA, 2023; pp. 133–155. [Google Scholar]
- Liu, S.; Zhang, X.; Asselin, E.; Li, Z. A new process for peracetic acid production from acetic acid and hydrogen peroxide based on kinetic modeling and distillation simulation. Ind. Eng. Chem. Res. 2021, 61, 339–348. [Google Scholar] [CrossRef]
- Hatch, G.L.; Lambert, J.L.; Fina, L.R. Some properties of the quaternary ammonium anion-exchange resin-triiodide disinfectant for water. Ind. Eng. Chem. Prod. Res. Dev. 1980, 19, 259–263. [Google Scholar] [CrossRef]
- Lambert, J.L.; Fina, G.T.; Fina, L.R. Preparation and properties of triiodide-, pentaiodide-, and heptaiodide-quaternary ammonium stron base anion-exchange resin disinfectants. Ind. Eng. Chem. Prod. Res. Dev. 1980, 19, 256–258. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lim, H.-J.; Kang, J.-Y.; Kim, G.-H.; Kwon, J.-H. Preparation of Triiodide Resin Using Potassium Iodide and Peracetic Acid: Application to Wastewater Treatment. Water 2025, 17, 1266. https://doi.org/10.3390/w17091266
Lim H-J, Kang J-Y, Kim G-H, Kwon J-H. Preparation of Triiodide Resin Using Potassium Iodide and Peracetic Acid: Application to Wastewater Treatment. Water. 2025; 17(9):1266. https://doi.org/10.3390/w17091266
Chicago/Turabian StyleLim, Hyun-Jin, Ji-Yeon Kang, Ga-Hyeon Kim, and Jong-Hee Kwon. 2025. "Preparation of Triiodide Resin Using Potassium Iodide and Peracetic Acid: Application to Wastewater Treatment" Water 17, no. 9: 1266. https://doi.org/10.3390/w17091266
APA StyleLim, H.-J., Kang, J.-Y., Kim, G.-H., & Kwon, J.-H. (2025). Preparation of Triiodide Resin Using Potassium Iodide and Peracetic Acid: Application to Wastewater Treatment. Water, 17(9), 1266. https://doi.org/10.3390/w17091266








