Abstract
Wastewater treatment plants (WWTPs) have been confirmed as reservoirs of antibiotic resistance genes (ARGs). This study systematically investigated the distribution patterns of ARGs across different treatment units in municipal WWTPs, along with the environmental drivers, dissemination characteristics, and exposure risks of aerosol-borne ARGs in aerated tank environments. The results revealed a high compositional similarity in aerosol-borne ARGs across the sampling sites, with multidrug ARGs predominating at an average relative abundance of 52%, followed sequentially by tetracycline (11%), MLS (10%), and glycopeptide resistance genes (7%). The diffusion of aerosol-borne ARGs is significantly influenced by environmental factors including temperature, relative humidity, wind speed, and total suspended particulate (TSP) concentration, with temperature being the most dominant factor affecting the dispersion of ARGs. The atmospheric dispersion model demonstrates that aerosol-borne ARGs decay with increasing downwind distance, showing potential for transport from aeration tanks to locations exceeding 1500 m along the prevailing wind direction. Both within wastewater treatment units and downwind areas, adult males had higher respiratory exposure doses but lower skin contact doses compared to females, with respiratory doses exceeding skin contact by 3–4 orders of magnitude. This study highlights the potential health risks posed by aerosol-borne ARG transmission from WWTP operations.
1. Introduction
Since the clinical application of penicillin in the 1940s, humans have developed over 200 antimicrobial agents through biological extraction and chemical synthesis. These compounds serve as critical therapeutic agents not only in human and veterinary medicine, but are also extensively employed in animal husbandry for disease prevention and growth performance regulation [1,2]. However, the excessive utilization of antibiotics has precipitated significant ecological and public health concerns. Studies demonstrate that 30–90% of administered antibiotics with insufficient bioavailability are excreted into environmental matrices through animal waste systems, resulting in persistent pharmaceutical residues [1,3]. Upon environmental release, these residual antibiotics exert substantial selective pressure on microbial communities, significantly increasing the likelihood of genetic mutations in environmentally exposed bacteria. This evolutionary pressure drives microbial adaptation through mechanisms that reduce antibiotic sensitivity or confer functional resistance, thereby accelerating the emergence and dissemination of ARGs [4]. The resultant multidrug-resistant organisms (MDROs) now represent a critical biosecurity threat, with projections indicating that antimicrobial resistance could cause approximately 10 million deaths annually by 2050 [5,6].
WWTPs are widely recognized as critical environmental reservoirs for ARGs, playing a pivotal role in their enrichment and dissemination. Converging evidence from multi-source investigations demonstrates that WWTPs exhibit significantly higher diversity and abundance of ARGs compared to other ecosystems [7,8]. Through the systematic analysis of wastewater samples, Ju et al. [9] identified over 20 distinct ARG types conferring resistance to aminoglycosides, β-lactams, chloramphenicol, fosfomycin, multidrug, sulfonamides, and tetracyclines. Furthermore, Guo et al. [10] conducted a comprehensive metagenomic analysis revealing 42 and 51 distinct ARG subtypes in activated sludge and digested sludge, respectively, while concurrently detecting significant enrichment of mobile genetic elements (MGEs), including plasmids, transposons, and integrons. Notably, Yu et al. [11] quantified extracellular DNA-associated ARGs in WWTP processes using quantitative PCR (qPCR), detecting >40 ARG subtypes with abundances ranging from (6.0 ± 0.7) × 105 to (1.0 ± 0.2) × 108 copies/mL, while demonstrating persistent ARG enrichment in effluent discharges. These findings collectively indicate that WWTPs serve as critical hotspots for both ARGs and MGEs, posing substantial risks for environmental transmission.
During wastewater treatment processes, mechanical agitation and aeration operations frequently induce substantial bubble generation. These bubbles migrate towards the liquid phase under gravitational forces and subsequently rupture due to hydrodynamic compression effects [12,13,14]. Upon collapse of the gaseous film encapsulating the bubbles, the surrounding liquid medium rapidly fills the resultant cavities, generating microjets that promote droplet atomization and subsequent suspension within the gaseous environment, ultimately forming bioaerosols. These bioaerosols serve as microbial vectors, containing not only antibiotic-resistant bacteria (ARB) carrying ARGs but also opportunistic pathogens [15]. Compared to aqueous and soil matrices, the atmospheric environment exhibits distinct high diffusivity that significantly enhances the migration efficiency of ARGs within aerosol vectors, thereby amplifying their ecological dissemination risks. The aerosolized microorganisms harboring ARGs may infiltrate human hosts via respiratory exposure pathways, posing potential biosafety threats to wastewater treatment workers and adjacent communities [6]. Notably, the presence of ARGs introduces substantial uncertainty in antibiotic therapeutic efficacy during clinical interventions. Current research on aerosol-borne ARGs in WWTPs has primarily focused on quantitative detection and characterization of ARGs and their host bacteria, while systematic investigations into their horizontal dispersion characteristics and associated exposure risks remain scarce [13,16].
Currently, atmospheric dispersion models are commonly utilized to characterize the dispersion of microbial aerosols, with predictions of dispersion processes derived from empirical measurements. Nguyen et al. [17] employed the atmospheric modeling tool (ADMS) to analyze the dispersion of a Legionella pneumophila outbreak, achieving precise identification of the pathogen’s source. A research team led by Li [18] simulated microbial aerosol concentrations downwind of rotating brush aerators in WWTPs using a refined Gaussian plume model. Their results indicated a rapid exponential decline in the mean ground-level bacterial concentration with increasing downwind distance. By coupling meteorological datasets with the Gaussian diffusion equation, Bai et al. [19] modeled the potential dispersion patterns of airborne bacteria from livestock housing. Their data demonstrated that airborne bacteria could propagate along the wind direction for distances exceeding 10 km. Collectively, these studies indicate that numerical simulation techniques grounded in atmospheric dispersion theory have emerged as a critical methodological framework for elucidating the dispersion dynamics of bioaerosols.
This study selected a WWTP employing multistage anaerobic-anoxic-oxic (A/A/O) and membrane bioreactor (MBR) processes as the research target. Sampling points were strategically established across different treatment units and at varying distances along the upwind/downwind directions of the aerobic tanks (AerT) to collect aerosol samples and detect ARGs. The investigation evaluated the influence of environmental factors on ARG dissemination and focused on characterizing horizontal dispersion patterns of aerosol-borne ARGs, with the subsequent development of dispersion models to quantitatively describe these patterns. Additionally, the study also conducted exposure risk assessments for personnel operating in key operational units.
2. Materials and Methods
2.1. Sampling Strategy
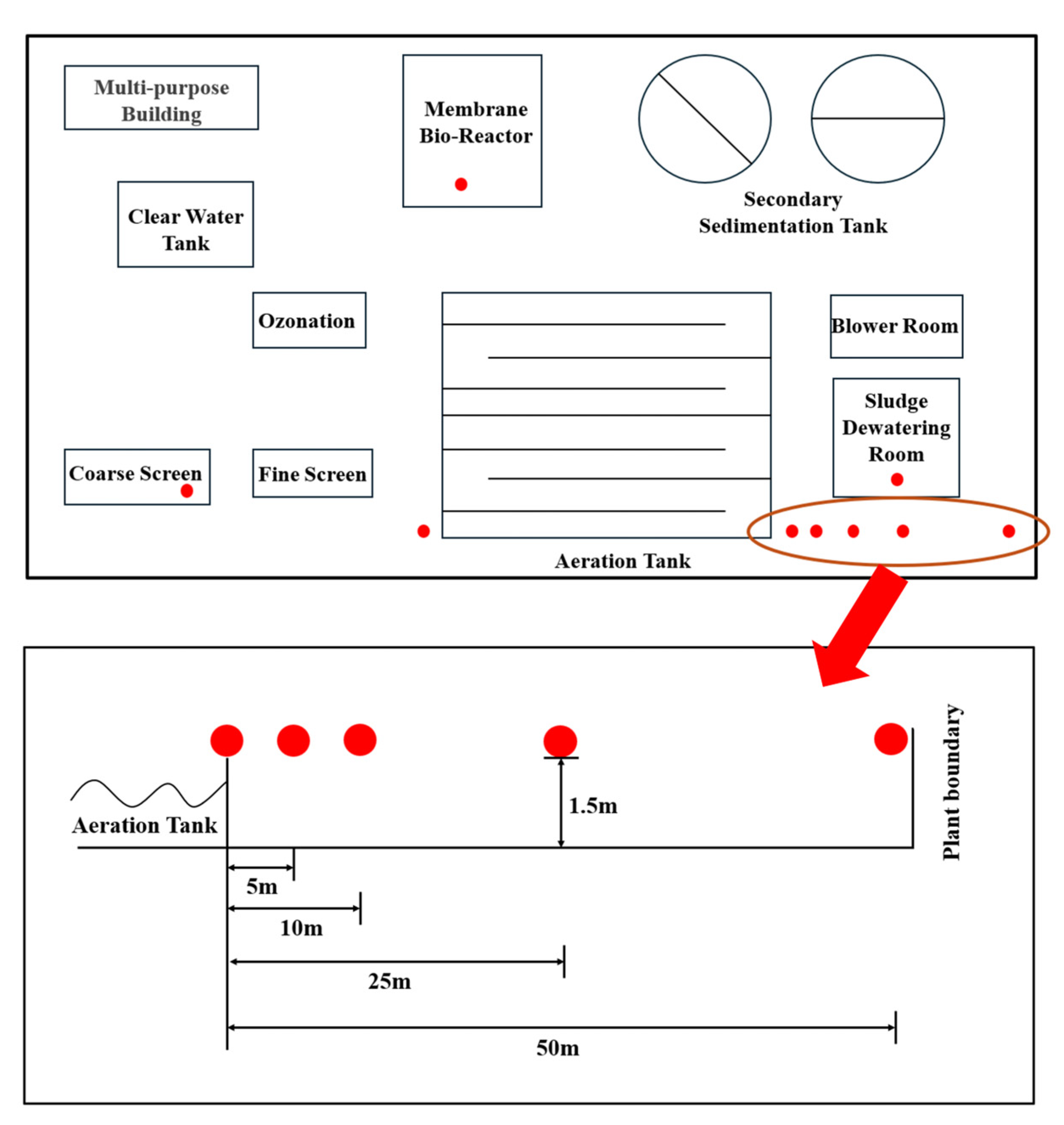
This study selected a municipal WWTP in Beijing employing a multistage A/A/O process coupled with MBR. The facility occupies 6.19 hectares, serves a catchment area of 70 km2 with a population of 100,000 residents, and processes 70,000 tons of municipal wastewater daily. The sampling was conducted from 8 to 12 November 2021. Specific details regarding the sampling schedule are provided in Supplementary Text S1. Sampling sites were selected at 0 m upwind and 0, 5, 10, 25, 50 m downwind of the AerT, along with the coarse screen (CS), MBR, and sludge dewatering room (SDR). The facility layout with geospatial sampling configuration is illustrated in Figure 1, while comprehensive sampling metadata are tabulated in Table S1.
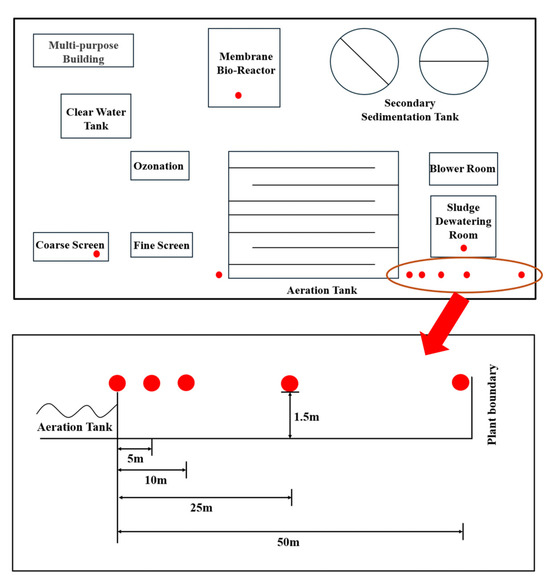
Figure 1.
Schematic diagram of the wastewater treatment plant layout and sampling points.
2.2. Sample Collection and Pre-Treatment
The meteorological conditions during the sampling period were as follows: clear sky, mean temperature of 5 °C, average wind speed of 1.3 m/s, and relative humidity of 45%. The TH-150 medium-flow air sampler (Wuhan, China) was deployed for total suspended particulate (TSP) collection at 100 L/min, utilizing 90 mm quartz fiber filters. To achieve optimal particulate loading while preserving microbial integrity, a 24 h sampling regimen was implemented across all sites, incorporating sterile filter replacement every 4 h [14,20]. Filter housings underwent disinfection with 75% ethanol prior to installation, with samplers positioned at 1.5 m elevation to mimic respiratory exposure zones. Post-sampling, filters were cryopreserved at −20 °C in sterilized containers. The collected filters were subsequently centrifuged and resuspended for enrichment in the laboratory following the pretreatment methodology described by the study [21].
2.3. DNA Extraction and Metagenomic Sequencing
The total biomass of composite samples was extracted using the MO-BIO Power Soil DNA Isolation Kit (MO BIO laboratories, Inc., Carlsbad, CA, USA). Nucleic acid purity and concentration were determined through micro-spectrophotometry (NanoDrop ND-1000, NanoDrop Technologies, Wilmington, DE, USA). DNA fragmentation was performed using Covaris M220 (Covaris, Inc., Woburn, MA, USA), followed by size selection of 400 bp fragments. Sequencing libraries were constructed with the NEXTFLEX Rapid DNA-Seq (Bioo Scientific, Austin, TX, USA) and subjected to metagenomic sequencing on the Illumina NovaSeq/HiSeq X Ten platform (Illumina, San Diego, CA, USA). ARG annotation was performed by aligning amino acid sequences from non-redundant gene catalogs against the CARD using DIAMOND software (http://www.diamondsearch.org (accessed on 28 May 2016), version 0.8.3) with BLASTP parameters set at an e-value threshold of 1 × 10−5 [10,22,23]. Functional abundance of ARGs was subsequently calculated based on the cumulative abundance of corresponding annotated resistance genes [24,25]. The metagenomic sequencing data related to this project have been archived in the NCBI Short Read Archive database (Accession number: PRJNA961672).
2.4. qPCR Quantification
The real-time quantitative PCR (qPCR) standard curve was constructed using recombinant plasmids harboring the 16S rRNA gene, which underwent 10-fold serial dilutions to generate a concentration gradient ranging from 10−2 to 10−7. The amplification protocol comprised an initial denaturation step at 95 °C for 3 min, followed by denaturation at 95 °C for 5 s, annealing at 58 °C for 30 s, and extension at 72 °C for 1 min. The melting curve was studied after 40 cycles. Reaction mixtures were aliquoted into 96-well plates according to the predefined protocol and analyzed using the ABI7300 fluorescence qPCR instrument (Thermo Fisher Scientific, Waltham, MA, USA) [26].
2.5. Atmospheric Dispersion Model
The dispersion of airborne bacteria along the wind direction was simulated using a Gaussian plume model [19,27,28,29]. Atmospheric stability was determined via the Pasquill classification scheme based on wind speed and solar radiation. The dispersion model is expressed as Equation (1).
The dispersion model represented by Equation (1) was calibrated to account for site-specific configurations of the WWTP and real-time meteorological conditions recorded during sampling. Detailed derivation procedures for these modifications are provided in Text S2, yielding the calibrated formulation designated as follows in Equation (2):
In the model formulation, C represents the pollutant concentration (copies/m3), x denotes the horizontal distance between the sampling point and emission source (m), y corresponds to the horizontal distance of the measurement point from the central line (m), He designates the effective emission height of the point source (m), Q0 quantifies the pollutant emission intensity (copies/s), u indicates the mean wind speed (m/s), σx and σz represent horizontal and vertical diffusion coefficients, respectively, z specifies the sampling height (m), I characterizes the environmental impact factor, λ defines the microbial decay coefficient, and t represents aerosol age—quantified as the ratio of propagation distance to wind speed. This study employs Equation (2) for dispersion modeling.
The environmental impact factor (I) and microbial decay coefficient (λ) were determined based on meteorological conditions during sampling and reference values from Supplementary Table S2. Horizontal (σx) and vertical (σz) diffusion coefficients were derived from meteorological conditions and stability using Pasquill-Gifford (P-G) curves (Supplementary Figure S1). Based on the above conditions, the following parameters were obtained: I = 0.210, λ = −0.004, σx = 0.425806x0.901074, σz = 1.12154x0.0799904, Q0 = 2.31 × 107 copies/s. The model validation protocol is detailed in Supplementary Text S3.
2.6. Exposure Assessment
A non-carcinogenic risk assessment model was employed to evaluate public health risks posed by aerosolized microorganisms and associated ARGs [18,19]. Exposure and health risk assessment can be conducted using the model developed by the United States Environmental Protection Agency (US EPA), which comprises two primary exposure pathways: respiration of aerosol-borne ARGs (ADDrespiration) and skin contact with aerosol-borne ARGs (ADDskin). The computational models for estimating exposure doses through these two pathways are presented in Equations (3) and (4).
Exposure dose calculations were performed using the following parameters: C denotes the abundance of aerosol-borne ARGs (copies/m3), IR represents the respiration rate (m3/d), EF quantifies the exposure frequency (d/a), ET indicates the exposure duration (a), BW designates the average body weight (kg), AT characterizes the averaging time for non-carcinogenic exposure (d), SA specifies the skin contact area (m2), and PC defines the permeability coefficient (m/h).
The study population was stratified by gender into male and female subgroups. Parameter values were determined according to the Exposure Factors Handbook of Chinese Population (Adults Volume), as detailed in Table S3.
2.7. Statistical Analysis
Statistical analyses were performed using SPSS 26.0 (SPSS Inc., Chicago, IL, USA). Percentage bar charts and line graphs were generated with Origin 9.0 (OriginLab, Northampton, MA, USA). Heatmap visualization and redundancy analysis (RDA) were implemented using the vegan package in R software (Version 4.0.2). Atmospheric dispersion modeling was conducted through MATLAB R2021a.
3. Results and Discussion
3.1. Distribution Characteristics of Aerosol-Borne Bacteria and ARGs in Different Functional Zones
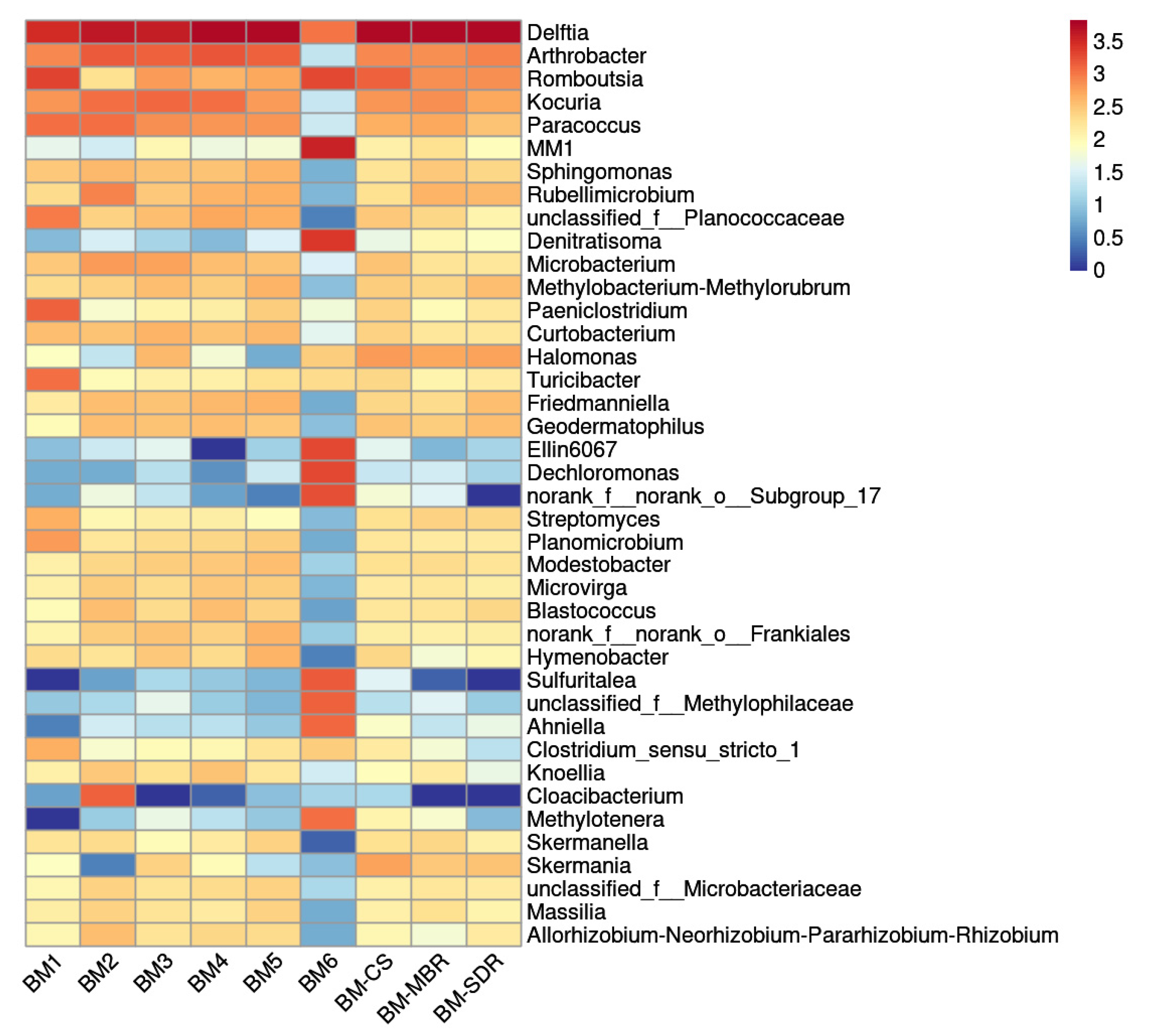
The bacterial community structures of aerosol-borne bacteria at the genus level across all sampling sites were analyzed using high-throughput sequencing, as illustrated in Figure 2.
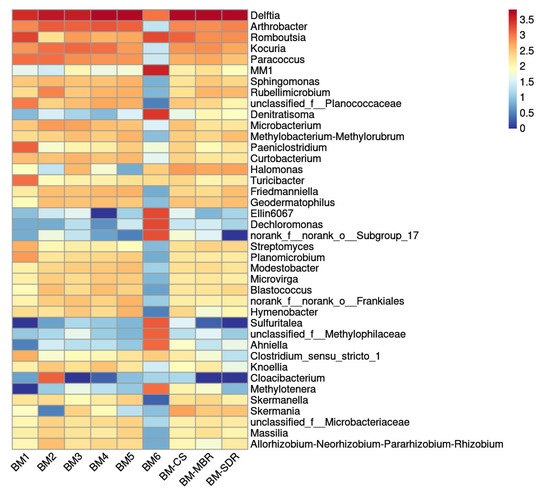
Figure 2.
Genus-level community composition of aerosol-borne bacteria in the WWTP.
Delftia predominated at all sampling sites, exhibiting absolute dominance. Romboutsia and Arthrobacter were significantly enriched in specific process units, including the AerT, MBR, CS, and SDR, where they emerged as dominant taxa. Notably, distinct aggregation of specialized genera such as MM1, Denitratisoma, Ellin6067, and Sulfuritalea was detected 50 m downwind of the AerT. These taxa exhibited relatively low abundances at other sampling sites, particularly in the SDR. The bacterial community compositions of aerosols collected at 0 m, 5 m, 10 m, and 25 m downwind of the AerT showed high similarity (Figure 2), indicating low spatial heterogeneity and significant convergence in the microbial community structure within this proximity range, along with high stability [30]. In contrast, the community composition at 50 m downwind diverged markedly from these proximal sites, with most genera displaying reduced abundances. This divergence may be attributed to the attenuation and restructuring of microbial communities during aerosol particle transport in outdoor environments, driven by the combined effects of meteorological factors such as wind speed, air temperature, and relative humidity over increasing diffusion distances [1].
The composition of ARGs in different bacterial communities exhibited significant variation, leading to spatial heterogeneity in ARG distribution across environmental samples [31].
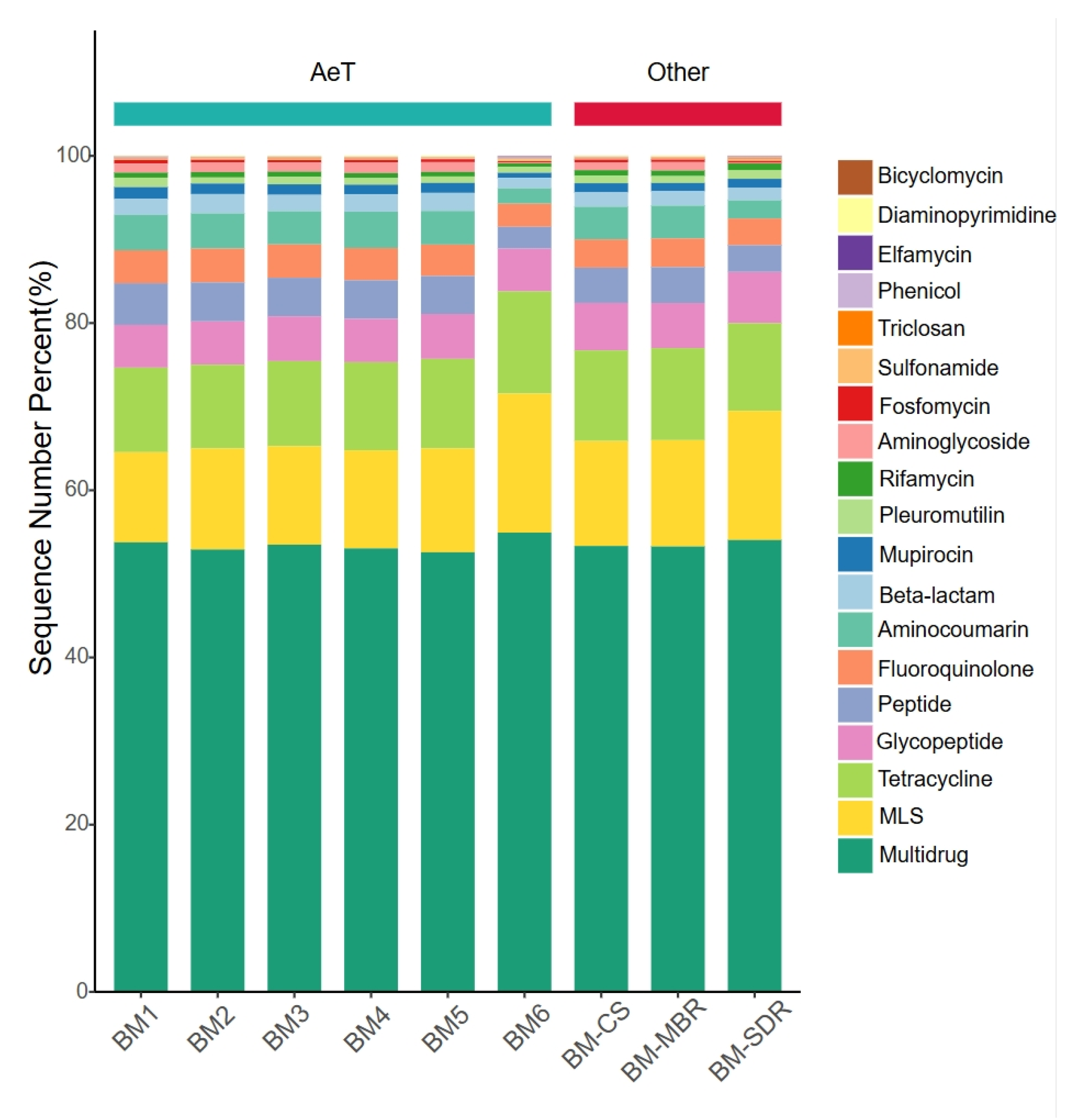
As shown in Figure 3, aerosol-borne ARG types (>1%) in the WWTP displayed highly similar compositional profiles across all sampling sites. Multidrug resistance genes dominated with an average relative abundance of 52%, followed by tetracycline (11%), MLS (10%), and glycopeptide (7%) resistance genes. This distribution pattern aligns with findings from the study [32] on aerosol-borne ARGs in a large-scale WWTP in Hong Kong and corroborates the ubiquitous enrichment of multidrug resistance genes in both aerial and aquatic environments reported by global studies. This phenomenon may be attributed to the widespread presence of multidrug antibiotics in environmental matrices, which exert co-selective pressure on microbial communities, driving the emergence of multidrug ARGs through adaptive mechanisms such as mutation [33,34]. Mechanistic studies reveal that multidrug ARGs frequently encode P-glycoprotein-like efflux pumps capable of expelling hydrophobic cytotoxic compounds, thereby conferring cross-resistance to multiple antibiotic classes and posing heightened risks to public health [35].
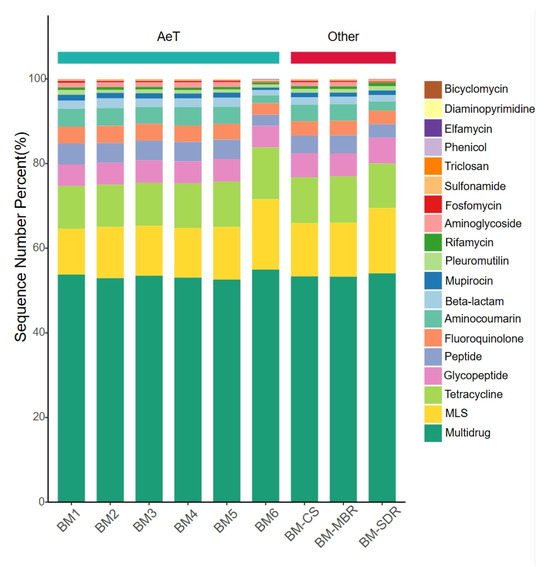
Figure 3.
Distribution of ARG types across sampling sites.
3.2. Effects of Environmental Factors on the Abundance of Aerosol-Borne ARGs
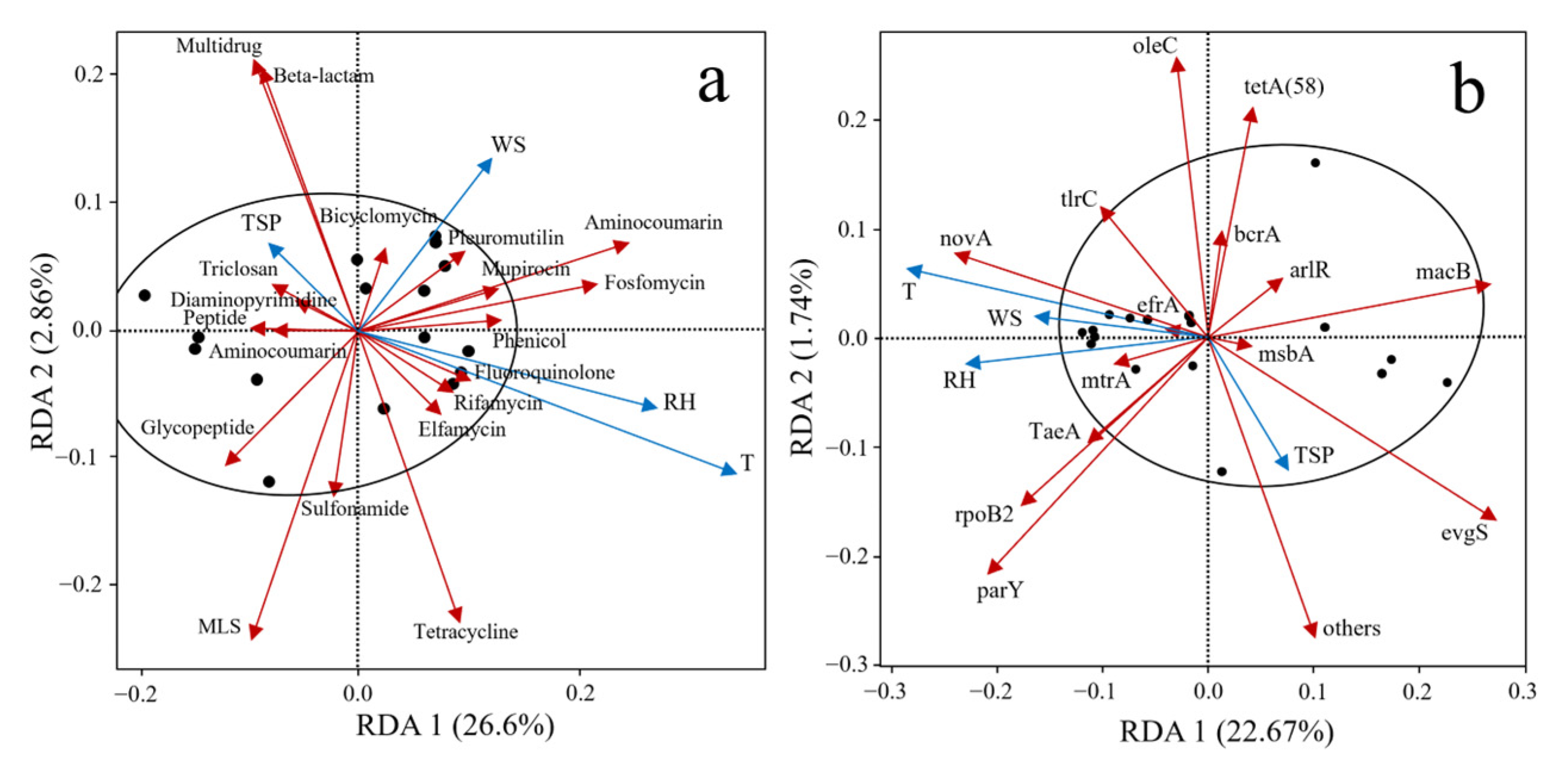
The relationships between the abundance of aerosol-borne ARGs and four environmental factors—temperature (T), relative humidity (RH), wind speed (WS), and total suspended particulate (TSP) concentration—in the WWTP were investigated, as illustrated in Figure 4.
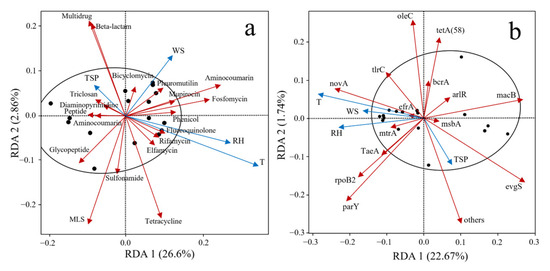
Figure 4.
RDA analysis of ARGs through aerosols and environmental factors. (a) ARG types, (b) ARG subtypes.
Figure 4a reveals significant correlations between different classes of ARGs and environmental factors. The abundances of multidrug ARGs and β-lactam ARGs exhibited significant positive correlations with TSP concentration and wind speed, with TSP concentration showing the most pronounced influence on their distribution. In contrast, the abundances of aminocoumarin, fosfomycin, and fluoroquinolone ARGs were positively associated with temperature, relative humidity, and wind speed. Among these factors, wind speed emerged as the primary driver for the distribution of aminocoumarin and fosfomycin resistance genes, while temperature and relative humidity predominantly regulated the abundances of fluoroquinolone and tetracycline ARGs. Furthermore, this study investigated the relationships between the top 15 subtype ARGs (based on abundance) and environmental factors (Figure 4b). Temperature, relative humidity, and wind speed showed positive correlations with subtype ARGs, such as novA, tlrC, mtrA, TaeA, rpoB2, and parY, while TSP concentration was identified as the dominant factor influencing evgS.
As shown in Figure 4, whether for ARG types or ARG subtypes, the primary environmental factors influencing ARG distribution across samples were temperature, relative humidity, and wind speed. Notably, temperature exerted the strongest impact on ARG abundance variations, while TSP concentration exhibited a relatively minor influence. The study revealed that as ambient temperature increases within a certain range, the plasmid-mediated conjugation frequency correspondingly elevates, thereby enhancing the horizontal transfer rate of ARGs and consequently influencing their dissemination abundance levels across different environments [36]. Furthermore, elevated air humidity intensifies the interaction frequency between exogenous chemical substances and airborne bacteria. This process affects the atmospheric dispersion concentration of ARGs through oxidative inactivation of ARG-carrying bacteria [37]. Van et al. [28] demonstrated that higher wind speeds accelerate the dilution of aerosol-borne ARGs within a short period, subsequently reducing localized concentrations. The aerosol-borne ARG abundance exhibits a positive correlation with TSP concentration, as heightened TSP levels provide greater attachment surface area, increased microbial attachment density, and extended survival duration for host microorganisms carrying ARGs [38,39].
This study demonstrates that environmental factors play a pivotal role in the dissemination of ARGs. Temperature emerged as the most significant determinant influencing the abundance of both ARG types and ARG subtypes, whereas TSP concentration exhibited a comparatively minor influence. These findings provide a critical theoretical foundation for predicting the environmental dissemination risks of antibiotic resistance.
3.3. Horizontal Dissemination Characteristics of Aerosol-Borne ARGs
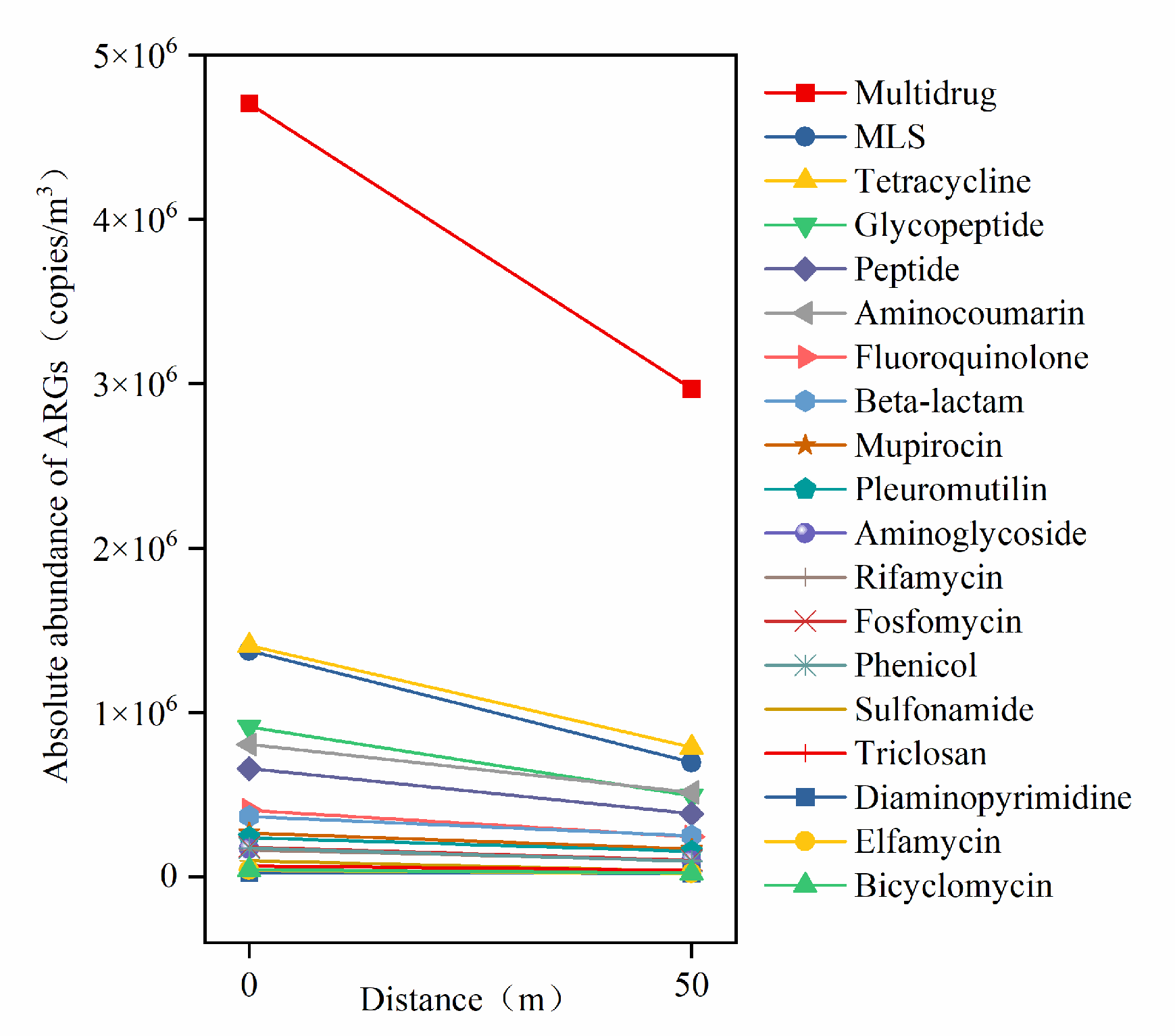
The AerT in the WWTP were selected as the emission source, and six sampling points were established at 0 m upwind and 0 m, 5 m, 10 m, 25 m, and 50 m downwind of the tanks for aerosol sample collection. To investigate the horizontal dissemination characteristics of ARGs, the relationship between the absolute abundance of ARG types and downwind distance is illustrated in Figure 5.
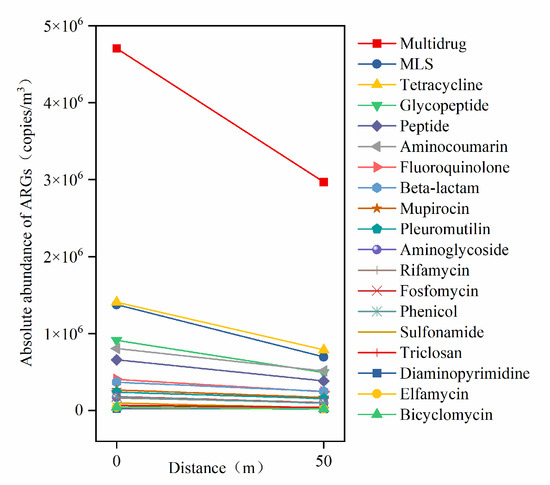
Figure 5.
Linear relationship between the absolute abundance of ARG types and downwind distance from aeration tank.
Multidrug ARGs, which exhibited the highest absolute abundance, showed the fastest attenuation rate (3.5 × 104 copies/m3/m) downwind, followed by tetracycline and MLS resistance genes. The absolute abundances of tetracycline and MLS ARGs were comparable, though the attenuation rate of MLS ARGs was slightly higher than that of tetracycline ARGs but significantly lower than multidrug ARGs. In contrast, ARGs with lower absolute abundances—such as triclosan, sulfonamide, and diaminopyrimidine resistance genes—displayed the slowest attenuation rates downwind. These findings indicate that the horizontal attenuation rates of ARGs are strongly associated with their emission abundance, with higher initial abundances correlating to faster attenuation rates.
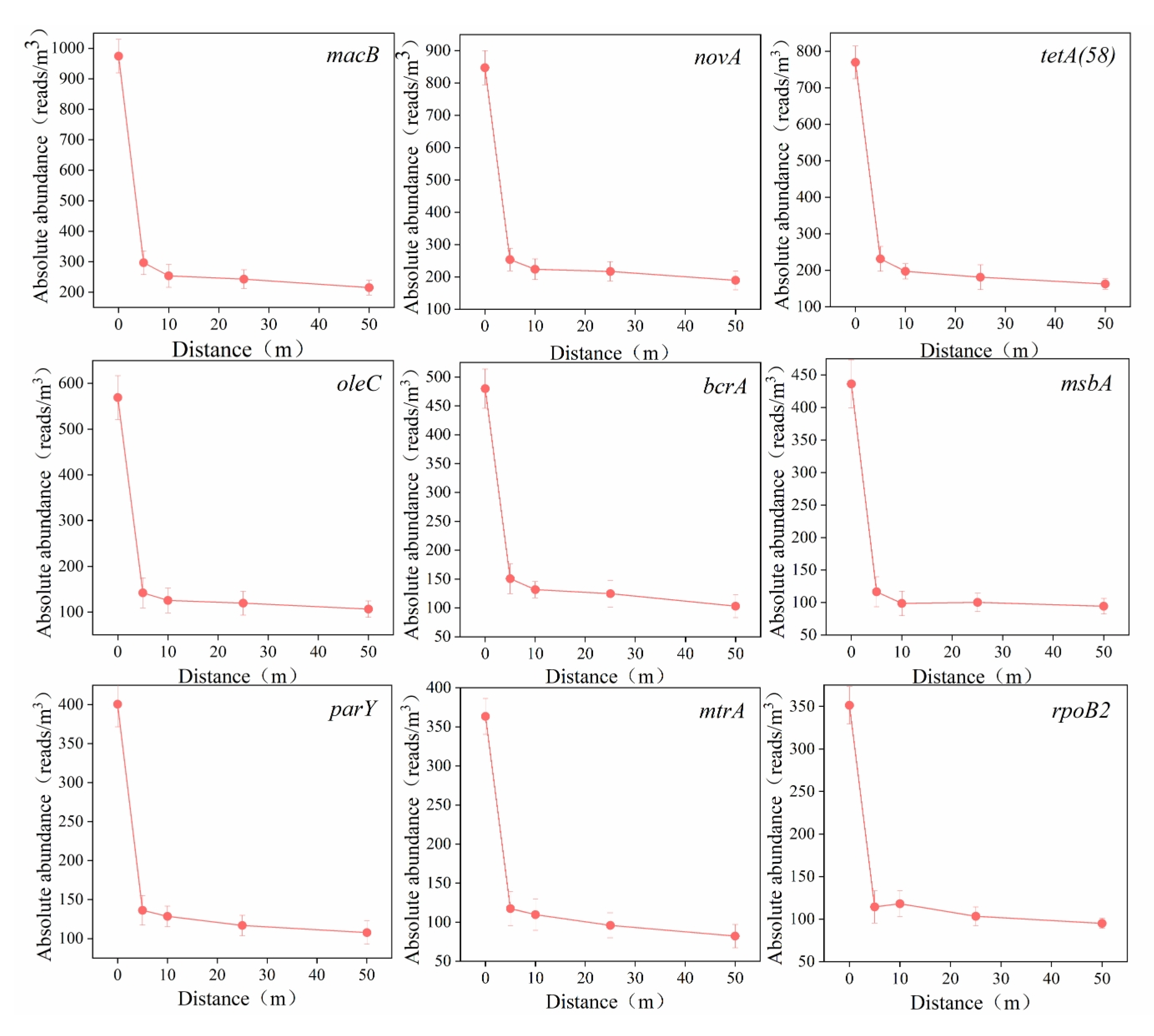
To clarify the horizontal dissemination characteristics of ARG subtypes, the nine most abundant subtype ARGs (macB, novA, tetA(58), oleC, bcrA, msbA, parY, mtrA, and rpoB2) were selected, and their variations in absolute abundance across horizontal distances are shown in Figure 6.
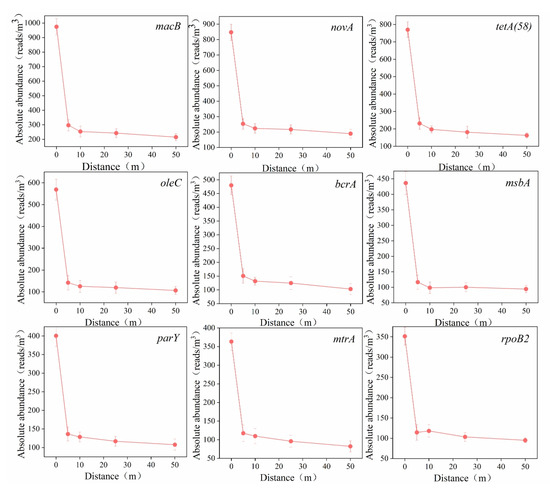
Figure 6.
Variation trend in ARG subtypes’ absolute abundance in downwind horizontal distance of aeration tank.
The nine ARG subtypes exhibited similar attenuation trends: a rapid decline within short distances, with the highest attenuation rate (42.54%) observed within 5 m, followed by gradually reduced decay rates, eventually entering a slow-decay phase. These findings suggest that the decay rates of ARG subtype abundances progressively decrease with increasing horizontal distance. Bai et al. [19] investigated horizontal dissemination patterns of ARGs emitted from poultry and dairy farms, reporting analogous trends: the absolute abundance of resistance bacteria decreased by approximately 95% within 20 m for poultry farms and 90% for dairy farms. Studies have demonstrated that the purines and pyrimidines in DNA can strongly absorb ultraviolet (UV) radiation, inducing DNA strand breaks and consequently triggering significant degradation of aerosol-borne ARGs under UV irradiation [40]. Following their release from AerT, the ARGs carried by aerosols are rapidly photodegraded by ambient UV radiation, resulting in a sharp decline in concentration within the initial 10 m range downstream of AerT.
In this study, multiple ARG subtypes displayed comparable attenuation trends, implying that ARG subtypes may share conserved horizontal dissemination mechanisms. Based on these observations, predictive models could be developed to characterize and forecast the horizontal dissemination dynamics of ARGs, enabling validation of their spatial abundance distribution.
3.4. Dissemination Patterns of Aerosol-Borne ARGs
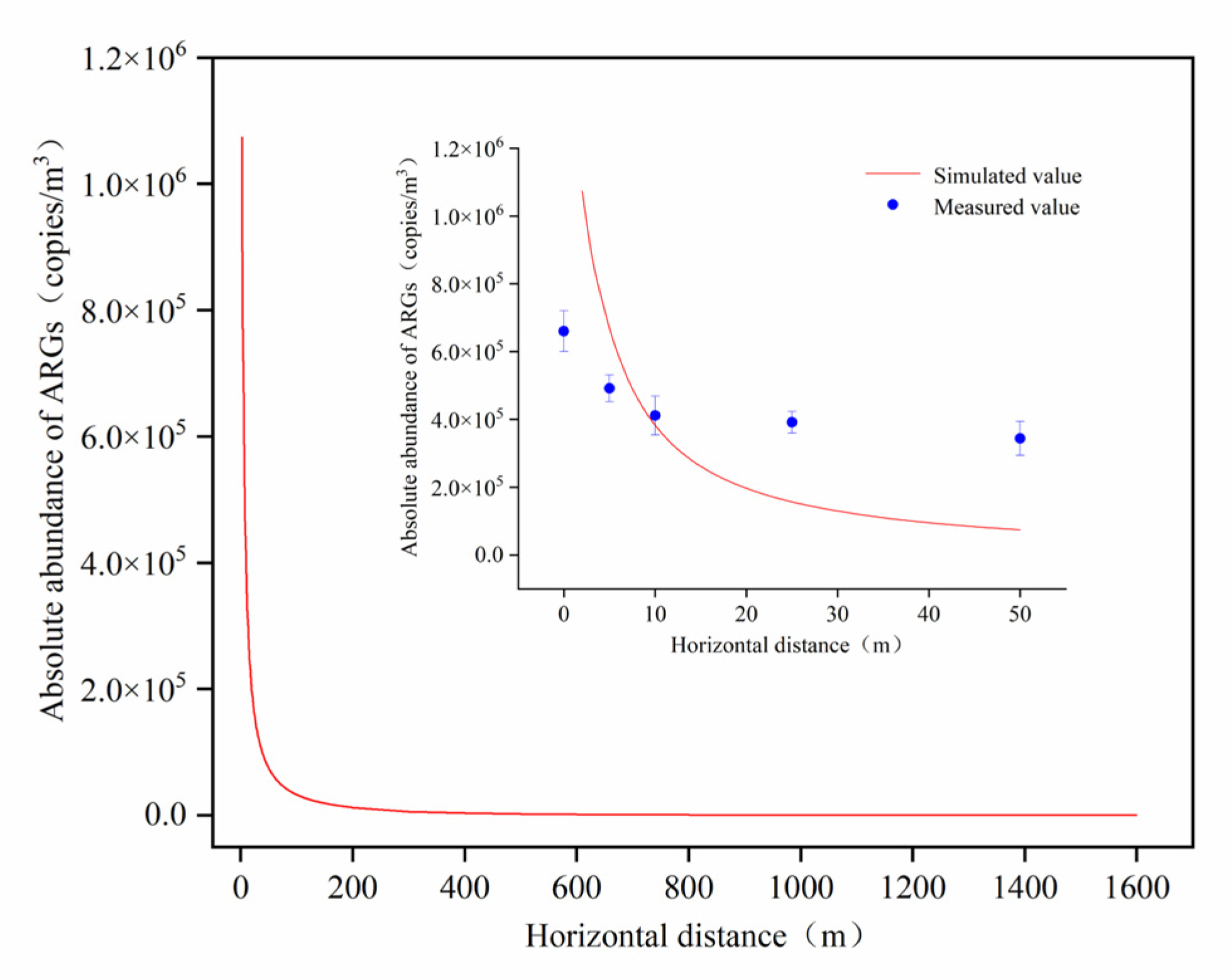
The horizontal dissemination of aerosol-borne ARGs was modeled using a modified Gaussian plume approach, with visualization results presented in Figure 7.
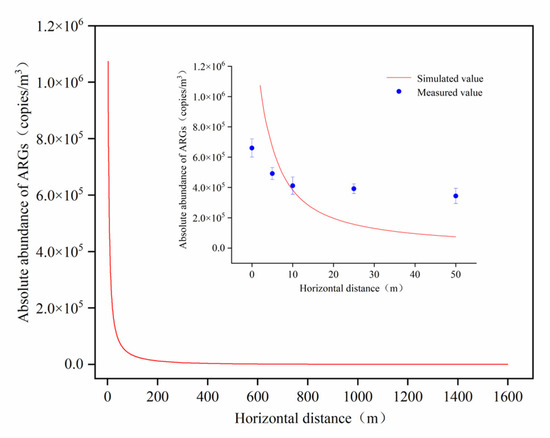
Figure 7.
Simulation and actual monitoring values of ARG abundance diffusion downwind of aeration tank.
While the simulated data generally reflected the downwind dispersion patterns of ARGs, deviations from field-monitored data increased with distance. Three key factors likely contributed to these discrepancies. Firstly, during the dispersal process of aerosol-borne ARGs, the suspended aerosol particles, acting as discrete particulate entities, may undergo deposition and adsorption behaviors, potentially leading to uncertainties in the diffusion process [41]. Secondly, in this study, although aerosol-borne ARGs in downwind areas primarily originated from the aeration tank, other potential sources (e.g., soil surfaces and phyllosphere) may also contribute to the atmospheric ARGs abundance through microbial release and associated gene dissemination [42]. Finally, aerosol-borne ARGs and co-transported microorganisms may exhibit distinct decay rates during atmospheric diffusion. Additionally, different ARG types could demonstrate varying attenuation velocities in air parcels due to differential environmental stress-resistance capacities. Current limitations in empirical data regarding ARGs’ decay coefficients and their interactions with environmental factors suggest that using microbial attenuation parameters as proxies for ARG persistence may introduce systematic bias. Therefore, the dispersal processes of aerosol-borne ARGs and their underlying mechanisms warrant in-depth investigation.
The modified Gaussian plume model revealed that aerosolized ARGs emitted from aeration tanks could be dispersed by wind over distances exceeding 1500 m. Given that the observed attenuation rates of ARGs were slower than model simulations, the actual dissemination range of ARGs may extend further, posing heightened potential health risks to WWTP workers and nearby residents. Numerous studies have demonstrated the long-range transmission of ARGs via aerosols. For instance, Bai et al. [19] applied a modified Gaussian plume model to simulate the dispersal of ARG-carrying bacteria from poultry and dairy farms, showing their transport to areas beyond 10 km. Wang et al. [43] identified atmospheric PM2.5 originating from the Yellow Sea as a key contributor to ARGs detected in PM2.5 samples in Handan City during summer, highlighting the role of cross-regional atmospheric transport in sustaining ARG levels. Similarly, Gaviria-Figueroa et al. [44] modeled the dispersal patterns of ARGs emitted from WWTP aeration tanks, estimating an emission rate of 10,620 copies/hour for targeted ARGs, with 83% attributed to subtypes QnrS, IMP12, and VEB. Their simulations predicted that even under minimal wind speeds, 2.2 × 105 copies of target ARGs could disseminate beyond 10 km and 5 × 10⁴ copies beyond 80 km after 24 h. These findings collectively underscore the critical need to address the long-distance dissemination of aerosol-borne ARGs as a significant environmental and public health concern.
The modified Gaussian plume model can generally characterize the downwind dissemination patterns of aerosol-borne ARGs; however, deviations between simulated data and empirical observations intensify with increasing distance. The model predicts that ARGs emitted from AerT can spread beyond 1500 m, yet the monitored attenuation rates of ARGs are lower than simulated values, implying an extended potential dissemination range. This discrepancy suggests a significantly elevated exposure risk for populations in the surroundings of WWTPs.
3.5. Exposure Risk Assessment of Aerosol-Borne ARGs
As emerging contaminants, the accumulation and widespread dissemination of ARGs in the environment have significantly increased the risk of human exposure through multiple pathways. Studies indicate that approximately 57% of PM2.5-associated ARGs in urban areas may originate from WWTPs [32]. Currently, the primary threat of ARGs to human health lies in the hazards posed by pathogenic bacteria carrying ARGs. These pathogens, once entering the human body and causing infections, often reduce susceptibility to clinical antibiotic treatments or render them ineffective, leading to higher morbidity and mortality rates [45]. Compared to ARGs in water and soil, aerosol-borne ARGs pose elevated health risks due to their ability to penetrate directly into the respiratory system [46].
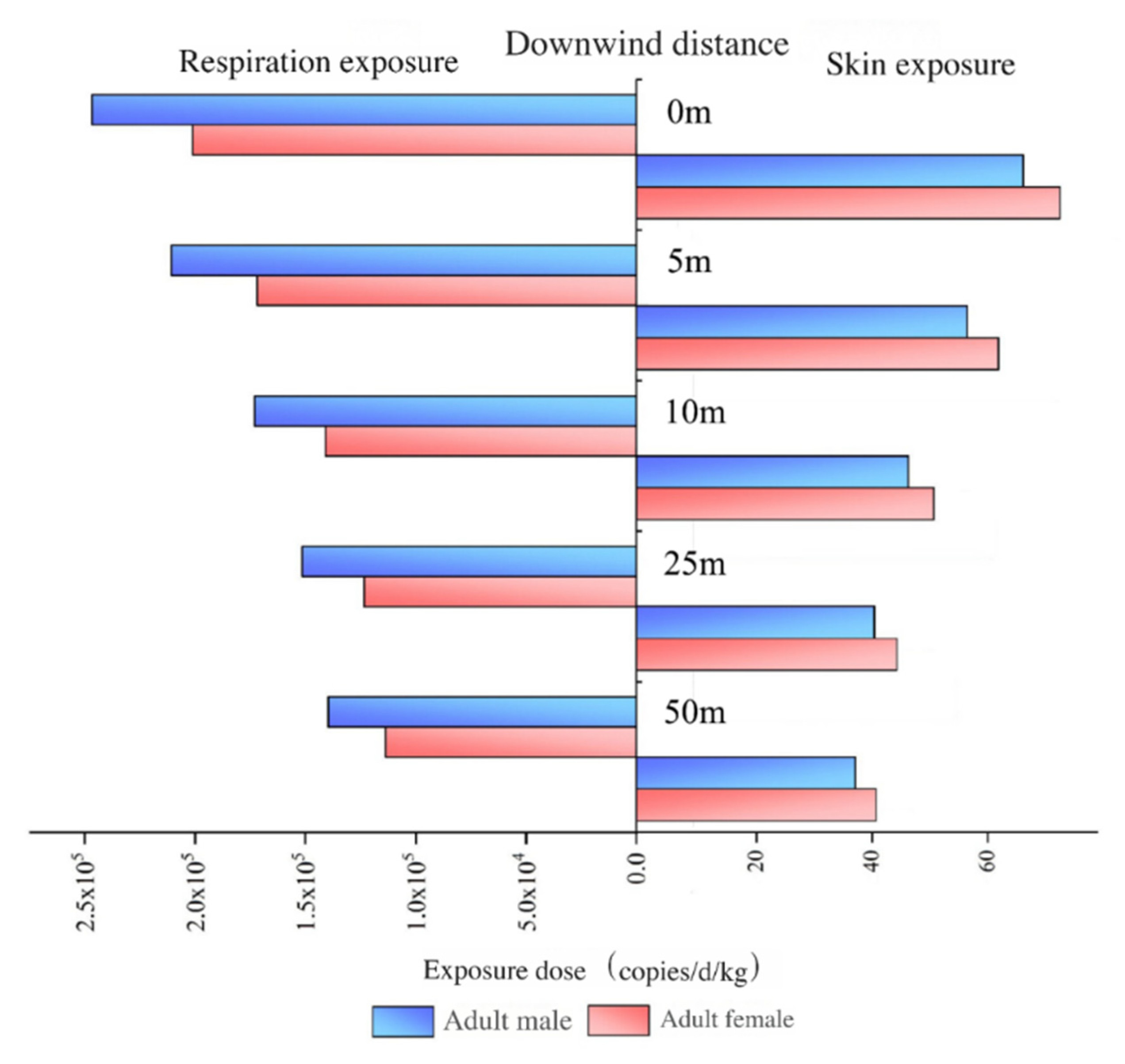
Aerosol-borne microbes and ARGs are generally classified as non-carcinogenic agents, with primary exposure pathways including respiration and skin contact. The estimated exposure doses of aerosol-borne ARGs at varying downwind distances from the aeration tanks in WWTPs are illustrated in Figure 8.
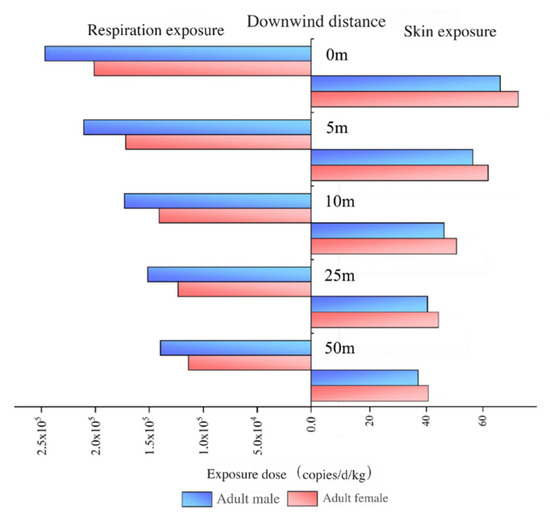
Figure 8.
Exposure doses of ARGs through aerosol for different distances downwind of aeration tanks.
As shown in Figure 8, the exposure doses of aerosol-borne ARGs via both respiration and skin contact pathways decreased with increasing downwind distance from the aeration tanks. When the distance increased from 0 m to 50 m, the respiration exposure dose for adult males declined from 2.47 × 105 copies/d/kg to 1.40 × 105 copies/d/kg, while the skin exposure dose decreased from 66.92 copies/d/kg to 37.86 copies/d/kg. For adult females, the respiration exposure dose decreased from 2.01 × 105 copies/d/kg to 1.14 × 105 copies/d/kg, and the skin exposure dose declined from 73.24 copies/d/kg to 41.44 copies/d/kg. Compared to the emission source (aeration tanks), the exposure doses of aerosol-borne ARGs at 50 m downwind decreased by 43.32% (respiration contact) and 43.43% (skin contact). These results demonstrate that the attenuation of aerosol-borne ARG abundances downwind directly drives the reduction in exposure doses with increasing distance.
Additionally, the exposure doses of aerosol-borne ARGs for different population groups across various treatment units in the WWTP were quantitatively assessed, as summarized in Table 1.

Table 1.
Exposure doses of aerosol-borne ARGs in various treatment units of the WWTP.
As summarized in Table 1, for the respiration pathway, the exposure doses of aerosol-borne ARGs for adult males across the various treatment units in the WWTP ranged from 2.11 × 105 to 1.38 × 106 copies/d/kg, while those for adult females ranged from 1.72 × 105 to 1.12 × 106 copies/d/kg. The exposure doses for adult males were consistently higher than those for adult females, aligning with findings reported by the study in [47]. This discrepancy primarily stems from higher respiratory rates and shorter average lifespans in males compared to females. For the skin contact pathway, the exposure doses of aerosol-borne ARGs ranged from 57.37 to 373.37 copies/d/kg for adult males and 62.78 to 408.61 copies/d/kg for adult females, indicating slightly higher exposure doses for adult females—a trend contrasting with the respiration pathway results. Notably, of the four treatment units analyzed, the highest exposure doses were observed in the SDR, while the lowest occurred in the MBR tank. This spatial variation is attributed to the enclosed indoor environment of the SDR, which experiences limited air exchange with the external atmosphere. The sludge treatment unit operates under relatively confined conditions, resulting in restricted dispersion and dilution of microbial aerosols. During sludge sedimentation and centrifugal dewatering processes, substantial amounts of microorganisms are released into the enclosed space. These aerosols cannot be effectively dispersed or diluted, leading to the accumulation of high-concentration aerosol-borne ARGs [26].
Both within wastewater treatment units and downwind areas, the exposure doses of aerosol-borne ARGs via respiration pathways were substantially higher than those via skin contact, with respiration doses exceeding skin contact by 3–4 orders of magnitude, consistent with prior studies. Wang et al. [47] quantified ARG exposure doses for diverse populations across six atmospheric environments, including WWTPs and hospitals, revealing that respiration exposure exceeded skin contact by 3–4 orders of magnitude, thus identifying respiration as the dominant exposure route for aerosol-loaded ARGs. Consequently, many studies have disregarded skin contact as a negligible contributor to overall exposure [19,47]. However, it is critical to emphasize that higher exposure doses do not directly equate to elevated health risks. The health risks from ARGs primarily depend on their host pathogens, particularly their pathogenicity, colonization capacity, and invasive potential. Furthermore, uncertainties in horizontal gene transfer (HGT) dynamics complicate the accurate assessment of ARG-associated health risks.
4. Conclusions
This study demonstrates that WWTPs are significant sources of ARGs in ambient air, with the potential for long-distance atmospheric transport. Across WWTP treatment units, aerosol-borne ARGs exhibited high compositional similarity, dominated by multidrug ARGs (average relative abundance: 52%), followed by tetracycline (11%), MLS (10%), and glycopeptide ARGs (7%). For ARG types, multidrug and β-lactam ARGs showed positive correlations with TSP concentration and wind speed, while aminocoumarin, fosfomycin, and fluoroquinolone resistance genes were positively associated with temperature, humidity, and wind speed. At the subtype level, temperature, relative humidity, and wind speed correlated positively with novA, tlrC, mtrA, TaeA, rpoB2, and parY, whereas TSP concentration was the primary factor influencing evgS. Horizontal attenuation rates of aerosol-borne ARGs were inversely proportional to their emission abundance. For example, multidrug ARGs (highest abundance) exhibited the fastest attenuation, followed by tetracycline and MLS resistance genes. ARG subtypes displayed similar attenuation trends, characterized by a rapid initial decline over short distances (e.g., ≤5 m) followed by a slow-decay phase, with attenuation rates progressively decreasing with distance. Modified Gaussian plume model simulations predicted that ARGs emitted from aeration tanks could disperse beyond 1500 m, with actual ranges likely to be greater, posing elevated health risks to WWTP workers and adjacent residents. Exposure risk assessments revealed gender-specific patterns. Adult males had higher respiration exposure doses but lower skin contact doses compared to females, with respiration doses exceeding skin contact by 3–4 orders of magnitude. These findings provide critical scientific evidence for optimizing ARG control strategies in wastewater treatment processes, offering significant implications for occupational health, community safety, and ecological security.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/w17091305/s1, Text S1. Details of the Sampling Schedule. Text S2. Modification of Gaussian plume models. Text S3. Model Validation. Table S1. Description of Sample Collection at the Wastewater Treatment Plant. Table S2. Microbial Attenuation Coefficient. Table S3. Calculation parameter of the model for exposure dose. Figure S1. P-G curves.
Author Contributions
Conceptualization, J.L. and J.S.; methodology, J.L., J.S. and M.C.; formal analysis, M.C.; investigation, D.D. and M.C.; resources, J.L.; data curation, D.D., L.L. and Z.R.; writing—original draft, D.D.; writing—review and editing, D.D., M.C. and J.L.; visualization, D.D., J.S. and M.C.; supervision, J.L.; project administration, J.L.; funding acquisition, J.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
Data are contained within the article and Supplementary Materials.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| WWTPs | Wastewater treatment plants |
| A/A/O | Anaerobic-anoxic-oxic |
| ARGs | Antibiotic resistance genes |
| TSP | Total suspended particulate |
| ARB | Antibiotic-resistant bacteria |
| AerT | Aerobic tank |
| CS | Coarse screen |
| MBR | Membrane bioreactor |
| SDR | Sludge dewatering room |
References
- Wang, Y.; Han, Y.P.; Li, L.; Liu, J.X.; Yan, X. Distribution, sources, and potential risks of antibiotic resistance genes in wastewater treatment plant: A review. Environ. Pollut. 2022, 310, 11. [Google Scholar] [CrossRef] [PubMed]
- Zhi, S.L.; Shen, S.Z.; Zhou, J.; Ding, G.Y.; Zhang, K.Q. Systematic analysis of occurrence, density and ecological risks of 45 veterinary antibiotics: Focused on family livestock farms in Erhai Lake basin, Yunnan, China. Environ. Pollut. 2020, 267, 14. [Google Scholar] [CrossRef] [PubMed]
- Cheng, D.M.; Feng, Y.; Liu, Y.W.; Li, J.P.; Xue, J.M.; Li, Z.J. Quantitative models for predicting adsorption of oxytetracycline, ciprofloxacin and sulfamerazine to swine manures with contrasting properties. Sci. Total Environ. 2018, 634, 1148–1156. [Google Scholar] [CrossRef] [PubMed]
- Zielinski, W.; Korzeniewska, E.; Harnisz, M.; Drzymala, J.; Felis, E.; Bajkacz, S. Wastewater treatment plants as a reservoir of integrase and antibiotic resistance genes—An epidemiological threat to workers and environment. Environ. Int. 2021, 156, 13. [Google Scholar] [CrossRef]
- Yang, T.; Wang, X.Y.; Hui, X.L.; Jiang, L.; Bi, X.J.; Ng, H.Y.; Zheng, X.; Huang, S.J.; Jiang, B.; Zhou, X.L. Antibiotic resistome associated with inhalable bioaerosols from wastewater to atmosphere: Mobility, bacterial hosts, source contributions and resistome risk. Water Res. 2023, 243, 12. [Google Scholar] [CrossRef]
- Yang, T.; Wang, X.Y.; Ng, H.Y.; Huang, S.J.; Zheng, X.; Bi, X.J. Airborne antibiotic resistome from sludge dewatering systems: Mobility, pathogen accessibility, cross-media migration propensity, impacting factors, and risks. Water Res. 2024, 267, 14. [Google Scholar] [CrossRef]
- Tavares, R.D.S.; Fidalgo, C.; Rodrigues, E.T.; Tacao, M.; Henriques, I. Integron-associated genes are reliable indicators of antibiotic resistance in wastewater despite treatment- and seasonality-driven fluctuations. Water Res. 2024, 258, 12. [Google Scholar] [CrossRef]
- Yin, X.L.; Li, L.G.; Chen, X.; Liu, Y.Y.; Lam, T.T.Y.; Topp, E.; Zhang, T. Global environmental resistome: Distinction and connectivity across diverse habitats benchmarked by metagenomic analyses. Water Res. 2023, 235, 10. [Google Scholar] [CrossRef]
- Ju, F.; Beck, K.; Yin, X.L.; Maccagnan, A.; McArdell, C.S.; Singer, H.P.; Johnson, D.R.; Zhang, T.; Bürgmann, H. Wastewater treatment plant resistomes are shaped by bacterial composition, genetic exchange, and upregulated expression in the effluent microbiomes. ISME J. 2019, 13, 346–360. [Google Scholar] [CrossRef]
- Guo, J.H.; Li, J.; Chen, H.; Bond, P.L.; Yuan, Z.G. Metagenomic analysis reveals wastewater treatment plants as hotspots of antibiotic resistance genes and mobile genetic elements. Water Res. 2017, 123, 468–478. [Google Scholar] [CrossRef]
- Yu, K.F.; Li, P.; He, Y.L.; Zhang, B.; Chen, Y.H.; Yang, J.H. Unveiling dynamics of size-dependent antibiotic resistome associated with microbial communities in full-scale wastewater treatment plants. Water Res. 2020, 187, 14. [Google Scholar] [CrossRef]
- Han, Y.P.; Yang, T.; Yan, X.; Li, L.; Liu, J.X. Effect of aeration mode on aerosol characteristics from the same wastewater treatment plant. Water Res. 2020, 170, 11. [Google Scholar] [CrossRef]
- Yang, T.; Jiang, L.; Bi, X.J.; Cheng, L.H.; Zheng, X.; Wang, X.D.; Zhou, X.L. Submicron aerosols share potential pathogens and antibiotic resistomes with wastewater or sludge. Sci. Total Environ. 2022, 821, 11. [Google Scholar] [CrossRef]
- Yang, T.; Jiang, L.; Cheng, L.H.; Zheng, X.; Bi, X.J.; Wang, X.D.; Zhou, X.L. Characteristics of size-segregated aerosols emitted from an aerobic moving bed biofilm reactor at a full-scale wastewater treatment plant. J. Hazard. Mater. 2021, 416, 11. [Google Scholar] [CrossRef]
- Burdsall, A.C.; Xing, Y.; Cooper, C.W.; Harper, W.F. Bioaerosol emissions from activated sludge basins: Characterization, release, and attenuation. Sci. Total Environ. 2021, 753, 7. [Google Scholar] [CrossRef]
- Osinska, A.; Jachimowicz, P.; Niestepski, S.; Harnisz, M.; Korzeniewska, E. The Effects of Season and Processing Technology on the Abundance of Antibiotic Resistance Genes in Air Samples from Municipal Wastewater Treatment and Waste Management Plants. Environ. Prot. Eng. 2021, 47, 101–114. [Google Scholar] [CrossRef]
- Nguyen, T.M.N.; IIef, D.; Jarraud, S.; Rouil, L.; Campese, C.; Che, D.; Haeghebaert, S.; Ganiayre, F.; Marcel, F.; Etienne, J. A community-wide outbreak of Legionnaires disease linked to industrial cooling towers: How far can contaminated aerosols spread? J. Infect. Dis. 2006, 193, 102–111. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, H.; Qiu, X.; Zhang, Y.; Wang, H. Dispersion and risk assessment of bacterial aerosols emitted from rotating-brush aerator during summer in a wastewater treatment plant of Xi’an, China. Aerosol Air Qual. Res. 2013, 13, 1807–1814. [Google Scholar] [CrossRef]
- Bai, H.; He, L.-Y.; Wu, D.-L.; Gao, F.-Z.; Zhang, M.; Zou, H.-Y.; Yao, M.-S.; Ying, G.-G. Spread of airborne antibiotic resistance from animal farms to the environment: Dispersal pattern and exposure risk. Environ. Int. 2022, 158, 106927. [Google Scholar] [CrossRef]
- Yang, T.; Jiang, L.; Han, Y.P.; Liu, J.W.; Wang, X.D.; Yan, X.; Liu, J.X. Linking aerosol characteristics of size distributions, core potential pathogens and toxic metal(loid)s to wastewater treatment process. Environ. Pollut. 2020, 264, 12. [Google Scholar] [CrossRef]
- Jiang, W.; Liang, P.; Wang, B.; Fang, J.; Lang, J.; Tian, G.; Jiang, J.; Zhu, T.F. Optimized DNA extraction and metagenomic sequencing of airborne microbial communities. Nat. Protoc. 2015, 10, 768–779. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Guo, W.Q.; Luo, H.C.; Xing, C.M.; Wang, H.Z.; Liu, B.H.; Si, Q.S.; Ren, N.Q. Deciphering the transfers of antibiotic resistance genes under antibiotic exposure conditions: Driven by functional modules and bacterial community. Water Res. 2021, 205, 11. [Google Scholar] [CrossRef]
- Zhao, X.; Wang, Z.M.; Xu, T.; Feng, Z.H.; Liu, J.; Luo, L.; He, Y.; Xiao, Y.L.; Peng, H.; Zhang, Y.Z.; et al. The fate of antibiotic resistance genes and their influential factors during excess sludge composting in a full-scale plant. Bioresour. Technol. 2021, 342, 10. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Li, B.; Ju, F.; Zhang, T. Exploring Variation of Antibiotic Resistance Genes in Activated Sludge over a Four-Year Period through a Metagenomic Approach. Environ. Sci. Technol. 2013, 47, 10197–10205. [Google Scholar] [CrossRef] [PubMed]
- Zhao, R.X.; Yu, K.; Zhang, J.Y.; Zhang, G.J.; Huang, J.; Ma, L.P.; Deng, C.F.; Li, X.Y.; Li, B. Deciphering the mobility and bacterial hosts of antibiotic resistance genes under antibiotic selection pressure by metagenomic assembly and binning approaches. Water Res. 2020, 186, 15. [Google Scholar] [CrossRef]
- Tian, H.Y.; Liu, J.W.; Sun, J.B.; Zhang, Y.X.; Li, T.G. Cross-media migration behavior of antibiotic resistance genes (ARGs) from municipal wastewater treatment systems (MWTSs): Fugitive characteristics, sharing mechanisms, and aerosolization behavior. Sci. Total Environ. 2023, 893, 13. [Google Scholar] [CrossRef]
- Pasalari, H.; Ataei-Pirkooh, A.; Aminikhah, M.; Jafari, A.J.; Farzadkia, M. Assessment of airborne enteric viruses emitted from wastewater treatment plant: Atmospheric dispersion model, quantitative microbial risk assessment, disease burden. Environ. Pollut. 2019, 253, 464–473. [Google Scholar] [CrossRef]
- Van Leuken, J.; Swart, A.; Havelaar, A.; Van Pul, A.; Van der Hoek, W.; Heederik, D. Atmospheric dispersion modelling of bioaerosols that are pathogenic to humans and livestock–A review to inform risk assessment studies. Microb. Risk Anal. 2016, 1, 19–39. [Google Scholar] [CrossRef]
- He, P.; Zheng, B.; Zheng, J. Urban PM_(2.5) Diffusion Analysis Based on the Improved Gaussian Smoke Plume Model and Support Vector Machine. Aerosol Air Qual. Res. 2018, 18, 3177–3186. [Google Scholar] [CrossRef]
- Yang, F.; Gao, Y.; Zhao, H.; Li, J.; Cheng, X.; Meng, L.; Dong, P.; Yang, H.; Chen, S.; Zhu, J. Revealing the distribution characteristics of antibiotic resistance genes and bacterial communities in animal-aerosol-human in a chicken farm: From one-health perspective. Ecotoxicol. Environ. Saf. 2021, 224, 112687. [Google Scholar] [CrossRef]
- Ma, L.; Xia, Y.; Li, B.; Yang, Y.; Li, L.-G.; Tiedje, J.M.; Zhang, T. Metagenomic assembly reveals hosts of antibiotic resistance genes and the shared resistome in pig, chicken, and human feces. Environ. Sci. Technol. 2016, 50, 420–427. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Jin, L.; Wu, D.; Pruden, A.; Li, X. Inhalable antibiotic resistome from wastewater treatment plants to urban areas: Bacterial hosts, dissemination risks, and source contributions. Environ. Sci. Technol. 2022, 56, 7040–7051. [Google Scholar] [CrossRef] [PubMed]
- He, P.; Wu, Y.; Huang, W.; Wu, X.; Lv, J.; Liu, P.; Bu, L.; Bai, Z.; Chen, S.; Feng, W. Characteristics of and variation in airborne ARGs among urban hospitals and adjacent urban and suburban communities: A metagenomic approach. Environ. Int. 2020, 139, 105625. [Google Scholar] [CrossRef] [PubMed]
- Osińska, A.; Harnisz, M.; Korzeniewska, E. Prevalence of plasmid-mediated multidrug resistance determinants in fluoroquinolone-resistant bacteria isolated from sewage and surface water. Environ. Sci. Pollut. Res. 2016, 23, 10818–10831. [Google Scholar] [CrossRef]
- McGrath, J.P.; Varshavsky, A. The yeast STE6 gene encodes a homologue of the mammalian multidrug resistance P-glycoprotein. Nature 1989, 340, 400–404. [Google Scholar] [CrossRef]
- Mishra, S.; Klümper, U.; Voolaid, V.; Berendonk, T.U.; Kneis, D. Simultaneous estimation of parameters governing the vertical and horizontal transfer of antibiotic resistance genes. Sci. Total Environ. 2021, 798, 149174. [Google Scholar] [CrossRef]
- Götz, A.; Lechner, M.; Mader, A.; von Bronk, B.; Frey, E.; Opitz, M. CsrA and its regulators control the time-point of ColicinE2 release in Escherichia coli. Sci. Rep. 2018, 8, 6537. [Google Scholar] [CrossRef]
- Kabelitz, T.; Ammon, C.; Funk, R.; Münch, S.; Biniasch, O.; Nübel, U.; Thiel, N.; Rösler, U.; Siller, P.; Amon, B.; et al. Functional relationship of particulate matter (PM) emissions, animal species, and moisture content during manure application. Environ. Int. 2020, 143, 105577. [Google Scholar] [CrossRef]
- Noda, J.; Tomizawa, S.; Takahashi, K.; Morimoto, K.; Mitarai, S. Air pollution and airborne infection with mycobacterial bioaerosols: A potential attribution of soot. Int. J. Environ. Sci. Technol. 2022, 19, 717–726. [Google Scholar] [CrossRef]
- Lee, B.U. Life comes from the air: A short review on bioaerosol control. Aerosol Air Qual. Res. 2011, 11, 921–927. [Google Scholar] [CrossRef]
- Wang, Y.; Lan, H.; Li, L.; Yang, K.; Qu, J.; Liu, J. Chemicals and microbes in bioaerosols from reaction tanks of six wastewater treatment plants: Survival factors, generation sources, and mechanisms. Sci. Rep. 2018, 8, 9362. [Google Scholar] [CrossRef] [PubMed]
- Lymperopoulou, D.S.; Adams, R.I.; Lindow, S.E. Contribution of vegetation to the microbial composition of nearby outdoor air. Appl. Environ. Microbiol. 2016, 82, 3822–3833. [Google Scholar] [CrossRef]
- Wang, Q.; Hou, Z.; Li, L.; Guo, S.; Liang, H.; Li, M.; Luo, H.; Wang, L.; Luo, Y.; Ren, H. Seasonal disparities and source tracking of airborne antibiotic resistance genes in Handan, China. J. Hazard. Mater. 2022, 422, 126844. [Google Scholar] [CrossRef] [PubMed]
- Gaviria-Figueroa, A.; Preisner, E.C.; Hoque, S.; Feigley, C.E.; Norman, R.S. Emission and dispersal of antibiotic resistance genes through bioaerosols generated during the treatment of municipal sewage. Sci. Total Environ. 2019, 686, 402–412. [Google Scholar] [CrossRef]
- Hernando-Amado, S.; Coque, T.M.; Baquero, F.; Martínez, J.L. Defining and combating antibiotic resistance from One Health and Global Health perspectives. Nat. Microbiol. 2019, 4, 1432–1442. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Jin, L.; Luo, X.; Zhao, Z.; Li, X. Seasonal disparities in airborne bacteria and associated antibiotic resistance genes in PM2. 5 between urban and rural sites. Environ. Sci. Technol. Lett. 2018, 5, 74–79. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, C.; Song, L. Distribution of antibiotic resistance genes and bacteria from six atmospheric environments: Exposure risk to human. Sci. Total Environ. 2019, 694, 133750. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).