Phytoremediation Potential of Four Native Plants in Soils Contaminated with Lead in a Mining Area
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area Description
2.2. Experimental Materials
2.2.1. Test Soil
2.2.2. Test Plants
2.3. Experimental Design
2.4. Indexes and Methods for Determination
2.4.1. Treatment and Determination of Soil Samples
- (1)
- Treatment of soil samples. The soil samples were air-dried, crushed, and ground with wooden rods, sieved (<0.149 mm), and stored until measurement [14] of Pb concentrations following the method outlined in GB/T 17141-1997.
- (2)
- Determination of soil samples. Soil samples were weighed, and weights were recorded (with an accuracy of one ten thousandth). Microwave digestion was used for pretreatment according to the USEPA method [15]. After mineralization, soil extracts were filtered (0.45 μm PTFE), diluted, and analyzed. Total Pb concentrations in the soil extracts were determined using an AAS Zeenit 700P atomic absorption spectrometer. The accuracy of the analytical procedure adopted for atomic absorption spectrometer analysis was checked by running standard solutions every 20 samples.
2.4.2. Treatment and Determination of Plant Samples
- (1)
- Treatment of plant samples. After harvesting, plant height, root length, and biomass were measured under different treatment conditions. Forty-eight samples of selected plants were further assigned to two sections (aboveground and underground organs) and thoroughly washed with tap water and deionized water. After weighing and recording the fresh weights, plant samples were placed in an oven at 105 ℃for approximately 15 min. Then, the temperature was adjusted to 75 ℃ until a constant plant weight was reached, which was taken as the dry weight (g) [4].
- (2)
- Determining the Pb concentrations of plant samples. Samples were milled using an agate mortar and sieved through a 0.25 mm nylon sieve. Weighed samples of approximately 0.1000 g were placed in a polytetrafluoroethylene digestion tank along with 65–68% nitric acid and 30% hydrogen peroxide for digestion. Plant samples were then acid-digested according to the USEPA method [14]. An AAS Zeenit 700P atomic absorption spectrometer was used to determine Pb concentrations in the aboveground and underground organs. Only analytical grade reagents were used for testing, with strict quality control [16].
2.5. Method for Evaluation of Coefficient
2.6. Data Processing
3. Results and Discussion
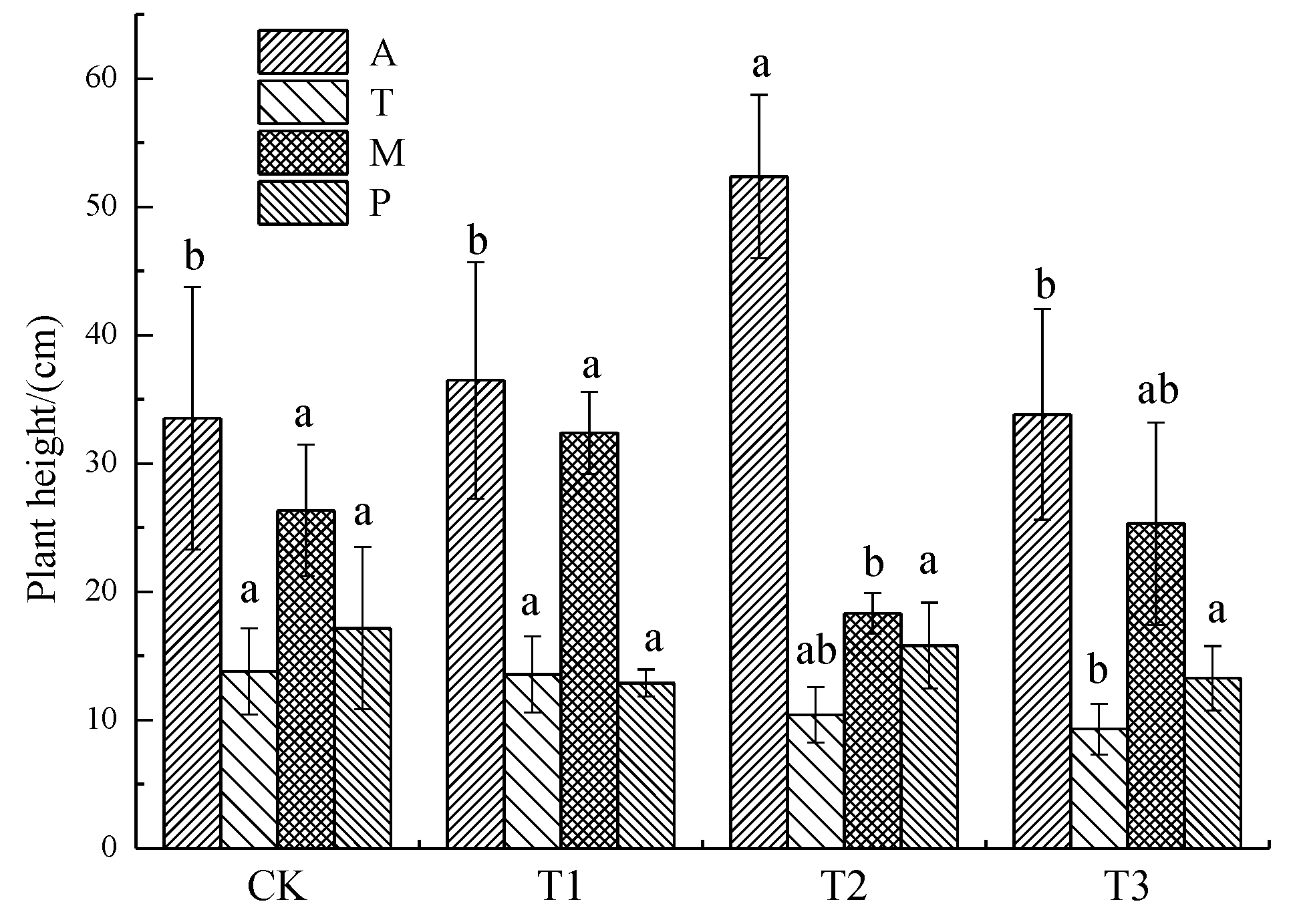
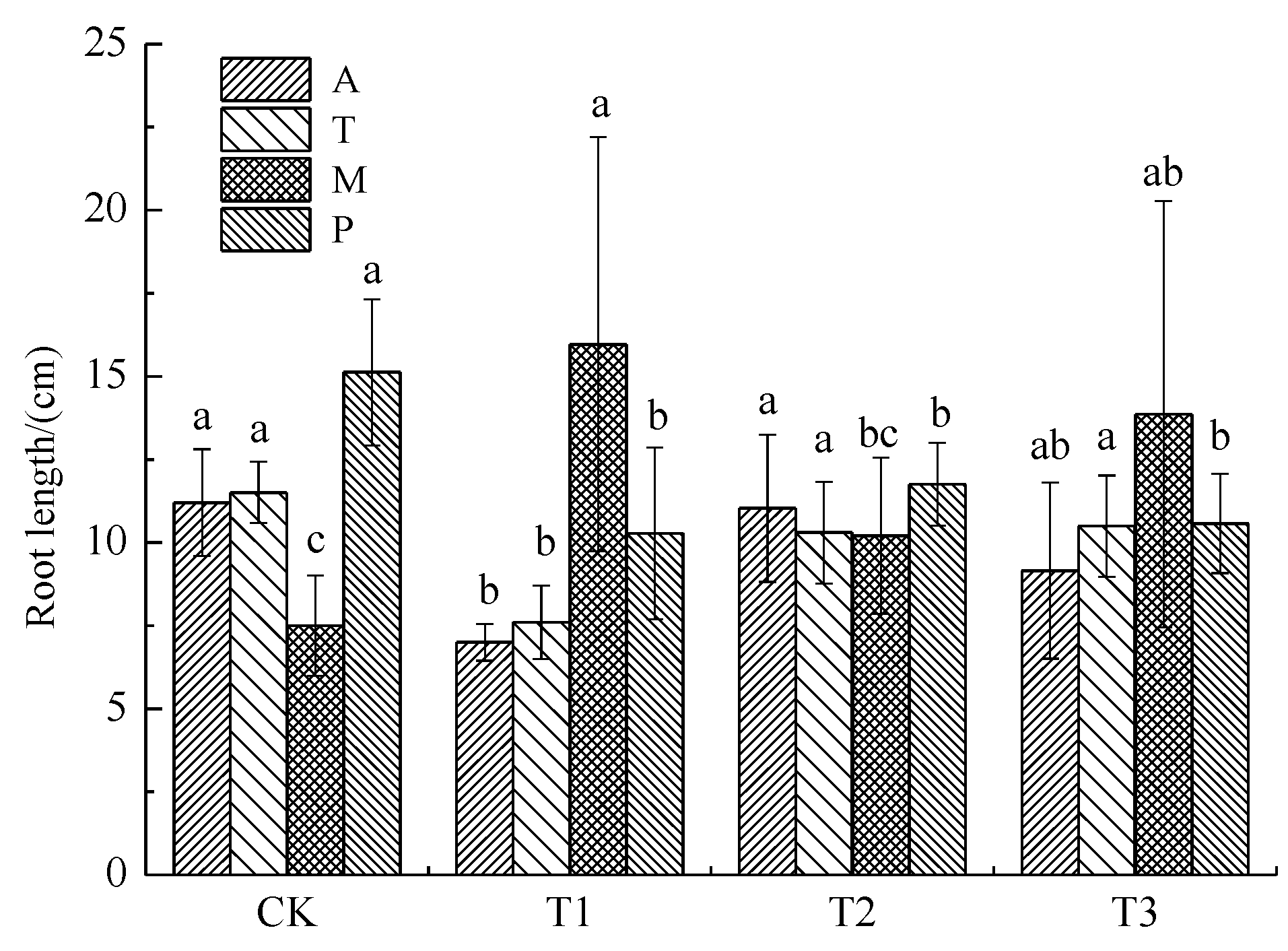
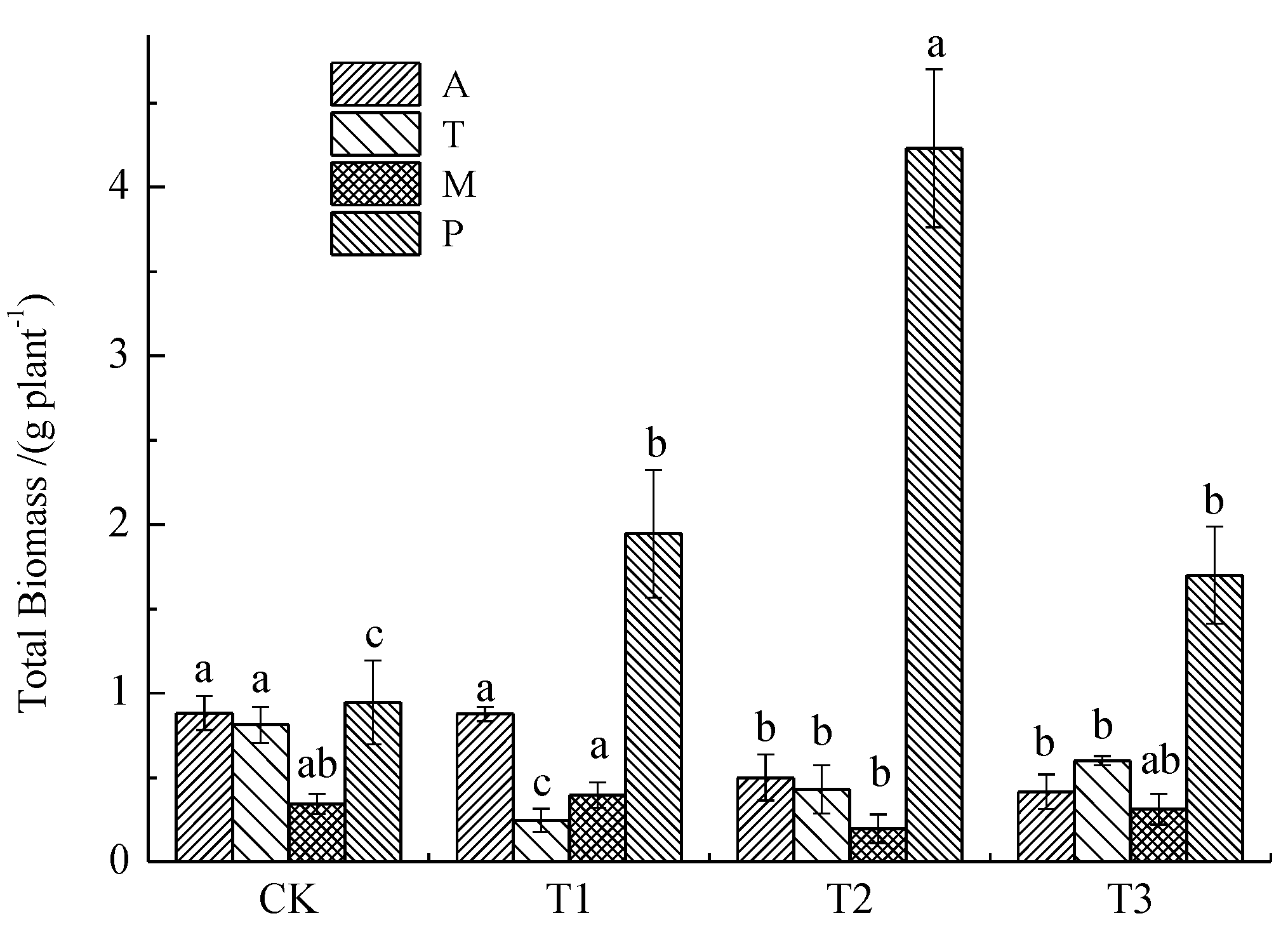
3.1. Effects of Soil Pb Pollution Level on Plant Growth
3.1.1. Plant Height
3.1.2. Root Length
3.1.3. Total Biomass
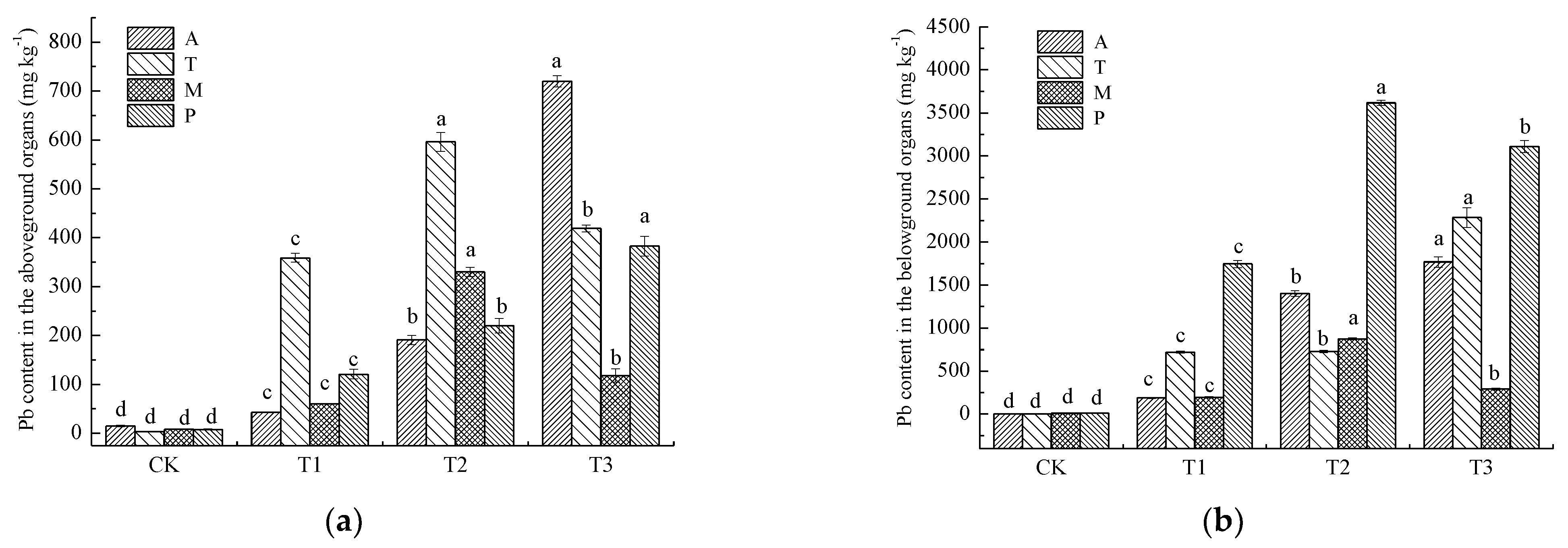
3.2. Pb Distributions and Concentrations in Various Plant Organs
3.3. Remediation Effects of Plants on Soil Pb
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sarwar, N.; Imran, M.; Shaheen, M.R.; Ishaque, W.; Kamran, M.A.; Matloob, A.; Rehim, A.; Hussain, S. Phytoremediation strategies for soils contaminated with heavy metals: Modifications and future perspectives. Chemosphere 2017, 171, 710–721. [Google Scholar] [CrossRef] [PubMed]
- Kumar, G.H.; Kumari, J.P. Heavy metal lead influative toxicity and its assessment in phytoremediating plants—A review. Water Air Soil Pollut. 2015, 226, 324. [Google Scholar] [CrossRef]
- Pourrut, B.; Shahid, M.; Dumat, C.; Winterton, P.; Pinelli, E. Lead uptake, toxicity, and detoxification in plants. In Reviews of Environmental Contamination and Toxicology; Whitacre, D., Ed.; Springer: New York, NY, USA, 2011; Volume 213, pp. 113–136. [Google Scholar]
- Luo, Y.; Tu, C. The research and development of technology for contaminated site remediation. In Twenty Years of Research and Development on Soil Pollution and Remediation in China; Springer: Singapore, Singapore, 2018; Volume Chapter 48, pp. 785–798. [Google Scholar]
- Wuana, R.A.; Okieimen, F.E. Heavy metals in contaminated soils: A review of sources, chemistry, risks and best available strategies for remediation. Int. Sch. Res. Not. 2011, 2011, 20. [Google Scholar] [CrossRef] [Green Version]
- Nouri, J.; Lorestani, B.; Yousefi, N.; Khorasani, N.; Hasani, A.H.; Seif, F.; Cheraghi, M. Phytoremediation potential of native plants grown in the vicinity of Ahangaran lead–zinc mine (Hamedan, Iran). Environ. Earth Sci. 2011, 62, 639–644. [Google Scholar] [CrossRef]
- Tseng, C.C.; Wang, J.Y.; Yang, L. Accumulation of copper, lead, and zinc by in situ plants inoculated with AM fungi in multicontaminated soil. Commun. Soil Sci. Plant Anal. 2009, 40, 3367–3386. [Google Scholar] [CrossRef]
- Reeves, R.D.; Brooks, R.R. Hyperaccumulation of lead and zinc by two metallophytes from mining areas of Central Europe. Environ. Pollut. Ser. A Ecol. Biol. 1983, 31, 277–285. [Google Scholar] [CrossRef]
- Kumar, P.N.; Dushenkov, V.; Motto, H.; Raskin, I. Phytoextraction: The use of plants to remove heavy metals from soils. Environ. Sci. Technol. 1995, 29, 1232–1238. [Google Scholar] [CrossRef]
- Salazar, M.J.; Pignata, M.L. Lead accumulation in plants grown in polluted soils. Screening of native species for phytoremediation. J. Geochem. Explor. 2014, 137, 29–36. [Google Scholar] [CrossRef] [Green Version]
- Malar, S.; Manikandan, R.; Favas, P.J.C.; Sahi, S.V.; Venkatachalam, P. Effect of lead on phytotoxicity, growth, biochemical alterations and its role on genomic template stability in Sesbania grandiflora: A potential plant for phytoremediation. Ecotoxicol. Environ. Saf. 2014, 108, 249–257. [Google Scholar] [CrossRef] [PubMed]
- Gupta, D.K.; Huang, H.G.; Corpas, F.J. Lead tolerance in plants: Strategies for phytoremediation. Environ. Sci. Pollut. Res. 2013, 20, 2150–2161. [Google Scholar] [CrossRef]
- Liu, D.; Li, S.; Islam, E.; Chen, J.R.; Wu, J.S.; Ye, Z.Q.; Peng, D.L.; Yan, W.B.; Lu, K.P. Lead accumulation and tolerance of Moso bamboo (Phyllostachys pubescens) seedlings: Applications of phytoremediation. J. Zhejiang Univ.-Sci. B 2015, 16, 123–130. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Y.Y.; Wang, J.; Gao, W.; Sun, X.J.; Xu, S.Y. Comprehensive analysis of heavy metals in soils from Baoshan District, Shanghai: A heavily industrialized area in China. Environ. Earth Sci. 2012, 67, 2331–2343. [Google Scholar] [CrossRef]
- US Environmental Protection Agency. Test Methods for Evaluating Solid Waste, Physical/Chemical Methods, SW-846, 3rd ed.; US Gov Print Office: Washington, DC, USA, 1995. [Google Scholar]
- Lu, N.; LI, G.; Hav, J.C.; Wang, H.Y.; Wei, Y.; Sun, Y.Y. Investigation of lead and cadmium contamination in mine soil and metal accumulation in selected plants growing in a gold mining area. Appl. Ecol. Environ. Res. 2019, 17, 10587–10597. [Google Scholar] [CrossRef]
- Bu-Olayan, A.H.; Thomas, B.V. Translocation and Bioaccumulation of Trace Metals in Desert Plants of Kuwait Governorates. Res. J. Environ. Sci. 2009, 3, 581–587. [Google Scholar] [CrossRef] [Green Version]
- Usman, K.; Al-Ghouti, M.A.; Abu-Dieyeh, M.H. The assessment of cadmium, chromium, copper, and nickel tolerance and bioaccumulation by shrub plant Tetraena qataranse. Sci. Rep. 2019, 9, 5658. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zayed, A.; Gowthaman, S.; Terry, N. Phytoaccumulation of trace elements by wetland plants: I. Duckweed. J. Environ. Qual. 1998, 27, 715–721. [Google Scholar] [CrossRef]
- Zhuang, P.; Yang, Q.W.; Wang, H.B.; Shu, W.S. Phytoextraction of heavy metals by eight plant species in the field. Water Air Soil Pollut. 2007, 184, 235–242. [Google Scholar] [CrossRef]
- Liu, Z.B.; Cai, J.; Wang, T.; Wu, L.T.; Chen, C.; Jiang, L.H.; Li, X.Y.; Zhang, W. Houttuynia cordata Hyperaccumulates Lead (Pb) and its Combination with Bacillus subtilis wb600 Improves Shoot Transportation. Int. J. Agric. Biol. 2018, 20, 621–627. [Google Scholar] [CrossRef]
- Shi, X.; Mee-Len, C. Arabidopsis ACBP1 over expressors are Pb(II)-tolerant and accumulate Pb(II). Plant Signal. Behav. 2008, 3, 693–694. [Google Scholar] [CrossRef] [Green Version]
- Islam, E.; Yang, X.; Li, T.Q.; Liu, D.; Jin, X.F.; Meng, F.H. Effect of Pb toxicity on root morphology, physiology and ultrastructure in the two ecotypes of Elsholtzia argyi. J. Hazard. Mater. 2007, 147, 806–816. [Google Scholar] [CrossRef]
- Shi, Y.; Huang, Z.B.; Liu, X.J.; Imran, S.Y.; Peng, L.C.; Dai, R.J.; Deng, Y.L. Environmental materials for remediation of soils contaminated with lead and cadmium using maize (Zea mays L.) growth as a bioindicator. Environ. Sci. Pollut. Res. 2016, 23, 6168–6178. [Google Scholar] [CrossRef] [PubMed]
- Chanu, L.B.; Gupta, A. Phytoremediation of lead using Ipomoea aquatica Forsk. in hydroponic solution. Chemosphere 2016, 156, 407–411. [Google Scholar] [CrossRef]
- Salazar, M.J.; Rodriguez, J.H.; Cid, C.V.; Bernardelli, C.E.; Donati, E.R.; Pignata, M.L. Soil variables that determine lead accumulation in Bidens pilosa L. and Tagetes minuta L. growing in polluted soils. Groderma 2016, 279, 97–108. [Google Scholar] [CrossRef]
- Shafigh, M.; Ghasemi-Fasaei, R.; Ronaghi, A. Influence of plant growth regulators and humic acid on the phytoremediation of lead by maize in a Pb-polluted calcareous soil. Arch. Agron. Soil Sci. 2016, 62, 1733–1740. [Google Scholar] [CrossRef]
- Jin, Y.; Wei, L.; Li, X.L.; Shen, S.G.; Liang, S.X.; Liu, C.Q.; Shan, L.Y. Nano-hydroxyapatite immobilized lead and enhanced plant growth of ryegrass in a contaminated soil. Ecol. Eng. 2016, 95, 25–29. [Google Scholar] [CrossRef]
- Kosobrukhov, A.; Knyazeva, I.; Mudrik, V. Plantago major plants responses to increase content of lead in soil: Growth and photosynthesis. Plant Growth Regul. 2004, 42, 145–151. [Google Scholar] [CrossRef]
- Pitchell, J.; Kuroiwa, K.; Sawyerr, H.T. Distribution of Pb, Cd, and Ba in soils and plants of two contaminated soils. Environ. Pollut. 1999, 110, 171–178. [Google Scholar] [CrossRef]
- Romeh, A.A.; Khamis, M.A.; Metwally, S.M. Potential of Plantago major L. for phytoremediation of Lead-contaminated soil and water. Water Air Soil Pollut. 2016, 227, 9. [Google Scholar] [CrossRef]
- Buscaroli, A. An overview of indexes to evaluate terrestrial plants for phytoremediation purposes. Ecol. Indic. 2017, 82, 367–380. [Google Scholar] [CrossRef]
- Gong, X.; Huang, D.; Liu, Y.; Zeng, G.; Wang, R.; Wei, J.; Huang, C.; Xu, P.; Wan, J.; Zhang, C. Pyrolysis and reutilization of plant residues after phytoremediation of heavy metals contaminated sediments: For heavy metals stabilization and dye adsorption. Bioresour. Technol. 2018, 253, 64–71. [Google Scholar] [CrossRef]

| Soil Samples | pH | Conductivity (dS·m−1) | Total Iron (mg·kg−1) | CaCO3 (%) | Soil Texture (USDA) |
| S1 | 9.23 | 0.359 | 1.42 | 11.21 | Silty loam |
| S2 | 9.11 | 0.433 | 1.13 | 10.23 | |
| S3 | 9.12 | 0.309 | 1.13 | 10.72 | |
| Mean ± SE | 9.15 ± 0.07 | 0.367 ± 0.062 | 1.23 ± 0.20 | 10.72 ± 0.49 | |
| Soil Samples | Organic Matter (g·kg−1) | Available Potassium (mg·kg−1) | Available Phosphorus (mg·kg−1) | TN (g·kg−1) | CEC (cmol·kg−1) |
| S1 | 6.12 | 92 | 3.30 | 0.404 | 3.88 |
| S2 | 5.59 | 79 | 3.50 | 0.375 | 3.49 |
| S3 | 5.48 | 86 | 2.90 | 0.401 | 3.70 |
| Mean ± SE | 5.73 ± 0.34 | 85.70 ± 6.51 | 3.23 ± 0.31 | 0.39 ± 0.02 | 3.69 ± 0.20 |
| Treatment | Target Content (‰) | Measured Content (‰) | Crops |
|---|---|---|---|
| CK | 0 | 0.06547 ± 0.00640 | A, T, M, and P |
| T1 | 2 | 2.23942 ± 0.08999 | |
| T2 | 3 | 3.25626 ± 0.14722 | |
| T3 | 5 | 5.10648 ± 0.10070 |
| Plant | Treatment | ||||
|---|---|---|---|---|---|
| CK | T1 | T2 | T3 | ||
| TF | A | 4.194 ± 0.426 a | 0.228 ± 0.014 b | 0.136 ± 0.010 b | 0.408 ± 0.016 b |
| T | 0.711 ± 0.050 b | 0.499 ± 0.010 c | 0.819 ± 0.020 a | 0.184 ± 0.007 d | |
| M | 0.759 ± 0.080 a | 0.310 ± 0.016 c | 0.377 ± 0.009 bc | 0.407 ± 0.045 b | |
| P | 0.593 ± 0.088 a | 0.092 ± 0.004 b | 0.069 ± 0.005 b | 0.061 ± 0.004 b | |
| BCF | Plant | Treatment | |||
|---|---|---|---|---|---|
| CK(Control) | T1 | T2 | T3 | ||
| roots | A | 0.160 ± 0.040 a | 0.121 ± 0.008 a | 0.539 ± 0.016 b | 0.509 ± 0.022 b |
| T | 0.248 ± 0.010 d | 0.349 ± 0.007 b | 0.303 ± 0.010 c | 0.474 ± 0.019 a | |
| M | 0.293 ± 0.030 b | 0.114 ± 0.006 c | 0.360 ± 0.014 a | 0.061 ± 0.002 d | |
| P | 0.462 ± 0.020 d | 1.062 ± 0.007 b | 1.430 ± 0.022 a | 0.931 ± 0.022 c | |
| stems | A | 0.662 ± 0.111 a | 0.028 ± 0.001 c | 0.074 ± 0.006 c | 0.207 ± 0.003 b |
| T | 0.177 ± 0.012 b | 0.174 ± 0.001 b | 0.248 ± 0.004 a | 0.087 ± 0.002 c | |
| M | 0.222 ± 0.019 a | 0.036 ± 0.001 c | 0.136 ± 0.007 b | 0.025 ± 0.003 c | |
| P | 0.274 ± 0.039 a | 0.074 ± 0.006 b | 0.087 ± 0.006 b | 0.086 ± 0.007 b | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, N.; Li, G.; Sun, Y.; Wei, Y.; He, L.; Li, Y. Phytoremediation Potential of Four Native Plants in Soils Contaminated with Lead in a Mining Area. Land 2021, 10, 1129. https://doi.org/10.3390/land10111129
Lu N, Li G, Sun Y, Wei Y, He L, Li Y. Phytoremediation Potential of Four Native Plants in Soils Contaminated with Lead in a Mining Area. Land. 2021; 10(11):1129. https://doi.org/10.3390/land10111129
Chicago/Turabian StyleLu, Nan, Gang Li, Yingying Sun, Yang Wei, Lirong He, and Yan Li. 2021. "Phytoremediation Potential of Four Native Plants in Soils Contaminated with Lead in a Mining Area" Land 10, no. 11: 1129. https://doi.org/10.3390/land10111129






