Assessing Soil-like Materials for Ecosystem Services Provided by Constructed Technosols
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials Used for Technosols’ Construction in Moscow
2.2. Integral Assessment of the Materials’ Quality
2.3. Chemical Analysis
2.4. Microbiological Analysis
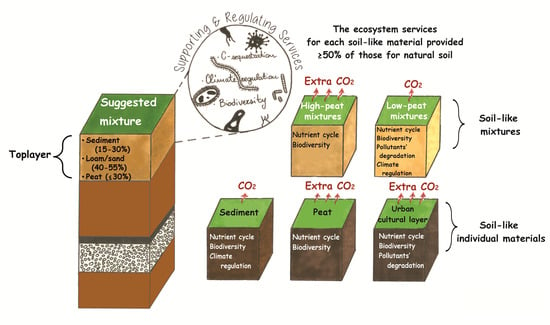
2.5. Interpretation of Soil-like Materials’ Properties from the Ecosystem Services’ Perspective
2.6. Statistical Analysis
3. Results
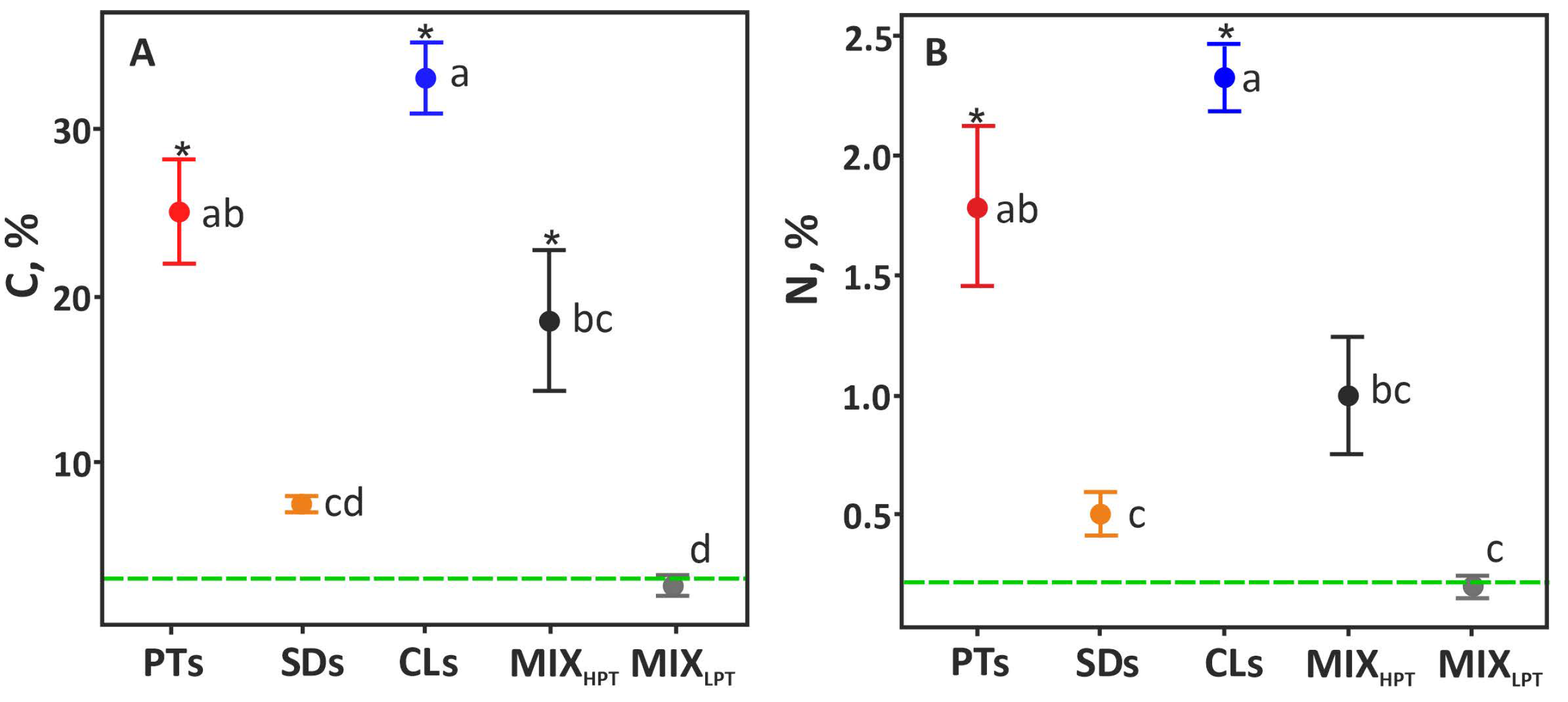
3.1. Chemical Properties
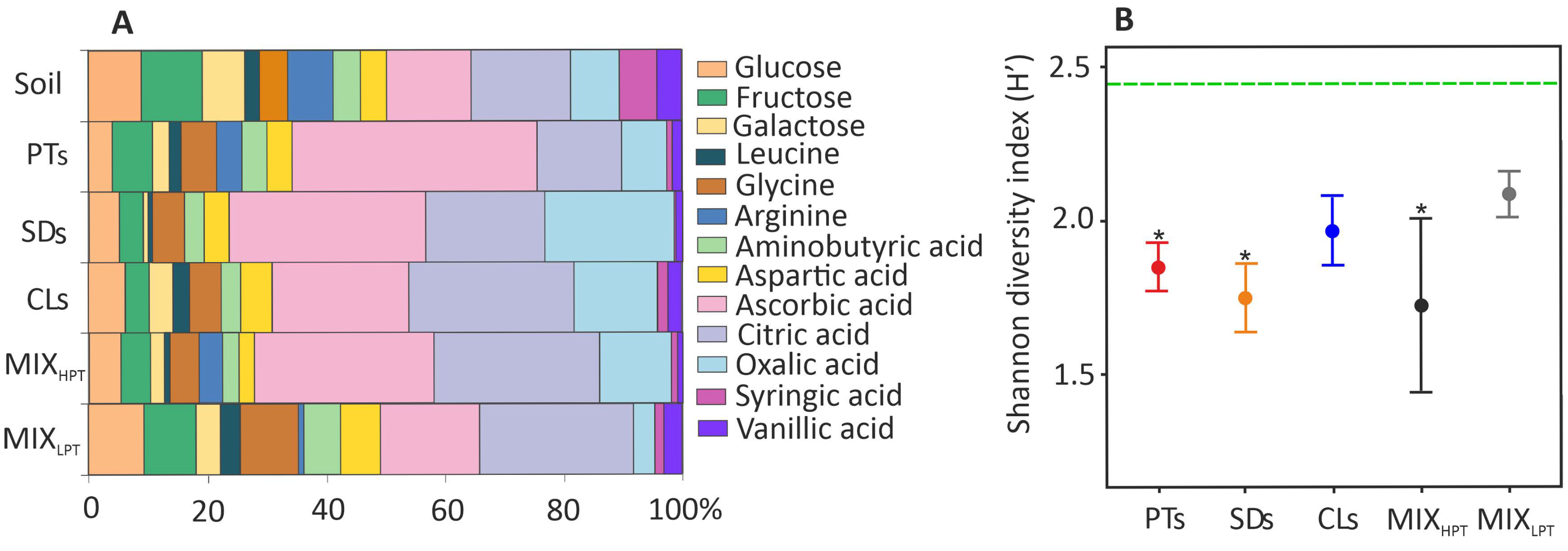
3.2. Microbial Properties
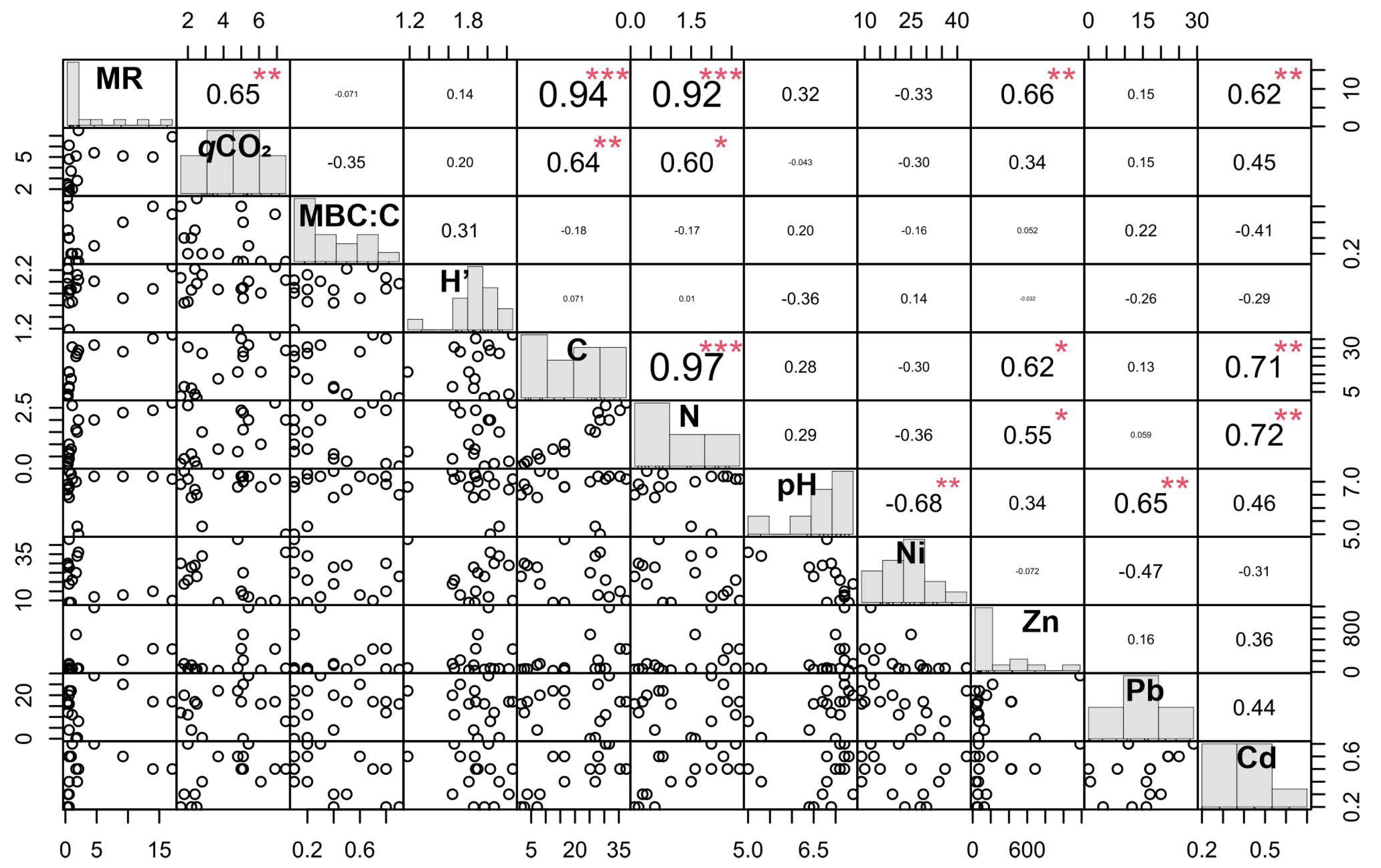
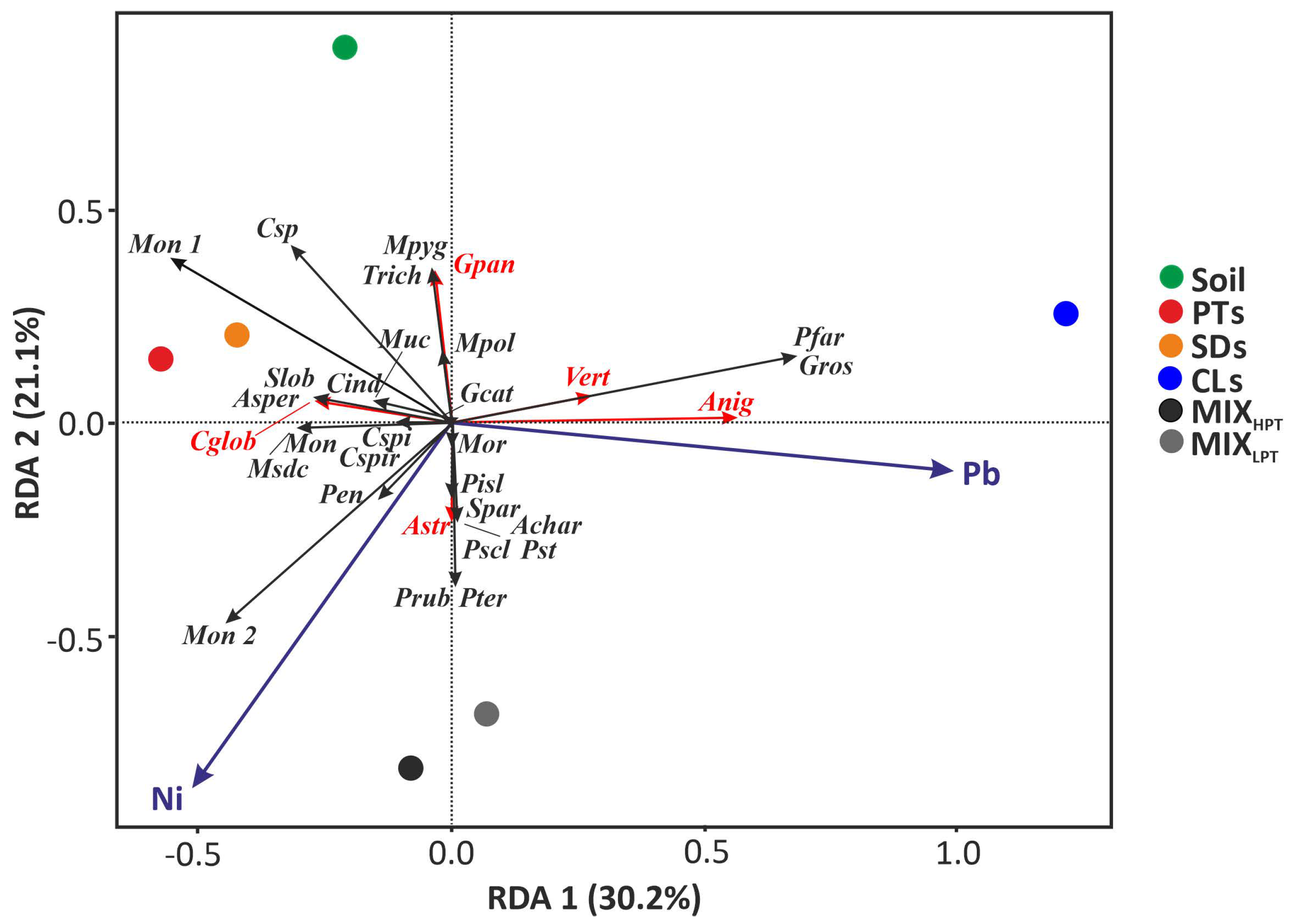
3.3. Relationships between Microbial and Chemical Properties
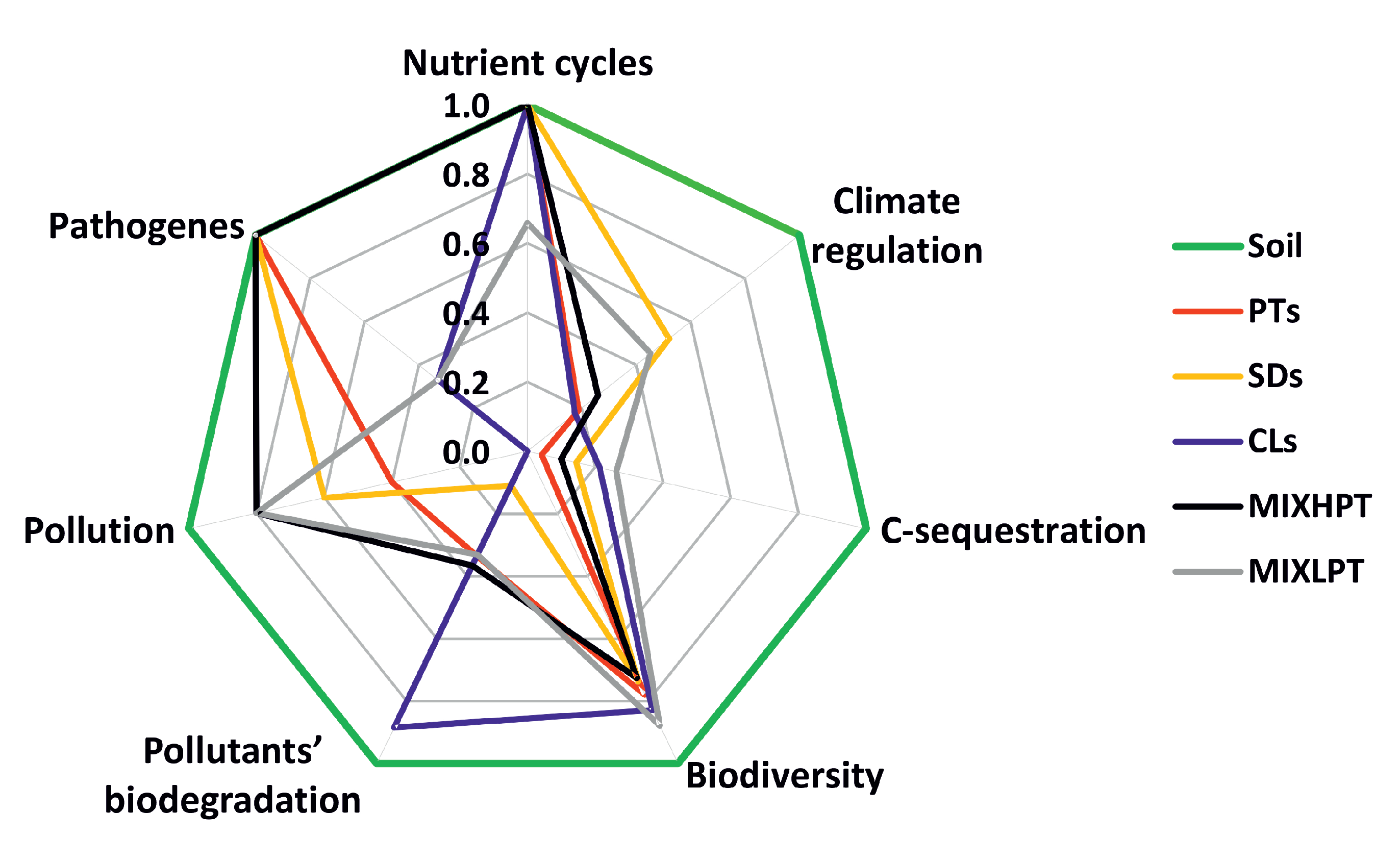
3.4. From Properties towards Ecosystem Services
4. Discussion
4.1. Advantages and Disadvantages of the Soil-like Materials from the Ecosystem Services’ Perspective
4.2. Microbial Properties of the Materials in Relation to Nutrients and PTE Contents
4.3. Perspectives of Microbial Indicators for the Materials Quality Control
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Grimm, N.B.; Faeth, S.H.; Golubiewski, N.E.; Redman, C.L.; Wu, J.; Bai, X.; Briggs, J.M. Global change and the ecology of cities. Science 2008, 319, 756–760. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pickett, S.T.A.; Cadenasso, M.L.; Grove, J.M.; Boone, C.G.; Groffman, P.M.; Irwin, E.; Kaushal, S.S.; Marshall, V.; McGrath, B.P.; Nilon, C.H.; et al. Urban ecological systems: Scientific foundations and a decade of progress. J. Environ. Manag. 2011, 92, 331–362. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Baggethun, E.; Barton, D.N. Classifying and valuing ecosystem services for urban planning. Ecol. Econ. 2013, 86, 235–245. [Google Scholar] [CrossRef]
- Lehmann, A.; Stahr, K. Nature and significance of anthropogenic urban soils. J. Soils Sediments 2007, 7, 247–260. [Google Scholar] [CrossRef]
- Brianskaia, I.P.; Vasenev, V.I.; Brykova, R.A.; Markelova, V.N.; Ushakova, N.V.; Gosse, D.D.; Gavrilenko, E.V.; Blagodatskaya, E.V. Analysis of volume and properties of imported soils for prediction of carbon stocks in soil constructions in the Moscow metropolis. Eurasian Soil Sci. 2020, 53, 1809–1817. [Google Scholar] [CrossRef]
- Deeb, M.; Groffman, P.M.; Blouin, M.; Egendorf, S.P.; Vergnes, A.; Vasenev, V.; Cao, D.L.; Walsh, D.; Morin, T.; Séré, G. Using constructed soils for green infrastructure—Challenges and limitations. Soil 2020, 6, 413–434. [Google Scholar] [CrossRef]
- Fabbri, D.; Pizzol, R.; Calza, P.; Malandrino, M.; Gaggero, E.; Padoan, E.; Ajmone-Marsan, F. Constructed technosols: A strategy toward a circular economy. Appl. Sci. (Switzerland) 2021, 11, 3432. [Google Scholar] [CrossRef]
- Smagin, A.V.; Sadovnikova, N.B. Creation of soil-like constructions. Eurasian Soil Sci. 2015, 48, 981–990. [Google Scholar] [CrossRef]
- Brose, D.A.; Hundal, L.S.; Oladeji, O.O.; Kumar, K.; Granato, T.C.; Cox, A.; Abedin, Z. Greening a steel mill slag brownfield with biosolids and sediments: A case study. J. Environ. Qual. 2016, 45, 53–61. [Google Scholar] [CrossRef] [PubMed]
- Vasenev, V.I.; Smagin, A.V.; Ananyeva, N.D.; Ivashchenko, K.V.; Gavrilenko, E.G.; Prokofeva, T.V.; Patlseva, A.; Stoorvogel, J.J.; Gosse, D.D.; Valentini, R. Urban soil’s functions: Monitoring, assessment, and management. In Adaptive Soil Management: From Theory to Practices; Rakshit, A., Abhilash, P., Singh, H., Ghosh, S., Eds.; Springer: Singapore, 2017; pp. 359–409. [Google Scholar]
- Slukovskaya, M.V.; Vasenev, V.I.; Ivashchenko, K.V.; Morev, D.V.; Drogobuzhskaya, S.V.; Ivanova, L.A.; Kremenetskaya, I.P. Technosols on mining wastes in the subarctic: Efficiency of remediation under Cu-Ni atmospheric pollution. Int. Soil Water Conserv. Res. 2019, 7, 297–307. [Google Scholar] [CrossRef]
- Séré, G.; Schwartz, C.; Ouvrard, S.; Sauvage, C.; Renat, J.C.; Morel, J.L. Soil construction: A step for ecological reclamation of derelict lands. J. Soils Sediments 2008, 8, 130–136. [Google Scholar] [CrossRef]
- Burghardt, W.; Morel, J.L.; Zhang, G.L. Development of the soil research about urban, industrial, traffic, mining and military areas (SUITMA). Soil Sci. Plant Nutr. 2015, 61, 3–21. [Google Scholar] [CrossRef]
- Kumar, K.; Hundal, L.S. Soil in the city: Sustainably improving urban soils. J. Environ. Qual. 2016, 45, 2–8. [Google Scholar] [CrossRef] [PubMed]
- Vidal-Beaudet, L.; Schwartz, C.; Séré, G. Using Wastes for Fertile Urban Soil Construction—The French Research Project SITERRE. In Soils within Cities; Levin, M.J., Kim, K.-H.J., Morel, J.L., Burghardt, W., Charzynski, P., Shaw, R.K., IUSS Working Group SUITMA, Eds.; Schweizerbart Science Publishers: Stuttgart, Germany, 2017; pp. 159–168. [Google Scholar]
- Municipality of Parma. Municipal Regulation of Public and Private Green Areas; Municipality of Parma: Parma, Italy, 2016. (In Italian) [Google Scholar]
- Malenkovska Todorova, M.; Donceva, R.; Bunevska, J. Road Regulation and Functional Classification of the Urban Streets of Rome Capital. Transp. Probl. 2009, 4, 97–104. [Google Scholar]
- Fourvel, G.; Vidal-Beaudet, L.; le Bocq, A.; Brochier, V.; Théry, F.; Landry, D.; Kumarasamy, T.; Cannavo, P. Early structural stability of fine dam sediment in soil construction. J. Soils Sediments 2018, 18, 2647–2663. [Google Scholar] [CrossRef]
- Morel, J.L.; Chenu, C.; Lorenz, K. Ecosystem services provided by soils of urban, industrial, traffic, mining, and military areas (SUITMAs). J. Soils Sediments 2015, 15, 1659–1666. [Google Scholar] [CrossRef]
- Vasenev, V.I.; van Oudenhoven, A.P.E.; Romzaykina, O.N.; Hajiaghaeva, R.A. The ecological functions and ecosystem services of urban and technogenic soils: From theory to practice (a review). Eurasian Soil Sci. 2018, 51, 1119–1132. [Google Scholar] [CrossRef] [Green Version]
- Saccá, M.L.; Caracciolo, A.B.; di Lenola, M.; Grenni, P. Ecosystem services provided by soil microorganisms. In Soil Biological Communities and Ecosystem Resilience; Springer: Cham, Switzerland, 2017. [Google Scholar] [CrossRef]
- Frey, S.D.; Drijber, R.; Smith, H.; Melillo, J. Microbial biomass, functional capacity, and community structure after 12 years of soil warming. Soil Biol. Biochem. 2008, 40, 2904–2907. [Google Scholar] [CrossRef]
- Diakhaté, S.; Gueye, M.; Chevallier, T.; Diallo, N.H.; Assigbetse, K.; Abadie, J.; Diouf, M.; Masse, D.; Sembène, M.; Ndour, Y.B.; et al. Soil microbial functional capacity and diversity in a millet-shrub intercropping system of semi-arid senegal. J. Arid. Environ. 2016, 129, 71–79. [Google Scholar] [CrossRef]
- Kumaresan, D.; Cross, A.T.; Moreira-Grez, B.; Kariman, K.; Nevill, P.; Stevens, J.; Allcock, R.J.N.; O’Donnell, A.G.; DIxon, K.W.; Whiteley, A.S. Microbial functional capacity is preserved within engineered soil formulations used in mine site restoration. Sci. Rep. 2017, 7, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Joergensen, R.G.; Emmerling, C. Methods for evaluating human impact on soil microorganisms based on their activity, biomass, and diversity in agricultural soils. J. Plant Nutr. Soil Sci. 2006, 169, 295–309. [Google Scholar] [CrossRef]
- Ritz, K.; Black, H.I.J.; Campbell, C.D.; Harris, J.A.; Wood, C. Selecting biological indicators for monitoring soils: A framework for balancing scientific and technical opinion to assist policy development. Ecol. Indic. 2009, 9, 1212–1221. [Google Scholar] [CrossRef] [Green Version]
- Marinari, S.; Bonifacio, E.; Moscatelli, M.C.; Falsone, G.; Antisari, L.V.; Vianello, G. Soil development and microbial functional diversity: Proposal for a methodological approach. Geoderma 2013, 192, 437–445. [Google Scholar] [CrossRef]
- Moscatelli, M.C.; Secondi, L.; Marabottini, R.; Papp, R.; Stazi, S.R.; Mania, E.; Marinari, S. Assessment of soil microbial functional diversity: Land use and soil properties affect CLPP-MicroResp and enzymes responses. Pedobiologia 2018, 66, 36–42. [Google Scholar] [CrossRef]
- Escalas, A.; Hale, L.; Voordeckers, J.W.; Yang, Y.; Firestone, M.K.; Alvarez-Cohen, L.; Zhou, J. Microbial functional diversity: From concepts to applications. Ecol. Evol. 2019, 9, 12000–12016. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marfenina, O.E.; Makarova, N.V.; Ivanova, A.E. Opportunistic fungi in soils and surface air of a megalopolis (for the Tushino Region, Moscow). Microbiology 2011, 80, 870–876. [Google Scholar] [CrossRef]
- Korneykova, M.V.; Myazin, V.A.; Fokina, N.V.; Chaporgina, A.A. Bioremediation of soil of the kola peninsula (Murmansk region) contaminated with diesel fuel. Geogr. Environ. Sustain. 2021, 14, 171–176. [Google Scholar] [CrossRef]
- Stroganova, M.N.; Myagkova, A.D.; Prokof’eva, T.V. The role of soils in urban ecosystems. Eurasian Soil Sci. 1997, 30, 82–86. [Google Scholar]
- Prokofyeva, T.V.; Martynenko, I.A.; Ivannikov, F.A. Classification of Moscow soils and parent materials and its possible inclusion in the classification system of Russian soils. Eurasian Soil Sci. 2011, 44, 561–571. [Google Scholar] [CrossRef]
- Prokhorov, I.S.; Karev, S.Y. Particularities of artificial soil-ground production for landscape and shade gardening. Agrochem. Her. 2012, 3, 21–25. (In Russian) [Google Scholar]
- Alexandrovskiy, A.L.; Dolgikh, A.V.; Alexandrovskaya, E.I. Pedogenetic features of habitation deposits in ancient towns of european russia and their alteration under different natural conditions. Bol. De La Soc. Geol. Mex. 2012, 64, 71–77. [Google Scholar] [CrossRef]
- Vasenev, V.I.; Stoorvogel, J.J.; Dolgikh, A.V.; Ananyeva, N.D.; Ivashchenko, K.V. Changes in soil organic carbon stocks by urbanization. In Advance in Soil Science “Urban Soils”; Lal, R., Stewart, B.A., Eds.; CRC Press Taylor and Francis Group: New York, NY, USA, 2017; pp. 61–93. [Google Scholar]
- Anderson, J.P.E.; Domsch, K.H. A physiological method for the quantitative measurement of microbial biomass in soils. Soil Biol. Biochem. 1978, 10, 215–221. [Google Scholar] [CrossRef]
- Ananyeva, N.D.; Susyan, E.A.; Chernova, O.V.; Wirth, S. Microbial respiration activities of soils from different climatic regions of European Russia. Eur. J. Soil Biol. 2008, 44, 147–157. [Google Scholar] [CrossRef]
- Creamer, R.E.; Schulte, R.P.O.; Stone, D.; Gal, A.; Krogh, P.H.; lo Papa, G.; Murray, P.J.; Pérès, G.; Foerster, B.; Rutgers, M.; et al. Measuring basal soil respiration across europe: Do incubation temperature and incubation period matter? Ecol. Indic. 2014, 36, 409–418. [Google Scholar] [CrossRef] [Green Version]
- Kroetsch, D.; Wang, C. Soil Sampling and Methods of Analysis, 2nd ed.; Carter, M.R., Gregorich, E.G., Eds.; CRC Press: Boca Raton, FL, USA, 2007. [Google Scholar] [CrossRef]
- ISO. Soil quality–laboratory methods for determination of microbial soil respiration; International Organization for Standardization: Geneva, Switzerland, 2002. [Google Scholar]
- Dilly, O. Microbial energetics in soils. In Microorganisms in Soils: Roles in Genesis and Functions; Springer: Berlin, Heidelberg, 2005. [Google Scholar] [CrossRef]
- Campbell, C.D.; Chapman, S.J.; Cameron, C.M.; Davidson, M.S.; Potts, J.M. A rapid microtiter plate method to measure carbon dioxide evolved from carbon substrate amendments so as to determine the physiological profiles of soil microbial communities by using whole soil. Appl. Environ. Microbiol. 2003, 69, 3593–3599. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shannon, C.; Weaver, W. The mathematical theory of comumunication; The University of Illinois Press: Champaign, IL, USA, 1964; 131p. [Google Scholar]
- Davet, P.; Rouxel, F. Detection and Isolation of Soil Fungi; Science Publishers: Plymouth, UK, 2000. [Google Scholar]
- Acea, M.J.; Carballas, T. Changes in physiological groups of microorganisms in soil following wildfire. FEMS Microbiol. Ecol. 1996, 20, 33–39. [Google Scholar] [CrossRef]
- Marfenina, O.E.; Danilogorskaya, A.A. Effect of elevated temperatures on composition and diversity of microfungal communities in natural and urban boreal soils, with emphasis on potentially pathogenic species. Pedobiologia 2017, 60, 11–19. [Google Scholar] [CrossRef]
- Seifert, K.A. Compendium of soil fungi—by Domsch, K.H.; Gams, W.; Anderson, T.H. Eur. J. Soil Sci. 2008, 59, 1007. [Google Scholar] [CrossRef]
- De Hoog, G.S.; Guarro, J.; Gené, J.; Ahmed, S.A.; Al-Hatmi, A.M.S.; Figueras, M.J.; Vitale, R.G. Atlas of Clinical Fungi, 4th ed.; Centraalbureau voor Schimmelcultures: Utrecht, The Netherlands, 2019. [Google Scholar]
- Thiele-Bruhn, S.; Schloter, M.; Wilke, B.M.; Beaudette, L.A.; Martin-Laurent, F.; Cheviron, N.; Mougin, C.; Römbke, J. Identification of new microbial functional standards for soil quality assessment. SOIL 2020, 6, 17–34. [Google Scholar] [CrossRef] [Green Version]
- Legendre, P.; Gallagher, E.D. Ecologically meaningful transformations for ordination of species data. Oecologia 2001, 129, 271–280. [Google Scholar] [CrossRef]
- TeamCore, R.C. Language and Environment for Statistical Computing: R Foundation for Statistical Computing; The R Development Core Team: Vienna, Austria, 2018. [Google Scholar]
- Wickham, H. Ggplot2 Elegant Graphics for Data Analysis (Use R!); Springer: New York, NY, USA, 2016. [Google Scholar]
- Vasenev, V.I.; Stoorvogel, J.J.; Vasenev, I.I. Urban soil organic carbon and its spatial heterogeneity in comparison with natural and agricultural areas in the Moscow region. Catena 2013, 107, 96–102. [Google Scholar] [CrossRef]
- Ivashchenko, K.; Ananyeva, N.; Vasenev, V.; Sushko, S.; Seleznyova, A.; Kudeyarov, V. Microbial C–availability and organic matter decomposition in urban soils of megapolis depend on functional zoning. Soil Environ. 2019, 38, 31–41. [Google Scholar] [CrossRef]
- Darmody, R.G.; Marlin, J.C.; Talbott, J.; Green, R.A.; Brewer, E.F.; Stohr, C. Dredged Illinois river sediments: Plant growth and metal uptake. J. Environ. Qual. 2004, 33, 458–464. [Google Scholar] [CrossRef] [PubMed]
- Vasbieva, M.T.; Kosolapova, A.I. Changes in fertility parameters and contents of heavy metals of soddy-podzolic soils upon the long-term application of sewage sludge. Eurasian Soil Sci. 2015, 48, 518–523. [Google Scholar] [CrossRef]
- Plekhanova, I.O. Self-purification of agrosoddy-podzolic sandy loamy soils fertilized with sewage sludge. Eurasian Soil Sci. 2017, 50, 491–497. [Google Scholar] [CrossRef]
- Shchegolkova, N.M.; Smagin, A.V.; Rybka, K.Y. Methodological aspects of soil engineering: Agrophysical properties (in Russian). Voda Khimiya Ekol. 2013, 7, 9–17. [Google Scholar]
- HS-514-11 Moscow Government Document Regulates Quality of the Materials Applied for Greening in Moscow. 2019.
- Khrenov, K.E.; Kozlov, M.N.; Shchegolkova, N.M.; Vanyushina, A.Y.; Grachev, V.A. Properties investigation of the created soils made from sediments of water treatment station (in Russian). Water Supply Sanit. Tech. 2011, 10, 20–25. [Google Scholar]
- Kitir, N.; Yildirim, E.; Şahin, Ü.; Turan, M.; Ekinci, M.; Ors, S.; Kul, R.; Ünlü, H.; Ünlü, H. Peat use in horticulture. In Peat; IntechOpen: London, UK, 2018. [Google Scholar] [CrossRef] [Green Version]
- Shchepeleva, A.S.; Vasenev, V.I.; Mazirov, I.M.; Vasenev, I.I.; Prokhorov, I.S.; Gosse, D.D. Changes of soil organic carbon stocks and CO2 emissions at the early stages of urban turf grasses’ development. Urban Ecosyst. 2017, 20, 309–321. [Google Scholar] [CrossRef]
- Smagin, A.V.; Sadovnikova, N.B.; Vasenev, V.I.; Smagina, M.V. Biodegradation of some organic materials in soils and soil constructions: Experiments, modeling and prevention. Materials 2018, 11, 1889. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vasenev, V.; Varentsov, M.; Konstantinov, P.; Romzaykina, O.; Kanareykina, I.; Dvornikov, Y.; Manukyan, V. Projecting urban heat island effect on the spatial-temporal variation of microbial respiration in urban soils of moscow megalopolis. Sci. Total. Environ. 2021, 786, 147457. [Google Scholar] [CrossRef]
- Kelly, J.J.; Favila, E.; Hundal, L.S.; Marlin, J.C. Assessment of soil microbial communities in surface applied mixtures of Illinois river sediments and biosolids. Appl. Soil Ecol. 2007, 36, 176–183. [Google Scholar] [CrossRef]
- Alexandrovskaya, E.I.; Alexandrovskiy, A.L. History of the cultural layer in moscow and accumulation of anthropogenic substances in it. Catena 2000, 41, 249–259. [Google Scholar] [CrossRef]
- Ivashchenko, K.V.; Ananyeva, N.D.; Vasenev, V.I.; Kudeyarov, V.N.; Valentini, R. Biomass and respiration activity of soil microorganisms in anthropogenically transformed ecosystems (Moscow region). Eurasian Soil Sci. 2014, 47, 892–903. [Google Scholar] [CrossRef]
- Ovsepyan, L.; Kurganova, I.; de Gerenyu, V.L.; Kuzyakov, Y. Recovery of organic matter and microbial biomass after abandonment of degraded agricultural soils: The influence of climate. Land Degrad. Dev. 2019, 30, 1861–1874. [Google Scholar] [CrossRef]
- Shahbaz, M.; Kuzyakov, Y.; Sanaullah, M.; Heitkamp, F.; Zelenev, V.; Kumar, A.; Blagodatskaya, E. Microbial decomposition of soil organic matter is mediated by quality and quantity of crop residues: Mechanisms and thresholds. Biol. Fertil. Soils 2017, 53, 287–301. [Google Scholar] [CrossRef]
- Karlen, D.L.; Mausbach, M.J.; Doran, J.W.; Cline, R.G.; Harris, R.F.; Schuman, G.E. Soil quality: A concept, definition, and framework for evaluation (a guest editorial). Soil Sci. Soc. Am. J. 1997, 61, 4–10. [Google Scholar] [CrossRef] [Green Version]
- Doran, J.W.; Zeiss, M.R. Soil health and sustainability: Managing the biotic component of soil quality. Appl. Soil Ecol. 2000, 15, 3–11. [Google Scholar] [CrossRef] [Green Version]
- Aqua Engineering, Inc. City of Evans Standard: Lawn and Grass Specification Standard-02930; Aqua Engineering, Inc.: City of Evans, CO, USA, 2000. [Google Scholar]
- British Standards Institution. BS 3882 Specification for Topsoil; British Standards Institution: London, UK, 2015. [Google Scholar]
- ISO. ISO 14240-1 Soil Quality—Determination of Soil Microbial Biomass—Part 1: Substrate-Induced Respiration Method; International Organization for Standardization: Geneva, Switzerland, 1997. [Google Scholar]
- ISO. ISO/TS 22939 (2019): Soil Quality—Measurement of Enzyme Activity Patterns in Soil Samples Using Fluorogenic Substrates in Micro-Well Plates; International Organization for Standardization: Geneva, Switzerland, 2019. [Google Scholar]
- ISO. ISO 14238 Soil Quality—Biological Methods—Determination of Nitrogen Mineralization and Nitrification in Soils and the Influence of Chemicals on These Processes; International Organization for Standardization: Geneva, Switzerland, 2012. [Google Scholar]
- Hafeez, F.; Martin-Laurent, F.; Béguet, J.; Bru, D.; Cortet, J.; Schwartz, C.; Morel, J.L.; Philippot, L. Taxonomic and functional characterization of microbial communities in technosols constructed for remediation of a contaminated industrial wasteland. J. Soils Sediments 2012, 12, 1396–1406. [Google Scholar] [CrossRef]
- Vidal-Beaudet, L.; Galopin, G.; Grosbellet, C. Effect of organic amendment for the construction of favourable urban soils for tree growth. Eur. J. Hortic. Sci. 2018, 83, 173–186. [Google Scholar] [CrossRef]


| Material | Origin | Suppliers | Implementation |
|---|---|---|---|
| PTs | peatlands | peat mining companies | high |
| SDs | water body | water management companies | low |
| CLs | urban subsoil | producers of soil mixtures for gardening and landscaping | low |
| MIXLPT | man-made | high | |
| MIXHPT | high |
| Mixture | Number | Composition | Volume Portion, % |
|---|---|---|---|
| Low peat content | I | peat/excavated urban topsoil/sand/excavated river valley topsoil | 30/30/30/10 |
| II | excavated urban topsoil/peat/compost/sand | 25/25/25/25 | |
| III | excavated urban topsoil/valley peat/sand | 50/30/20 | |
| High peat content | IV | peat/sand | 75/25 |
| V | peat/compost/sand | 80/10/10 | |
| VI | peat/sand | 95/5 |
| PRP | PTs (n = 4) | SDs (n = 2) | CLs (n = 4) | MIXHPT (n = 3) | MIXLPT (n = 3) | THL |
|---|---|---|---|---|---|---|
| pH | 6.4 ± 0.5 a | 6.9 ± 0.5 a | 7.2 ± 0.0 a | 6.5 ± 0.6 a | 6.7 ± 0.1 a | 6.0–7.5 |
| Ni | 23 ± 6 a | 23 ± 5 a | 12 ± 1 b | 29 ± 10 a | 27 ± 2 a | 80 |
| Zn | 215 ± 158 b | 140 ± 12 b | 563 ± 212 a | 54 ± 14 c | 53 ± 3 c | 220 |
| Pb | 9 ± 3 a | 12 ± 8 a | 22 ± 3 a | 15 ± 7 a | 15 ± 2 a | 130 |
| Cd | 0.5 ± 0.1 a | 0.3 ± 0.0 b | 0.6 ± 0.0 a | 0.5 ± 0.1 a | 0.2 ± 0.0 b | 2.0 |
| Fungi | Abbreviation | Health Risks | Soil | PTs | CLs | SDs | MIXHPT | MIXLPT |
|---|---|---|---|---|---|---|---|---|
| Acremonium strictum Gams | Astr | pulmonary, pleuritis, fungemia | - | - | - | - | - | ++ |
| Aspergillus niger Tiegh. | Anig | otomycosis, aspergillosis | - | - | ++ | - | - | + |
| Aspergillus sp. | Asper | - | + | - | - | - | - | |
| Acremonium charticola Lindau | Achar | - | - | - | - | - | ++ | |
| Chaetomium globosum Kunze | Cglob | onychomycosis, cutaneous lesions | - | + | - | + | - | - |
| Chaetomium indicum Corda | Cind | - | + | - | - | - | - | |
| Chaetomium spiralliforum Bainier | Cspi | - | - | - | + | - | - | |
| Chaetomium spirale Zopf | Cspir | - | - | - | + | - | - | |
| Chaetomium sp. | Csp | ++ | + | - | + | |||
| Geomyces pannorum Link | Gpan | onychomycosis | ++ | - | - | - | - | - |
| Gliocladium catenulatum Gilman & Abbott | Gcat | - | - | - | + | - | - | |
| Gliocladium roseum Bainier | Gros | - | - | +++ | - | - | - | |
| Monocillium sp. | Mon | - | + | - | - | - | - | |
| Monocillium pygmaea Chalab. | Mpyg | ++ | - | - | - | - | - | |
| Mortierella polycephala Coem. | Mpol | + | - | - | - | - | - | |
| Mortierella sp. | Mor | + | - | - | - | - | ++ | |
| Mucor sp. | Muc | - | + | - | - | - | - | |
| Paecilomyces farinosus Holm | Pfar | - | - | +++ | - | - | - | |
| Penicillium islandicum Sopp | Pisl | - | - | - | - | + | - | |
| Penicillium steckii Zaleski | Pst | - | - | - | - | + | - | |
| Penicillium sclerotiorum Beyma | Pscl | - | - | - | - | - | ++ | |
| Penicillium rubrum Stoll | Prub | - | - | - | - | ++ | - | |
| Penicillium terlikowskii Zaleski | Pter | - | - | - | - | ++ | - | |
| Penicillium sp. | Pen | - | + | - | - | - | ++ | |
| Stachybotrys parvispora Hughes | Spar | - | - | - | - | + | - | |
| Stachybotrys lobulatus Berk. | Slob | - | + | - | + | - | - | |
| Trichoderma sp. | Trich | ++ | - | - | - | - | - | |
| Verticillium sp. | Vert | plant diseases | - | - | + | - | - | - |
| Moniliaceae sp.1 | Mon1 | +++ | +++ | - | + | + | - | |
| Moniliaceae sp.2 | Mon2 | + | ++ | - | + | +++ | +++ | |
| Micelia sterilia dark-colored | Msdc | - | ++ | - | - | - | + |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ivashchenko, K.; Lepore, E.; Vasenev, V.; Ananyeva, N.; Demina, S.; Khabibullina, F.; Vaseneva, I.; Selezneva, A.; Dolgikh, A.; Sushko, S.; et al. Assessing Soil-like Materials for Ecosystem Services Provided by Constructed Technosols. Land 2021, 10, 1185. https://doi.org/10.3390/land10111185
Ivashchenko K, Lepore E, Vasenev V, Ananyeva N, Demina S, Khabibullina F, Vaseneva I, Selezneva A, Dolgikh A, Sushko S, et al. Assessing Soil-like Materials for Ecosystem Services Provided by Constructed Technosols. Land. 2021; 10(11):1185. https://doi.org/10.3390/land10111185
Chicago/Turabian StyleIvashchenko, Kristina, Emanuela Lepore, Viacheslav Vasenev, Nadezhda Ananyeva, Sofiya Demina, Fluza Khabibullina, Inna Vaseneva, Alexandra Selezneva, Andrey Dolgikh, Sofia Sushko, and et al. 2021. "Assessing Soil-like Materials for Ecosystem Services Provided by Constructed Technosols" Land 10, no. 11: 1185. https://doi.org/10.3390/land10111185
APA StyleIvashchenko, K., Lepore, E., Vasenev, V., Ananyeva, N., Demina, S., Khabibullina, F., Vaseneva, I., Selezneva, A., Dolgikh, A., Sushko, S., Marinari, S., & Dovletyarova, E. (2021). Assessing Soil-like Materials for Ecosystem Services Provided by Constructed Technosols. Land, 10(11), 1185. https://doi.org/10.3390/land10111185