Electrochemical and Structural Modifications of Humic Acids in Aerobically and Anaerobically Incubated Peat
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
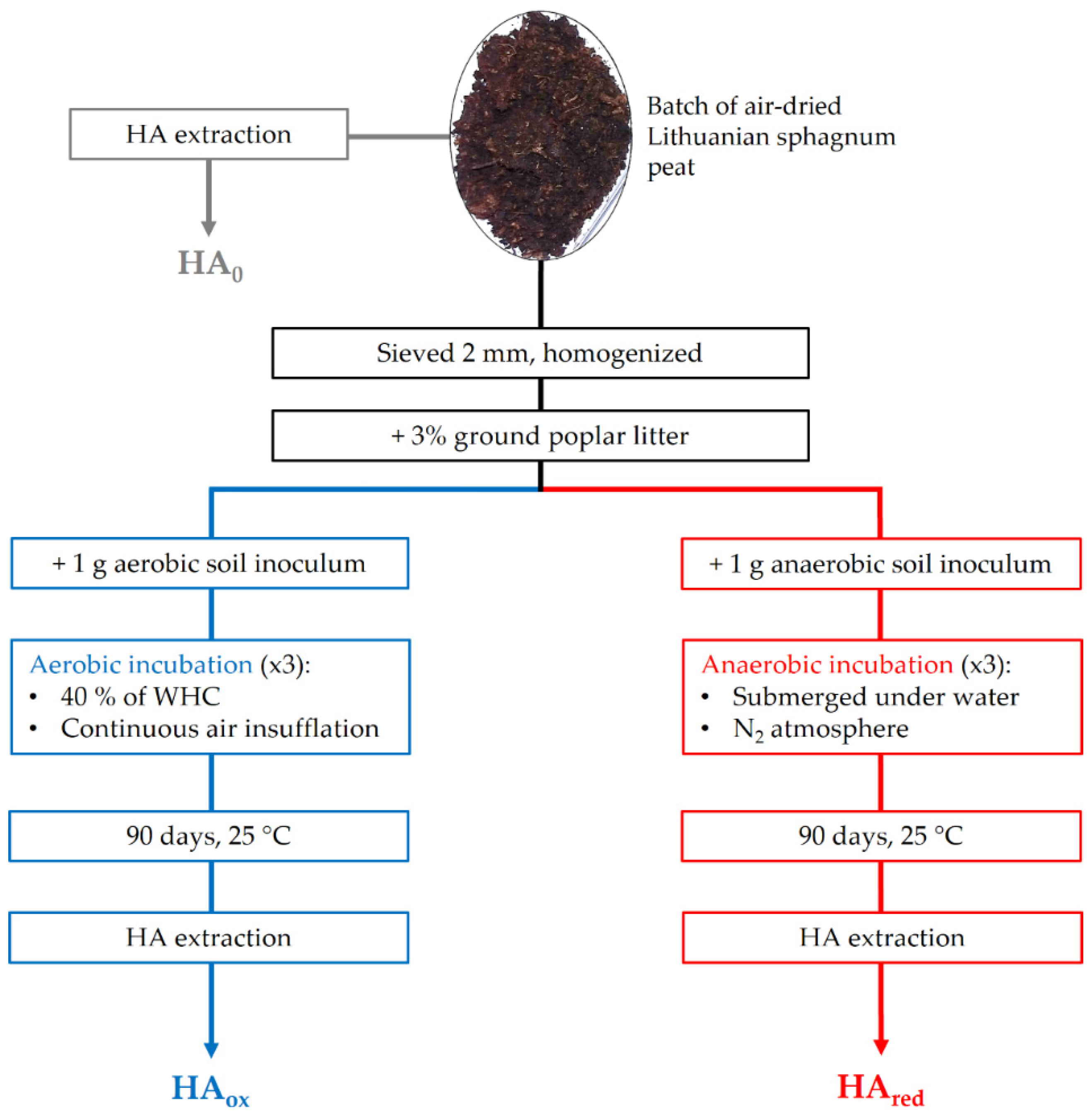
2.2. Experiment Layout
2.3. HA Extraction
2.4. HA Characterization
2.5. Electrochemical Measurements
2.6. Statistics
3. Results
3.1. HA Composition
3.2. Electrochemical Behavior of HAs
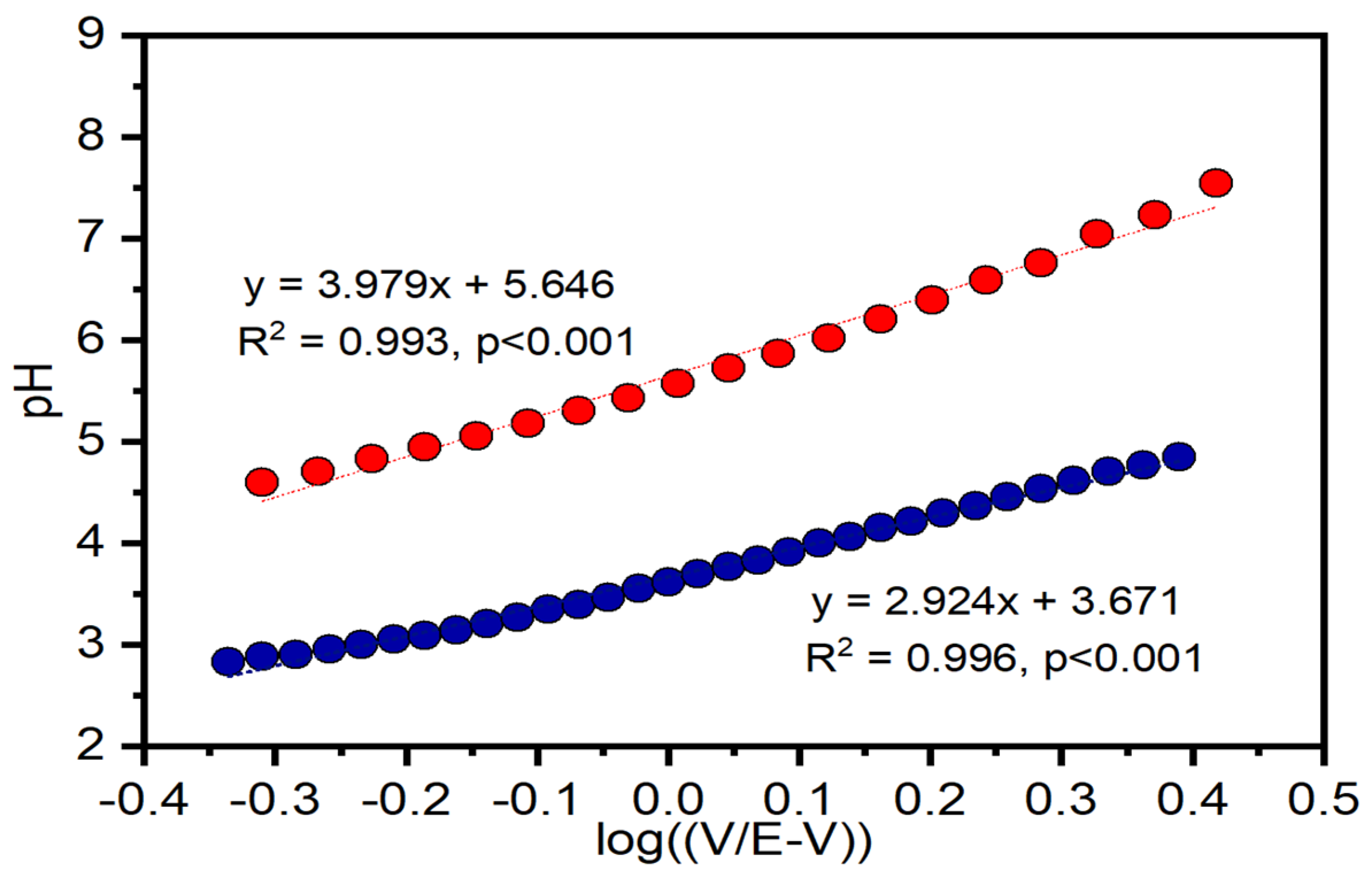
3.3. Electron Transfer Kinetic Constants from CV
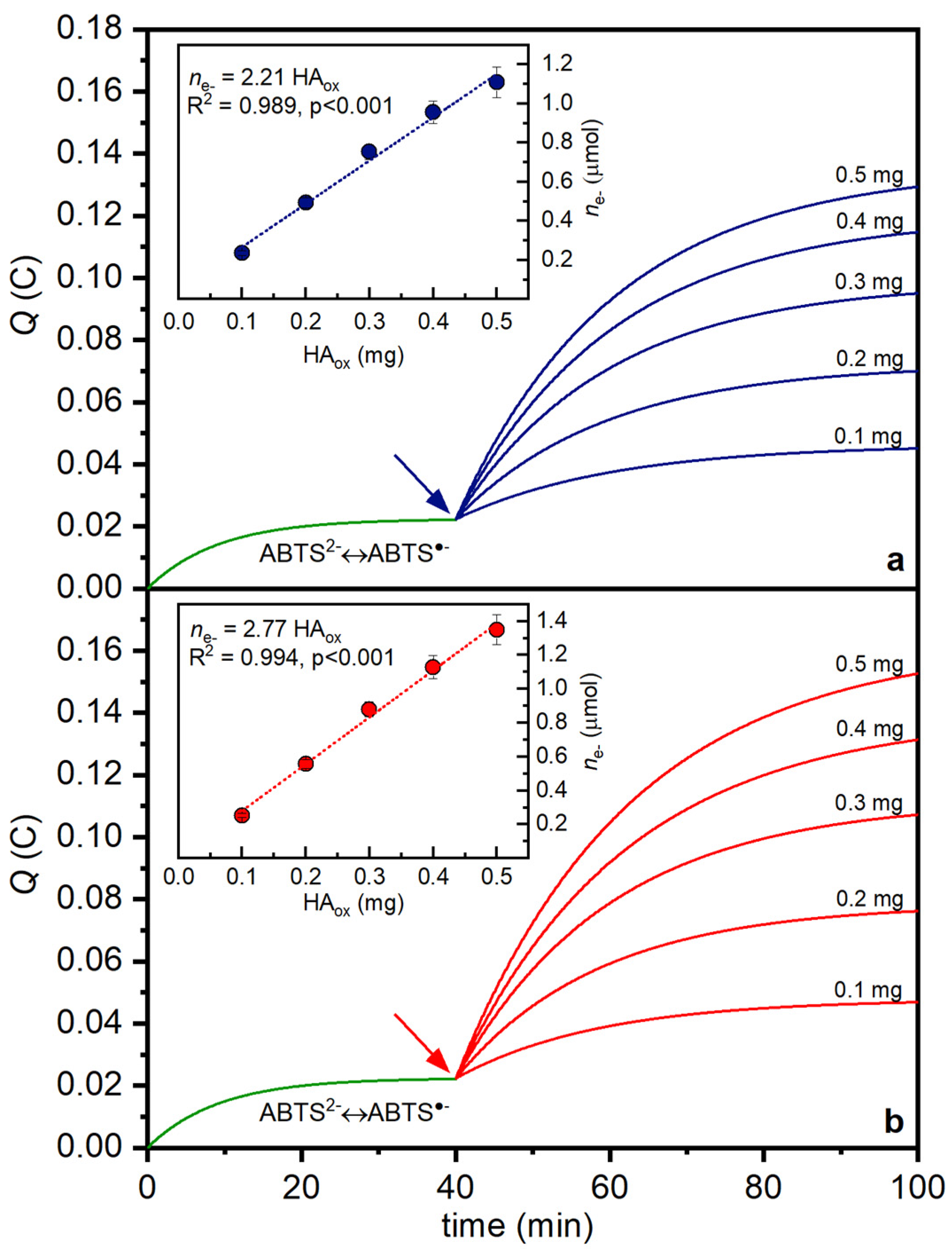
3.4. Quantification of Redox Changes
3.5. Structural Changes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
List of Abbreviations
References
- Keiluweit, M.; Nico, P.S.; Kleber, M.; Fendorf, S. Are oxygen limitations under recognized regulators of organic carbon turnover in upland soils? Biogeochemistry 2016, 127, 157–171. [Google Scholar] [CrossRef] [Green Version]
- Boye, K.; Noël, V.; Tfaily, M.; Bone, S.E.; Williams, K.H.; Bargar, J.R.; Fendorf, S. Thermodynamically controlled preservation of organic carbon in floodplains. Nat. Geosci. 2017, 10, 415–419. [Google Scholar] [CrossRef] [Green Version]
- Drake, H.L.; Horn, M.A.; Wüst, P.K. Intermediary ecosystem metabolism as a main driver of methanogenesis in acidic wetlands soil. Environ. Microbiol. Rep. 2009, 1, 307–318. [Google Scholar] [CrossRef] [PubMed]
- Bridgham, S.D.; Cadillo-Quiroz, H.; Keller, J.K.; Zhuang, Q. Methane emissions from wetlands: Biogeochemical, microbial, and modeling perspectives from local to global scales. Glob. Chang. Biol. 2013, 19, 1325–1346. [Google Scholar] [CrossRef] [PubMed]
- Davidson, E.A.; Janssens, I.A. Temperature sensitivity of soil carbon decomposition and feedbacks to climate change. Nature 2006, 440, 165–173. [Google Scholar] [CrossRef] [PubMed]
- Ise, T.; Moorcroft, P.R. The global-scale temperature and moisture dependencies of soil organic carbon decomposition: An analysis using a mechanistic decomposition model. Biogeochemistry 2006, 80, 217–231. [Google Scholar] [CrossRef]
- Guo, X.; Meng, M.; Zhang, J.; Chen, H.Y.H. Vegetation change impacts on soil organic carbon chemical composition in subtropical forests. Sci. Rep. 2016, 6, 29607. [Google Scholar] [CrossRef]
- Keller, J.K.; Bridgham, S.D. Pathways of anaerobic carbon cycling across an ombrotrophic-minerotrophic peatland gradient. Limnol. Oceanogr. 2007, 52, 96–107. [Google Scholar] [CrossRef] [Green Version]
- Ye, R.; Jin, Q.; Bohannan, B.; Keller, J.K.; Bridgham, S.D. Homoacetogenesis: A potentially underappreciated carbon pathway in peatlands. Soil Biol. Biochem. 2014, 68, 385–391. [Google Scholar] [CrossRef]
- Mikaloff Fletcher, S.E.; Tans, P.P.; Bruhwiler, L.M.; Miller, J.B.; Heimann, M. CH4 sources estimated from atmospheric observations of CH4 and its 13C/12C isotopic ratios: 1. Inverse modeling of source processes. Glob. Biogeochem. Cycles. 2004, 18, GB4004. [Google Scholar] [CrossRef] [Green Version]
- Keller, J.K.; Takagi, K.K. Solid-phase organic matter reduction regulates anaerobic decomposition in bog soil. Ecosphere 2013, 4, 1–10. [Google Scholar] [CrossRef]
- Vile, M.A.; Bridgham, S.D.; Wieder, R.K. Response of anaerobic carbon mineralization rates to sulfate amendments in a boreal peatland. Ecol. Appl. 2003, 13, 720–734. [Google Scholar] [CrossRef]
- Galand, P.E.; Yrjälä, K.; Conrad, R. Stable carbon isotope fractionation during methanogenesis in three boreal peatland ecosystems. Biogeosciences 2010, 7, 3893–3900. [Google Scholar] [CrossRef] [Green Version]
- Lovley, D.R.; Coates, J.D.; Blunt-Harris, E.L.; Phillips, E.J.P.; Woodward, J.C. Humic substances as electron acceptors for microbial respiration. Nature 1996, 382, 445–448. [Google Scholar] [CrossRef]
- Cervantes, F.J.; van der Velde, S.; Lettinga, G.; Field, J.A. Competition between methanogenesis and quinone respiration for ecologically important substrates in anaerobic consortia. FEMS Microbiol. Ecol. 2000, 34, 161–171. [Google Scholar] [CrossRef]
- Keller, J.K.; Weisenhorn, P.B.; Megonigal, J.P. Humic acids as electron acceptors in wetland decomposition. Soil Biol. Biochem. 2009, 41, 1518–1522. [Google Scholar] [CrossRef]
- Klüpfel, L.; Piepenbrock, A.; Kappler, A.; Sander, M. Humic substances as fully regenerable electron acceptors in recurrently anoxic environments. Nat. Geosci. 2014, 7, 195–200. [Google Scholar] [CrossRef]
- Ye, R.; Keller, J.K.; Jin, Q.; Bohannan, B.J.M.; Bridgham, S.D. Peatland types influence the inhibitory effects of a humic substance analog on methane production. Geoderma 2016, 265, 131–140. [Google Scholar] [CrossRef] [Green Version]
- Valenzuela, E.I.; Avendaño, K.A.; Balagurusamy, N.; Arriaga, S.; Nieto-Delgado, C.; Thalasso, F.; Cervantes, F.J. Electron shuttling mediated by humic substances fuels anaerobic methane oxidation and carbon burial in wetland sediments. Sci. Total Environ. 2019, 650, 2674–2684. [Google Scholar] [CrossRef] [PubMed]
- Peiffer, S.; Kappler, A.; Haderlein, S.B.; Schmidt, C.; Byrne, J.M.; Kleindienst, S.; Vogt, C.; Richnow, H.H.; Obst, M.; Angenent, L.T.; et al. A biogeochemical–hydrological framework for the role of redox-active compounds in aquatic systems. Nat. Geosci. 2021, 14, 264–272. [Google Scholar] [CrossRef]
- Leng, L.Y.; Ahmed, O.H.; Jalloh, M.B. Brief review on climate change and tropical peatlands. Geosci. Front. 2019, 10, 373–380. [Google Scholar] [CrossRef]
- Morris, P.J. Wetter is better for peat carbon. Nat. Clim. Chang. 2021, 11, 561–562. [Google Scholar] [CrossRef]
- Huang, Y.; Ciais, P.; Luo, Y.; Zhu, D.; Wang, Y.; Qiu, C.; Goll, D.S.; Guenet, B.; Makowski, D.; De Graaf, I.; et al. Tradeoff of CO2 and CH4 emissions from global peatlands under water-table drawdown. Nat. Clim. Chang. 2021, 11, 618–622. [Google Scholar] [CrossRef]
- Hoyos-Santillan, J.; Lomax, B.H.; Large, D.; Turner, B.L.; Boom, A.; Lopez, O.R.; Sjögersten, S. Quality not quantity: Organic matter composition controls of CO2 and CH4 fluxes in neotropical peat profiles. Soil Biol. Biochem. 2016, 103, 86–96. [Google Scholar] [CrossRef]
- Ise, T.; Dunn, A.L.; Wofsy, S.C.; Moorcroft, P.R. High sensitivity of peat decomposition to climate change through water-table feedback. Nat. Geosci. 2008, 1, 763–766. [Google Scholar] [CrossRef]
- Gao, C.; Sander, M.; Agethen, S.; Knorr, K.H. Electron accepting capacity of dissolved and particulate organic matter control CO2 and CH4 formation in peat soils. Geochim. Cosmochim. Acta 2018, 245, 266–277. [Google Scholar] [CrossRef]
- Roden, E.E.; Kappler, A.; Bauer, I.; Jiang, J.; Paul, A.; Stoesser, R.; Konishi, H.; Xu, H. Extracellular electron transfer through microbial reduction of solid-phase humic substances. Nat. Geosci 2010, 3, 417–421. [Google Scholar] [CrossRef]
- Coles, J.R.P.; Yavitt, J.B. Linking belowground carbon allocation to anaerobic CH4 and CO2 production in a forested peatland, New York State. Geomicrobiol. J. 2004, 21, 445–455. [Google Scholar] [CrossRef]
- Yavitt, J.B.; Williams, C.J. Linking tree species identity to anaerobic microbial activity in a forested wetland soil via leaf litter decomposition and leaf carbon fractions. Plant. Soil 2015, 390, 293–305. [Google Scholar] [CrossRef]
- Barak, P.; Chen, Y. Equivalent radii of humic macromolecules from acid-base titration. Soil Sci. 1992, 152, 184–195. [Google Scholar] [CrossRef]
- Inbar, Y.; Chen, Y.; Hadar, Y. Solid-state carbon-13 nuclear magnetic resonance and infrared spectroscopy of composted organic matter. Soil Sci. Soc. Am. J. 1989, 53, 1695–1701. [Google Scholar] [CrossRef]
- Bravo, C.; De Nobili, M.; Gambi, A.; Martin-Neto, L.; Nascimento, O.R.; Toniolo, R. Kinetics of electron transfer reactions by humic substances: Implications for their biogeochemical roles and determination of their electron donating capacity. Chemosphere 2022, 286, 131755. [Google Scholar] [CrossRef] [PubMed]
- Aeschbacher, M.; Sander, M.; Schwarzenbach, R.P. Novel electrochemical approach to assess the redox properties of humic substances. Environ. Sci. Technol. 2010, 44, 87–93. [Google Scholar] [CrossRef] [PubMed]
- Miller, J.C.; Miller, J.N. Statistics and Chemometrics for Analytical Chemistry, 6th ed.; Pearson: Harlow, UK, 2010. [Google Scholar]
- R Development Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2018. [Google Scholar]
- Nicholson, R.S.; Shain, I. Theory of Stationary Electrode Polarography. Single Scan and Cyclic Methods Applied to Reversible, Irreversible, and Kinetic Systems. Anal. Chem. 1964, 36, 706–723. [Google Scholar] [CrossRef]
- Chin, Y.P.; Aiken, G.; O’Loughlin, E. Molecular Weight, Polydispersity, and Spectroscopic Properties of Aquatic Humic Substances. Environ. Sci. Technol. 1994, 28, 1853–1858. [Google Scholar] [CrossRef]
- Del Vecchio, R.; Blough, N.V. On the origin of the optical properties of humic substances. Environ. Sci. Technol. 2004, 38, 3885–3891. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Senesi, N.; Schnitzer, M. Information provided on humic substances by E4/E6 ratios. Soil Sci. Soc. Am. J. 1977, 41, 352–358. [Google Scholar] [CrossRef]
- Szajdak, L.; Szatylowicz, J. Impact of drainage on hydrophobicity of fen peat-moorsh soils. Mires Peat. 2010, 6, 158–174. [Google Scholar]
- Aeschbacher, M.; Graf, C.; Schwarzenbach, R.P.; Sander, M. Antioxidant properties of Humic Substances. Environ. Sci. Technol. 2012, 46, 4916–4925. [Google Scholar] [CrossRef]
- Nurmi, J.T.; Tratnyek, P.G. Electrochemical properties of natural organic matter (NOM), fractions of NOM, and model biogeochemical electron shuttles. Environ. Sci. Technol. 2002, 36, 617–624. [Google Scholar] [CrossRef]
- Maurer, F.; Christl, I.; Kretzschmar, R. Reduction and reoxidation of humic acid: Influence on spectroscopic properties and proton binding. Environ. Sci. Technol. 2010, 44, 5787–5792. [Google Scholar] [CrossRef] [PubMed]
- Boyle, E.S.; Guerriero, N.; Thiallet, A.; Del Vecchio, R.; Blough, N.V. Optical properties of humic substances and CDOM: Relation to structure. Environ. Sci. Technol. 2009, 43, 2262–2268. [Google Scholar] [CrossRef] [PubMed]
- Macalady, D.; Walton-Day, K. New light on a dark subject: On the use of fluorescence data to deduce redox states of natural organic matter (NOM). Aquat. Sci. 2009, 71, 135–143. [Google Scholar] [CrossRef]
- Zaccone, C.; Cocozza, C.; D’Orazio, V.; Plaza, C.; Cheburkin, A.; Miano, T.M. Influence of extractant on quality and trace elements content of peat humic acids. Talanta 2007, 73, 820–830. [Google Scholar] [CrossRef] [PubMed]
- Zykova, M.V.; Schepetkin, I.A.; Belousov, M.V.; Krivoshchekov, S.V.; Logvinova, L.A.; Bratishko, K.A.; Yusubov, M.S.; Romanenko, S.V.; Quinn, M.T. Physicochemical Characterization and Antioxidant Activity of Humic Acids Isolated from Peat of Various Origins. Molecules 2018, 23, 753. [Google Scholar] [CrossRef] [Green Version]



| OC (%) | Ntot (%) | C/N | δ13C (‰) 1 | |
|---|---|---|---|---|
| Original Peat | 45.9 ± 1.0 a | 1.10 ± 0.06 a | 41.7 a | −27.04 ± 0.05 a |
| HA0 | 48.8 ± 0.2 b | 1.96 ± 0.20 b | 24.9 b | −26.98 ± 0.04 a |
| HAox | 48.8 ± 0.5 b | 2.22 ± 0.12 b | 22.0 c | −27.08 ± 0.02 a |
| HAred | 48.9 ± 0.2 b | 1.93 ± 0.14 b | 25.3 b | −26.97 ± 0.04 a |
| 1 SUVA254 | 1 E4/E6 | 1 SA400 | 2 Peak A | 2 Peak B | |||
|---|---|---|---|---|---|---|---|
| (l mg−1 cm−1) | (l mg−1 cm−1) | Ex/Em | F.I. | Ex/Em | F.I. | ||
| HAred | 4.66 ± 0.16 a | 5.52 ± 0.04 a | 0.98 a | 255/470 | 0.71 a | 340/470 | 0.58 a |
| HAox | 4.77 ± 0.12 a | 5.83 ± 0.05 b | 1.14 b | 260/470 | 0.65 a | 340/470 | 0.53 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bravo, C.; Toniolo, R.; Contin, M.; Nobili, M.D. Electrochemical and Structural Modifications of Humic Acids in Aerobically and Anaerobically Incubated Peat. Land 2021, 10, 1189. https://doi.org/10.3390/land10111189
Bravo C, Toniolo R, Contin M, Nobili MD. Electrochemical and Structural Modifications of Humic Acids in Aerobically and Anaerobically Incubated Peat. Land. 2021; 10(11):1189. https://doi.org/10.3390/land10111189
Chicago/Turabian StyleBravo, Carlo, Rosanna Toniolo, Marco Contin, and Maria De Nobili. 2021. "Electrochemical and Structural Modifications of Humic Acids in Aerobically and Anaerobically Incubated Peat" Land 10, no. 11: 1189. https://doi.org/10.3390/land10111189







