Effects of Understory Vegetation Heterogeneity on Soil Organic Carbon Components in Cunninghamia lanceolata Plantation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Site
2.2. Plot Establishment
2.3. Soil Sample Collection and Measurement
2.4. Statistical Analyses
3. Results
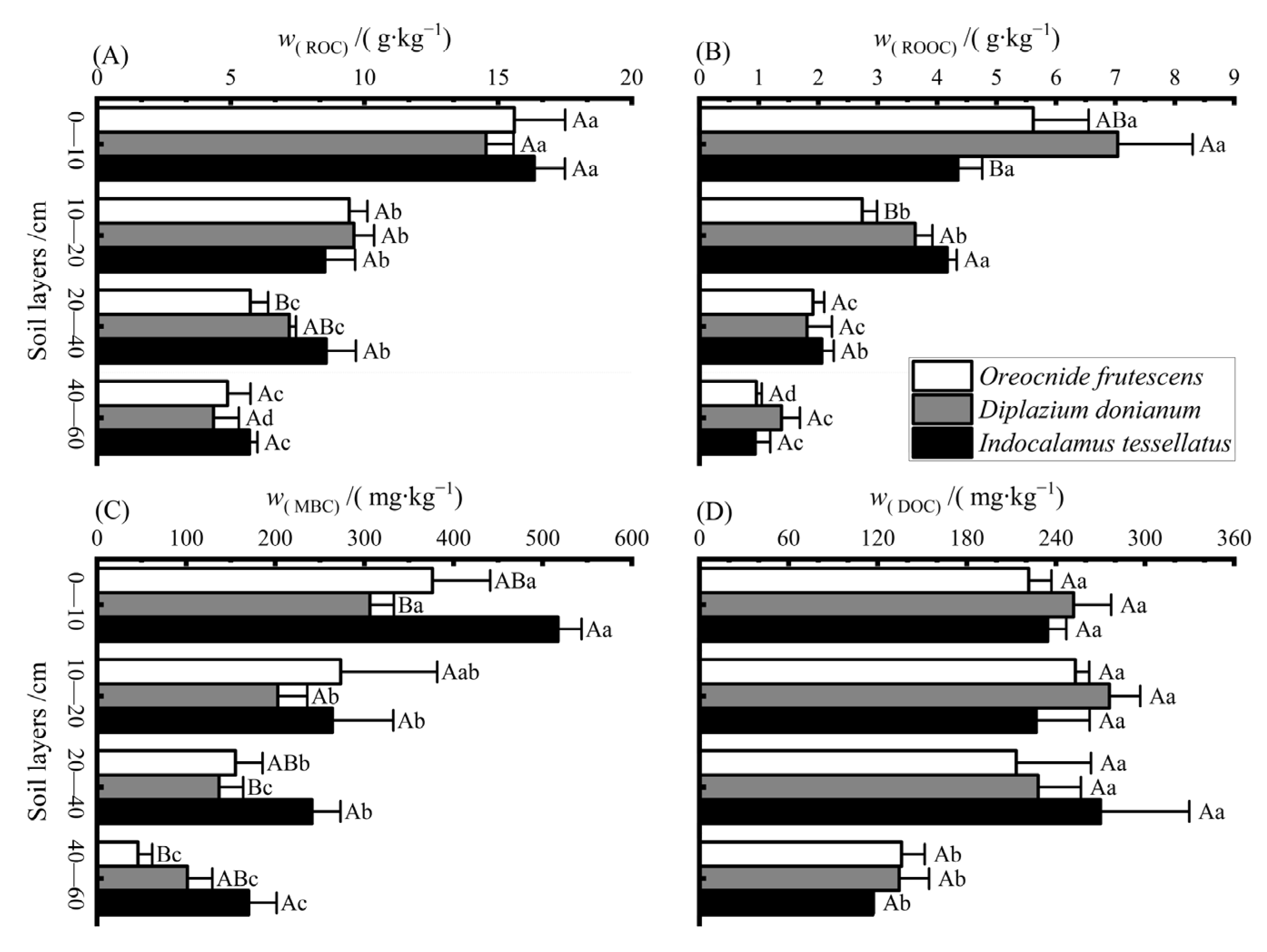
3.1. Mass Fraction Characteristics of Soil TOC
3.2. Mass Fraction Characteristics of Soil Organic Carbon Components
3.3. The Percentage of Soil Organic Carbon Composition to TOC
3.4. Correlation Analysis of Soil Organic Carbon Components with Physicochemical Factors
4. Discussion
4.1. Characteristics of Changes in Soil TOC in Different Understory Vegetation
4.2. Characteristics of Changes in Soil Organic Carbon Components in Different Understory Vegetation
4.3. Relationship between Soil Organic Carbon Components and Soil Physicochemical Factors
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liu, S.R.; Wang, H.; Luan, J.W. A review of research progress and future prospective of forest soil carbon stock and soil carbon process in China. Acta Ecol. Sin. 2011, 31, 5437–5448. [Google Scholar]
- Lai, R. Soil carbon sequestration impacts on global climate change and food security. Science 2004, 304, 1623–1627. [Google Scholar]
- Parton, W.J.; Schimel, D.S.; Cole, C.V. Analysis of factors controlling soil organic matter levels in Great Plains grasslands. Soil Sci. Soc. Am. J. 1987, 51, 1173–1179. [Google Scholar] [CrossRef]
- Li, Y.C.; Li, Y.F.; Chang, S.X. Linking soil fungal community structure and function to soil organic carbon chemical composition in intensively managed subtropical bamboo forests. Soil Biol. Biochem. 2017, 107, 19–31. [Google Scholar] [CrossRef]
- Zhao, S.X.; Ta, N.; Li, Z.H. Varying pyrolysis temperature impacts application effects of biochar on soil labile organic carbon and humic fractions. Appl. Soil Ecol. 2018, 123, 484–493. [Google Scholar] [CrossRef]
- Zhu, H.Y.; Wang, Z.F.; Lu, C. Characteristics of changes in soil reactive organic carbon and carbon pool under five planting covers in Jinyun Mountain. Soils 2021, 53, 354–360. [Google Scholar]
- Xi, D.; Kuang, Y.W. Characteristics of soil organic carbon and its components in subtropical broadleaf evergreen forests on an urbanization gradient. Chin. J. Appl. Ecol. 2018, 29, 2149–2155. [Google Scholar]
- Du, X.; Wang, H.Y. Organic carbon activity fraction in Chinese forest soils and its influencing factors. World For. Res. 2022, 35, 76–81. [Google Scholar]
- Ravindran, A.; Yang, S. Effect of vegetation type on microbial biomass carbon and nitrogen in subalpine mountain forest soils. J. Microbiol. Immunol. Infect. 2015, 45, 362–369. [Google Scholar] [CrossRef]
- Wang, D.; Wang, B.; Niu, X. Effects of natural forest types on soil carbon fractions in North-East China. J. Trop. For. Sci. 2014, 26, 362–370. [Google Scholar]
- Inoue, T.; Fukuzawa, K.; Watanabe, T. Spatial pattern of soil nitrogen availability and its relationship to stand structure in a coniferous-broadleaved mixed forest with a dense dwarf bamboo understory in northern Japan. Ecol. Res. 2017, 32, 227–241. [Google Scholar] [CrossRef]
- Zhang, J.Y.; Yu, T.; E, X.W. Effects of understory vegetation management on soil microbial biomass carbon and nitrogen and extracellular enzyme activities in the early stages of plantation. Acta Ecol. Sin. 2021, 41, 9898–9909. [Google Scholar]
- Dai, X.Q.; Fu, X.L.; Kou, L. C: N: P stoichiometry of rhizosphere soils differed significantly among overstory trees and understory shrubs in plantations in subtropical China. Can. J. For. Res. 2018, 48, 1398–1405. [Google Scholar] [CrossRef]
- Mo, X.L.; Dai, X.Q.; Wang, H.M. Rhizosphere effects of overstory tree and understory shrub species in central subtropical plantations—A case study at Qianyanzhou, Taihe, Jiangxi, China. Chin. J. Plant Ecol. 2018, 42, 723–733. [Google Scholar]
- Wang, X.P.; Yang, X.; Yang, N. Effects of litter diversity and composition on litter decomposition characteristics and soil microbial community. Acta Ecol. Sin. 2019, 39, 6264–6272. [Google Scholar]
- Gao, Y.Q.; Dai, X.Q.; Wang, J.L. Characteristics of soil enzymes stoichiometry in rhizosphere of understory vegetation in subtropical forest plantations. Chin. J. Plant Ecol. 2019, 43, 258–272. [Google Scholar] [CrossRef]
- Wang, T.; Xu, Q.; Gao, D. Effects of thinning and understory removal on the soil water-holding capacity in Pinus massoniana plantations. Sci. Rep. 2021, 11, 13029. [Google Scholar] [CrossRef]
- Xi, D.; Weng, H.D.; Hu, Y.L. Effect of canopy nitrogen addition and understory vegetation removal on soil organic carbon fractions in a Chinese fir plantation. Acta Ecol. Sin. 2021, 41, 8525–8534. [Google Scholar]
- Pan, P.; Zhao, F.; Ouyang, X.Z.; Zang, H.; Ning, J.K.; Guo, R. Characteristics of soil carbon and nitrogen and relationship with litter quality under different understory vegetation in Pinus massoniana plantations. Acta Ecol. Sin. 2018, 38, 3988–3997. [Google Scholar]
- Zhao, F.; Ouyang, X.Z. Assessing relative contributions of various influencing factors to soil organic carbon in aerially—Seeded Pinus massoniana plantations. Acta Ecol. Sin. 2016, 36, 2637–2645. [Google Scholar]
- Xu, H.T.; Sun, Y.J.; Liu, S.Z. Stand biomass and carbon storage distribution of Chinese fir plantation in Subtropical China: A case study of China Lanceolata plantation in Jiangle County, Fujian Province. J. Cent. South Univ. For. Technol. 2015, 35, 94–99. [Google Scholar]
- Xia, L.D.; Yu, J.D.; Deng, L.L. Researches on soil decline of Chinese fir plantation. World For. Res. 2012, 31, 37–42. [Google Scholar]
- Fei, Y.C.; Wu, Q.Z.; Zhang, X. Effects of different undergrowth vegetation management measures on soil characteristics and timber outturn of a large-diameter, timber plantation of Cunninghamia lanceolata. Chin. J. Appl. Environ. Biol. 2020, 26, 626–634. [Google Scholar]
- Wang, Y.Z.; Zheng, J.Q.; Liu, X.; Yan, Q.; Hu, Y.L. Short-term impact of fire-deposited charcoal on soil microbial community abundance and composition in a subtropical plantation in China—ScienceDirect. Geoderma 2020, 359, 113992. [Google Scholar] [CrossRef]
- Brookes, P.C.; Landman, A.; Pruden, G.; Jenkinson, D.S. Chloroform fumigation and the release of soil nitro-gen: A rapid direct extraction method to measure microbial biomass nitrogen in soil. Soil Biol. Biochem. 1985, 17, 837–842. [Google Scholar] [CrossRef]
- Jenkinson, D.S.; Powlson, D.S. The effects of biocidal treatments on metabolism in soil. Soil Biol. Biochem. 1976, 8, 209–213. [Google Scholar] [CrossRef]
- Fang, H.; Cheng, S.; Yu, G.; Xu, M.; Wang, Y.; Li, L. Experimental nitrogen deposition alters the quantity and quality of soil dissolved organic carbon in an alpine meadow on the Qinghai-Tibetan Plateau. Appl. Soil Ecol. 2014, 81, 1–11. [Google Scholar] [CrossRef]
- Lu, R.K. Methods for Agricultural Chemical Analysis of Soil; China Agricultural Science and Technology Press: Beijing, China, 1999. (In Chinese) [Google Scholar]
- Ma, H.Y.; Li, X.Z.; Ma, X.Y.; Gong, L. Soil organic carbon components and their influencing factors under different vegetation types in the middle part of northern Tianshan Mountains, Xinjiang. Ecol. Environ. Sci. 2022, 31, 1124–1131. [Google Scholar]
- Xiang, H.M.; Wen, D.Z.; Zhang, L.L. Changes in soil active and inert carbon along an elevation gradient in Dinghu Mountain forests. Acta Ecol. Sin. 2015, 35, 6089–6099. [Google Scholar]
- Jobbagy, E.G.; Jackson, R.B. The vertical distribution of soil organic carbon and its relation to climate and vegetation. Ecol. Appl. 2000, 10, 423–436. [Google Scholar] [CrossRef]
- Wang, D.; Geng, Z.C.; Yu, D. Soil organic carbon stocks and vertical distribution of carbon and nitrogen in typical forest stands in the Qinling Mountains. Acta Ecol. Sin. 2015, 35, 5421–5429. [Google Scholar]
- Sauheitl, L.; Glaser, B.; Dippold, M. Amino acid fingerprint of a grassland soil reflects changes in plant species richness. Plant Soil 2010, 334, 353–363. [Google Scholar] [CrossRef]
- Yang, L.W.; Luo, T.X.; Wu, S.T. Comparison of belowground biomass and carbon and nitrogen stocks in different successional stages of primitive broadleaf red pine forests in Changbai Mountain. Chin. J. Appl. Ecol. 2005, 7, 1195–1199. [Google Scholar]
- Knorr, W.; Prentice, I.C.; House, J.I. Long-term sensitivity of soil carbon turnover to warming. Nature 2005, 433, 298–301. [Google Scholar] [CrossRef] [PubMed]
- Sylvain, T.; Miguel, A. Changes in soil organic carbon pools along a chronosequence of land abandonment in southern Spain. Geoderma 2016, 268, 14–21. [Google Scholar]
- Zhang, X.Y.; Chen, X.M.; Wei, H. Effects of urbanization on soil organic carbon components and carbon pool management index of persistent evergreen broad-leaved forest in the Pearl River Delta. J. Soil Water Conserv. 2017, 31, 184–190. [Google Scholar]
- Wu, Y.C.; Li, Z.C.; Cheng, C.F. Effects of understory removal on soil labile organic carbon pool in a Cinnamomum camphora plantation. Chin. J. Appl. Ecol. 2013, 24, 3341–3346. [Google Scholar]
- Ma, Y.D.; Jiang, H.; Yu, S.Q.; Dou, R.P.; Guo, P.P.; W, B. Decomposition characteristics of plant leaf litter of different origin times in the central subtropics. Acta Ecol. Sin. 2009, 29, 5237–5245. [Google Scholar]
- Cui, D.; Yan, J.J.; Liu, H.J. Differences in soil labile organic carbon fractions and their contents in different types of wetlands in the Ili River Valley. Chin. J. Ecol. 2019, 38, 2087–2093. [Google Scholar]
- Li, P.Q.; Fang, X.M.; Chen, F.S. Variability of soil water soluble organic carbon content and its response to temperature change in green spaces along urban-to-rural gradient of Nanchang, China. Chin. J. Appl. Ecol. 2015, 26, 3398–3404. [Google Scholar]




| Undergrowth Vegetation | Indocalamus tessellatus | Diplazium donianum | Oreocnide frutescens |
|---|---|---|---|
| Height (cm) | 44.2 ± 1.4 | 108.3 ± 13.0 | 130.8 ± 4.6 |
| Ground diameter (cm) | 0.53 ± 0.1 | 5.80 ± 1.1 | 1.7 ± 0.68 |
| Slope aspect | Southwest | Southwest | Southwest |
| Slope position | Downslope | Mid-slope | Upslope |
| Temperature (°C) | 18.96 | 19.05 | 18.91 |
| Altitude (masl) | 110 ± 3 | 125 ± 3 | 130 ± 3 |
| Abundance (%) | 90.3 ± 0.7 | 83.1 ± 2.5 | 79.0 ± 5.7 |
| Associated understory vegetation | Phoebe bournei (Hemsl.) Yang, Lophatherum gracile Brongn., Diplazium donianum. | Callicarpa kochiana Makino, Phoebe bournei, Indocalamus tessellatus, Oreocnide frutescens, Dicranopteris linearis (Burm.) Underw. | Phoebe bournei, Indocalamus tessellatus, Diplazium donianum, Embelia rudis Hand.-Mazz., Callicarpa kochiana, Dicranopteris linearis, Melastoma dodecandrum Lour. |
| Index | Soil Layers (cm) | Indocalamus tessellatus | Diplazium donianum | Oreocnide frutescens |
|---|---|---|---|---|
| PROC/TOC | 0–10 | 81.46 ± 7.79 Aa | 74.39 ± 14.69 Aa | 74.89 ± 12.29 Aa |
| 10–20 | 64.548 ± 9.56 Aa | 72.07 ± 9.02 Aa | 74.13 ± 7.25 Aa | |
| 20–40 | 83.53 ± 8.48 Aa | 70.35 ± 11.95 Aa | 68.65 ± 19.40 Aa | |
| 40–60 | 86.04 ± 15.02 Aa | 72.72 ± 10.62 Aa | 76.42 ± 25.85 Aa | |
| PROOC/TOC | 0–10 | 21.66 ± 1.03 Ba | 35.46 ± 4.13 Aa | 27.35 ± 5.46 Ba |
| 10–20 | 31.96 ± 2.53 Aa | 27.35 ± 4.72 ABab | 21.76 ± 4.72 Ba | |
| 20–40 | 20.27 ± 2.20 Aa | 17.19 ± 6.74 Ab | 22.84 ± 5.77 Aa | |
| 40–60 | 13.58 ± 4.85 Aa | 23.47 ± 11.38 Aa | 14.50 ± 2.17 Ab | |
| PMBC/TOC | 0–10 | 2.59 ± 0.35 Aa | 1.54 ± 0.20 Ba | 1.84 ± 0.71 ABa |
| 10–20 | 2.02 ± 0.84 Aa | 1.54 ± 0.56 Aa | 2.08 ± 1.48 Aa | |
| 20–40 | 2.35 ± 0.29 Aa | 1.31 ± 0.48 Ba | 1.82 ± 0.50 ABa | |
| 40–60 | 1.90 ± 0.90 Aa | 1.75 ± 0.98 Aa | 0.69 ± 0.46 Aa | |
| PDOC/TOC | 0–10 | 1.17 ± 0.03 Ab | 1.29 ± 0.34 Ab | 1.08 ± 0.21 Ab |
| 10–20 | 1.74 ± 0.43 Aa | 2.08 ± 0.35 Aa | 2.00 ± 0.20 Aa | |
| 20–40 | 2.63 ± 0.81 Aa | 2.19 ± 0.42 Aa | 2.50 ± 0.93 Aa | |
| 40–60 | 3.21 ± 2.27 Aa | 2.23 ± 0.46 Aa | 2.13 ± 0.79 Aa |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, R.; Han, X.; Yang, Q.; Zheng, Z.; Xi, D. Effects of Understory Vegetation Heterogeneity on Soil Organic Carbon Components in Cunninghamia lanceolata Plantation. Land 2022, 11, 2300. https://doi.org/10.3390/land11122300
Song R, Han X, Yang Q, Zheng Z, Xi D. Effects of Understory Vegetation Heterogeneity on Soil Organic Carbon Components in Cunninghamia lanceolata Plantation. Land. 2022; 11(12):2300. https://doi.org/10.3390/land11122300
Chicago/Turabian StyleSong, Ruipeng, Xiaomeng Han, Qifan Yang, Zhiheng Zheng, and Dan Xi. 2022. "Effects of Understory Vegetation Heterogeneity on Soil Organic Carbon Components in Cunninghamia lanceolata Plantation" Land 11, no. 12: 2300. https://doi.org/10.3390/land11122300




