The Rapid Effects of Yellow-Legged Gull (Larus michahellis) Colony on Dune Habitats and Plant Landscape in the Atlantic Islands National Park (NW Spain)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Floristic Inventories and Soil Sampling
2.3. Study of Vegetation Cover by Image Analysis
2.4. Soil Analysis
2.5. Statistical Analysis
3. Results
3.1. Floristic Inventories and Plant Cover
3.2. Study of Plant Cover Using Satellite Images
3.3. Soil Properties and Nutrient Concentration
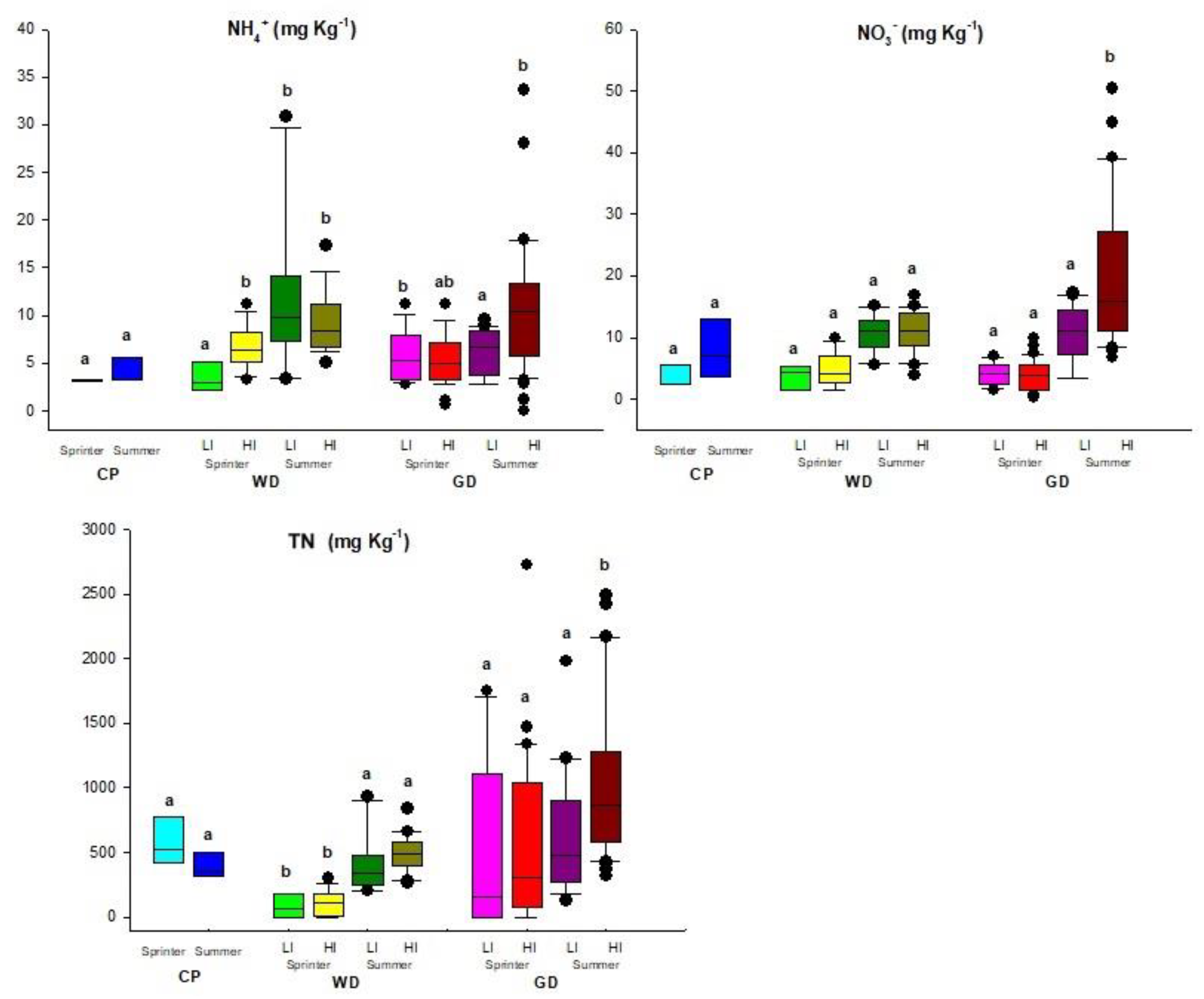
3.3.1. Total Nitrogen (TN) and Inorganic Nitrogen (N-NH4+, N-NO3−)
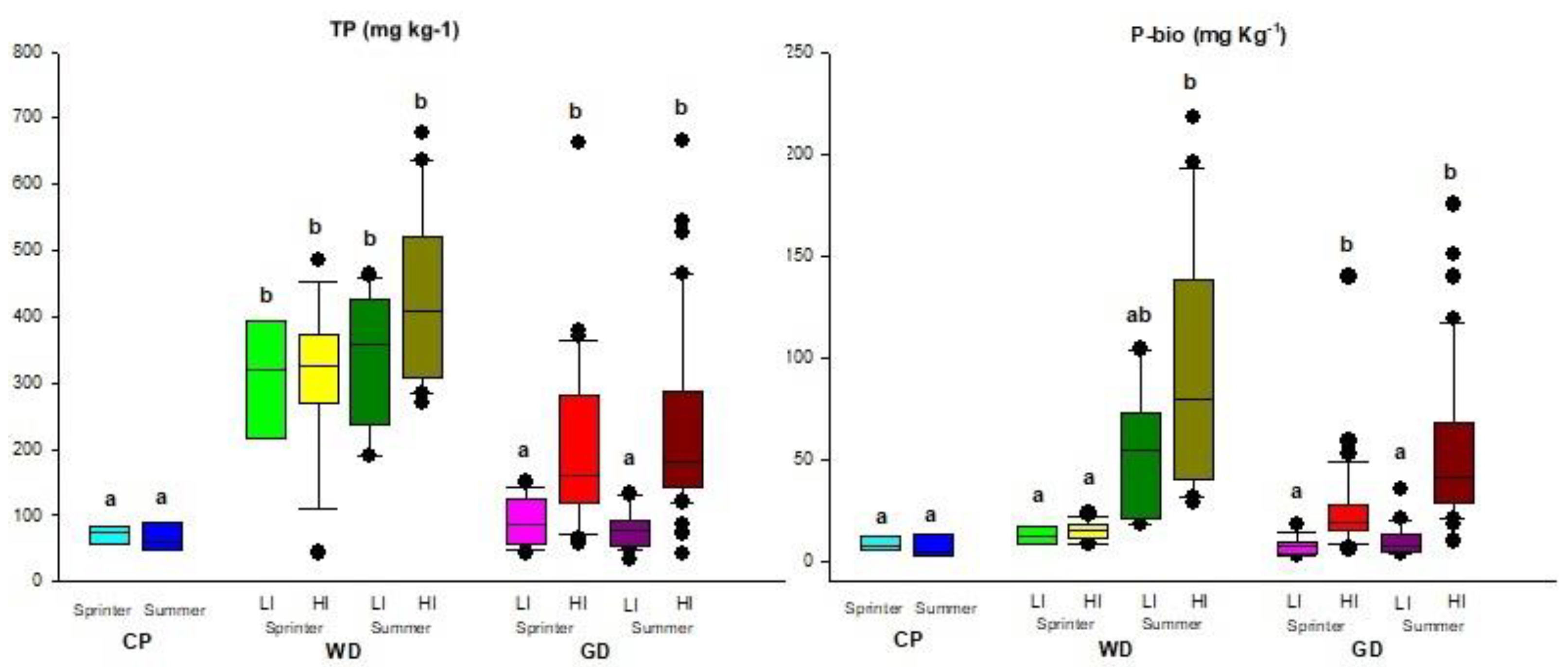
3.3.2. Total Phosphorus (TP) and Bioassimilable Phosphorus (P-Bio)
3.4. Interaction between Soil Conditions and Vegetation
4. Discussion
4.1. Influence of the Yellow-Legged Gull Colony on Dune Vegetation and Habitats
4.2. The Effect of Seabird Colony on Nutrient Availability
4.3. Interaction between Soil Conditions and Vegetation
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Otero, X.L.; Tejada, O.; Martín-Pastor, M.; De la Peña, S.; Ferreira, T.O.; Pérez-Alberti, A. Phosphorus in seagull colonies and the effect on the habitats. The case of yellow-legged gulls (Larus michahellis) in the Atlantic Islands National Park (Galicia-NW Spain). Sci. Total Environ. 2015, 532, 383–397. [Google Scholar] [CrossRef] [PubMed]
- Otero Pérez, X.L.; De La Peña-Lastra, S.; Pérez-Alberti, A.; Ferreira, T.O.; Huerta-Díaz, M.A. Seabird colonies as new global drivers in the nitrogen and phosphorus cycles. Nat. Commun. 2018, 9, 246. [Google Scholar] [CrossRef] [PubMed]
- Zmudczynska-Skarbek, K.; Barcikowski, M.; Drobniak, S.M.; Gwiazdowicz, D.J.; Richard, P.; Skubała, P.; Stempniewicz, L. Transfer of ornithogenic influence through different trophic levels of the Arctic terrestrial ecosystem of Bjørnøya (Bear Island), Svalbard. Soil Biol. Biochem. 2017, 115, 475–489. [Google Scholar] [CrossRef]
- Magnusson, B.; Magnusson, S.H. Vegetation succession on Surtsey, Iceland, during 1990–1998 under the influence of breeding gulls. Surtsey Res. 2000, 11, 9–20. [Google Scholar] [CrossRef] [Green Version]
- Ellis, J.C. Marine birds on land: A review of plant biomass, species richness, and community composition in seabird colonies. Plant Ecol. 2005, 181, 227–241. [Google Scholar] [CrossRef]
- Miller, G.C. Changes in Plant Distribution and Island Size Accompanying White Pelican Nesting. Colon. Waterbirds 1982, 5, 73–78. [Google Scholar] [CrossRef]
- Sánchez-Piñero, F.; Polis, G.A. Bottom-up dynamics of allochthonous input: Direct and indirect effects of seabirds on islands. Ecology 2000, 81, 3117–3132. [Google Scholar] [CrossRef]
- Grime, J.P. Evidence for the existence of three primary strategies in plants and its relevance to ecological and evolutionary theory. Am. Nat. 1977, 111, 1169–1194. [Google Scholar] [CrossRef]
- Hogg, E.H.; Morton, J.K. The effect of nesting gulls on the vegetation and soil of islands in the Great Lakes. Can. J. Bot. 1983, 61, 3240–3254. [Google Scholar] [CrossRef]
- Hogg, E.H.; Morton, J.K.; Venn, J.M. Biogeography of island floras in the Great Lakes. I. Species richness and composition in relation to gull nesting activities. Can. J. Bot. 1989, 67, 961–969. [Google Scholar] [CrossRef]
- Vidal, E.; Jouventin, P.; Frenot, Y. Contribution of alien and indigenous species to plant-community assemblages near penguin rookeries at Crozet archipelago. Polar Biol. 2003, 26, 432–437. [Google Scholar] [CrossRef]
- Baumberger, T.; Affre, L.; Torre, F.; Vidal, E.; Dumas, P.-J.; Tatoni, T. Plant community changes as ecological indicator of seabird colonies’ impacts on Mediterranean Islands. Ecol. Indic. 2012, 15, 76–84. [Google Scholar] [CrossRef]
- Hawke, D.J.; Holdaway, R.N.; Causer, J.E.; Ogden, S. Soil indicators of pre-European seabird breeding in New Zealand at sites identified by predator deposits. Aust. J. Soil Res. 1999, 37, 103–113. [Google Scholar]
- Guitián, J.; Guitián, P. A paisaxe vexetal das illas Cíes; Xunta de Galicia: Santiago de Compostela, Spain, 1990; p. 127. [Google Scholar]
- Zwolicki, A.; Zmudczyńska-Skarbek, K.; Richard, P.; Stempniewicz, L. Importance of Marine-Derived Nutrients Supplied by Planktivorous Seabirds to High Arctic Tundra Plant Communities. PLoS ONE 2016, 11, e0154950. [Google Scholar] [CrossRef]
- MeteoGalicia. Consellería de Medio Ambiente, Territorio e Infraestruturas-Xunta de Galicia. 2018. Available online: www.meteogalicia.gal (accessed on 10 October 2021).
- Pérez, C.; Barros, Á.; Velando, A.; Munilla, I. Seguimento das Poboacións Reproductoras de Corvo Mariño (Phalacrocorax aristotelis) e Gaivota Patimarela (Larus michahellis) do Parque Nacional das Illas Atlánticas de Galicia; Universidade de Santiago de Compostela, Universidade de Vigo, Ministerio de Medio Ambiente: Vigo, Spina, 2012; p. 41. [Google Scholar]
- Barros, A. Censo da Poboación Reprodutora de Corvo Mariño Cristado (Phalacrocorax aristotelis), Gaivota Patiamarela (Larus michahellis), Gaivota Escura (Larus fuscus) e Gaivotón (Larus marinus) no Parque Nacional Marítimo-Terrestre das Illas Atlánticas de Galicia: Resultados de 2015. 2015; 21, unpublished report. [Google Scholar]
- Munilla, I. Seabird monitoring at the National Park of the Atlantic islands of Galicia. Results of 2017; Parque Nacional Marítimo e Terrestre das illas Atlánticas de Galicia: Galicia, Spain, 2017; p. 43. [Google Scholar]
- Braun-Blanquet, J. Fitosociología: Bases para el Estudio de las Comunidades Vegetales; Blume Ediciones: Madrid, Spain, 1979; p. 820. [Google Scholar]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef]
- Ridler, T.W.; Calvard, S. Picture thresholding using an iterative selection method. IEEE Trans. Syst. Man Cybern. 1978, 8, 630–632. [Google Scholar] [CrossRef]
- Buurman, P.; Van Lagen, B.; Velthorst, E.J. Manual for Soil and Water Analysis; Backhuys Publishers: Leiden, The Netherlands, 1996; p. 214. [Google Scholar]
- Mulvaney, R.L. Nitrogen-Inorganic forms. In Methods of Soil Análisis. Part 3. Chemical Methods; Sparks, D.L., Page, A.L., Helmke, P.A., Loeppert, R.H., Eds.; SSSA Book Ser. 5.3; SSSA, ASA: Madison, WI, USA, 1996; pp. 1123–1184. [Google Scholar]
- Mehlich, A. Mehlich 3 soil test extractant: A modification of the mehlich 2 extractant. Commun. Soil Sci. Plant Anal. 1984, 15, 1409–1416. [Google Scholar] [CrossRef]
- Ter Braak, C.J.F. Canonical Correspondence Analysis: A new eigenvector technique for multivariate direct gradient analysis. Ecology 1986, 67, 1167–1179. [Google Scholar] [CrossRef] [Green Version]
- Dray, S.; Dufour, A.B.; Chessel, D. The ade4 package-II: Two-table and K-table methods. R News 2007, 7, 47–52. [Google Scholar]
- Oksanen, J.; Blanchet, F.G.; Friendly, M.; Kindt, R.; Legendre, P.; McGlinn, D.; Minchin, P.R.; O’Hara, R.B.; Simpson, G.L.; Solymos, P.; et al. Vegan: Community Ecology Package. R Package Version 2.4-6. 2018. Available online: https://CRAN.R-project.org/package=vegan (accessed on 1 October 2021).
- Ishida, A. Effects of the common cormorant, Phalacrocorax carbo, on evergreen forests in two nest sites at Lake Biwa, Japan. Ecol. Res. 1996, 11, 193–200. [Google Scholar] [CrossRef]
- Sobey, G.G.; Kenworthy, J.B. The relationship between herring gulls and the vegetation of their breeding colonies. J. Ecol. 1979, 67, 469–496. [Google Scholar] [CrossRef]
- Otero, X.L.; Fernández-Balado, C.; Ferreira, T.O.; Pérez-Alberti, A.; Revilla, G. Soil eutrophication in seabird colonies affects cell wall composition: Implications for the conservation of rare plant species 2021. Mar. Pollut. Bull. 2021, 168, 112469. [Google Scholar] [CrossRef]
- Miller, T.E.; Gornish, E.S.; Buckley, H.L. Climate and coastal dune vegetation: Disturbance, recovery and succession. Plant Ecol. 2010, 206, 97–104. [Google Scholar] [CrossRef]
- García-Novo, F.; Diaz-Barradas, M.C.; Zunzunegui, M.; García-Mora, R.; Gallego Fernández, J.B. Plant Functional Types in Coastal Dune Habitats. In Coastal Dunes: Ecology and Conservation. Ecological Studies; Martínez, M.L., Psuty, N.P., Eds.; Springer: Heidelberg/Berlin, Germany, 2004; Volume 171, pp. 155–169. [Google Scholar]
- Antunes, C.; Pereira, A.J.; Fernandes, P.; Ramos, M.; Ascensão, L.; Correia, O.; Máguas, C. Understanding plant drought resistance in a Mediterranean coastal sand dune ecosystem: Differences between native and exotic invasive species. J. Plant Ecol. 2018, 11, 26–38. [Google Scholar] [CrossRef] [Green Version]
- Coastal Dunes: Ecology and Conservation. In Ecological Studies; Martínez, M.L.; Psuty, N.P. (Eds.) Springer: Berlin, Germany, 2008; Volume 171. [Google Scholar]
- Pérez-Alberti, A.; Gómez-Pazo, A.; Otero, X.L. Natural and Anthropogenic Variations in the Large Shifting Dune in the Corrubedo Natural Park, NW Iberian Peninsula (1956–2017). Appl. Sci. 2021, 11, 34. [Google Scholar] [CrossRef]
- Tilman, D. Resources, competition and the dynamics of plant communities. In Plant Ecology, 1st ed.; Crawley, M., Ed.; Blackwell Scientific Publications: Oxford, UK, 1986; pp. 51–75. [Google Scholar]
- Hill, P.; Farrar, J.; Roberts, P.; Farrell, M.; Grant, H.; Newsham, K.; Hopkins, D.W.; Bardgett, R.D.; Jones, D.L. Vascular plant success in a warming Antarctic may be due to efficient nitrogen acquisition. Nat. Clim. Change 2011, 1, 50–53. [Google Scholar] [CrossRef]
- Willis, A.J. The Influence of Mineral Nutrients on the Growth of Ammophila Arenaria. J. Ecol. 1965, 53, 735–745. [Google Scholar] [CrossRef]
- Kooijman, A.M.; Dopheide, J.C.R.; Sevink, J.; Takken, I.; Verstraten, J.M. Nutrient limitations and their implications on the effects of atmospheric deposition in coastal dunes; Lime-poor and lime-rich sites in the Netherlands. J. Ecol. 1998, 86, 511–526. [Google Scholar] [CrossRef]
- Ligeza, S.; Smal, H. Accumulation of nutrients in soils affected by perennial colonies of piscivorous birds with reference to biogeochemical cycles of elements. Chemosphere 2003, 52, 595–602. [Google Scholar] [CrossRef]
- Kardos, L.T. Soil Fixation of Plant Nutrients. In Chemistry of the Soils; Bear, F.E., Ed.; Reinhold Publishing Corporation: New York, NY, USA, 1995; pp. 177–199. [Google Scholar]
- Mizutani, H.; Hasegawa, H.; Wada, E. High nitrogen isotope ratio for soils of seabird rookeries. Biogeochemistry 1986, 2, 221–247. [Google Scholar] [CrossRef]
- Mizota, C. Temporal variations in the concentration and isotopic signature of ammonium- and nitrate–nitrogen in soils under a breeding colony of Black-tailed Gulls (Larus crassirostris) on Kabushima Island, northeastern Japan. Appl. Geochem. 2009, 24, 328–332. [Google Scholar] [CrossRef]
- Mizota, C. Nitrogen isotopic patterns of vegetation as affected by breeding activity of Black-tailed Gull (Larus crassiostris): A coupled analysis of feces, inorganic soil nitrogen and flora. Appl. Geochem. 2009, 24, 2027–2033. [Google Scholar] [CrossRef]
- Lindeboom, H.J. The nitrogen pathway in a penguin rookery. Ecology 1984, 65, 269–277. [Google Scholar] [CrossRef]
- Blackall, T.D.; Wilson, L.J.; Bull, J.; Theobald, M.R.; Bacon, P.J.; Hamer, K.C.; Wanless, S.; Sutton, M.A. Temporal variation in atmospheric ammonia concentrations above seabird colonies. Atmos. Environ. 2008, 42, 6942–6950. [Google Scholar] [CrossRef] [Green Version]
- Riddick, S.N.; Dragosits, U.; Blackall, T.D.; Daunt, F.; Wanless, S.; Sutton, M.A. The global distribution of ammonia emissions from seabird colonies. Atmos. Environ. 2012, 55, 319–327. [Google Scholar] [CrossRef] [Green Version]
- Otero, X.L.; De La Peña-Lastra, S.; Pérez Alberti, A.; Macías, F. Variabilidad Espacio-Temporal de las Formas de N y P en Suelos de las Colonias de Gaviota Patiamarilla (Larus michahellis) en el Parque Nacional de las Islas Atlánticas de Galicia. In Proyectos de Investigación en Parques Nacionales: 2011–2014; Organismo Autónomo Parques Nacionales: Madrid, Spain, 2016; pp. 123–140. [Google Scholar]
- Hutchinson, G.E. The biogeochemistry of vertebrate excretion. Bull. Am. Mus. Nat. Hist. 1950, 96, 1–554. [Google Scholar] [CrossRef]
- Loder, I.T.C.; Ganning, B.; Love, J.A. Ammonia nitrogen dynamics in coastal rockpools affected by gull guano. J. Exp. Mar. Biol. Ecol. 1996, 196, 113–129. [Google Scholar] [CrossRef]
- De La Peña-Lastra, S.; Gómez-Rodríguez, C.; Pérez-Alberti, A.; Torre, F.; Otero, X.L. Effects of a yellow legged gull (Larus michahellis) colony on soils and cliff vegetation in the Atlantic Islands of Galicia National Park (NW Spain). Catena 2021, 199, 105115. [Google Scholar] [CrossRef]
- De la Peña-Lastra, S.; Affre, F.; Otero, X.L. Soil nutrient dynamics in colonies of the yellow-legged seagull (Larus michahellis) in different biogeographical zones. Geoderma 2020, 361, 114109. [Google Scholar] [CrossRef]




| Plot | Coordinates ETRS89 | Type of Vegetation | Seagull Influence |
|---|---|---|---|
| White Dune (WDLI) | 508439 4675059 | Otantho-Ammophiletum australis | Low |
| White Dune (WDHI) | 508440 4675073 | Otantho-Ammophiletum australis | High |
| Gray Dune (GDLI) (two plots) | 508243 4675121; 508249 4675052 | Scrophulario-Vulpietum alopecuroidis; Ulici-Coremetum albae | Low |
| Gray Dune (GDHI) (two plots) | 508248 4675072; 508294 4675120 | Scrophulario-Vulpietum alopecuroidis | High |
| Control Plot (CP) | 508109 4673837 | Scrophulario-Vulpietum alopecuroidis | Absence |
| Pearson Correlation | GDLI | GDHI | WDLI | WDHI |
| Seagull influence | −0.220 | −0.521 | −0.361 | −0.224 |
| GDLI | 0.698 | 0.861 | 0.855 | |
| GDHI | 0.521 | 0.482 | ||
| WDLI | 0.958 | |||
| WDHI | ||||
| p-value | GDLI | GDHI | WDLI | WDHI |
| seagulls | 0.723 | 0.368 | 0.551 | 0.717 |
| GDLI | 0.036 | 0.003 | 0.003 | |
| GDHI | 0.151 | 0.189 | ||
| WDLI | <0.0001 | |||
| WDHI |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peña-Lastra, S.D.L.; Torre, F.; Carballeira, R.; Santiso, M.J.; Pérez-Alberti, A.; Otero, X.L. The Rapid Effects of Yellow-Legged Gull (Larus michahellis) Colony on Dune Habitats and Plant Landscape in the Atlantic Islands National Park (NW Spain). Land 2022, 11, 258. https://doi.org/10.3390/land11020258
Peña-Lastra SDL, Torre F, Carballeira R, Santiso MJ, Pérez-Alberti A, Otero XL. The Rapid Effects of Yellow-Legged Gull (Larus michahellis) Colony on Dune Habitats and Plant Landscape in the Atlantic Islands National Park (NW Spain). Land. 2022; 11(2):258. https://doi.org/10.3390/land11020258
Chicago/Turabian StylePeña-Lastra, Saúl De La, Franck Torre, Rafael Carballeira, María José Santiso, Augusto Pérez-Alberti, and Xosé Lois Otero. 2022. "The Rapid Effects of Yellow-Legged Gull (Larus michahellis) Colony on Dune Habitats and Plant Landscape in the Atlantic Islands National Park (NW Spain)" Land 11, no. 2: 258. https://doi.org/10.3390/land11020258








