Forest Ecosystem Fragmentation in Ecuador: Challenges for Sustainable Land Use in the Tropical Andean
Abstract
:1. Introduction
2. Materials and Methods
2.1. Dataset for the Ecosystems and Land Cover Classification
- Cloud mountain forest: The height of trees reaches 15–25 m. The underwood is very rich, and epiphytes and mosses are very abundant. Persistent presence of fog at the vegetation level significantly reduces incident solar radiation and evapotranspiration [55].
- Lower mountain forest: The height of canopy can reach 20–35 m and trees of 40 m are sporadically located. The forest is composed of different layers such as canopy, sub-canopy, shrub, and herbaceous species [34].
- Foothill forest: Forest transition occurs between the foothills of eastern and western ranges and Amazonian forests. Substrate is mainly composed of volcanic rock and sediment of recent origin. The height of the canopy reaches 30 m and sub-canopy and understory are very dense [56].
| Main Land Use | No. | Land Cover Type | Description | Source |
|---|---|---|---|---|
| Forest | 1 | Native forest | Vegetation with native tree species, including higher mountain forest, cloud mountain forest, lower mountain forest, and foothill forest. | [44,57] |
| Shrub/Grassland | 2 | Shrubland | Area with a substantial component of non-tree native woody species. It includes degraded areas in transition to dense shrub layer. | [44,57] |
| 3 | Grassland | Native grassland with spontaneous growth which do not receive special care, and use for sporadic grazing or protection. | [44,58] | |
| 4 | Páramo | Typical ecosystem of the tropical Andes, located above 3400 m.a.s.l. Vegetation can reach 50 cm in height. | [44,58] | |
| Industrial plantation | 5 | Industrial plantation (PLT) | Vegetation with exotic/non-native species, including young and harvested plantations. | [58] |
| Pasture | 6 | Pasture (PST) | Cultivated grassland, dominated by introduced species of Gramineas and Legunimosas, for feeding livestock. | [58] |
| Agriculture | 7 | Annual crops (AFM) | Cultivated land for annual crops. | [58] |
| 8 | Permanent crops (PFM) | Mainly orchards and permanent crops and vegetables. | [58] | |
| 9 | Semi-permanent crops (SFM) | Cultivated land for 2- or 3-year-cycle crops. | [58] | |
| Urban | 10 | Inhabited area (HBT) | Land mainly occupied by housing and buildings for communities and public services. | [58] |
| 11 | Infrastructure (IFR) | Land occupied by roads, industry, and other anthropogenic surfaces (e.g., shrimp fishery). | [58] | |
| Others | 12 | Natural water | Land occupied by natural water bodies such as small lakes and ponds. | [58] |
| 13 | Artificial water | Land or flowing water associated with anthropic activities and water resource management. | [58] | |
| 14 | Bare ground | Cleared land, rocks, and river beds. | [58] | |
| 15 | Glacier | Snow and ice. | [58] |
2.2. Deforestation Rate, Land Cover Change Rate, and Forest Fragmentation
2.3. Analysis
3. Results
3.1. Land Use Change in Forest Ecosystems
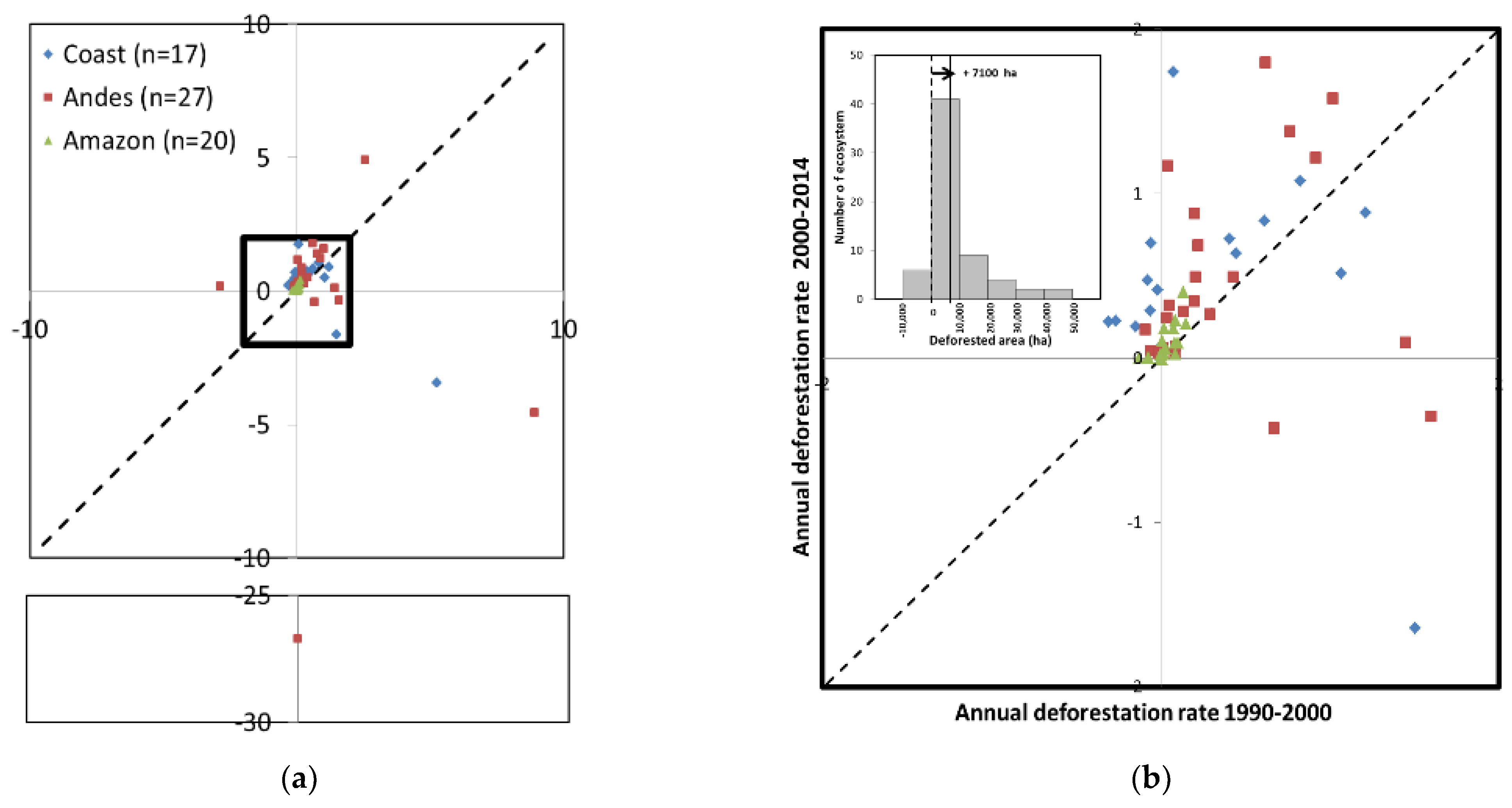
3.2. Degree of Forest Fragmentation
3.3. Relationship between Forest Fragmentation and Human Land Use
4. Discussion
4.1. Forest Fragmentation at Regional Level
4.2. Patterns of Forest Ecosystem Changes
4.3. Conservation Implications and Recommendations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Millennium Ecosystem Assessment. Ecosytems and Human Well-Being: Policy Responses; Island Press: Washington, DC, USA, 2005. [Google Scholar]
- Díaz, S.; Settele, J.; Brondízio, E.S.; Ngo, H.T.; Agard, J.; Arneth, A.; Balvanera, P.; Brauman, K.A.; Butchart, S.H.M.; Chan, K.M.A.; et al. Pervasive human-driven decline of life on Earth points to the need for transformative change. Science 2019, 366, eaax3100. [Google Scholar] [CrossRef] [Green Version]
- Hobbs, R.J.; Higgs, E.; Harris, J.A. Novel ecosystems: Implications for conservation and restoration. Trends Ecol. Evol. 2009, 24, 599–605. [Google Scholar] [CrossRef]
- Holl, K.D.; Aide, T.M. When and where to actively restore ecosystems? For. Ecol. Manag. 2011, 261, 1558–1563. [Google Scholar] [CrossRef]
- Laurance, W.F.; Lovejoy, T.E.; Vasconcelos, H.L. Ecosystem Decay of Amazonian Forest Fragments: A 22-Year Investigation. Conserv. Biol. 2002, 16, 605–618. [Google Scholar] [CrossRef] [Green Version]
- Letcher, S.G.; Chazdon, R.L. Lianas and self-supporting plants during tropical forest succession. For. Ecol. Manag. 2009, 257, 2150–2156. [Google Scholar] [CrossRef]
- Turner, M.G. Disturbance and landscape dynamics in a changing world. Ecology 2010, 91, 2833–2849. [Google Scholar] [CrossRef] [Green Version]
- Noh, J.K.; Echeverria, C.; Kleemann, J.; Koo, H.; Fürst, C.; Cuenca, P. Warning about conservation status of forest ecosystems in tropical Andes: National assessment based on IUCN criteria. PLoS ONE 2020, 15, e0237877. [Google Scholar] [CrossRef]
- Cuenca, P.; Robalino, J.; Arriagada, R.; Echeverría, C. Are government incentives effective for avoided deforestation in the tropical Andean forest? PLoS ONE 2018, 13, e0203545. [Google Scholar] [CrossRef] [Green Version]
- Taubert, F.; Fischer, R.; Groeneveld, J.; Lehmann, S.; Müller, M.S.; Rödig, E.; Wiegand, T.; Huth, A. Global patterns of tropical forest fragmentation. Nature 2018, 554, 519–522. [Google Scholar] [CrossRef]
- Duraiappah, A.K.; Naeem, S. The Role of Soil Microbiology in Restoration. Millennium Ecosystem Assessment; Cranfield University: Bedford, UK, 2005. [Google Scholar]
- Wilcove, D.S.; McLellan, C.H.; Dobson, A.P. Habitat fragmentation in the temperate zone. Conserv. Biol. 1986, 6, 237–256. [Google Scholar]
- Wang, X.; Blanchet, F.G.; Koper, N. Measuring habitat fragmentation: An evaluation of landscape pattern metrics. Methods Ecol. Evol. 2014, 5, 634–646. [Google Scholar] [CrossRef]
- Midha, N.; Mathur, P. Assessment of forest fragmentation in the conservation priority Dudhwa landscape, India using FRAGSTATS computed class level metrics. J. Indian Soc. Remote Sens. 2010, 38, 487–500. [Google Scholar] [CrossRef]
- Hargis, C.D.; Bissonette, J.A.; David, J.L. The behavior of landscape metrics commonly used in the study of habitat fragmentation. Landsc. Ecol. 1998, 13, 167–186. [Google Scholar] [CrossRef]
- Fahrig, L. Effect of Habitat Fragmentation on the Extinction Threshold: A Synthesis. Ecol. Appl. 2002, 12, 346–353. [Google Scholar] [CrossRef]
- Echeverria, C.; Coomes, D.; Salas, J.; Rey-Benayas, J.M.; Lara, A.; Newton, A. Rapid deforestation and fragmentation of Chilean Temperate Forests. Biol. Conserv. 2006, 130, 481–494. [Google Scholar] [CrossRef]
- Cain, D.H.; Riitters, K.; Orvis, K. A multi-scale analysis of landscape statistics. Landsc. Ecol. 1997, 12, 199–212. [Google Scholar] [CrossRef]
- Hermosilla, T.; Wulder, M.A.; White, J.C.; Coops, N.C.; Pickell, P.D.; Bolton, D.K. Impact of time on interpretations of forest fragmentation: Three-decades of fragmentation dynamics over Canada. Remote Sens. Environ. 2019, 222, 65–77. [Google Scholar] [CrossRef]
- Fischer, R.; Taubert, F.; Müller, M.S.; Groeneveld, J.; Lehmann, S.; Wiegand, T.; Huth, A. Accelerated forest fragmentation leads to critical increase in tropical forest edge area. Sci. Adv. 2021, 7, eabg7012. [Google Scholar] [CrossRef]
- Ibáñez, I.; Katz, D.S.W.; Peltier, D.; Wolf, S.M.; Connor Barrie, B.T. Assessing the integrated effects of landscape fragmentation on plants and plant communities: The challenge of multiprocess–multiresponse dynamics. J. Ecol. 2014, 102, 882–895. [Google Scholar] [CrossRef] [Green Version]
- Arroyo-Rodríguez, V.; Melo, F.P.L.; Martínez-Ramos, M.; Bongers, F.; Chazdon, R.L.; Meave, J.A.; Norden, N.; Santos, B.A.; Leal, I.R.; Tabarelli, M. Multiple successional pathways in human-modified tropical landscapes: New insights from forest succession, forest fragmentation and landscape ecology research. Biol. Rev. 2017, 92, 326–340. [Google Scholar] [CrossRef]
- Batar, A.K.; Watanabe, T.; Kumar, A. Assessment of Land-Use/Land-Cover Change and Forest Fragmentation in the Garhwal Himalayan Region of India. Environments 2017, 4, 34. [Google Scholar] [CrossRef] [Green Version]
- de Oliveira, B.R.; Carvalho-Ribeiro, S.M.; Maia-Barbosa, P.M. Rio Doce State Park buffer zone: Forest fragmentation and land use dynamics. Environ. Dev. Sustain. 2021, 23, 8365–8376. [Google Scholar] [CrossRef]
- Keith, D.A.; Rodríguez, J.P.; Rodríguez-Clark, K.M.; Nicholson, E.; Aapala, K.; Alonso, A.; Asmussen, M.; Bachman, S.; Basset, A.; Barrow, E.G.; et al. Scientific Foundations for an IUCN Red List of Ecosystems. PLoS ONE 2013, 8, e62111. [Google Scholar] [CrossRef] [Green Version]
- Butler, B.J.; Swenson, J.J.; Alig, R.J. Forest fragmentation in the Pacific Northwest: Quantification and correlations. For. Ecol. Manag. 2004, 189, 363–373. [Google Scholar] [CrossRef]
- Abdullah, S.A.; Nakagoshi, N. Forest fragmentation and its correlation to human land use change in the state of Selangor, peninsular Malaysia. For. Ecol. Manag. 2007, 241, 39–48. [Google Scholar] [CrossRef]
- Wu, J. Landscape sustainability science: Ecosystem services and human well-being in changing landscapes. Landsc. Ecol. 2013, 28, 999–1023. [Google Scholar] [CrossRef]
- Armenteras, D.; Gast, F.; Villareal, H. Andean forest fragmentation and the representativeness of protected natural areas in the eastern Andes, Colombia. Biol. Conserv. 2003, 113, 245–256. [Google Scholar] [CrossRef]
- Armenteras, D.; Rudas, G.; Rodriguez, N.; Sua, S.; Romero, M. Patterns and causes of deforestation in the Colombian Amazon. Ecol. Indic. 2006, 6, 353–368. [Google Scholar] [CrossRef]
- Gómez Mora, A.M.; Anaya, J.A.; Álvarez Dávila, E. Análisis de fragmentación de los ecosistemas boscosos en una región de la cordillera central de los andes colombianos. Rev. Ing. Univ. Medellín 2005, 4, 13–27. [Google Scholar]
- Rodríguez Eraso, N.; Armenteras-Pascual, D.; Alumbreros, J.R. Land use and land cover change in the Colombian Andes: Dynamics and future scenarios. J. Land Use Sci. 2013, 8, 154–174. [Google Scholar] [CrossRef]
- León, J.D.; González, M.I.; Gallardo, J.F. Ciclos biogeoquímicos en bosques naturales y plantaciones de coníferas en ecosistemas de alta montaña de Colombia. Rev. Biol. Trop. 2011, 59, 1883–1894. [Google Scholar] [CrossRef]
- Valencia, R.; Balslev, H.; Paz, Y.; Miño, C.G. High tree alpha-diversity in Amazonian Ecuador. Biodivers. Conserv. 1994, 3, 21–28. [Google Scholar] [CrossRef]
- Olson, D.M.; Dinerstein, E. The Global 200: Priority Ecoregions for Global Conservation. Ann. Mo. Bot. Gard. 2002, 89, 199–224. [Google Scholar] [CrossRef]
- Cuenca, P.; Arriagada, R.; Echeverría, C. How much deforestation do protected areas avoid in tropical Andean landscapes? Environ. Sci. Policy 2016, 56, 56–66. [Google Scholar] [CrossRef]
- Mena, C.F. Trajectories of Land-use and Land-cover in the Northern Ecuadorian Amazon: Temporal Composition, Spatial Configuration, and Probability of Change. Photogramm. Eng. Remote Sens. 2008, 6, 737–751. [Google Scholar] [CrossRef]
- Sierra, R.; Stallings, J. The Dynamics and Social Organization of Tropical Deforestation in Northwest Ecuador, 1983–1995. Hum. Ecol. 1998, 26, 135–161. [Google Scholar] [CrossRef]
- Tapia-Armijos, M.F.; Homeier, J.; Espinosa, C.I.; Leuschner, C.; de la Cruz, M. Deforestation and Forest Fragmentation in South Ecuador since the 1970s–Losing a Hotspot of Biodiversity. PLoS ONE 2015, 10, e0133701. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cadilhac, L.; Torres, R.; Calles, J.; Vanacker, V.; Calderón, E. Desafíos para la investigación sobre el cambio climático en Ecuador. Neotrop. Biodivers. 2017, 3, 168–181. [Google Scholar] [CrossRef]
- Cuenca, P.; Echeverria, C. How do protected landscapes associated with high biodiversity and population levels change? PLoS ONE 2017, 12, e0180537. [Google Scholar] [CrossRef] [Green Version]
- Cuesta, F.; Peralvo, M.; Merino-Viteri, A.; Bustamante, M.; Baquero, F.; Freile, J.F.; Muriel, P.; Torres-Carvajal, O. Priority areas for biodiversity conservation in mainland Ecuador. Neotrop. Biodivers. 2017, 3, 93–106. [Google Scholar] [CrossRef]
- Lessmann, J.; Muñoz, J.; Bonaccorso, E. Maximizing species conservation in continental Ecuador: A case of systematic conservation planning for biodiverse regions. Ecol. Evol. 2014, 4, 2410–2422. [Google Scholar] [CrossRef]
- Ministerio del Ambiente del Ecuador. Sistema de Clasificación de los Ecosistemas del Ecuador Continental; Subsecretaría de Patrimonio Natural: Quito, Ecuador, 2013.
- Sierra, R.; Palacios, W.; Cern, C.; Valencia, R. Las Formaciones Naturales de la Amazonía del Ecuador; EcoCiencia: Illes Balears, Spania, 1999. [Google Scholar] [CrossRef]
- Josse, C.; Navarro, G.; Comer, P.; Evans, R.; Faber, D. Ecological Systems of Latin America and the Caribbean a Working Classification of Terrestrial Systems; NatureServe: Arlington County, VA, USA, 2003. [Google Scholar]
- MAE. Fragmentación de los Ecosistemas del Ecuador Continental; Ministerio del Ambiente de Ecuador: Quito, Ecuador, 2015.
- MAE. Línea Base de Deforestación del Ecuador Continental; Ministerio del Ambiente de Ecuador: Quito, Ecuador, 2012; p. 32.
- MAGAP-MAE. Cobertura del Uso Actual Actual del Suelo del Ecuador Continental 1990–2000–2008–2014; Subsecretaría de Patrimonio Natural: Quito, Ecuador, 2015.
- MAE. Estadística de Patrimonio Natural; Ministerio del Ambiente de Ecuador: Quito, Ecuador, 2015.
- IGM. Geoportales y Visores Geográficos; Subsecretaría de Patrimonio Natural: Quito, Ecuador, 2018.
- Bremer, L.L.; Farley, K.A.; Lopez-Carr, D.; Romero, J. Conservation and livelihood outcomes of payment for ecosystem services in the Ecuadorian Andes: What is the potential for ‘win–win’? Ecosyst. Serv. 2014, 8, 148–165. [Google Scholar] [CrossRef]
- Viteri Salazar, O.; Ramos-Martín, J.; Lomas, P.L. Livelihood sustainability assessment of coffee and cocoa producers in the Amazon region of Ecuador using household types. J. Rural. Stud. 2018, 62, 1–9. [Google Scholar] [CrossRef]
- Buytaert, W.; Iiguez, V.; Bivre, B.D. The effects of afforestation and cultivation on water yield in the Andean páramo. For. Ecol. Manag. 2007, 251, 22–30. [Google Scholar] [CrossRef]
- Célleri, R.; Feyen, J. The Hydrology of Tropical Andean Ecosystems: Importance, Knowledge Status, and Perspectives. Mt. Res. Dev. 2009, 29, 350–355. [Google Scholar] [CrossRef]
- Bendix, J.; Trachte, K.; Cermak, J.; Rollenbeck, R.; Naub, T. Formation of convective clouds at the foothills of the tropical eastern Andes (South Ecuador). J. Appl. Meteorol. Climatol. 2009, 48, 1682–1695. [Google Scholar] [CrossRef]
- MAE. Informe del Aporte del Programa Socio Bosque en la Conservación; Ministerio del Ambiente: Quito, Ecuador, 2016.
- MAGAP. Mapa de Cobertura y Uso de la TIerra del Ecuador Continental Escala 1:100 000: Temporalidad: 2013–2014; 2016. Available online: http://ide.ambiente.gob.ec/geonetwork/srv/spa/catalog.search;jsessionid=66D82E3F6D48F054363C8A0447861702#/search?facet.q=topicCat%2FimageryBaseMapsEarthCover (accessed on 21 August 2021).
- Puyravaud, J.-P. Standardizing the calculation of the annual rate of deforestation. For. Ecol. Manag. 2003, 177, 593–596. [Google Scholar] [CrossRef]
- Pontius, R.G.; Shusas, E.; McEachern, M. Detecting important categorical land changes while accounting for persistence. Agric. Ecosyst. Environ. 2004, 101, 251–268. [Google Scholar] [CrossRef]
- Riitters, K.H.; Wickham, J.D. Decline of forest interior conditions in the conterminous United States. Sci. Rep. 2012, 2, 653. [Google Scholar] [CrossRef] [Green Version]
- Riitters, K.H.; Wickham, J.D.; O’Neill, R.V.; Jones, K.B.; Smith, E.R.; Coulston, J.W.; Wade, T.G.; Smith, J.H. Fragmentation of continental United States forests. Ecosystems 2002, 5, 815–822. [Google Scholar] [CrossRef]
- Vogt, P. Measuring Forest Area Density to Quantify Forest Fragmentation. (Available in the Free JRC Software GuidosToolbox); European Commission: Brussels, Belgium, 2018. [Google Scholar]
- Noh, J.K. Effects of forest fragmentation on biodiversity in the Andes region. PH. D. Thesis, Universidad de Concepción, Concepción, Chile, 2019. [Google Scholar]
- Lambin, E.F.; Geist, H.J.; Lepers, E. Dynamics of Land-Use and Land-Cover Change in Tropical Regions. Annu. Rev. Environ. Resour. 2003, 28, 205–241. [Google Scholar] [CrossRef] [Green Version]
- Lessmann, J.; Fajardo, J.; Muoz, J.; Bonaccorso, E. Large expansion of oil industry in the Ecuadorian Amazon: Biodiversity vulnerability and conservation alternatives. Ecol. Evol. 2016, 6, 4997–5012. [Google Scholar] [CrossRef] [Green Version]
- Sáenz, M.A.O. Preguntas Clave: Reporte de los Ecosistemas Terrestres Ecuatorianos. Indicadores de Biodiversidad para Uso Nacional; Ministerio del Ambiente del Ecuador/Fundación EcoCiencia: Quito, Ecuador, 2005; p. 61.
- Mittermeier, R.A.; Myers, N.; Tliomsen, J.B.; Olivieri, S. Biodiversity hotspots and major tropical wilderness areas: Approaches to setting conservation priorities. Conserv. Biol. 1998, 12, 516–520. [Google Scholar] [CrossRef]
- Rodriguez, J.P.; Keith, D.A.; Rodrguez-Clark, K.M.; Murray, N.J.; Nicholson, E.; Regan, T.J.; Miller, R.M.; Barrow, E.G.; Bland, L.M.; Boe, K.; et al. A practical guide to the application of the IUCN Red List of Ecosystems criteria. Philos. Trans. R. Soc. B Biol. Sci. 2015, 370, 20140003. [Google Scholar] [CrossRef] [PubMed]
- Brandt, J.S.; Townsend, P.A. Land use—Land cover conversion, regeneration and degradation in the high elevation Bolivian Andes. Landsc. Ecol. 2006, 21, 607–623. [Google Scholar] [CrossRef]
- Wunder, S. Deforestation: The Poor Man’s Lot? In The Economics of Deforestation; Macmillan: London, UK, 2000; pp. 141–163. [Google Scholar]
- Sierra, R.; Campos, F.; Chamberlin, J. Assessing biodiversity conservation priorities: Ecosystem risk and representativeness in continental Ecuador. Landsc. Urban Plan. 2002, 59, 95–110. [Google Scholar] [CrossRef]
- Balmford, A.; Mace, G.M.; Ginsberg, J.R. The challenges to conservation in a changing world: Putting processes on the map. In Conservation in a Changing World; Cambridge University Press: Cambridge, UK, 1998; pp. 1–28. [Google Scholar]
- Suding, K.N.; Hobbs, R.J. Threshold models in restoration and conservation: A developing framework. Trends Ecol. Evol. 2009, 24, 271–279. [Google Scholar] [CrossRef] [PubMed]
- Brown, D.; Schreckenberg, K. Shifting Cultivators as Agents of Deforestation: Assessing the Evidence; Overseas Development Institute: London, UK, 1998. [Google Scholar]
- Amelung, T.; Diehl, M. Deforestation of Tropical Rainforests: Economic Causes and Impact on Development; Institut für Weltwirtschaft: Kiel, Germany, 1992. [Google Scholar]
- Holl, K.D.; Loik, M.E.; Lin, E.H.V.; Samuels, I.A. Tropical montane forest restoration in Costa Rica: Overcoming barriers to dispersal and establishment. Restor. Ecol. 2000, 8, 339–349. [Google Scholar] [CrossRef]
- Pendrill, F.; Persson, U.M.; Godar, J.; Kastner, T. Deforestation displaced: Trade in forest-risk commodities and the prospects for a global forest transition. Environ. Res. Lett. 2019, 14, 055003. [Google Scholar] [CrossRef]
- Gaglio, M.; Aschonitis, V.G.; Mancuso, M.M.; Reyes Puig, J.P.; Moscoso, F.; Castaldelli, G.; Fano, E.A. Changes in land use and ecosystem services in tropical forest areas: A case study in Andes mountains of Ecuador. Int. J. Biodivers. Sci. Ecosyst. Serv. Manag. 2017, 13, 264–279. [Google Scholar] [CrossRef] [Green Version]
- Cubina, A.; Aide, T.M. The Effect of Distance from Forest Edge on Seed Rain and Soil Seed Bank in a Tropical Pasture. Biotropica 2001, 33, 260–267. [Google Scholar] [CrossRef]
- Myster, R.W. Post-Agricultural Invasion, Establishment, and Growth of Neotropical Trees. Bot. Rev. 2004, 70, 381–402. [Google Scholar] [CrossRef]
- Zahawi, R.A.; Augspurger, C.K. Early plant succession in abandoned pastures in Ecuador. Biotropica 1999, 31, 540–552. [Google Scholar] [CrossRef]
- MAE. Políticas y Plan Estratégico del Sistema Nacional de Áreas Protegidas del Ecuador 2007–2016; Ministerio del Ambiente del Ecuador, Proyecto GEF: Sistema Nacional de Áreas Protegidas: Quito, Ecuador, 2006; p. 154.
- Suding, K.N.; Gross, K.L.; Houseman, G.R. Alternative states and positive feedbacks in restoration ecology. Trends Ecol. Evol. 2004, 19, 46–53. [Google Scholar] [CrossRef] [PubMed]
- MAGAP. Decreto Ejecutivo No 1042. Reglamento Para Normar el Pago Por Calidad de la Leche y Sanidad Animal. Acuerdo Ministerial 077; Ministerio de Agricultura, Ganadería, Acuacultura y Pesca: Quito, Ecuador, 2008.
- Contero, R.; Requelme, N.; Cachipuendo, C.; Acurio, D. Calidad de la Leche Cruda i Sistema de Pago por Calidad en el Ecuador. LA GRANJA Rev. Cienc. Vida 2021, 33, 31–43. [Google Scholar] [CrossRef]



| Region | Ecoregion | Code | Forest Ecosystems (Forest Vegetation Type) | Altitudinal Range (m) |
|---|---|---|---|---|
| COAST | Equatorial-Chocó | E1 | Flood alluvial plain forest of the Equatorial Chocó | 50–200 |
| E2 | Equatorial Chocó mangrove | 0–20 | ||
| E3 | Evergreen forest of the Equatorial Chocó lowland | 0–300 | ||
| E4 | Flood intertidal plain forest of the Equatorial Chocó | 0–50 | ||
| E5 | Seasonal evergreen forest of Equatorial Chocó lowlands | 0–300 | ||
| E6 | Piedmont seasonal evergreen forest of the Chocó coastal range | 200–400 | ||
| E7 | Low montane evergreen forest of Chocó coastal range | >400 | ||
| Equatorial-Pacific | E8 | Semideciduous forest of the Jama-Zapotillo lowland | 0–300 | |
| E9 | Semideciduous forest of the Equatorial Pacific coastal range | >200 | ||
| E10 | Low forest and deciduous shrubland of the Jama-Zapotillo lowland | 0–400 | ||
| E11 | Piedmont seasonal evergreen forest of the Equatorial Pacific coastal range | 200–400 | ||
| E12 | Low montane seasonal evergreen forest of the Equatorial Pacific coastal range | 400–860 | ||
| E13 | Seasonal evergreen forest of the Jama-Zapotillo lowland | 0–400 | ||
| E14 | Deciduous forest of the Jama-Zapotillo lowland | 0–400 | ||
| E15 | Deciduous forest of the Equatorial Pacific coastal range | >200 | ||
| E16 | Jama-Zapotillo mangrove | 0–10 | ||
| E17 | Seasonal flood alluvial plain evergreen forest of the Jama-Zapotillo | 0–300 | ||
| ANDES | Western-range | E18 | Piedmont evergreen forest of the western Andean range | 300–1400 |
| E19 | Low montane evergreen forest of the western Andean range | 1400–2000 | ||
| E20 | Montane evergreen forest of the western Andean range | 2000–3100 | ||
| E21 | High montane evergreen forest of the western Andean range | 3100–3600 | ||
| E22 | Piedmont seasonal evergreen forest of the western Andean range | 300–1400 | ||
| E23 | Piemontano seasonal evergreen forest of the Catamayo-Alamor | 400–1600 | ||
| E24 | Low montane seasonal evergreen forest of the Catamayo-Alamor | 1600–2000 | ||
| E25 | Montane evergreen forest of the Catamayo-Alamor | 2200–2900 | ||
| E26 | High montane evergreen forest of the Catamayo-Alamor | 2900–3400 | ||
| E27 | Low montane evergreen forest of the Catamayo- Alamor | 1600–2200 | ||
| E28 | Piedmont evergreen forest of the Catamayo-Alamor | 400–1600 | ||
| E29 | Piedmont semideciduous forest of the Catamayo-Alamor | 400–1600 | ||
| E30 | Low montane semideciduous forest of the Catamayo-Alamor | 1600–2200 | ||
| E31 | Piedmont deciduous forest of the Catamayo-Alamor | 400–1600 | ||
| E32 | Low montane deciduous forest of the Catamayo- Alamor | 1600–2200 | ||
| Valley, Alpine | E33 | Semideciduous forest and shrubland of the North Valleys | 1200–2600 | |
| E34 | Semideciduous forest and shrubland of the South Valleys | 1200–2000 | ||
| E35 | Páramo evergreen forest | 3200–4100 | ||
| Eastern-range | E36 | High montane evergreen forest of the north-eastern Andean range | 3000–3700 | |
| E37 | Montane evergreen forest of the north-eastern Andean range | 2000–3000 | ||
| E38 | Low montane evergreen forest of the north-eastern Andean range | 1200–2000 | ||
| E39 | Piedmont evergreen forest of the north-eastern Andean range | 400–1200 | ||
| E40 | Low montane evergreen forest of the south-eastern Andean range | 1660–2200 | ||
| E41 | Montane evergreen forest of the south-eastern Andean range | 2200–3000 | ||
| E42 | High montane evergreen forest of the south-eastern Andean range | 3000–3400 | ||
| E43 | Piedmont evergreen forest of the south-eastern Andean range | 400–1650 | ||
| E44 | Piedmont semideciduous forest of the south-eastern Andean range | 500–1300 | ||
| AMAZON | Range | E45 | Low montane evergreen forest of Galeras | 1300–1700 |
| E46 | Piedmont evergreen forest of Galeras | 600–1300 | ||
| E47 | Piedmont evergreen forest of the Cóndor-Kutukú range | 350–1400 | ||
| E48 | Low montane evergreen forest of the Cóndor-Kutukú range | 1400–1900 | ||
| E49 | Montane evergreen forest of the Cóndor-Kutukú range | 1900–2400 | ||
| E50 | Piedmont evergreen forest on sandstone plateaus of the Cóndor-Kutukú range | 350–1400 | ||
| E51 | Montane evergreen forest on sandstone plateaus of the Cóndor range | 1900–2700 | ||
| E52 | Piedmont evergreen forest on limestone outcrops of the Amazonian range | 600–1400 | ||
| E53 | Low montane evergreen forest on sandstone plateaus of the Cóndor-Kutukú range | 1400–1900 | ||
| E54 | Evergreen forest on sandstone plateaus of the Cóndor range in the lower Ecuadorian Amazon | 243–550 | ||
| Plain | E55 | Evergreen forest of the Aguarico-Putumayo-Caquetá lowland | 168–350 | |
| E56 | Flood alluvial plain palm forest of the Amazon | 171–350 | ||
| E57 | Flood river (originated in the Andean and Amazonian ranges) alluvial-plain forest | 164–350 | ||
| E58 | Lowland evergreen forest of the Napo-Curaray | 170–350 | ||
| E59 | Flood alluvial plain forest of the Amazon | 158–350 | ||
| E60 | Flood forest and lacustrine-riparian vegetation of the Amazonian black water | 170–350 | ||
| E61 | Flood river (originated in the Amazon) alluvial plain forest | 158–350 | ||
| E62 | Evergreen bamboo forest of the Amazonian lowland | 196–500 | ||
| E63 | Evergreen forest of the Tigre-Pastaza lowland | 166–350 | ||
| E64 | Evergreen forest of the Pastaza fan-shaped lowland | 197–350 |
| FAD Class | Color | FAD Range | |
|---|---|---|---|
| 1 | Rare |  | FAD < 10% |
| 2 | Patchy |  | 10% ≤ FAD < 40% |
| 3 | Transitional |  | 40% ≤ FAD < 60% |
| 4 | Dominant |  | 60% ≤ FAD < 90% |
| 5 | Interior |  | 90% ≤ FAD ≤ 100% |
| Human Land Use/Cover | All | Coast | Andes | Amazon |
|---|---|---|---|---|
| (n = 64, p < 0.001, R2 = 0.646) | (n = 17, p < 0.05, R2 = 0.572) | (n = 27, p < 0.01, R2 = 0.854) | (n = 20, p < 0.01, R2 = 0.003) | |
| PLT | 2.52 | 0.126 | 0.7 | - |
| PST | 1.30 *** | −0.47 | 1.31 *** | 1.08 ** |
| AFM | 1.45 | −5.64 | 0.44 | −26.97 |
| PFM | 0.18 | 2.58 . | −4.1 | −9.53 |
| SFM | 1.23 | 4.61 . | 3.67 | 16 |
| HBT | 59.78 *** | 59.74 . | 106.48 ** | −87.06 |
| IFR | 6.57 | 54.47 | −358.21 | −98.74 |
| Human Land Use/Cover | Low | Moderate | High |
|---|---|---|---|
| (n = 22, p < 0.001, R2 = 0.936) | (n = 31, p < 0.1, R2 = 0.302) | (n = 11, p < 0.01, R2 = 0.995) | |
| PLT | −2.08 * | 1.9 | 9.23 |
| PST | 1.20 *** | 0.63 * | 0.37 * |
| AFM | 46.67 *** | 1.55 . | 0.43 |
| PFM | −3.51 | 0.43 | −0.61 * |
| SFM | 3.33 | 0.34 | 1.28 . |
| HBT | −63.78 | 4.92 | 10.3 |
| IFR | 0.88 | 8.21 | 93.33 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Noh, J.K.; Echeverria, C.; Gaona, G.; Kleemann, J.; Koo, H.; Fürst, C.; Cuenca, P. Forest Ecosystem Fragmentation in Ecuador: Challenges for Sustainable Land Use in the Tropical Andean. Land 2022, 11, 287. https://doi.org/10.3390/land11020287
Noh JK, Echeverria C, Gaona G, Kleemann J, Koo H, Fürst C, Cuenca P. Forest Ecosystem Fragmentation in Ecuador: Challenges for Sustainable Land Use in the Tropical Andean. Land. 2022; 11(2):287. https://doi.org/10.3390/land11020287
Chicago/Turabian StyleNoh, Jin Kyoung, Cristian Echeverria, Gabriel Gaona, Janina Kleemann, Hongmi Koo, Christine Fürst, and Pablo Cuenca. 2022. "Forest Ecosystem Fragmentation in Ecuador: Challenges for Sustainable Land Use in the Tropical Andean" Land 11, no. 2: 287. https://doi.org/10.3390/land11020287
APA StyleNoh, J. K., Echeverria, C., Gaona, G., Kleemann, J., Koo, H., Fürst, C., & Cuenca, P. (2022). Forest Ecosystem Fragmentation in Ecuador: Challenges for Sustainable Land Use in the Tropical Andean. Land, 11(2), 287. https://doi.org/10.3390/land11020287








