Seasonal Dynamics of Organic Carbon and Nitrogen in Biomasses of Microorganisms in Arable Mollisols Affected by Different Tillage Systems
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Site and Sampling
2.2. Soil MB Analyses
2.3. Statistical Analyses
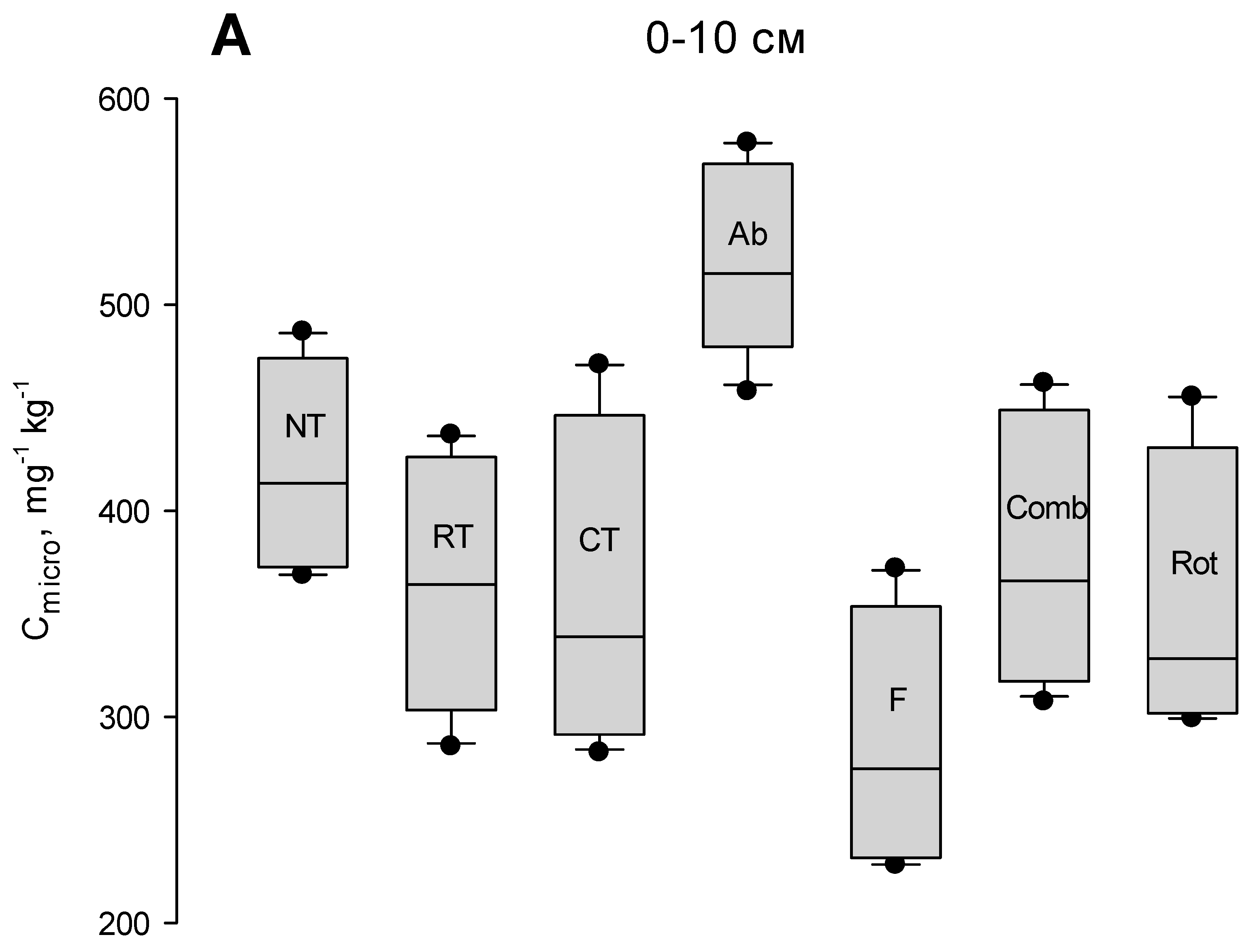
3. Results
3.1. Seasonal Cmicro Changes
3.2. Seasonal Nmicro and Cmicro:Nmicro Changes
3.3. A Correlation Analysis and Determination of the Coefficients of Linear Correlation of K. Pearson
4. Discussion
4.1. Temperature and Moisture Effect on the Soil Microbial Biomass Amount
4.2. CO2-C Emission in Mollisol under Different Tillage Systems
4.3. Tillage Effect on Dynamics of Soil Microbial Biomass
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chernov, T.I.; Semenov, M.V. Management of Soil Microbial Communities: Opportunities and Prospects (a Review). Eurasian Soil Sci. 2021, 54, 1888–1902. [Google Scholar] [CrossRef]
- Dalal, R.C. Soil microbial biomass–what do the numbers really mean? Aust. J. Experim. Agric. 1998, 38, 649–665. [Google Scholar] [CrossRef]
- Jenkinson, D.S.; Ladd, J.N. Microbial biomass in soil: Measurement and turnover. Soil Biochem. 1991, 5, 415–471. [Google Scholar]
- Paul, A. The nature and dynamics of soil organic matter: Plant inputs, microbial transformations, and organic matter stabilization. Soil Biol. Biochem. 2016, 98, 109–126. [Google Scholar] [CrossRef] [Green Version]
- Fry, S. The spatial distribution of soil biota. In Soil Microbiol., Ecol. and Biochem., 4th ed.; Paul, E., Ed.; Academic Press: San Diego, CA, USA, 2015; pp. 223–244. [Google Scholar]
- Butenschoen, O.; Poll, C.; Langel, R. Endogeic earthworms alter carbon translocation by fungi at the soil–litter interface. Soil Biol. Biochem. 2007, 39, 2854–2864. [Google Scholar] [CrossRef]
- Cotrufo, F.; Wallenstein, M.; Denef, K.; Paul, E. The molecular efficiency-matrix stabilization (MEMS) framework integrates plant litter decomposition with soil organic matter stabilization. Glob Change Biol. 2013, 19, 988–995. [Google Scholar] [CrossRef] [Green Version]
- Nicolardot, B.; Bouziri, L.; Bastian, F.; Ranjard, L. A microcosm experiment to evaluate the influence of location and quality of plant residues on residue decomposition and genetic structure of soil microbial communities. Soil Biol. Biochem. 2007, 39, 1631–1644. [Google Scholar] [CrossRef]
- Helgason, B.L.; Walley, F.L.; Germida, J.J. Fungal and bacterial abundance in long-term no-till and intensive-till soils of the Northern Great Plains. Soil Sci. Soc. Am. J. 2009, 73, 120–127. [Google Scholar] [CrossRef]
- Demoling, F.; Nilsson, L.; Baath, E. Bacterial and fungal response to nitrogen fertilization in three coniferous forest soils. Soil Biol. Biochem. 2008, 40, 370–379. [Google Scholar] [CrossRef]
- de Vries, F.T.; Hoffland, E.; van Eekeren, N.; Brussaard, L.; Bloem, J. Fungal bacterial ratios in grasslands with contrasting nitrogen management. Soil Biol. Biochem. 2006, 38, 2092–2103. [Google Scholar] [CrossRef] [Green Version]
- Bailey, V.L.; Smith, J.L.; Bolton, H. Fungal: Bacterial ratios in soils investigated for enhanced C sequestration. Soil Biol. Biochem. 2002, 34, 997–1007. [Google Scholar] [CrossRef]
- Sparling, G.P. Ratio of microbial biomass carbon to soil organic carbon as a sensitive indicator of changes in soil organic matter. Aust. J. Soil Res. 1992, 30, 195–207. [Google Scholar] [CrossRef]
- Roy, J.; Guillerm, J.; Navas, M.; Dhillion, S. 9—Responses to Elevated CO2 in Mediterranean Old-Field Microcosms: Species, Community, and Ecosystem Components. Physiol. Ecol. 1996, 123–138. [Google Scholar] [CrossRef]
- Karlen, D.L.; Mausbach, M.J.; Doran, J.W.; Cline, R.G.; Harris, R.F.; Schuman, G.E. Soil quality: A concept, definition and framework for evaluation. Soil Sci. Soc. Am. J. 1997, 61, 4–10. [Google Scholar] [CrossRef] [Green Version]
- Holmes, W.; Zak, R. Soil Microbial Biomass Dynamics and Net Nitrogen Mineralization in Northern Hardwood Ecosystems. Soil Sci. Soc. Am. J. 1994, 58, 238–243. [Google Scholar] [CrossRef] [Green Version]
- Powlson, D.S.; Brookes, P.C.; Christensen, B.T. Measurement of soil microbial biomass provides an early indication of changes in total soil organic matter due to straw incorporation. Soil Biol. Biochem. 1987, 19, 159–164. [Google Scholar] [CrossRef]
- Anderson, J.F.; Domsch, K.H. Quantities of plant nutrients in the microbial biomass of selected soils. Soil Sci. 1980, 130, 211–216. [Google Scholar] [CrossRef]
- Schmidt, S.K.; Costello, E.K.; Nemergut, D.R.; Cleveland, C.C.; Reed, S.C.; Weintraub, M.N.; Meyer, A.F.; Martin, A.M. Biogeochemical consequences of rapid microbial turnover and seasonal succession in soil. Ecology 2007, 88, 1379–1385. [Google Scholar] [CrossRef]
- Powlson, D.S.; Jenkinson, D.S. A comparison of the organic matter, biomass, adenosine triphosphate and mineralizable nitrogen contents of ploughed and direct-drilled soils. J. Agric. Sci. 1981, 97, 713–721. [Google Scholar] [CrossRef]
- Wright, A.L.; Hons, F.M.; Lemon, R.G. Microbial activity and soil C sequestration for reduced and conventional tillage cotton. Appl. Soil Ecol. 2008, 38, 168–173. [Google Scholar] [CrossRef]
- Semenov, M.; Krasnov, G.; Semenov, V.; Bruggen, A. Mineral and Organic Fertilizers Distinctly Affect Fungal Communities in the Crop Rhizosphere. J. Fungi 2022, 8, 251. [Google Scholar] [CrossRef] [PubMed]
- Das, S.; Lee, J.; Cho, S.; Song, H.; Kim, P. Silicate Fertilizer Amendment Alters Fungal Communities and Accelerates Soil Organic Matter Decomposition. Front. Microbiol. 2019, 10, 2950. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thiessen, S.; Gleixner, G.; Wutzler, T.; Reichstein, M. Both priming and temperature sensitivity of soil organic matter decomposition depend on microbial biomass–an incubation study. Soil Biol. Biochem. 2013, 57, 739–748. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.; Xu, W.; Hu, G.; Dai, W.; Jiang, P.; Bai, E. The priming effect of soluble carbon inputs in organic and mineral soils from a temperate forest. Oecologia 2015, 178, 1239–1250. [Google Scholar] [CrossRef]
- Murphy, C.J.; Baggs, E.M.; Morley, N.; Wall, D.P.; Paterson, E. Rhizosphere priming can promote mobilisation of N-rich compounds from soil organic matter. Soil Biol. Biochem. 2015, 81, 236–243. [Google Scholar] [CrossRef]
- Rousk, J.; Hill, P.; Jones, D. Priming of the decomposition of ageing soil organic matter: Concentration dependence and microbial control. Function. Ecol. 2015, 29, 285–296. [Google Scholar] [CrossRef] [Green Version]
- Shahbaz, M.; Kuzyakov, Y.; Heitkamp, F. Decrease of soil organic matter stabilization with increasing inputs: Mechanisms and controls. Geoderma 2017, 304, 76–82. [Google Scholar] [CrossRef]
- Hamilton, S.K.; Doll, J.E.; Robertson, G.P. The Ecology of Agricultural Landscapes: Long-Term Research on the Path to Sustainability; Oxford University Press: New York, NY, USA, 2015. [Google Scholar]
- Selyaninov, G.T. About climate agricultural estimation. Proc. Agric. Meteorol. 1928, 20, 165–177. (In Russian) [Google Scholar]
- Soil Survey Staff. Illustrated Guide to Soil Taxonomy: Version 1.1.; USDA-NRCS: Lincoln, Nebraska, 2015. [Google Scholar]
- SSTU ISO. ISO 10694: 1995, IDT; 10694-2001 Soil Quality. Determination of Organic and Total Carbon Content by Dry Combustion (Elemental Analysis). Stateconsumerstandard of Ukraine: Kyiv, Ukraine, 2003. (In Ukrainian)
- Vance, E.D.; Brookes, P.C.; Jenkinson, D.S. An extraction method for measuring soil microbial biomass C. Soil Biol. Biochem. 1987, 19, 703–707. [Google Scholar] [CrossRef]
- Voroney, R.; Winter, J.; Beyaert, R. Soil microbial biomass C and N. In Soil Sampling and Methods of Analysis; Carter, M.R., Ed.; Canadian Society of Soil Science: Lewis, Chelsea, 1993; pp. 277–286. [Google Scholar]
- Wu, J.; Joergensen, R.G.; Pommering, B.; Chaussod, R.; Brookes, P.C. Measurement of soil microbial biomass C–an automated procedure. Soil Biol. Biochem. 1990, 22, 1167–1169. [Google Scholar] [CrossRef]
- Brookes, P.; Landman, A.; Pruden, G.; Jenkinson, D.S. Chloroform fumigation and the release of soil nitrogen: A rapid direct extraction method to measure microbial biomass nitrogen in soil. Soil Biol. Biochem. 1985, 17, 837–842. [Google Scholar] [CrossRef]
- Jenkinson, D.S. The determination of microbial biomass carbon and nitrogen in soil. In Advances in Nitrogen Cycling in Agricultural Ecosystems; Wilson, J.R., Ed.; CAB International: Wallingford, UK, 1988; pp. 368–386. [Google Scholar]
- Mueller, T.; Jensen, L.; Nielsen, N.; Magid, J. Turnover of carbon and nitrogen in a sandy loam soil following incorporation of chopped maize plants, barley straw and blue grass in the field. Soil Biol. Biochem. 1998, 30, 561–571. [Google Scholar] [CrossRef]
- Devi, N.B.; Yadava, P.S. Seasonal dynamics in soil microbial biomass C, N and P in a mixed-oak forest ecosystem of Manipur, North-east India. Appl. Soil Ecol. 2006, 31, 220–227. [Google Scholar] [CrossRef]
- Edwards, K.A.; Jefferies, R.L. Inter-annual and seasonal dynamics of soil microbial biomass and nutrients in wet and dry low-Arctic sedge meadows. Soil Biol. Biochem. 2013, 57, 83–90. [Google Scholar] [CrossRef]
- Chernova, T.I.; Zhelezova, A.D. Dynamics of soil microbial communities in different time ranges (review). Soil Sci. 2020, 5, 590–600. (In Russian) [Google Scholar]
- Lauber, C.L.; Ramirez, K.S.; Aanderud, Z.; Lennon, J.; Fierer, N. Temporal variability in soil microbial communities across land-use types. ISME J. 2013, 7, 1641–1650. [Google Scholar] [CrossRef]
- Sui, Y.Y.; Jiao, X.G.; Gao, C.S.; Chen, W.; Zhang, X.Y.; Liu, X.B. The Relationship among Organic Matter Content and Soil Microbial Biomass and Soil Enzyme Activities. Chin. J. Soil Res. 2009, 40, 1036–1039. [Google Scholar]
- Lou, Y.; Xu, M.; Wang, W.; Sun, X.; Zhao, K. Return rate of straw residue affects soil organic C sequestration by chemical fertilization. Soil Till Res. 2011, 113, 70–73. [Google Scholar] [CrossRef]
- McGill, W.; Cannon, K.R.; Robertson, J.A. Dynamics of Soil Microbial Biomass and Water-Soluble Organic C in Breton L After 50 Years of Cropping to Two Rotations. Can. J. Soil Res. 1986, 66, 1–19. [Google Scholar] [CrossRef]
- Sun, L.; Song, F.; Liu, S.; Cao, Q.; Liu, F.; Zhu, X. Integrated agricultural management practice improves soil quality in Northeast China. Arch. Agron. Soil Sci. 2018, 64, 1932–1943. [Google Scholar] [CrossRef]
- Luo, X.; Wang, M.K.; Hu, G.; Weng, B. Seasonal Change in Microbial Diversity and its Relationship with Soil Chemical Properties in an Orchard. PLoS ONE 2019, 14, e0215556. [Google Scholar] [CrossRef] [PubMed]
- Moraru, P.; Rusu, T. Effect of tillage systems on soil moisture, soil temperature, soil respiration and production of wheat, maize and soybean crops. J. Food Agric. Environ. 2012, 10, 445–448. [Google Scholar]
- Pagliai, M.; Raglione, M.; Panini, T.; Maletta, M.; La Marca, M. The structure of two alluvial soils in Italy after 10 years of conventional and minimum. Soil Tillage Res. 1995, 34, 209–223. [Google Scholar] [CrossRef]
- Anderson, S.; Gantzer, C.; Brown, J. Soil physical properties after 100 years of continuous cultivation. J. Soil Water Conserv. 1990, 45, 117–121. [Google Scholar]
- Sarauskis, E.; Romaneckas, K.; Buragiene, S. Impact of conventional and sustainable soil tillage and sowing technologies on physical-mechanical soil properties. Environ. Res. Eng. Manag. 2009, 49, 36–43. [Google Scholar]
- Josa, R.; Ginovart, M.; Solé, A. Effects of two tillage techniques on soil macroporosity in sub-humid environment. Agrophysics 2010, 24, 139–147. [Google Scholar]
- Mestelan, S.; Smeck, N.; Durkalski, J.; Dick, W. Changes in soil profile properties as affected by 44 years of continuous no-tillage. In Proceedings of the 17th ISTRO Conference, Kiel, Germany, 28 August–3 September 2006; pp. 1135–1140. [Google Scholar]
- NaNagara, T.; Phillips, R.; Leggett, J. Diffusion and mass flow of nitrate nitrogen into corn roots grown under field conditions. Agron. J. 1976, 68, 67–72. [Google Scholar] [CrossRef]
- Piccolo, A. Carbon Sequestration in Agricultural Soils: A Multidisciplinary Approach to Innovative Methods; Springer: Berlin/Heidelberg, Germany, 2012; 307p. [Google Scholar]
- Piccolo, A.; Spaccini, R.; Cozzolino, V.; Nuzzo, A.; Drosos, M.; Zavattaro, L.; Grignani, C.; Puglisi, E.; Trevisan, M. Effective carbon sequestration in Italian agricultural soils by in situ polymerization of soil organic matter under biomimetic photocatalysis. Land Degrad Dev. 2018, 29, 485–494. [Google Scholar] [CrossRef]
- Nebbioso, A.; Piccolo, A. Basis of a Humeomics Science: Chemical Fractionation and Molecular Characterization of Humic Biomacromolecules. Biomacromolecules 2011, 12, 1187–1199. [Google Scholar] [CrossRef]
- Nuzzo, A.; Spaccini, R.; Cozzolino, V. In situ polymerization of soil organic matter by oxidative biomimetic catalysis. Chem. Biol. Technol. Agric. 2017, 4, 12. [Google Scholar] [CrossRef] [Green Version]
- Tsybulko, N. SOM sequestration and mineralization in different soils of Belorussia, Belorussia State University. Ecology 2018, 2, 110–117. (In Ukrainian) [Google Scholar]
- Zabolotniy, O.; Zabolotna, A. Soil microbiological activity under Merlin herbicide usage. Yang Sci. 2014, 2, 16–20. (In Ukrainian) [Google Scholar]
- Keshalava, R. Simazine and karagard effect on soil biological activity. Plant Prot. Quarant. 2000, 9, 49. (In Russian) [Google Scholar]
- Fontaine, S.; Mariotti, A.; Abbadie, L. The priming effect of organic matter: A question of microbial competition? Soil Biol. Biochem. 2003, 35, 837–843. [Google Scholar] [CrossRef]
- Chimitdorzhieva, E.; Chimitdorzhieva, G. Carbon dynamics of microbial biomass of virgin steppe and dry steppe soils of Transbaikalia. Bull. KSU Name N. A. Nekrasova 2012, 3, 16–20. (In Russian) [Google Scholar]
- Kuprechenkov, M. Seasonal dynamics of chemical and agrochemical properties of bio- and agro Chernozem. Agric. Sci. Tech. Achiev. 2013, 7, 67–68. (In Russian) [Google Scholar]
- Bulygin, S.; Velichko, V.; Demidenko, O. Agrogenesis of Chernozem. Kyiv, Agrar. Sci. 2016, 356. (In Ukrainian) [Google Scholar]
- Kravchenko, Y.; Rogovska, N.; Petrenko, L.; Zhang, X.; Song, C.; Chen, Y. Quality and dynamics of soil organic matter in Typical Chernozem of Ukraine under different soil tillage systems. Can. J. Soil Sci. 2012, 92, 429–438. [Google Scholar] [CrossRef] [Green Version]
- Geisseler, D.; Horwath, W.; Joergensen, R.; Ludwig, B. Pathways of nitrogen utilization by soil microorganisms–A review. Soil Biol. Biochem. 2010, 42, 2058–2067. [Google Scholar] [CrossRef]
- Bonde, T.; Schnurer, J.; Rosswall, T. Microbial biomass as a fraction of potentially mineralizable nitrogen in soils from longterm field experiments. Soil Biol Biochem. 1988, 20, 447–452. [Google Scholar] [CrossRef]
- Salinas-Garcia, J.; Hons, F.; Matocha, J.; Zuberer, D. Soil carbon and nitrogen dynamics as affected by long-term tillage and nitrogen fertilization. Biol Fertil Soils 1997, 25, 182–188. [Google Scholar] [CrossRef]
- Kuhnert, R.; Oberkofler, I.; Peintner, U. Fungal growth and biomass development is boosted by plants in snow-covered soil. Microb. Ecol. 2012, 64, 79–90. [Google Scholar] [CrossRef] [PubMed]
- Frey, S.D.; Elliot, E.T.; Paustian, K. Bacterial and fungal abundance and biomass in conventional and no-tillage agroecosystems along two climatic gradients. Soil Biol. Biochem. 1999, 31, 573–585. [Google Scholar] [CrossRef]
- Frey, S.D.; Six, J.; Elliott, E.T. Reciprocal transfer of carbon and nitrogen by decomposer fungi at the soil–litter interface. Soil Biol. Biochem. 2003, 35, 1001–1004. [Google Scholar] [CrossRef]
- Jiang, X.; Alan, L.; Wright, X.; Liang, F. Tillage-induced changes in fungal and bacterial biomass associated with soil aggregates: A long-term field study in a subtropical rice soil in China. Appl. Soil Ecol. 2011, 48, 168–173. [Google Scholar] [CrossRef]
- Cleveland, C.C.; Liptzin, D. C:N:P stoichiometry in soils: Is there a “Redfield ratio” for the microbial biomass? Biogeochemistry 2007, 85, 235–252. [Google Scholar] [CrossRef]
- Hernández-Hernández, R.M.; Lopez-Hernández, D. Microbial biomass, mineral nitrogen and carbon content in savanna soil aggregates under conventional and no-tillage. Soil Biol. Biochem. 2002, 34, 1563–1570. [Google Scholar] [CrossRef]
- Schadt, C.W.; Martin, A.P.; Lipson, D.A.; Schmidt, S.K. Seasonal dynamics of previously unknown fungal lineages in tundra soils. Science 2003, 301, 1359–1361. [Google Scholar] [CrossRef] [Green Version]
- Anderson, T.H.; Domsch, K.H. Ratio of microbial biomass carbon to total organic carbon in arable soils. Soil Biol. Biochem. 1989, 21, 471–479. [Google Scholar] [CrossRef]








| Parameters | NT CTOC | RT CTOC | CT C CTOC | Ab C CTOC | F CTOC | Comb CTOC | Rot CTOC |
|---|---|---|---|---|---|---|---|
| 0–10 cm | |||||||
| NT Cmicro | −0.418 | −0.395 | −0.351 | −0.143 | −0.789 * | −0.142 | −0.678 * |
| RT Cmicro | −0.571 * | −0.550 * | −0.558 | −0.371 | −0.884 * | −0.323 | −0.792 * |
| CT Cmicro | −0.289 | −0.285 | −0.278 | −0.0686 | −0.757 * | −0.0173 | −0.597 * |
| Ab Cmicro | −0.463 | −0.437 | −0.393 | −0.201 | −0.797 * | −0.186 | −0.701 * |
| F Cmicro | −0.269 | −0.255 | −0.212 | 0.00751 | −0.700 * | 0.0125 | −0.580 * |
| Comb Cmicro | −0.446 | −0.441 | −0.381 | −0.167 | −0.802 * | −0.187 | −0.700 * |
| Rot Cmicro | −0.177 | −0.165 | −0.158 | 0.0506 | −0.680 * | 0.103 | −0.509 |
| 10–20 cm | |||||||
| NT Cmicro | −0.697 * | −0.664 * | −0.708 * | −0.604 * | −0.877 * | −0.787 * | −0.731 * |
| RT Cmicro | −0.229 | −0.405 | −0.324 | −0.157 | −0.636 * | −0.630 * | −0.654 * |
| CT Cmicro | −0.221 | −0.544 * | −0.522 | −0.135 | −0.680 * | −0.820 * | −0.714 * |
| Ab Cmicro | −0.258 | −0.411 | −0.503 | −0.182 | −0.585 * | −0.747 * | −0.549 * |
| F Cmicro | −0.0173 | −0.639 * | −0.484 | −0.0712 | −0.609 * | −0.758 * | −0.780 * |
| Comb Cmicro | −0.169 | −0.482 | −0.268 | −0.0977 | −0.618 * | −0.598 * | −0.665 * |
| Rot Cmicro | −0.312 | −0.479 | −0.425 | −0.148 | −0.622 * | −0.736 * | −0.552 * |
| 20–40 cm | |||||||
| NT Cmicro | −0.660 * | −0.644 * | −0.591 * | −0.174 | −0.135 | −0.553 * | −0.175 |
| RT Cmicro | −0.788 * | −0.605 * | −0.907 * | −0.866 * | −0.413 | −0.850 * | −0.875 * |
| CT Cmicro | −0.654 * | −0.492 | −0.859 * | −0.798 * | −0.358 | −0.735 * | −0.824 * |
| Ab Cmicro | −0.405 | −0.141 | −0.630 * | −0.753 * | −0.440 | −0.508 | −0.752 * |
| F Cmicro | −0.615 * | −0.703 * | −0.682 * | −0.308 | −0.0644 | −0.578 * | −0.363 |
| Comb Cmicro | −0.506 | −0.363 | −0.746 * | −0.685 * | −0.347 | −0.589 * | −0.704 * |
| Rot Cmicro | −0.865 * | −0.671 * | −0.942 * | −0.845 * | −0.424 | −0.871* | −0.836 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kravchenko, Y.S.; Zhang, X.; Song, C.; Hu, W.; Yarosh, A.V.; Voitsekhivska, O.V. Seasonal Dynamics of Organic Carbon and Nitrogen in Biomasses of Microorganisms in Arable Mollisols Affected by Different Tillage Systems. Land 2022, 11, 486. https://doi.org/10.3390/land11040486
Kravchenko YS, Zhang X, Song C, Hu W, Yarosh AV, Voitsekhivska OV. Seasonal Dynamics of Organic Carbon and Nitrogen in Biomasses of Microorganisms in Arable Mollisols Affected by Different Tillage Systems. Land. 2022; 11(4):486. https://doi.org/10.3390/land11040486
Chicago/Turabian StyleKravchenko, Yuriy S., Xingyi Zhang, Chunyu Song, Wei Hu, Anna V. Yarosh, and Olena V. Voitsekhivska. 2022. "Seasonal Dynamics of Organic Carbon and Nitrogen in Biomasses of Microorganisms in Arable Mollisols Affected by Different Tillage Systems" Land 11, no. 4: 486. https://doi.org/10.3390/land11040486






