Plant Conservation in the Midst of Energy Transition: Can Regional Governments Rise to the Challenge?
Abstract
:1. Introduction
2. Materials and Methods
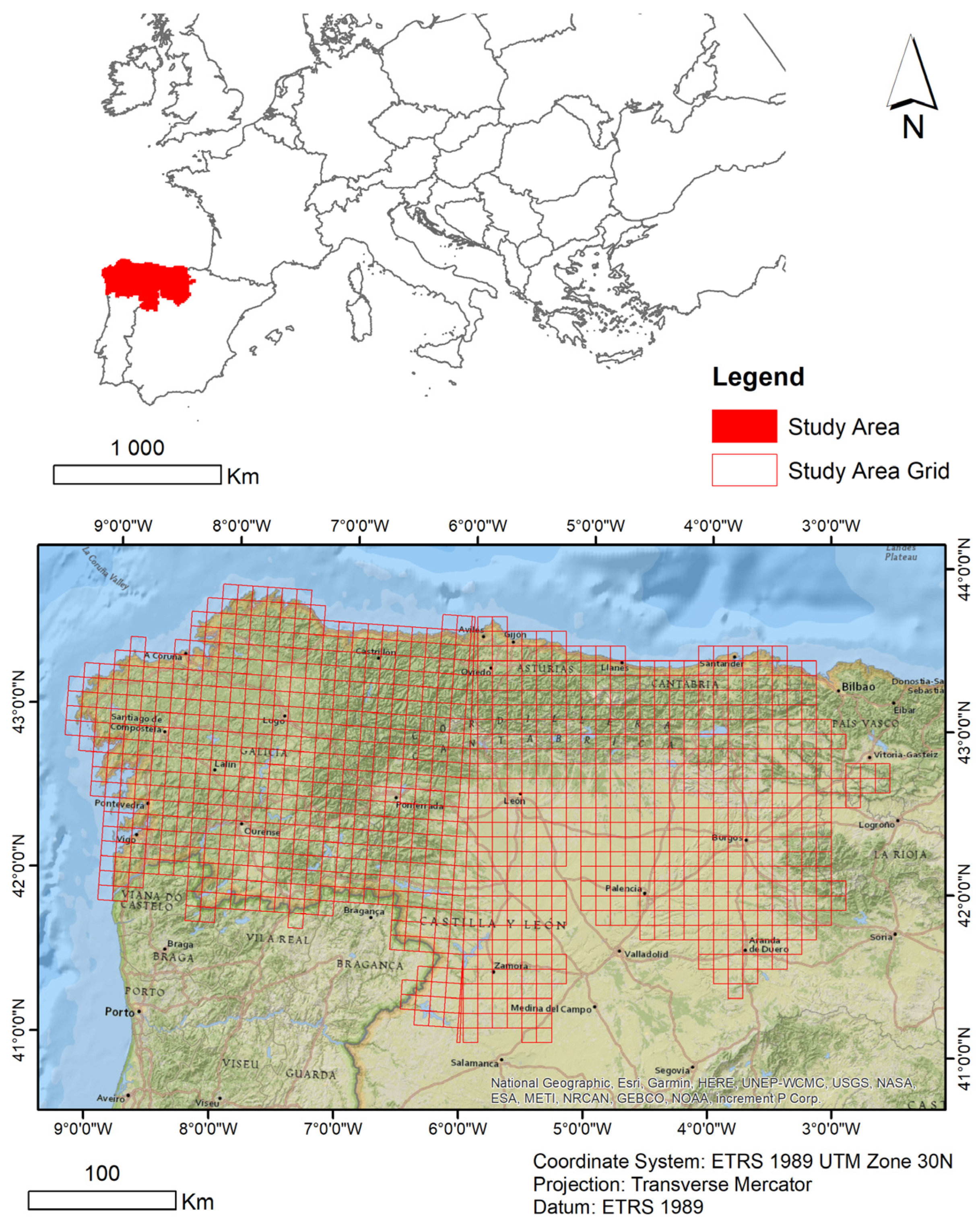
2.1. Study Area
2.2. Methods
2.2.1. Species
Legal Protection Category
Threat Category
Vascular Flora Location
Bryophytes Location
Rarity
- -
- Vascular plants (Three categories based on the calculation of quartiles):
- Rare: 0.94018–0.97572
- Very rare: 0.97572–0.98952
- Extremely rare: 0.98952–0.99984
- -
- Bryophytes (Three categories based on grid numbers due to the low number of grids occupied by all species, mostly smaller than 10):
- Rare: >20 UTMs 10 × 10 km
- Very rare: >10 to <20 UTMs 10 × 10 km
- Extremely rare: <10 UTMs 10 × 10 km
Endemicity
Ranking Assignment
2.2.2. Habitats
2.2.3. Wind Projects Database
2.2.4. Data Analysis
- -
- Total Value is the total sum that includes the maximum protection or threat value for plants and Habitats, rarity value, and endemicity value across all UTM grid cells.
- -
- n is the total number of UTM grid cells.
- -
- Protection Valuei is the protection value in grid cell i.
- -
- Threat Valuei is the threat value in grid cell i.
- -
- Protection HICs Valuei is the habitats of community interest protection value in grid cell i.
- -
- Threat HICs Valuei is the habitats of community interest threat value in grid cell i.
- -
- Threat Valuei is the threat value in grid cell i.
- -
- Endemicity Valuei is the threat value in grid cell i.
- -
- Rarity Valuei is the threat value in grid cell i.
- -
- Protection Valuei is the protection value in grid cell i.
- -
- Protection HICs Valuei is the habitats of community interest protection value in grid cell i.
3. Results
3.1. Wind Projects vs. Space Networks
3.2. Protection vs. Threatened
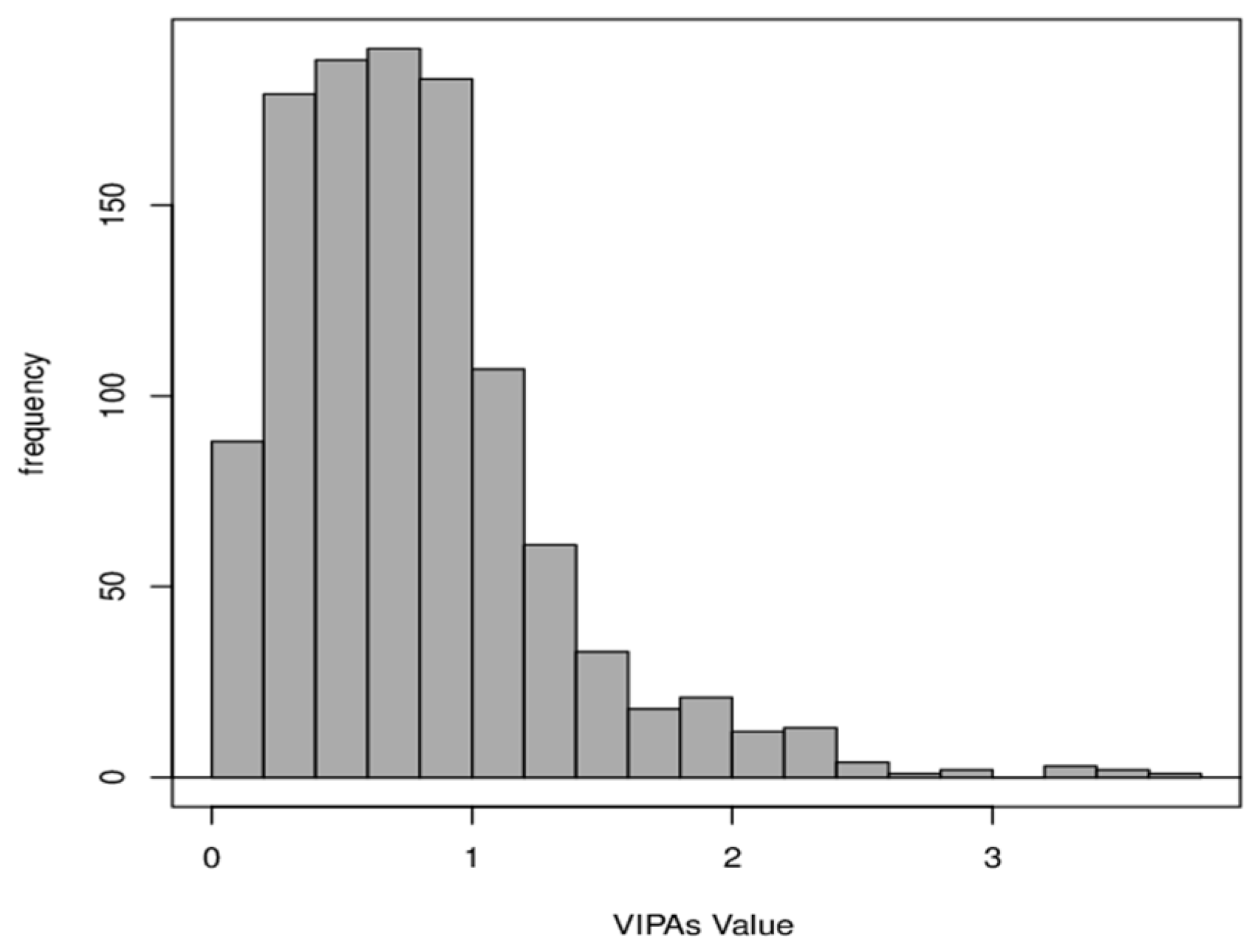
3.3. VIPAs
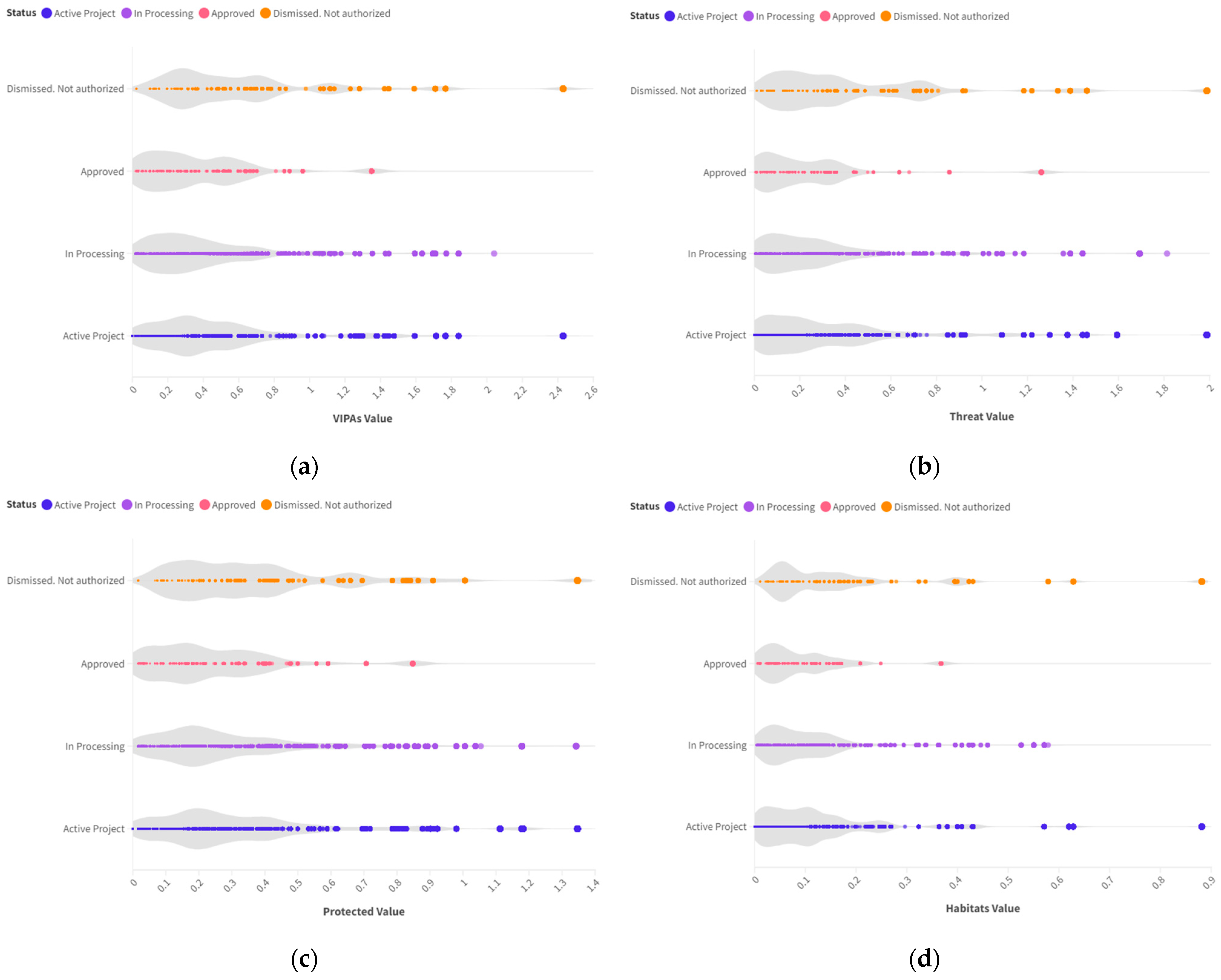
3.4. VIPAs vs. Wind Projects
4. Discussion
- -
- The interaction between scientists and managers needs enhancement [110,113], enabling the application of current knowledge in practical territorial management rather than staying confined to theoretical documents. Generating data for species like certain bryophytes, which are already in decline or vanishing, while simultaneously permitting severe impacts on their populations, serves little purpose.
- -
- There is a need for more streamlined legislation capable of updating lists of protected species in accordance with scientific knowledge. It should be noted that, according to the results obtained, more than one-third of the most endangered species do not have European, national, or regional protection. Time and resources are required to improve and update information on the distribution and status of threatened species [110].
- -
- Management and conservation plans should be developed for species lacking them. In the absence of such plans, efforts should be made to find solutions for environmental zoning that address the current shortcomings, which result in the exclusion of some taxa at high risk of extinction.
- -
- The principle of precaution requires a non-intervention of the vulnerable spaces, even less so when all the possibilities of implementation of production of energy have not been exhausted, complying with the basic requirements of the land use planning of maximum suitability, minimum impact.
- -
- The identification of priority areas in multiple taxonomic groups would help guide decisions and the expansion of protected areas [99]. It is imperative to ensure “minimal overlap” between renewable energy projects and critical biodiversity conservation zones [45,50]. Failure to achieve this could result in substantial, possibly irreversible risks due to an imbalanced information base.
- -
- It is very important that such management is addressed by all administrative levels working together and not that the private energy-developer companies are the ones who select the locations, without speeding up or relaxing the processes related to the environmental impact study or the process of submission to public information.
- -
- Environmental impact studies should be based in a strong fieldwork and capable of adhering to quality criteria [110]. Administrative experts must be proficient in the disciplines they assess, or they should rely on a group of expert scientists advisors. This would prevent, in many cases, the burden of omissions in environmental impact studies from falling on citizens’ objections.
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dasgupta, S.P. The Economics of Biodiversity: The Dasgupta Review Full Report. Environ. Econ. 2021, 1–610. [Google Scholar]
- Díaz, S.; Settele, J.; Brondízio, E.S.; Ngo, H.T.; Agard, J.; Arneth, A.; Balvanera, P.; Brauman, K.A.; Butchart, S.H.M.; Chan, K.M.A.; et al. Pervasive Human-Driven Decline of Life on Earth Points to the Need for Transformative Change. Science 2019, 366, eaax3100. [Google Scholar] [CrossRef] [PubMed]
- Brockett, C.; Woolaston, K.; Deane, F.; Humphries, F.; Kumar, E.; Kennedy, A.; Bell-James, J. Best Practice Mechanisms for Biodiversity Conservation Law and Policy. Camb. Prism. Extinction 2023, 1, e16. [Google Scholar] [CrossRef]
- Bruner, A.G.; Gullison, R.E.; Rice, R.E.; da Fonseca, G.A.B. Effectiveness of Parks in Protecting Tropical Biodiversity. Science 2001, 291, 125–128. [Google Scholar] [CrossRef]
- Geldmann, J.; Barnes, M.; Coad, L.; Craigie, I.D.; Hockings, M.; Burgess, N.D. Effectiveness of Terrestrial Protected Areas in Reducing Habitat Loss and Population Declines. Biol. Conserv. 2013, 161, 230–238. [Google Scholar] [CrossRef]
- Geldmann, J.; Coad, L.; Barnes, M.D.; Craigie, I.D.; Woodley, S.; Balmford, A.; Brooks, T.M.; Hockings, M.; Knights, K.; Mascia, M.B.; et al. A Global Analysis of Management Capacity and Ecological Outcomes in Terrestrial Protected Areas. Conserv. Lett. 2018, 11, e12434. [Google Scholar] [CrossRef]
- Gray, C.L.; Hill, S.L.L.; Newbold, T.; Hudson, L.N.; Börger, L.; Contu, S.; Hoskins, A.J.; Ferrier, S.; Purvis, A.; Scharlemann, J.P.W. Local Biodiversity Is Higher inside than Outside Terrestrial Protected Areas Worldwide. Nat. Commun. 2016, 7, 12306. [Google Scholar] [CrossRef]
- Younge, A.; Fowkes, S. The Cape Action Plan for the Environment: Overview of an Ecoregional Planning Process. Biol. Conserv. 2003, 112, 15–28. [Google Scholar] [CrossRef]
- Coad, L.; Watson, J.E.; Geldmann, J.; Burgess, N.D.; Leverington, F.; Hockings, M.; Knights, K.; Di Marco, M. Widespread Shortfalls in Protected Area Resourcing Undermine Efforts to Conserve Biodiversity. Front. Ecol. Environ. 2019, 17, 259–264. [Google Scholar] [CrossRef]
- Di Minin, E.; Toivonen, T. Global Protected Area Expansion: Creating More than Paper Parks. BioScience 2015, 65, 637–638. [Google Scholar] [CrossRef]
- Jaureguiberry, P.; Titeux, N.; Wiemers, M.; Bowler, D.E.; Coscieme, L.; Golden, A.S.; Guerra, C.A.; Jacob, U.; Takahashi, Y.; Settele, J.; et al. The Direct Drivers of Recent Global Anthropogenic Biodiversity Loss. Sci. Adv. 2022, 8, eabm9982. [Google Scholar] [CrossRef] [PubMed]
- Fahrig, L. Effects of Habitat Fragmentation on Biodiversity. Annu. Rev. Ecol. Evol. Syst. 2003, 34, 487–515. [Google Scholar] [CrossRef]
- Heywood, V.H. Global Biodiversity Assessment; Cambridge University Press: Cambridge, UK, 1995; Volume 1140. [Google Scholar]
- Langhammer, P.F. Identification and Gap Analysis of Key Biodiversity Areas: Targets for Comprehensive Protected Area Systems; IUCN: Gland, Switzerland, 2007; ISBN 978-2-8317-0992-5. [Google Scholar]
- Eken, G.; Bennun, L.; Brooks, T.M.; Darwall, W.; Fishpool, L.D.C.; Foster, M.; Knox, D.; Langhammer, P.; Matiku, P.; Radford, E.; et al. Key Biodiversity Areas as Site Conservation Targets. BioScience 2004, 54, 1110–1118. [Google Scholar] [CrossRef]
- Donald, P.F.; Fishpool, L.D.C.; Ajagbe, A.; Bennun, L.A.; Bunting, G.; Burfield, I.J.; Butchart, S.H.M.; Capellan, S.; Crosby, M.J.; Dias, M.P.; et al. Important Bird and Biodiversity Areas (IBAs): The Development and Characteristics of a Global Inventory of Key Sites for Biodiversity. Bird Conserv. Int. 2019, 29, 177–198. [Google Scholar] [CrossRef]
- Cuena-Lombraña, A.; Fois, M.; Cogoni, A.; Bacchetta, G. Where We Come from and Where to Go: Six Decades of Botanical Studies in the Mediterranean Wetlands, with Sardinia (Italy) as a Case Study. Wetlands 2021, 41, 69. [Google Scholar] [CrossRef]
- Darbyshire, I.; Anderson, S.; Asatryan, A.; Byfield, A.; Cheek, M.; Clubbe, C.; Ghrabi, Z.; Harris, T.; Heatubun, C.D.; Kalema, J.; et al. Important Plant Areas: Revised Selection Criteria for a Global Approach to Plant Conservation. Biodivers. Conserv. 2017, 26, 1767–1800. [Google Scholar] [CrossRef]
- Di Marco, M.; Brooks, T.; Cuttelod, A.; Fishpool, L.D.C.; Rondinini, C.; Smith, R.J.; Bennun, L.; Butchart, S.H.M.; Ferrier, S.; Foppen, R.P.B.; et al. Quantifying the relative irreplaceability of important bird and biodiversity areas. Conserv. Biol. 2016, 30, 392–402. [Google Scholar] [CrossRef]
- Donald, P.F.; Buchanan, G.M.; Balmford, A.; Bingham, H.; Couturier, A.R.; de la Rosa, G.E., Jr.; Gacheru, P.; Herzog, S.K.; Jathar, G.; Kingston, N.; et al. The Prevalence, Characteristics and Effectiveness of Aichi Target 11′s “Other Effective Area-Based Conservation Measures” (OECMs) in Key Biodiversity Areas. Conserv. Lett. 2019, 12, e12659. [Google Scholar] [CrossRef]
- Anderson, S. Identifying Important Plant Areas; Plantlife International: Salisbury, UK, 2002. [Google Scholar]
- Alcaraz, F.; Barreno, E.; Blanca, G.; Blanco, E.; Bolós, O.; Cabezudo, B.; Camarasa, J.M.; Castroviejo, S.; Currás, R.; Fernández Prieto, J.A.; et al. Criterios Para Definir Las Áreas Importantes Para La Flora y La Vegetación. Ecología 1989, 3, 3–5. [Google Scholar]
- Del Valle, E.; Maldonado, F.J.; Sainz, H.; Sánchez de Dios, R. Áreas importantes para la flora amenazada Española. In Atlas y Libro Rojo de la Flora Vascular Amenazada de España; Bañares, A., Blanca, G., Güemes, J., Ortiz, J.C.M.Y.S., Eds.; Dirección General de Conservación de la Naturaleza: Madrid, Spain, 2004; pp. 979–1007. [Google Scholar]
- Muñoz-Rodríguez, P.; Draper Munt, D.; Moreno Saiz, J.C. Global Strategy for Plant Conservation: Inadequate In Situ Conservation of Threatened Flora in Spain. Isr. J. Plant Sci. 2016, 63, 297–308. [Google Scholar] [CrossRef]
- Sánchez de Dios, R.; Cabal Ruano, C.; Domínguez Lozano, F.; Sainz Ollero, H.; Moreno Saiz, J.C. The Role of Criteria in Selecting Important Areas for Conservation in Biodiversity-Rich Territories. Divers. Distrib. 2017, 23, 368–380. [Google Scholar] [CrossRef]
- Infante, M.; Heras, P. Red preliminar de áreas importantes para los briófitos (IBrA). In Atlas y Libro Rojo de Los Briófitos Amenazados de España; Garilleti, R., Albertos, B., Eds.; Organismo Autònomo de Parques Nacionales: Madrid, Spain, 2012. [Google Scholar]
- Infante, M.; Heras, P. Bryophytes in the Spanish Law. Bol. Soc. Esp. Briol. 2005, 5–16. [Google Scholar]
- González-Mancebo, J.M.; Dirkse, G.M.; Patiño, J.; Romaguera, F.; Werner, O.; Ros, R.M.; Martín, J.L. Applying the IUCN Red List Criteria to Small-Sized Plants on Oceanic Islands: Conservation Implications for Threatened Bryophytes in the Canary Islands. Biodivers. Conserv. 2012, 21, 3613–3636. [Google Scholar] [CrossRef]
- Sérgio, C.; Brugués, M.; Cros, R.M.; Casas, C.; Garcia, C. The 2006 Red List and an Updated Checklist of Bryophytes of the Iberian Peninsula (Portugal, Spain and Andorra). Lindbergia 2007, 31, 109–125. [Google Scholar]
- Atienza, V.; Segarra-Moragues, J. Preliminary Red List of the Lichens of the Valencia Community (Eastern Spain). For. Snow Landsc. Res. 2000, 75, 391–400. [Google Scholar]
- Martinez, I.; Aragón, G.; Sarrión, F.J.; Escudero, A.; Burgaz, A.R.; Coppins, B.J. Threatened Lichens in Central Spain (Saxicolous Species Excluded). Cryptogam. Mycol. 2003, 24, 73–97. [Google Scholar]
- Nascimbene, J.; Nimis, P.; Ravera, S. Evaluating the Conservation Status of Epiphytic Lichens of Italy: A Red List. Plant Biosyst. 2013, 147, 898–904. [Google Scholar] [CrossRef]
- Czyżewska, K.; Cieśliński, S. Czerwona Lista Porostów Zagrożonych w Puszczy Białowieskiej [Red List of Threatened Lichens in the Białowieża Old-Growth Forest]. Monogr. Bot. 2014, 91, 107–119. [Google Scholar] [CrossRef]
- Randlane, T.; Jüriado, I.; Suija, A.; Lõhmus, P.; Leppik, E. Lichens in the New Red List of Estonia. Folia Cryptogam. Est. 2008, 44, 113–120. [Google Scholar]
- Lõhmus, P.; Marmor, L.; Jüriado, I.; Suija, A.; Oja, E.; Degtjarenko, P.; Randlane, T. Red List of Estonian Lichens: Revision in 2019. Folia Cryptogam. Est. 2019, 56, 63–76. [Google Scholar] [CrossRef]
- Dahlberg, A.; Mueller, G.M. Applying IUCN Red-Listing Criteria for Assessing and Reporting on the Conservation Status of Fungal Species. Fungal Ecol. 2011, 4, 147–162. [Google Scholar] [CrossRef]
- European Council for the Conservation of Fungi. Available online: http://www.eccf.eu/ (accessed on 6 January 2023).
- Gómez-Bolea, A.; Burgaz, A.; Atienza, V.; Dumitru, O.C.; Chesa, M.; Chiva, S.; Force, L.; Muriel, S.; Prieto, M.; Rico, V.; et al. Checklist of the Lichens and Lichenicolous Fungi of Sierra Nevada (Spain). Bot. Complut. 2021, 45, e74427. [Google Scholar] [CrossRef]
- Marcos Laso, B.; Velasco Santos, J.M.; García Blanco, A.; García Vicente, A.; Fernández Toirán, M.; Ágreda Cabo, T.; Martínez Peña, F.; Hergueta Perlado, A. Boletín Micológico de FAMCAL; Junta de Castilla y León: León, Spain, 2006; pp. 83–94. [Google Scholar]
- Alfaro-Saiz, E.; Alonso Villadangos, Y.; Acedo, C. El futuro es hoy: El reto de una transición energética compatible con la conservación vegetal. Conserv. Veg. 2022, 26, 17–21. [Google Scholar]
- Chico, G.; Clutterbuck, B.; Clough, J.; Lindsay, R.; Midgley, N.G.; Labadz, J.C. Geo-Hydromorphological Assessment of Europe’s Southernmost Blanket Bogs. Earth Surf. Process. Landf. 2020, 45, 2747–2760. [Google Scholar] [CrossRef]
- Kuvlesky, W.P.; Brennan, L.A.; Morrison, M.L.; Boydston, K.K.; Ballard, B.M.; Bryant, F.C. Wind Energy Development and Wildlife Conservation: Challenges and Opportunities. J. Wildl. Manag. 2007, 71, 2487–2498. [Google Scholar] [CrossRef]
- Serrano, D.; Margalida, A.; Pérez-García, J.M.; Juste, J.; Traba, J.; Valera, F.; Carrete, M.; Aihartza, J.; Real, J.; Mañosa, S.; et al. Renewables in Spain Threaten Biodiversity. Science 2020, 370, 1282–1283. [Google Scholar] [CrossRef]
- Santangeli, A.; Toivonen, T.; Pouzols, F.M.; Pogson, M.; Hastings, A.; Smith, P.; Moilanen, A. Global Change Synergies and Trade-Offs between Renewable Energy and Biodiversity. GCB Bioenergy 2016, 8, 941–951. [Google Scholar] [CrossRef]
- Rehbein, J.A.; Watson, J.E.M.; Lane, J.L.; Sonter, L.J.; Venter, O.; Atkinson, S.C.; Allan, J.R. Renewable Energy Development Threatens Many Globally Important Biodiversity Areas. Glob. Change Biol. 2020, 26, 3040–3051. [Google Scholar] [CrossRef]
- Northrup, J.M.; Wittemyer, G. Characterising the Impacts of Emerging Energy Development on Wildlife, with an Eye towards Mitigation. Ecol. Lett. 2013, 16, 112–125. [Google Scholar] [CrossRef]
- Gasparatos, A.; Doll, C.N.H.; Esteban, M.; Ahmed, A.; Olang, T.A. Renewable Energy and Biodiversity: Implications for Transitioning to a Green Economy. Renew. Sustain. Energy Rev. 2017, 70, 161–184. [Google Scholar] [CrossRef]
- Sánchez-Zapata, J.A.; Clavero, M.; Carrete, M.; DeVault, T.L.; Hermoso, V.; Losada, M.A.; Polo, M.J.; Sánchez-Navarro, S.; Pérez-García, J.M.; Botella, F.; et al. Effects of renewable energy production and infrastructure on wildlife. In Current Trends in Wildlife Research; Mateo, R., Arroyo, B., Garcia, J.T., Eds.; Wildlife Research Monographs; Springer International Publishing: Cham, Switzerland, 2016; pp. 97–123. ISBN 978-3-319-27912-1. [Google Scholar]
- European Commission. Guidance Document on Wind Energy Developments and EU Nature Legislation; Directorate-General for Environment (European Commission): Brussels, Belgium; Publications Office of the European Union: Luxembourg, 2020; ISBN 978-92-76-27290-8. [Google Scholar]
- Pérez-García, J.M.; Morant, J.; Arrondo, E.; Sebastián-González, E.; Lambertucci, S.A.; Santangeli, A.; Margalida, A.; Sánchez-Zapata, J.A.; Blanco, G.; Donázar, J.A.; et al. Priority Areas for Conservation Alone Are Not a Good Proxy for Predicting the Impact of Renewable Energy Expansion. Proc. Natl. Acad. Sci. USA 2022, 119, e2204505119. [Google Scholar] [CrossRef] [PubMed]
- Ministerio para la Transición Ecológica y el Reto Demográfico. Zonificación Ambiental para Energías Renovables: Eólica y Fotovoltaica. Available online: https://www.miteco.gob.es/es/calidad-y-evaluacion-ambiental/temas/evaluacion-ambiental/zonificacion_ambiental_energias_renovables.aspx (accessed on 5 July 2022).
- Del Olmo, A. Real Decreto Ley 6/2022, de 29 de Marzo, Por El Que Se Adoptan Medidas Urgentes En El Marco Del Plan Nacional de Respuesta a Las Consecuencias Económicas y Sociales de La Guerra En Ucrania; Fundación Dialnet: Logroño, Spain, 2022. [Google Scholar]
- De la Calzada, J. Análisis de la Legislación Relativa a Los Planes de Gestión de Taxones Amenazados en España. Master’s Thesis, Universidad de Huelva, Huelva, Spain, 2019. [Google Scholar]
- Ministerio para la Transición Ecológica y el Reto Demográfico. Estrategia de Conservación y de Lucha Contra las Amenazas de Plantas Protegidas en Ambientes Rupículas; Ministerio para la Transición Ecológica y el Reto Demográfico: Madrid, Spain, 2018. [Google Scholar]
- Ministerio para la Transición Ecológica y el Reto Demográfico. Estrategia de Conservación y de Lucha Contra Amenazas de Plantas Protegidas de Altas Cumbres; Ministerio para la Transición Ecológica y el Reto Demográfico: Madrid, Spain, 2019. [Google Scholar]
- Ministerio para la Transición Ecológica y el Reto Demográfico. Estrategia de Conservación y de Lucha Contra Amenazas de Plantas Protegidas Ligadas al Agua; Ministerio para la Transición Ecológica y el Reto Demográfico: Madrid, Spain, 2019. [Google Scholar]
- Junta de Castilla y León Criterios para la Implantación de Renovables. Available online: https://energia.jcyl.es/web/es/energias-renovables-ordenacion-energetica/documentos-definitivos.html (accessed on 25 January 2022).
- Consejería de Sostenibilidad. Decreto 63/2007, de 14 de Junio, Por El Que Se Crean El Catálogo de Flora Protegida de Castilla y León y La Figura de Protección Denominada Microrreserva de Flora; Consejería de Sostenibilidad: Madrid, Spain, 2007. [Google Scholar]
- Quevedo, M. Algunos impactos sobre fauna cantábrica. In I Jornadas de Medio Natural y Transición Energética; Universidad de León: León, Spain, 2022. [Google Scholar]
- Venter, O.; Sanderson, E.W.; Magrach, A.; Allan, J.R.; Beher, J.; Jones, K.R.; Levy, M.A.; Watson, J.E. Last of the Wild Project, Version 3 (LWP-3): 2009 Human Footprint; NASA: Washington, DC, USA, 2018.
- Jiménez-Alfaro, B.; Carlón, L.; Fernández-Pascual, E.; Acedo, C.; Alfaro-Saiz, E.; Alonso Redondo, R.; Cires, E.; Del Egido Mazuelas, F.; Del Río, S.; Díaz Gonz’alez, T.E.; et al. Checklist of the Vascular Plants of the Cantabrian Mountains. Mediterr. Bot. 2021, 42, 1–59. [Google Scholar] [CrossRef]
- Aedo, C.; Buira, A.; Medina, L.; Fernández-Albert, M. The Iberian vascular flora: Richness, endemicity and distribution patterns. In The Vegetation of the Iberian Peninsula; Loidi, J., Ed.; Plant and Vegetation; Springer International Publishing: Cham, Switzerland, 2017; Volume 1, pp. 101–130. ISBN 978-3-319-54784-8. [Google Scholar]
- Llamas, F.; Acedo, C.; Lence, C.; Redondo, R.; Molina, A.; Castro, V. Flora Cantábrica de Interés En Castilla y León. Nat. Cantab. 2007, 3, 57–78. [Google Scholar]
- Romero Buján, M.I. Catálogo Da Flora de Galicia; Alonso, J.L.B., Ed.; Jolube Consultor Botánico y Editor: Jaca, Spain, 2008; Volume 1. [Google Scholar]
- Fernández Prieto, J.A.; Cires, E.; Bueno, A.; Vazquez, V.M.; Nava, H. Catálogo de Las Plantas Vasculares Del Principado de Asturias. Doc. Jardín Bot. Atlántico Gijón 2014, 11, 7–267. [Google Scholar]
- Durán Gómez, J.A. Catálogo de la Flora Vascular de Cantabria; Alonso, J.L.B., Ed.; Jolube Consultor Botánico y Editor: Jaca, Spain, 2014; Volume 13, ISBN 978-84-941996-2-2. [Google Scholar]
- Ramos-Gutiérrez, I.; Lima, H.; Pajarón, S.; Romero-Zarco, C.; Sáez, L.; Pataro, L.; Molina-Venegas, R.; Rodriguez, M.; Moreno Saiz, J. Atlas of the Vascular Flora of the Iberian Peninsula Biodiversity Hotspot (AFLIBER). Glob. Ecol. Biogeogr. 2021, 30, 1951–1957. [Google Scholar] [CrossRef]
- Xunta de Galicia. Decreto 88/2007 de 19 de Abril, Por El Que Se Regula El Catálogo Gallego de Especies Amenazadas; Xunta de Galicia: Coruña, Spain, 2007.
- Ministerio de Transición Ecológica. Decreto 65/1995, de 27 de Abril Por El Que Se Crea El Catálogo Regional de Especies Amenazadas de La Flora Del Principado de Asturias y Se Dictan Normas Para Su Protección; Ministerio de Transición Ecológica: Madrid, Spain, 1995. [Google Scholar]
- Ministerio de Transición Ecológica. Decreto 120/2008, de 4 de Diciembre Por El Que Se Regula El Catálogo Regional de Especies Amenazadas de Cantabria; Ministerio de Transición Ecológica: Madrid, Spain, 2008. [Google Scholar]
- Ministerio de Transición Ecológica. Ley 4/2015, de 24 de Marzo, Del Patrimonio Natural de Castilla y León; Ministerio de Transición Ecológica: Madrid, Spain, 2015. [Google Scholar]
- Catalogue of Life COL Checklist, 2023-09-14. Available online: https://www.catalogueoflife.org/ (accessed on 6 September 2023).
- Plants of the World Online: POWO. Facilitated by the Royal Botanic Gardens, Kew. Available online: https://powo.science.kew.org/ (accessed on 6 May 2022).
- European Parliament. Directive 92/43/CEE of the Council of 21 May 1992 on the Conservation of Natural Habitats and of Wild Fauna and Flora (Habitat Directive); European Parliament: Strasbourg, France, 1992. [Google Scholar]
- European Parliament. Real Decreto 139/2011, de 4 de Febrero, Para el Desarrollo del Listado de Especies Silvestres En Régimen de Protección Especial y Del Catálogo Español de Especies Amenazadas; European Parliament: Strasbourg, France, 2011. [Google Scholar]
- Bañares, A.; Blanca, G.; Güemes, J.; Moreno, J.C.; Ortiz, S. Atlas y Libro Rojo de La Flora Vascular Amenazada de España; Dirección General de Conservación de la Naturaleza: Madrid, Spain, 2004; ISBN 84-8014-521-8. [Google Scholar]
- Bañares, A.; Blanca, G.; Güemes, J.; Moreno, J.C.; Ortiz, S. Atlas y Libro Rojo de La Flora Vascular Amenazada de España: Adenda 2006; Dirección General para la Biodiversidad-Sociedad Española de Biología de la Conservación de Plantas: Madrid, Spain, 2006; ISBN 978-84-8014-706-4. [Google Scholar]
- Bañares, A.; Blanca, G.; Güemes, J.; Moreno, J.C.; Ortiz, S. Atlas y Libro Rojo de La Flora Vascular Amenazada de España: Adenda 2008; Dirección General de Medio Natural y Política Forestal (Ministerio de Medio Ambiente, y Medio Rural y Marino)—Sociedad Española de Biología de la Conservación de Plantas: Madrid, Spain, 2008; ISBN 978-84-8014-741-5. [Google Scholar]
- Bañares, A.; Blanca, G.; Güemes, J.; Moreno, J.C.; Ortiz, S. Atlas y Libro Rojo de La Flora Vascular Amenazada de España: Adenda 2010; Dirección General de Medio Natural y Política Forestal (Ministerio de Medio Ambiente, y Medio Rural y Marino)—Sociedad Española de Biología de la Conservación de Plantas: Madrid, Spain, 2010. [Google Scholar]
- Moreno Saiz, J.C.; Iriondo Alegría, G.; Martínez García, F.; Martínez Rodríguez, J.; Salazar Mendías, C. Atlas y Libro Rojo de La Flora Vascular Amenazada de España. Adenda 2017; Ministerio para la Transición Ecológica-Sociedad Española de Biología de la Conservación de Plantas: Madrid, Spain, 2019. [Google Scholar]
- Brugués, M.; González, M. Lista Roja de Los Briófitos Amenazados de España; Garilleti, R., Albertos, B., Eds.; Atlas de Los Briófitos Amenazados de España; Publicado En Línea El 04/07/2014; Universitat de València: València, Spain, 2014. [Google Scholar]
- Garilleti, R.; Albertos, B. ABrA Atlas de Los Briófitos Amenazados de España; Universitat de València: València, Spain, 2017. [Google Scholar]
- GBIF. The Global Biodiversity Information Facility. Available online: www.gbif.org (accessed on 14 October 2023).
- GBIF.org. (6 October 2023) GBIF Occurrence; GBIF: Copenhagen, Denmark, 2023; Available online: https://www.gbif.org/occurrence/download/0004644-231002084531237 (accessed on 14 October 2023).
- Llamas García, F.; Acedo, C.; Lence Paz, C.; Molina González, A.M. Prioridades de conservación de flora cantábrica de interés en Castilla y León. In Botánica Pirenaico-Cantábrica en el Siglo XXI; Universidad de León: León, Spain, 2009; pp. 191–218. ISBN 978-84-9773-471-4. [Google Scholar]
- Jiménez-Alfaro, B.; Bueno Sánchez, Á.; Fernández-Prieto, J.A. Valoración de plantas de interés para la conservación en Asturias a través de un Índice de Responsabilidad. Nat. Cantab. 2007, 3, 25–36. [Google Scholar]
- Schatz, B.; Gauthier, P.; Debussche, M.; Thompson, J.D. A Decision Tool for Listing Species for Protection on Different Geographic Scales and Administrative Levels. J. Nat. Conserv. 2014, 22, 75–83. [Google Scholar] [CrossRef]
- Krigas, N.; Karapatzak, E.; Panagiotidou, M.; Sarropoulou, V.; Samartza, I.; Karydas, A.; Damianidis, C.K.; Najdovski, B.; Teofilovski, A.; Mandzukovski, D.; et al. Prioritizing Plants around the Cross-Border Area of Greece and the Republic of North Macedonia: Integrated Conservation Actions and Sustainable Exploitation Potential. Diversity 2022, 14, 570. [Google Scholar] [CrossRef]
- Vargas, P. Exploring ‘Endangered Living Fossils’ (ELFs) among Monotypic Genera of Plants and Animals of the World. Front. Ecol. Evol. 2023, 11, 570. [Google Scholar] [CrossRef]
- Ministerio para la Transición Ecológica y el Reto Demográfico. Informe Sobre La Aplicación de La Directiva Hábitats En España 2013–2018. Available online: https://www.miteco.gob.es/es/biodiversidad/temas/espacios-protegidos/red-natura-2000/rn_cons_seguimiento_art17_inf_2013_2018.html (accessed on 1 February 2023).
- Ministerio para la Transición Ecológica y el Reto Demográfico. Hábitats de Interés Comunitario Del Anexo I de La Directiva 92/43/CEE. Available online: https://www.miteco.gob.es/es/biodiversidad/servicios/banco-datos-naturaleza/informacion-disponible/habitat.html (accessed on 1 February 2023).
- Tsiripidis, I.; Piernik, A.; Janssen, J.A.M.; Tahvanainen, T.; Molina, J.A.; Giusso del Galdo, G.; Gardfjell, H.; Dimopoulos, P.; Šumberová, K.; Acosta, A.; et al. European Red List of Habitats. Part 2, Terrestrial and Freshwater Habitats; European Commission, Environment General Direction, Publications Office: Luxembourg, 2016; ISBN 978-92-79-61588-7. [Google Scholar]
- EUNIS. Database. European Nature Information System; European Environment Agency: Copenhagen, Denmark, 2019; Available online: https://eunis.eea.europa.eu/index.jsp (accessed on 6 October 2023).
- Ramírez Rodríguez, R.; Bañuelos Martínez, M.J.; Cires Rodríguez, E.; Álvarez Arbesú, R.L.; Valderrábano Luque, J.; García Manteca, P.; González Rodríguez, G.; Marquínez García, J.L.; Fernández Prieto, J.A. Hábitats EUNIS Terrestres y Litorales del Principado de Asturias. Nat. Cantab. 2021, 76, 1–138. [Google Scholar]
- Ramírez Rodríguez, R.; Bañuelos Martínez, M.J.; Cires Rodríguez, E.; Álvarez Arbesú, R.L.; Valderrábano Luque, J.; García Manteca, P.; González Rodríguez, G.; Marquínez García, J.L.; Fernández Prieto, J.A. Hábitats terrestres de interés comunitario y prioritarios presentes en el Principado de Asturias. Nat. Cantab. 2021, 9, 139–219. [Google Scholar]
- FJDCC. Fondo para la Defensa Jurídica de la Cordillera Cantábrica. Available online: https://fdjcc.org/ (accessed on 4 July 2023).
- R Software A Language and Environment for Statistical Computing. R Foundation for Statistical Computing. Available online: https://www.r-project.org/ (accessed on 14 October 2023).
- Fox, J.; Bouchet-Valat, M.; Andronic, L.; Ash, M.; Boye, T.; Calza, S.; Chang, A.; Grosjean, P.; Heiberger, R.; Pour, K.K.; et al. Package “Rcmdr”; R Project: Vienna, Austria, 2020. [Google Scholar]
- Lim, D.Y.H.; Starnes, T.; Plumptre, A.J. Global Priorities for Biodiversity Conservation in the United Kingdom. Biol. Conserv. 2023, 277, 109798. [Google Scholar] [CrossRef]
- Gibson, L.; Wilman, E.N.; Laurance, W.F. How Green Is ‘Green’ Energy? Trends Ecol. Evol. 2017, 32, 922–935. [Google Scholar] [CrossRef] [PubMed]
- Swift, T.L.; Hannon, S.J. Critical Thresholds Associated with Habitat Loss: A Review of the Concepts, Evidence, and Applications. Biol. Rev. 2010, 85, 35–53. [Google Scholar] [CrossRef] [PubMed]
- Templeton, A.R.; Shaw, K.; Routman, E.; Davis, S.K. The Genetic Consequences of Habitat Fragmentation. Ann. Mo. Bot. Gard. 1990, 77, 13–27. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, Z.; Li, B.; Guan, J. The Impact of Photovoltaic Projects on Ecological Corridors through the Least-Cost Path Model. Glob. Ecol. Conserv. 2023, 42, e02381. [Google Scholar] [CrossRef]
- Ramil Rego, P.; Santamarina, J.; Muñoz Benito, L.; San Martín Segura, D.; Rodriguez Guitián, M.A.; Ferreiro da Costa, J.; Gómez Orellana, L.; López Castro, H.; Oreiro Rey, C. Informe Sobre La Viabilidad de Las Instalaciones Eólicas, Fotovoltaicas y Termo Solares, Incluida La Repotenciación e Infraestructuras Asociadas En Los Territorios Declarados Reservas de Biosfera En España; Concejo Científico Del Comité Español de Programa M & B: Madrid, Spain, 2022. [Google Scholar]
- Chico, G.; Clutterbuck, B.; Lindsay, R.; Midgley, N.; Labadz, J. Identification and Classification of Unmapped Blanket Bogs in the Cordillera Cantábrica, Northern Spain. Mires Peat 2019, 24, 1–12. [Google Scholar] [CrossRef]
- Parish, F.; Sirin, A.A.; Charman, D.; Joosten, H.; Minaeva, T.Y.; Silvius, M. Assessment on Peatlands, Biodiversity and Climate Change; Wetlands International: Ede, The Netherlands, 2008. [Google Scholar]
- Smith, J.; Nayak, D.R.; Smith, P. Wind Farms on Undegraded Peatlands Are Unlikely to Reduce Future Carbon Emissions. Energy Policy 2014, 66, 585–591. [Google Scholar] [CrossRef]
- Chico, G.; Clewer, T.; Midgley, N.G.; Gallego-Anex, P.; Ramil-Rego, P.; Ferreiro, J.; Whayman, E.; Goeckeritz, S.; Stanton, T. The Extent of Windfarm Infrastructures on Recognised European Blanket Bogs. Sci. Rep. 2023, 13, 3919. [Google Scholar] [CrossRef]
- Heras, P.; Infante, M. La Turbera Cobertor Del Zalama (Burgos—Vizcaya): Un Enclave Único En Riesgo de Desaparición. Estud. Mus. Cienc. Nat. Alava 2004, 18–19, 49–57. [Google Scholar]
- Valera, F.; Bolonio, L.; Calle, A.L.; Moreno, E. Deployment of Solar Energy at the Expense of Conservation Sensitive Areas Precludes Its Classification as an Environmentally Sustainable Activity. Estud. Mus. Cienc. Nat. Álava 2022, 18, 2330. [Google Scholar] [CrossRef]
- Durá-Alemañ, C.J.; Moleón, M.; Pérez-García, J.M.; Serrano, D.; Sánchez-Zapata, J.A. Climate Change and Energy Crisis Drive an Unprecedented EU Environmental Law Regression. Conserv. Lett. 2023, 16, e12958. [Google Scholar] [CrossRef]
- Jefatura del Estado. Ley 7/2021, de 20 de Mayo, de Cambio Climático y Transición Energética; Jefatura del Estado: Madrid, Spain, 2021. [Google Scholar]
- Jager, H.I.; Efroymson, R.A.; McManamay, R.A. Renewable Energy and Biological Conservation in a Changing World. Biol. Conserv. 2021, 263, 109354. [Google Scholar] [CrossRef]


| 9 (Very High) | 6 (High) | 1 (Medium High) | ||
|---|---|---|---|---|
| Legal Protection Category | Europe (C.D. 92/43/CEE) | Annex II * | Annex II | Annex V |
| Annex IV | ||||
| Spain (R.D. 139/2011) | Endangered (CEEA) | Vulnerable (CEEA) | LERSPE | |
| Asturias (Decreto 65/1995) | Endangered | Sensitive to Habitat Alteration and Vulnerable | Special Interest | |
| Cantabria (Decreto 120/2008) | Endangered | Vulnerable | - | |
| Galicia (Decreto 88/2007) | Endangered | Vulnerable | - | |
| Castilla y León (Ley 4/2015) | Endangered | Vulnerable | Priority attention and Regulated use | |
| Threat Category | IUCN | Critically Endangered | Endangered | Vulnerable |
| Rarity | Extremely rare | Very rare | Rare | |
| Endemicity | Cantabrian Mountains | Cantabrian Mountains + Pyrenees | Iberian Peninsula | |
| Taxon Name | Endemicity | European Directive 92/43/CEE | Spanish Royal Decree 139/2011 | Regional Protection | Taxonomic Group |
|---|---|---|---|---|---|
| Geranium dolomiticum Rothm. | Iberian Peninsula | EN (CyL) | VP | ||
| Gyrocaryum oppositifolium Valdés | Iberian Peninsula | EN (CyL) | VP | ||
| Hibiscus palustris L. | EN (Cantabria) | VP | |||
| Hydrocharis morsus-ranae L. | CEEA-EN | EN (Galicia) | VP | ||
| Eleocharis mamillata subsp. austriaca (Hayek) Strandh. | VP | ||||
| Quercus pauciradiata Penas et al. | Cantabrian range | PAt (CyL) | VP | ||
| Ranunculus montserratii Grau | Cantabrian range | PAt (CyL) | VP | ||
| Santolina melidensis (Rodr.Oubiña and S.Ortiz) Rodr.Oubiña & S.Ortiz | Iberian Peninsula | EN (Galicia) | VP | ||
| Silene marizii Samp. | Iberian Peninsula | VU (Galicia) | VP | ||
| Iris boissieri Henriq. | Iberian Peninsula | Anexo IV | LESRPE | EN (CyL) | VP |
| Orchis spitzelii Saut. ex W.D.J.Koch | VP | ||||
| Tragopogon pseudocastellanus Blanca and C.Díaz | VP | ||||
| Breutelia chrysocoma Lindberg | Bryo | ||||
| Gymnomitrion crenulatum Gottsche ex Carrington | Bryo | ||||
| Jungermannia handelii (Schiffner) Amak. | LESRPE | Bryo | |||
| Neoorthocaulis binsteadii (Kaal.) L. Söderstr., De Roo and Hedd. | VU (Galicia) | Bryo | |||
| Orthotrichum patens Bruch and Bridel | Bryo | ||||
| Polytrichastrum longisetum G.L.Smith | Bryo | ||||
| Radula holtii Spruce | VU (Galicia) | Bryo | |||
| Zygodon stirtonii Schimp. | Bryo |
| Variable | Correlation Coefficient (tau) | z-Value | p-Value |
|---|---|---|---|
| Maximum protection | 0.1280161 | 4.074 | 0.00005405 ** |
| European protection | −0.06356556 | −1.893 | 0.05839 * |
| Spanish protection | 0.0695387 | 2.037 | 0.04167 ** |
| Regional protection | 0.1888842 | 5.907 | 3.485 × 10−9 ** |
| Asturias protection | 0.2075477 | 2.788 | 0.005311 ** |
| Cantabria protection | 0.1587965 | 1.301 | 0.1932 |
| CyL protection | 0.5299932 | 12.840 | <2.2 × 10−16 ** |
| Galicia protection | 0.07441219 | 0.974 | 0.3302 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alfaro-Saiz, E.; Fernández-Salegui, A.B.; Acedo, C. Plant Conservation in the Midst of Energy Transition: Can Regional Governments Rise to the Challenge? Land 2023, 12, 2003. https://doi.org/10.3390/land12112003
Alfaro-Saiz E, Fernández-Salegui AB, Acedo C. Plant Conservation in the Midst of Energy Transition: Can Regional Governments Rise to the Challenge? Land. 2023; 12(11):2003. https://doi.org/10.3390/land12112003
Chicago/Turabian StyleAlfaro-Saiz, Estrella, Ana Belén Fernández-Salegui, and Carmen Acedo. 2023. "Plant Conservation in the Midst of Energy Transition: Can Regional Governments Rise to the Challenge?" Land 12, no. 11: 2003. https://doi.org/10.3390/land12112003
APA StyleAlfaro-Saiz, E., Fernández-Salegui, A. B., & Acedo, C. (2023). Plant Conservation in the Midst of Energy Transition: Can Regional Governments Rise to the Challenge? Land, 12(11), 2003. https://doi.org/10.3390/land12112003







