Coastal Biodiversity Assessment Aided by Citizen Science Volunteers: A Look at the Italian Central Adriatic
Abstract
:1. Introduction
2. Materials and Methods
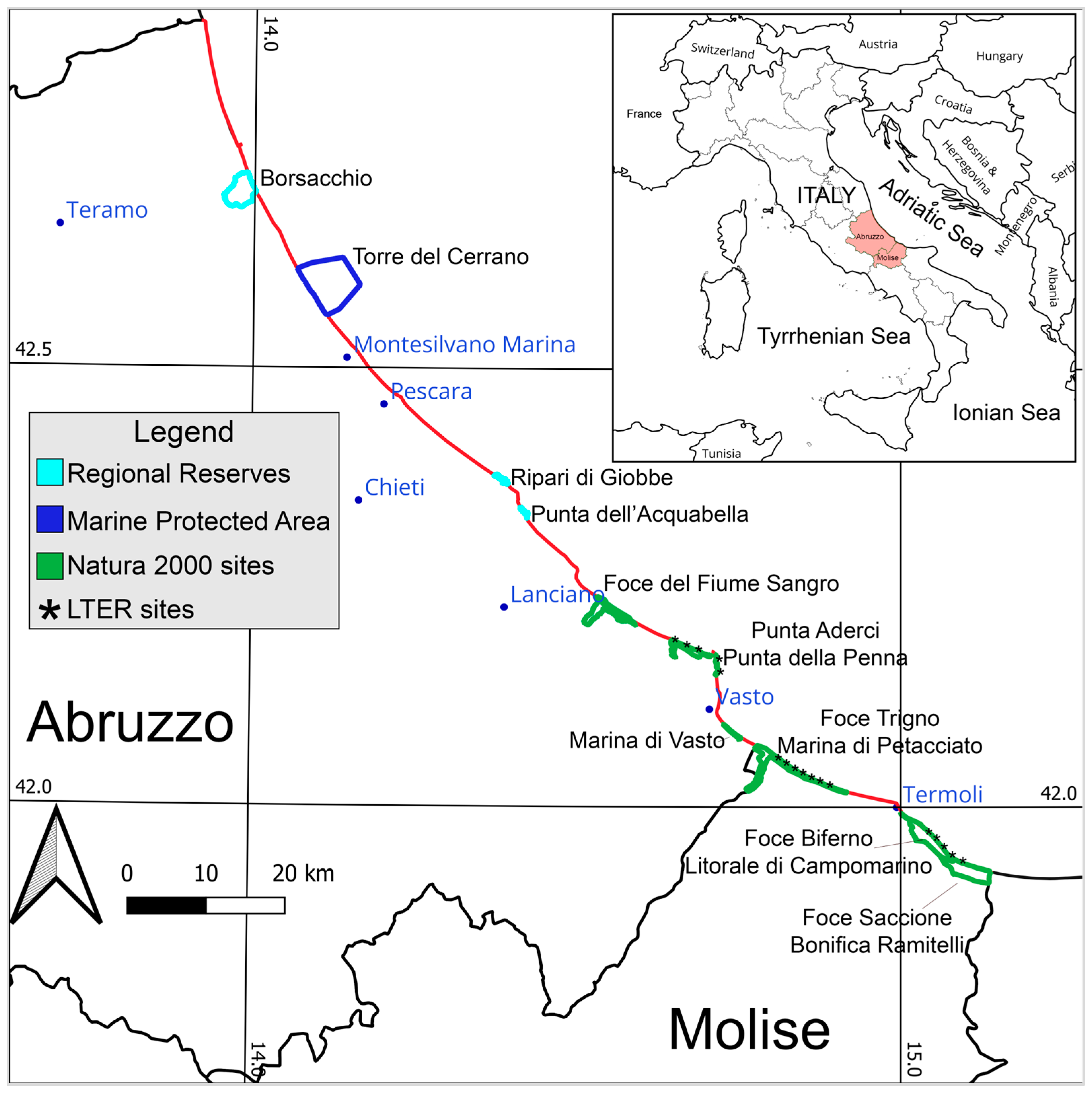
2.1. Study Area
2.2. Data Collection
2.3. Data Storage and Organization
2.4. Data Extraction and Analysis
3. Results and Discussion
3.1. Species of Conservation Concern
| Species | GLOBAL IUCN | Italian IUCN | HD Ann. | No. of Obs. | iNaturalist Category |
|---|---|---|---|---|---|
| Pinna nobilis | CR | CR | IV | 3 | Mollusks |
| Cladocora caespitosa | EN | LC | 9 | Other animals | |
| Chelonia mydas | EN | NE | IV | 3 | Reptiles |
| Testudo hermanni | EN | EN | II; IV | 8 | Reptiles |
| Caretta caretta | VU | EN | II; IV | 239 | Reptiles |
| Passer italiae | VU | VU | 8 | Birds | |
| Streptopelia turtur | VU | LC | 5 | Birds | |
| Palinurus elephas | VU | NE | 2 | Other animals | |
| Balaenoptera physalus | VU | EN | IV | 1 | Mammals |
| Epinephelus marginatus | VU | EN | 2 | Fishes | |
| Aythya ferina | VU | VU | 1 | Birds | |
| Cerambyx cerdo | VU | LC | II; IV | 1 | Insects |
| Haematopus ostralegus | NT | VU | 3 | Birds | |
| Calidris ferruginea | NT | NE | 2 | Birds | |
| Aythya nyroca | NT | EN | 1 | Birds | |
| Raja asterias | NT | NE | 2 | Other animals | |
| Sciaena umbra | NT | VU | 1 | Fishes |
3.2. EU Habitats
| Species | Habitat Code and Name |
|---|---|
| Cymodocea nodosa (Ucria) Asch. | 1110: Sandbanks which are slightly covered by seawater all the time |
| Cakile maritima Scop. subsp. Maritima | 1210: Annual vegetation of drift lines |
| Salsola kali L. | |
| Salsola soda L. | |
| Crithmum maritimum L. | 1240: Vegetated Sea cliffs of the Mediterranean coasts with endemic Limonium spp. |
| Calystegia soldanella (L.) R. Br. | 2110: Embryonic shifting dunes |
| Elymus farctus (Viv.) Runemark ex Melderis | |
| Eryngium maritimum L. | |
| Euphorbia paralias L. | |
| Euphorbia peplis L. | |
| Lotus creticus L. | |
| Medicago marina L. | |
| Polygonum maritimum L. | |
| Achillea maritima (L.) Ehrend. et Y.P. Guo | 2120: Shifting dunes along the shoreline with Ammophila arenaria (white dunes) |
| Ammophila arenaria LK. | |
| Anthemis maritima L. | |
| Cyperus capitatus Vand. | |
| Echinophora spinosa L. | |
| Eryngium maritimum L. | |
| Lotus creticus L. | |
| Pancratium maritimum L. | |
| Sixalix atropurpurea (L.) Greuter and Burdet | |
| Glaucium flavum Crantz | 3250: Constantly flowing Mediterranean rivers with Glaucium flavum |
| Euphorbia terracina L. | 2130 *: Fixed coastal dunes with herbaceous vegetation (grey dunes) |
| Verbascum niveum subsp. garganicum (Ten.) Murb. | |
| Artemisia campestris L. | 2230: Malcolmietalia dune grasslands |
| Erodium laciniatum (Cav.) Willd. | |
| Lagurus ovatus L. | |
| Malcolmia ramosissima (Desf.) Thell. | |
| Matthiola incana (L.) R. Br. | |
| Ononis variegata L. | |
| Silene colorata Poir. | |
| Verbascum niveum subsp. garganicum (Ten.) Murb. | |
| Asparagus acutifolius L. | 2250 *: Coastal dunes with Juniperus spp. |
| Juniperus macrocarpa Sm. | |
| Myrtus communis L. | |
| Phillyrea angustifolia L. | |
| Pistacia lentiscus L. | |
| Rhamnus alaternus L. | |
| Smilax aspera L. | |
| Asparagus acutifolius L. | 2260: Cisto-Lavanduletalia dune sclerophyllous scrubs |
| Cistus creticus L. | |
| Cistus salviifolius L. | |
| Phillyrea angustifolia L. | |
| Pistacia lentiscus L. | |
| Rhamnus alaternus L. | |
| Rosmarinus officinalis L. | |
| Smilax aspera L. | |
| Phillyrea angustifolia L. | 2270 *: Wooded dunes with Pinus pinea and/or Pinus pinaster |
| Pinus halepensis Mill. | |
| Pinus pinea L. | |
| Pistacia lentiscus L. | |
| Atriplex prostrata Boucher ex DC. | 1410: Mediterranean salt meadows (Juncetalia maritimi) |
| Blackstonia perfoliata (L.) Huds. | |
| Juncus acutus L. | |
| Limbarda crithmoides (L.) Dumort. | |
| Sarcocornia fruticosa (L.) A.J. Scott | 1420: Mediterranean and thermo-Atlantic halophilous scrubs (Sarcocornietea fruticosi) |
| Schoenus nigricans L. | 6420: Mediterranean tall humid herb grasslands of the Molinio-Holoschoenion |
| Scirpoides holoschoenus (L.) Soják | |
| Tripidium ravennae (L.) H. Scholz |
3.3. Alien Species
| Species | No. | Family | Life Form | Origin |
|---|---|---|---|---|
| Xanthium orientale subsp. italicum (Moretti) GreuterNI | 11 | Asteraceae | Annual | North America |
| Acacia saligna (Labill.) H.L. Wendl.NI | 9 | Fabaceae | Tree | Western Australia |
| Oxalis pes-caprae L.NI | 7 | Oxalidaceae | Herb | South Africa |
| Carpobrotus acinaciformis (L.) L. BolusNI | 5 | Aizoaceae | Dwarf | South Africa |
| Carpobrotus edulis (L.) N.E. Br.NI | 5 | Aizoaceae | Dwarf | South Africa |
| Erigeron bonariensis L.NI | 3 | Asteraceae | Annual | South America |
| Opuntia stricta (Haw.) Haw.NI | 3 | Cactaceae | Shrub | Central America |
| Robinia pseudoacacia L.NI | 3 | Fabaceae | Tree | North America |
| Symphyotrichum squamatum (Spreng.) G.L. NesomNI | 3 | Asteraceae | Annual | South America |
| Ailanthus altissima (Mill.) SwingleNI | 2 | Simaroubaceae | Tree | China |
| Datura stramonium L.NI-TX | 2 | Solanaceae | Annual | Central/North Am. |
| Euphorbia maculata L.NI | 2 | Euphorbiaceae | Annual | North America |
| Oxalis articulata SavignyNI | 2 | Oxalidaceae | Herb | South America |
| Agave americana L.NI | 1 | Asparagaceae | Shrub | Central/North Am. |
| Amorpha fruticosa L.NI | 1 | Fabaceae | Shrub | North America |
| Erigeron canadensis L.NI | 1 | Asteraceae | Annual | North America |
| Helianthus annuus L.NC | 1 | Asteraceae | Annual | North America |
| Mirabilis jalapa L.NI | 1 | Nyctaginaceae | Herb | South America |
| Nicotiana glauca GrahamNI-TX | 1 | Solanaceae | Shrub | South America |
| Oenothera glazioviana MicheliNI | 1 | Onagraceae | Herb | North America |
| Opuntia ficus-indica (L.) Mill.NI | 1 | Cactaceae | Shrub | Central America |
| Paspalum dilatatum Poir.NI | 1 | Poaceae | Herb | South America |
| Pittosporum tobira (Thunb.) W.T. AitonNN | 1 | Pittosporaceae | Shrub | Eastern Asia |
| Senecio inaequidens DCNI-TX | 1 | Asteraceae | Herb | South Africa |
| Yucca gloriosa L.NI | 1 | Asparagaceae | Shrub | North America |
| Species | No. | iNaturalist Taxon | Origin |
|---|---|---|---|
| Myocastor coypus (Molina, 1782) | 13 | Mammals | South America |
| Trachemys scripta (Schoepff, 1792) | 4 | Reptiles | North America |
| Hystrix cristata (Linnaeus, 1758) | 2 | Mammals | Africa |
| Magallana gigas (Thunberg, 1793) | 2 | Molluscs | Asia |
| Paysandisia archon (Burmeister, 1880) | 2 | Insects | South America |
| Rapana venosa (Valenciennes, 1846) | 2 | Molluscs | Asia |
| Sceliphron caementarium (Drury, 1773) | 2 | Insects | North America |
| Threskiornis aethiopicus (Latham, 1790) | 2 | Birds | Africa; Asia |
| Anadara transversa (Say, 1822) | 1 | Molluscs | North America |
| Blatta orientalis (Linnaeus, 1758) | 1 | Insects | Asia; Africa |
| Cairina moschata (Linnaeus, 1758) | 1 | Birds | South America |
| Complex Pelophylax ridibundus (Pallas, 1771) | 1 | Amphibians | Europe; Asia |
| Gambusia holbrooki (Girard, 1859) | 1 | Actinopterygii | North America |
| Harmonia axyridis (Pallas, 1773) | 1 | Insects | Asia |
| Isodontia mexicana (de Saussure, 1867) | 1 | Insects | North America |
| Leptoglossus occidentalis (Heidemann, 1910) | 1 | Insects | North America |
| Rhynchophorus ferrugineus (Olivier, 1790) | 1 | Insects | Asia |
| Steatoda nobilis (Thorell, 1875) | 1 | Arachnids | Macaronesia |
| Trichopoda pictipennis (Fabricius, 1781) | 1 | Insects | South America |
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Walker, S.; Wilson, J.B.; Steel, J.B.; Rapson, G.L.; Smith, B.; King, W.M.; Cottam, Y.H. Properties of ecotones: Evidence from five ecotones objectively determined from a coastal vegetation gradient. J. Veg. Sci. 2003, 14, 579–590. [Google Scholar] [CrossRef]
- Martínez, M.L.; Psuty, N.P.; Lubke, R.A. A Perspective on Coastal Dunes. In Coastal Dunes; Martínez, M.L., Psuty, N.P., Eds.; Ecology and Conservation; Springer: Berlin/Heidelberg, Germany, 2004; pp. 3–10. [Google Scholar] [CrossRef]
- Carpenter, S.R. Ecosystems and Human Well-Being: Scenarios; Findings of the Scenarios Working Group; Island Press: Washington, DC, USA, 2005; Volume 2. [Google Scholar]
- Drius, M.; Jones, L.; Marzialetti, F.; De Francesco, M.C.; Stanisci, A.; Carranza, M.L. Not just a sandy beach. The multi-service value of Mediterranean coastal dunes. Sci. Total. Environ. 2019, 668, 1139–1155. [Google Scholar] [CrossRef]
- Schlacher, T.A.; Dugan, J.; Schoeman, D.S.; Lastra, M.; Jones, A.; Scapini, F.; McLachlan, A.; Defeo, O. Sandy beaches at the brink. Divers. Distrib. 2007, 13, 556–560. [Google Scholar] [CrossRef]
- Defeo, O.; McLachlan, A.; Schoeman, D.S.; Schlacher, T.A.; Dugan, J.; Jones, A.; Lastra, M.; Scapini, F. Threats to sandy beach ecosystems: A review. Estuar. Coast. Shelf Sci. 2009, 81, 1–12. [Google Scholar] [CrossRef]
- Hesp, P.A.; Martínez, M.L. Disturbance Processes and Dynamics in Coastal Dunes. In Disturbance Ecology: The Process and the Response; Johnson, E.A., Miyanishi, K., Eds.; Elsevier: Amsterdam, The Netherlands, 2007; pp. 215–247. [Google Scholar] [CrossRef]
- Malavasi, M.; Bartak, V.; Carranza, M.L.; Simova, P.; Acosta, A.T.R. Landscape pattern and plant biodiversity in Mediterranean coastal dune ecosystems: Do habitat loss and fragmentation really matter? J. Biogeogr. 2018, 45, 1367–1377. [Google Scholar] [CrossRef]
- CBD 1992. Convention on Biological Diversity. Available online: http://www.cbd.int/doc/legal/cbd-en.pdf (accessed on 1 August 2023).
- Gigante, D.; Acosta, A.T.R.; Agrillo, E.; Armiraglio, S.; Assini, S.; Attorre, F.; Bagella, S.; Buffa, G.; Casella, L.; Giancola, C.; et al. Habitat conservation in Italy: The state of the art in the light of the first European Red List of Terrestrial and Freshwater Habitats. Rend. Lincei. Sci. Fis. E Nat. 2018, 29, 251–265. [Google Scholar] [CrossRef]
- La Mesa, G.; Paglialonga, A.; Tunesi, L. Manuali per il Monitoraggio di Specie e Habitat di Interesse Comunitario (Direttiva 92/43/CEE e Direttiva 09/147/CE) in Italia: Ambiente Marino; ISPRA, Serie Manuali e Linee Guida: Rome, Italy, 2019.
- Goldsmith, G.R. The field guide, rebooted Identifying species in the field? There’s an app for that. Science 2015, 349, 594. [Google Scholar] [CrossRef]
- Ellwood, E.R.; Crimmins, T.M.; Miller-Rushing, A.J. Citizen science and conservation: Recommendations for a rapidly moving field. Biol. Conserv. 2017, 208, 1–4. [Google Scholar] [CrossRef]
- Maccherini, S.; Bacaro, G.; Tordoni, E.; Bertacchi, A.; Castagnini, P.; Foggi, B.; Gennai, M.; Mugnai, M.; Sarmati, S.; Angiolini, C. Enough Is Enough? Searching for the Optimal Sample Size to Monitor European Habitats: A Case Study from Coastal Sand Dunes. Diversity 2020, 12, 138. [Google Scholar] [CrossRef]
- Marzialetti, F.; Giulio, S.; Malavasi, M.; Sperandii, M.G.; Acosta, A.T.R.; Carranza, M.L. Capturing Coastal Dune Natural Vegetation Types Using a Phenology-Based Mapping Approach: The Potential of Sentinel-2. Remote Sens. 2019, 11, 1506. [Google Scholar] [CrossRef]
- Silvertown, J. A new dawn for citizen science. Trends Ecol. Evol. 2009, 24, 467–471. [Google Scholar] [CrossRef] [PubMed]
- Lemmens, R.; Antoniou, V.; Hummer, P.; Potsiou, C. Citizen Science in the Digital World of Apps. In The Science of Citizen Science; Vohland, K., Land-Zandstra, A., Ceccaroni, L., Lemmens, R., Perelló, J., Ponti, M., Samson, R., Wagenknecht, K., Eds.; Springer: Cham, Switzerland, 2021. [Google Scholar] [CrossRef]
- Peter, M.; Diekötter, T.; Kremer, K. Participant Outcomes of Biodiversity Citizen Science Projects: A Systematic Literature Review. Sustainability 2019, 11, 2780. [Google Scholar] [CrossRef]
- Peter, M.; Diekötter, T.; Höffler, T.; Kremer, K. Biodiversity citizen science: Outcomes for the participating citizens. People Nat. 2021, 3, 294–311. [Google Scholar] [CrossRef]
- Conrad, C.C.; Hilchey, K.G. A review of citizen science and community-based environmental monitoring: Issues and opportunities. Environ. Monit. Assess. 2010, 176, 273–291. [Google Scholar] [CrossRef] [PubMed]
- Steger, C.; Butt, B.; Hooten, M.B. Safari Science: Assessing the reliability of citizen science data for wildlife surveys. J. Appl. Ecol. 2017, 54, 2053–2062. [Google Scholar] [CrossRef]
- de Groot, M.; Pocock, M.J.O.; Bonte, J.; Fernandez-Conradi, P.; Valdés-Correcher, E. Citizen Science and Monitoring Forest Pests: A Beneficial Alliance? Curr. For. Rep. 2022, 9, 15–32. [Google Scholar] [CrossRef]
- Bergami, C.; Campanaro, A.; Davis, C.; L’astorina, A.; Pugnetti, A.; Oggioni, A. Environmental citizen science practices in the ILTER community: Remarks from a case study at global scale. Front. Environ. Sci. 2023, 11, 1130020. [Google Scholar] [CrossRef]
- L’astorina, A.; Davis, C.; Pugnetti, A.; Campanaro, A.; Oggioni, A.; Bergami, C. Scientists’ attitudes about citizen science at Long-Term Ecological Research (LTER) sites. Front. Environ. Sci. 2023, 11, 1130022. [Google Scholar] [CrossRef]
- Mwango’mbe, M.G.; Spilsbury, J.; Trott, S.; Nyunja, J.; Wambiji, N.; Collins, T.; Gomes, I.; Pérez-Jorge, S. Cetacean Research and Citizen Science in Kenya. Front. Mar. Sci. 2021, 8, 642399. [Google Scholar] [CrossRef]
- Rosa, R.M.; Cavallari, D.C.; Salvador, R.B. iNaturalist as a tool in the study of tropical molluscs. PLoS ONE 2022, 17, e0268048. [Google Scholar] [CrossRef]
- Seregin, A.P.; Bochkov, D.A.; Shner, J.V.; Garin, E.V.; Pospelov, I.N.; Prokhorov, V.E.; Golyakov, P.V.; Mayorov, S.R.; Svirin, S.A.; Khimin, A.N.; et al. “Flora of Russia” on iNaturalist: A dataset. Biodivers. Data J. 2020, 8, e59249. [Google Scholar] [CrossRef] [PubMed]
- dos Santos, P.V.R.; Bessa, E. Dolphin conservation can profit from tourism and Citizen science. Environ. Dev. 2019, 32, 100467. [Google Scholar] [CrossRef]
- La Sorte, F.A.; Somveille, M. Survey completeness of a global citizen-science database of bird occurrence. Ecography 2020, 43, 34–43. [Google Scholar] [CrossRef]
- Aristeidou, M.; Herodotou, C.; Ballard, H.L.; Young, A.N.; Miller, A.E.; Higgins, L.; Johnson, R.F. Exploring the participation of young citizen scientists in scientific research: The case of iNaturalist. PLoS ONE 2021, 16, e0245682. [Google Scholar] [CrossRef]
- Echeverria, A.; Ariz, I.; Moreno, J.; Peralta, J.; Gonzalez, E.M. Learning Plant Biodiversity in Nature: The Use of the Citizen–Science Platform iNaturalist as a Collaborative Tool in Secondary Education. Sustainability 2021, 13, 735. [Google Scholar] [CrossRef]
- Forti, L.R. Students as citizen scientists: Project-based learning through the iNaturalist platform could provide useful biodiversity data. Biodiversity 2023, 24, 76–78. [Google Scholar] [CrossRef]
- Wilson, J.S.; Pan, A.D.; General, D.E.M.; Koch, J.B. More eyes on the prize: An observation of a very rare, threatened species of Philippine Bumble bee, Bombus irisanensis, on iNaturalist and the importance of citizen science in conservation biology. J. Insect Conserv. 2020, 24, 727–729. [Google Scholar] [CrossRef]
- Barbato, D.; Benocci, A.; Guasconi, M.; Manganelli, G. Light and shade of citizen science for less charismatic invertebrate groups: Quality assessment of iNaturalist nonmarine mollusc observations in central Italy. J. Molluscan Stud. 2021, 87, eyab033. [Google Scholar] [CrossRef]
- Hiller, T.; Haelewaters, D. A case of silent invasion: Citizen science confirms the presence of Harmonia axyridis (Coleoptera, Coccinellidae) in Central America. PLoS ONE 2019, 14, e0220082. [Google Scholar] [CrossRef]
- Dimson, M.; Fortini, L.B.; Tingley, M.W.; Gillespie, T.W. Citizen science can complement professional invasive plant surveys and improve estimates of suitable habitat. Divers. Distrib. 2023, 29, 1141–1156. [Google Scholar] [CrossRef]
- Callaghan, C.T.; Ozeroff, I.; Hitchcock, C.; Chandler, M. Capitalizing on opportunistic citizen science data to monitor urban biodiversity: A multi-taxa framework. Biol. Conserv. 2020, 251, 108753. [Google Scholar] [CrossRef]
- Mesaglio, T.; Callaghan, C.T. An overview of the history, current contributions and future outlook of iNaturalist in Australia. Wildl. Res. 2021, 48, 289–303. [Google Scholar] [CrossRef]
- Gorta, S.B.Z.; Callaghan, C.T.; Samonte, F.; Ooi, M.K.J.; Mesaglio, T.; Laffan, S.W.; Cornwell, W.K. Multi-taxon biodiversity responses to the 2019–2020 Australian megafires. Glob. Chang. Biol. 2023. online ahead of print. [Google Scholar] [CrossRef] [PubMed]
- iNaturalist. Available online: http://www.inaturalist.org (accessed on 30 September 2023).
- Pimm, S.L.; Jenkins, C.N.; Abell, R.; Brooks, T.M.; Gittleman, J.L.; Joppa, L.N.; Raven, P.H.; Roberts, C.M.; Sexton, J.O. The biodiversity of species and their rates of extinction, distribution, and protection. Science 2014, 344, 1246752. [Google Scholar] [CrossRef] [PubMed]
- Bowser, A.; Wiggins, A.; Shanley, L.; Preece, J.; Henderson, S. Sharing data while protecting privacy in citizen science. Interactions 2014, 21, 70–73. [Google Scholar] [CrossRef]
- Miccadei, E.; Mascioli, F.; Piacentini, T.; Ricci, F. Geomorphological Features of Coastal Dunes along the Central Adriatic Coast (Abruzzo, Italy). J. Coast. Res. 2011, 277, 1122–1136. [Google Scholar] [CrossRef]
- Drius, M.; Carranza, M.L.; Stanisci, A.; Jones, L. The role of Italian coastal dunes as carbon sinks and diversity sources. A multi-service perspective. Appl. Geogr. 2016, 75, 127–136. [Google Scholar] [CrossRef]
- Di Paola, G.; Amodio, A.M.; Dilauro, G.; Rodriguez, G.; Rosskopf, C.M. Shoreline Evolution and Erosion Vulnerability Assessment along the Central Adriatic Coast with the Contribution of UAV Beach Monitoring. Geosciences 2022, 12, 353. [Google Scholar] [CrossRef]
- Capotondi, L.; Ravaioli, M.; Acosta, A.; Chiarini, F.; Lami, A.; Stanisci, A.; Tarozzi, L.; Mazzocchi, M.G. (Eds.) La Rete Italiana per la Ricerca Ecologica di Lungo Termine. Lo Studio della Biodiversità e dei Cambiamenti; CNR Edizioni: Roma, Italy, 2011; ISBN 978-88-8080-214-3. [Google Scholar]
- IUCN. Red List of Threatened Species. Available online: http://www.iucnredlist.org (accessed on 30 September 2023).
- Rondinini, C.; Battistoni, A.; Teofili, C. Lista Rossa IUCN dei Vertebrati Italiani 2022; Comitato Italiano IUCN e Ministero dell’Ambiente e della Sicurezza Energetica: Roma, Italy, 2022. [Google Scholar]
- Pignatti, S.; Guarino, R.; La Rosa, M. Flora d’Italia; Edagricole: Bologna, Italy, 2017. [Google Scholar]
- Santoro, R.; Carboni, M.; Carranza, M.L.; Acosta, A.T.R. Focal species diversity patterns can provide diagnostic information on plant invasions. J. Nat. Conserv. 2012, 20, 85–91. [Google Scholar] [CrossRef]
- Biondi, E.; Blasi, C.; Allegrezza, M.; Anzellotti, I.; Azzella, M.M.; Carli, E.; Casavecchia, S.; Copiz, R.; Del Vico, E.; Facioni, L.; et al. Plant communities of Italy: The Vegetation Prodrome. Plant Biosyst. Int. J. Deal. Asp. Plant Biol. 2014, 148, 728–814. [Google Scholar] [CrossRef]
- European Commission DG Enviroment. Interpretation Manual of European Union Habitats. [Eur 28.Nature ENV B.3]. 2013. Available online: https://eunis.eea.europa.eu/references/2435 (accessed on 30 September 2023).
- EUR-Lex Access to European Union Law. Available online: http://data.europa.eu/eli/reg_impl/2022/1203/oj (accessed on 30 September 2023).
- Galasso, G.; Conti, F.; Peruzzi, L.; Ardenghi, N.M.G.; Banfi, E.; Celesti-Grapow, L.; Albano, A.; Alessandrini, A.; Bacchetta, G.; Ballelli, S.; et al. An updated checklist of the vascular flora alien to Italy. Plant Biosyst. Int. J. Deal. Asp. Plant Biol. 2018, 152, 556–592. [Google Scholar] [CrossRef]
- Loy, A.; Aloise, G.; Ancillotto, L.; Angelici, F.M.; Bertolino, S.; Capizzi, D.; Castiglia, R.; Colangelo, P.; Contoli, L.; Cozzi, B.; et al. Mammals of Italy: An annotated checklist. Hystrix It. J. Mamm. 2019, 30, 87–106. [Google Scholar] [CrossRef]
- Bartolucci, F.; Galasso, G.; Peruzzi, L.; Conti, F. Report 2020 on plant biodiversity in Italy: Native and alien vascular flora. Nat. Hist. Sci. 2021, 8, 41–54. [Google Scholar] [CrossRef]
- Bartolucci, F.; Galasso, G.; Peruzzi, L.; Conti, F. Report 2021 on plant biodiversity in Italy: Native and alien vascular flora. Nat. Hist. Sci. 2022, 10, 41–50. [Google Scholar] [CrossRef]
- Mazza, G.; Tricarico, E. Invasive Species and Human Health; CABI: Oxfordshire, UK, 2018. [Google Scholar]
- Aristeidou, M.; Herodotou, C.; Ballard, H.L.; Higgins, L.; Johnson, R.F.; Miller, A.E.; Young, A.N.; Robinson, L.D. How Do Young Community and Citizen Science Volunteers Support Scientific Research on Biodiversity? The Case of iNaturalist. Diversity 2021, 13, 318. [Google Scholar] [CrossRef] [PubMed]
- Casale, P.; Broderick, A.C.; Camiñas, J.A.; Cardona, L.; Carreras, C.; Demetropoulos, A.; Fuller, W.J.; Godley, B.J.; Hochscheid, S.; Kaska, Y.; et al. Mediterranean Sea turtles: Current knowledge and priorities for conservation and research. Endanger. Species Res. 2018, 36, 229–267. [Google Scholar] [CrossRef]
- Gregory, R.D.; Eaton, M.A.; Burfield, I.J.; Grice, P.V.; Howard, C.; Klvaňová, A.; Noble, D.; Šilarová, E.; Staneva, A.; Stephens, P.A.; et al. Drivers of the changing abundance of European birds at two spatial scales. Philos. Trans. R. Soc. B Biol. Sci. 2023, 378, 20220198. [Google Scholar] [CrossRef]
- Acosta, A.; Blasi, C.; Carranza, M.L.; Ricotta, C.; Stanisci, A. Quantifying ecological mosaic connectivity and hemeroby with a new topoecological index. Phytocoenologia 2003, 33, 623–631. [Google Scholar] [CrossRef]
- Degli, E.I.; Defeo, O.; Scapini, F. Arthropodofauna richness and abundance across beach–dune systems with contrasting morphodynamics. Reg. Stud. Mar. Sci. 2021, 44, 101722. [Google Scholar] [CrossRef]
- Hochmair, H.H.; Scheffrahn, R.H.; Basille, M.; Boone, M. Evaluating the data quality of iNaturalist termite records. PLoS ONE 2020, 15, e0226534. [Google Scholar] [CrossRef]
- Koo, K.-S.; Oh, J.-M.; Park, S.-J.; Im, J.-Y. Accessing the Accuracy of Citizen Science Data Based on iNaturalist Data. Diversity 2022, 14, 316. [Google Scholar] [CrossRef]
- Campbell, C.J.; Barve, V.; Belitz, M.W.; Doby, R.J.; White, E.; Seltzer, C.; Di Cecco, G.; Hurlbert, A.H.; Guralnick, R. Identifying the identifiers: How iNaturalist facilitates collaborative, research-relevant data generation and why it matters for biodiversity science. BioScience 2023, 73, 533–541. [Google Scholar] [CrossRef]
- Pinna nobilis. The IUCN Red List of Threatened Species 2019. Available online: https://www.iucnredlist.org/species/160075998/160081499 (accessed on 11 August 2023).
- Lattos, A.; Papadopoulos, D.K.; Giantsis, I.A.; Feidantsis, K.; Georgoulis, I.; Karagiannis, D.; Carella, F.; Michaelidis, B. Investigation of the highly endangered Pinna nobilis’ mass mortalities: Seasonal and temperature patterns of health status, antioxidant and heat stress responses. Mar. Environ. Res. 2023, 188, 105977. [Google Scholar] [CrossRef]
- Nebot-Colomer, E.; Álvarez, E.; Belando, M.D.; Deudero, S.; Catanese, G.; Bernardeau-Esteller, J.; García-Muñoz, R.; Ramos-Segura, A.; Ruiz, J.M.; Vázquez-Luis, M. Living under threat: Will one of the last Pinna nobilis populations be able to survive? Aquat. Conserv. Mar. Freshw. Ecosyst. 2022, 32, 1–13. [Google Scholar] [CrossRef]
- Corti, C.; Bassu, L.; Biaggini, M.; Bressi, N.; Capula, M.; Di Cerbo, A.R.; Vanni, S. Updated distribution of Testudo hermanni hermanni in Italy. In Proceedings of the International Workshop on the Management and Restoration of Hermann’s Tortoise Habitats and Populations, Soptom, Gonfaron, France, 18–20 September 2013. [Google Scholar]
- Berardo, F.; Carranza, M.L.; Frate, L.; Stanisci, A.; Loy, A. Seasonal habitat preference by the flagship species Testudo hermanni: Implications for the conservation of coastal dunes. Comptes Rendus Biol. 2015, 338, 343–350. [Google Scholar] [CrossRef]
- Cheylan, M.; Corti, C.; Carpaneto, G.M.; Mazzotti, S.; Zuffi, M.A.L. Testudo hermanni Gmelin, 1789. In Fauna d’Italia; Capula, M., Corti, C., Luiselli, L., Razzetti, E., Sindaco, R., Eds.; Reptilia, Edizioni Calderini: Bologna, Italy, 2011; Volume XLV, pp. 188–199. [Google Scholar]
- Seminoff, J.A.; Allen, C.D.; Balazs, G.H.; Dutton, P.H.; Eguchi, T.; Haas, H.L.; Hargrove, S.A.; Jensen, M.P.; Klemm, D.L.; Lauritsen, A.M.; et al. Green Turtle (Chelonia mydas) Status Review under the U.S. Endangered Species Act; NOAA Technical Memorandum, NOAA-NMFS-SWFSC: La Jolla, CA, USA, 2015; pp. 539–571.
- Chelonia mydas. The IUCN Red List of Threatened Species 2004. Available online: https://www.iucnredlist.org/species/pdf/11037468/attachmenten (accessed on 11 August 2023).
- IUCN Liste Rosse Italiane. Available online: https://www.iucn.it/liste-rosse-italiane.php (accessed on 30 September 2023).
- Casado de Amezua, P.; Kersting, D.; Linares, C.L.; Bo, M.; Caroselli, E.; Garrabou, J.; Cerrano, C.; Ozalp, B.; Terrón-Sigler, A.; Betti, F. Cladocora caespitosa. In The IUCN Red List of Threatened Species; International Union for Conservation of Nature: Gland, Switzerland, 2015. [Google Scholar] [CrossRef]
- Roveta, C.; Coppari, M.; Calcinai, B.; Di Camillo, C.G.; Marrocco, T.; Mantas, T.P.; Puce, S.; Torsani, F.; Valisano, L.; Cerrano, C. What’s the key for success? Translocation, growth and thermal stress mitigation in the Mediterranean coral Cladocora caespitosa (Linnaeus, 1767). Front. Mar. Sci. 2023, 10, 1199048. [Google Scholar] [CrossRef]
- El Kateb, A.; Stalder, C.; Neururer, C.; Pisapia, C.; Spezzaferri, S. Correlation between pollution and decline of Scleractinian Cladocora caespitosa (Linnaeus, 1758) in the Gulf of Gabes. Heliyon 2016, 2, e00195. [Google Scholar] [CrossRef] [PubMed]
- Kersting, D.K.; Cebrian, E.; Casado, C.; Teixidó, N.; Garrabou, J.; Linares, C. Experimental evidence of the synergistic effects of warming and invasive algae on a temperate reef-builder coral. Sci. Rep. 2015, 5, 18635. [Google Scholar] [CrossRef]
- Kružić, P.; Požar-Domac, A. Impact of tuna farming on the banks of the coral Cladocora caespitosa in the Adriatic Sea. Coral Reefs 2007, 26, 665. [Google Scholar] [CrossRef]
- Cladocora caespitosa. The IUCN Red List of Threatened Species 2015. Available online: https://www.iucnredlist.org/species/133142/165739749 (accessed on 11 August 2023).
- Garofalo, L.; Mastrogiacomo, A.; Casale, P.; Carlini, R.; Eleni, C.; Freggi, D.; Gelli, D.; Knittweis, L.; Mifsud, C.; Mingozzi, T.; et al. Genetic characterization of central Mediterranean stocks of the loggerhead turtle (Caretta caretta) using mitochondrial and nuclear markers, and conservation implications. Aquat. Conserv. Mar. Freshw. Ecosyst. 2013, 23, 868–884. [Google Scholar] [CrossRef]
- Giacoma, C.; Balletto, E.; Bentivegna, F.; Guarino, F.M.; Hochscheid, S.; Maio, N.; Mingozzi, A.T.; Piovano & Scaravelli, D. Caretta caretta (Linnaeus, 1758). In Fauna d’Italia; Corti, C., Capula, M., Luiselli, L., Razzetti, E., Sindaco, R., Eds.; Reptilia: Edizioni Calderini: Bologna, Italy, 2011; Volume XLV. [Google Scholar]
- Di Renzo, L.; Mascilongo, G.; Berti, M.; Bogdanović, T.; Listeš, E.; Brkljača, M.; Notarstefano, V.; Gioacchini, G.; Giorgini, E.; Olivieri, V.; et al. Potential Impact of Microplastics and Additives on the Health Status of Loggerhead Turtles (Caretta caretta) Stranded along the Central Adriatic Coast. Water Air Soil Pollut. 2021, 232, 98. [Google Scholar] [CrossRef]
- Fontaine, A.; Simard, A.; Brunet, N.; Elliott, K.H. Scientific contributions of citizen science applied to rare or threatened animals. Conserv. Biol. 2022, 36, e13976. [Google Scholar] [CrossRef] [PubMed]
- Stanisci, A.; Acosta, A.T.R.; Carranza, M.L.; de Chiro, M.; Del Vecchio, S.; Di Martino, L.; Frattaroli, A.R.; Fusco, S.; Izzi, C.F.; Pirone, G.; et al. EU habitats monitoring along the coastal dunes of the LTER sites of Abruzzo and Molise (Italy). Plant Sociol. 2014, 51, 51–56. [Google Scholar] [CrossRef]
- de Francesco, M.C.; Cerrano, C.; Pica, D.; D’Onofrio, D.; Stanisci, S. Characterization of Teatina Coast Marine Habitats (Central Adriatic Sea) toward an Integrated Coastal Management. Oceanogr. Fish. Open Access J. 2017, 5, 7–10. [Google Scholar] [CrossRef]
- de Francesco, M.C.; Tozzi, F.P.; Buffa, G.; Fantinato, E.; Innangi, M.; Stanisci, A. Identifying Critical Thresholds in the Impacts of Invasive Alien Plants and Dune Paths on Native Coastal Dune Vegetation. Land 2022, 12, 135. [Google Scholar] [CrossRef]
- Biondi, E.; Blasi, C.; Burrascano, S.; Casavecchia, S.; Copiz, R.; Del Vico, E.; Galdenzi, D.; Gigante, D.; Lasen, C.; Spampinato, G.; et al. Manuale Italiano di Interpretazione degli Habitat della Direttiva 92/43/CEE; Ministero dell’Ambiente e della Tutela del Territorio e del Mare, Direzione per la Protezione della Natura: Rome, Italy, 2009; pp. 1–16.
- Carranza, M.L.; Drius, M.; Malavasi, M.; Frate, L.; Stanisci, A.; Acosta, A.T.R. Assessing land take and its effects on dune carbon pools. An insight into the Mediterranean coastline. Ecol. Indic. 2018, 85, 951–955. [Google Scholar] [CrossRef]
- Arianoutsou, M.; Leone, V.; Moya, D.; Lovreglio, R.; Delipetrou, P.; de las Heras, J. Management of threatened, high conservation value, forest hotspots under changing fire regimes. In Post-Fire Management and Restoration of Southern European Forests, Managing Forest Ecosystems; Moreira, F., Arianoutsou, M., Corona, P., de las Heras, J., Eds.; Springer: Amsterdam, The Netherlands, 2012; pp. 257–291. [Google Scholar]
- Adam, P. Salt marsh restoration. In Coastal Wetlands, 2nd ed.; Perillo, G.M.E., Wolanski, E., Cahoon, D.R., Hopkinson, C.S., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 817–861. [Google Scholar] [CrossRef]
- Tozzi, F.P.; Varricchione, M.; de Francesco, M.C.; Carranza, M.L.; Stanisci, A. Vegetation Dynamics on a Restored salt Marsh Mosaic: A Re-Visitation Study in a Coastal Wetland in Central Italy. Wetlands 2022, 42, 101. [Google Scholar] [CrossRef]
- Perennou, C.; Gaget, E.; Galewski, T.; Geijzendorffer, I.; Guelmami, A. Evolution of wetlands in Mediterranean region. In Water Resources in the Mediterranean Region; Zribi, M., Brocca, L., Tramblay, Y., Molle, F., Eds.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 297–320. [Google Scholar]
- Casas, E.; Martín-García, L.; Otero-Ferrer, F.; Tuya, F.; Haroun, R.; Arbelo, M. Economic mapping and assessment of Cymodocea nodosa meadows as nursery grounds for commercially important fish species. A case study in the Canary Islands. One Ecosyst. 2021, 6, e70919. [Google Scholar] [CrossRef]
- Da Ros, Z.; Corinaldesi, C.; Dell’Anno, A.; Gambi, C.; Torsani, F.; Danovaro, R. Restoration of Cymodocea nodosa seagrass meadows: Efficiency and ecological implications. Restor. Ecol. 2021, 29, e13313. [Google Scholar] [CrossRef]
- Angelini, P.; Casella, L.; Grignetti, A.; Genovesi, P. Manuali per il Monitoraggio di Specie e Habitat di Interesse Comunitario (Direttiva 92/43/CEE) in Italia: Habitat; ISPRA Serie Manuali e Linee Guida: Rome, Italy, 2016.
- Fristoe, T.S.; Chytrý, M.; Dawson, W.; Essl, F.; Heleno, R.; Kreft, H.; Maurel, N.; Pergl, J.; Pyšek, P.; Seebens, H.; et al. Dimensions of invasiveness: Links between local abundance, geographic range size, and habitat breadth in Europe’s alien and native floras. Proc. Natl. Acad. Sci. USA 2021, 118, e2021173118. [Google Scholar] [CrossRef]
- Lazzaro, L.; Bolpagni, R.; Buffa, G.; Gentili, R.; Lonati, M.; Stinca, A.; Acosta, A.T.R.; Adorni, M.; Aleffi, M.; Allegrezza, M.; et al. Impact of invasive alien plants on native plant communities and Natura 2000 habitats: State of the art, gap analysis and perspectives in Italy. J. Environ. Manag. 2020, 274, 111140. [Google Scholar] [CrossRef] [PubMed]
- Marzialetti, F.; Frate, L.; De Simone, W.; Frattaroli, A.R.; Acosta, A.T.R.; Carranza, M.L. Unmanned Aerial Vehicle (UAV)-Based Mapping of Acacia saligna Invasion in the Mediterranean Coast. Remote Sens. 2021, 13, 3361. [Google Scholar] [CrossRef]
- Espenschied-Reilly, A.L.; Runkle, J.R. Distribution and changes in abundance of Ailanthus altissima (Miller) Swingle in a Southwest Ohio Woodlot. Ohio J. Sci. 2008, 108, 16–22. [Google Scholar]
- Castro-Díez, P.; Fierro-Brunnenmeister, N.; González-Muñoz, N.; Gallardo, A. Effects of exotic and native tree leaf litter on soil properties of two contrasting sites in the Iberian Peninsula. Plant Soil 2011, 350, 179–191. [Google Scholar] [CrossRef]
- Guarino, R.; Chytrý, M.; Attorre, F.; Landucci, F.; Marcenò, C. Alien plant invasions in Mediterranean habitats: An assessment for Sicily. Biol. Invasions 2021, 23, 3091–3107. [Google Scholar] [CrossRef]
- Cocchi, R.; Bertolino, S. Piano di Gestione Nazionale della Nutria (Myocastor coypus); Manuali ISPRA: Rome, Italy, 2020.
- Standfuss, B.; Lipovšek, G.; Fritz, U.; Vamberger, M. Threat or fiction: Is the pond slider (Trachemys scripta) really invasive in Central Europe? A case study from Slovenia. Conserv. Genet. 2016, 17, 557–563. [Google Scholar] [CrossRef]
- Macchi, S.; Scali, S.; Bisi, F.; Martinoli, A.; Alonzi, A.; Carnevali, L. Piano Nazionale per la Gestione della Testuggine Palustre Americana (Trachemys scripta); Manuali ISPRA: Rome, Italy, 2020.
- Lozano, V.; Di Febbraro, M.; Brundu, G.; Carranza, M.L.; Alessandrini, A.; Ardenghi, N.M.G.; Barni, E.; Bedini, G.; Celesti-Grapow, L.; Cianfaglione, K.; et al. Plant invasion risk inside and outside protected areas: Propagule pressure, abiotic and biotic factors definitively matter. Sci. Total. Environ. 2023, 877, 162993. [Google Scholar] [CrossRef]
- Di Febbraro, M.; Bosso, L.; Fasola, M.; Santicchia, F.; Aloise, G.; Lioy, S.; Tricarico, E.; Ruggieri, L.; Bovero, S.; Mori, E.; et al. Different facets of the same niche: Integrating citizen science and scientific survey data to predict biological invasion risk under multiple global change drivers. Glob. Chang. Biol. 2023, 29, 5509–5523. [Google Scholar] [CrossRef]
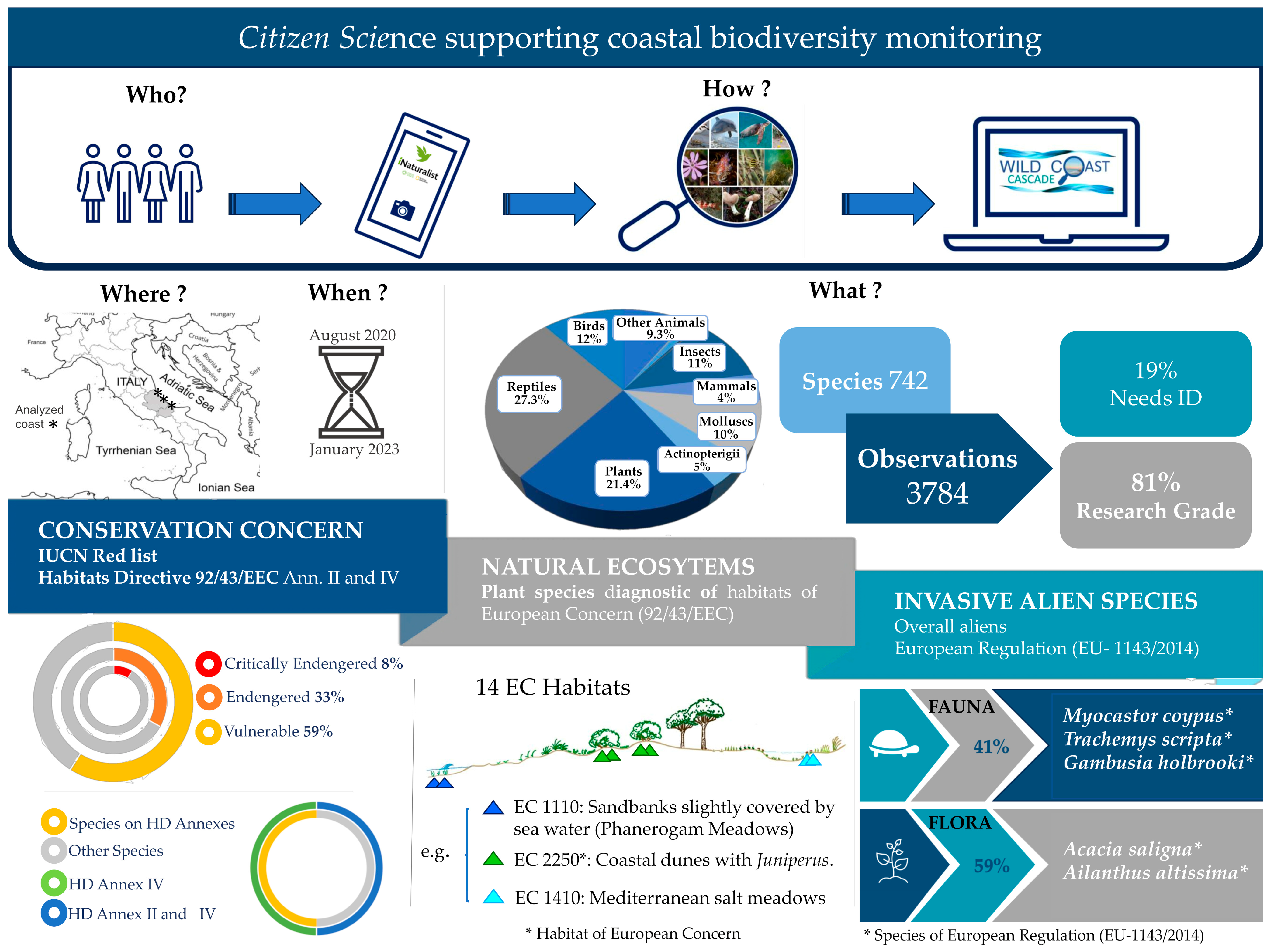
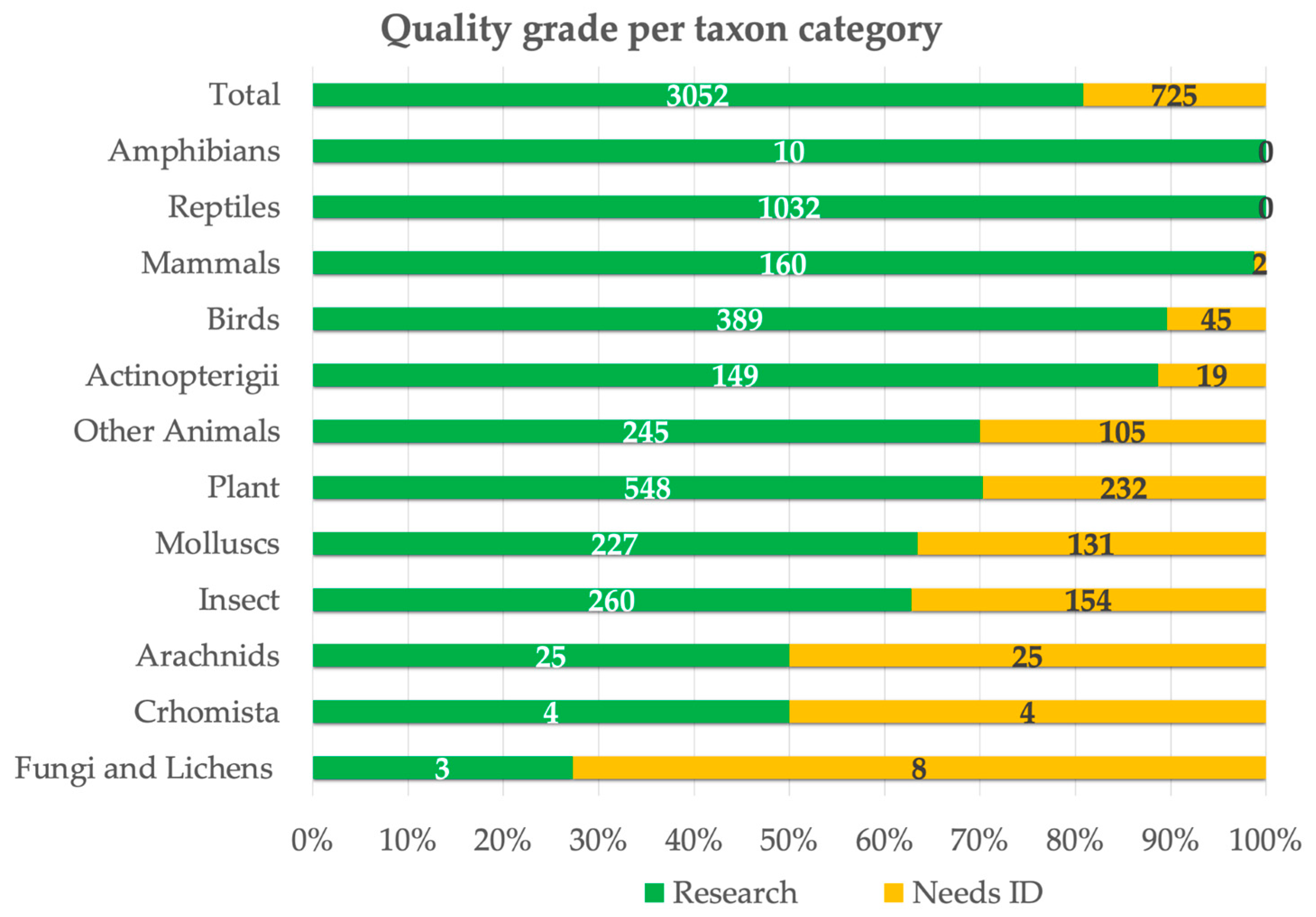
| iNaturalist Macro-Categories | Number of Observations | % of Observations | Number of Species | % of Species |
|---|---|---|---|---|
| Actinopterygii | 168 | 5% | 53 | 7% |
| Amphibians | 10 | <1% | 3 | <1% |
| Arachnids | 50 | 1% | 18 | 3% |
| Birds | 434 | 12% | 82 | 11% |
| Chromists | 8 | <1% | 2 | <1% |
| Fungi and lichens | 11 | <1% | 2 | <1% |
| Insects | 415 | 11% | 161 | 22% |
| Mammals | 162 | 4% | 8 | 1% |
| Molluscs | 358 | 10% | 79 | 11% |
| Other animals | 350 | 9% | 94 | 13% |
| Plants | 784 | 21% | 230 | 31% |
| Reptiles | 1034 | 27% | 10 | 1% |
| Total | 3784 | 742 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Compagnone, F.; Varricchione, M.; Innangi, M.; Di Febbraro, M.; Loy, A.; Stanisci, A.; de Francesco, M.C.; Matteucci, G.; Carranza, M.L. Coastal Biodiversity Assessment Aided by Citizen Science Volunteers: A Look at the Italian Central Adriatic. Land 2023, 12, 2023. https://doi.org/10.3390/land12112023
Compagnone F, Varricchione M, Innangi M, Di Febbraro M, Loy A, Stanisci A, de Francesco MC, Matteucci G, Carranza ML. Coastal Biodiversity Assessment Aided by Citizen Science Volunteers: A Look at the Italian Central Adriatic. Land. 2023; 12(11):2023. https://doi.org/10.3390/land12112023
Chicago/Turabian StyleCompagnone, Federica, Marco Varricchione, Michele Innangi, Mirko Di Febbraro, Anna Loy, Angela Stanisci, Maria Carla de Francesco, Giorgio Matteucci, and Maria Laura Carranza. 2023. "Coastal Biodiversity Assessment Aided by Citizen Science Volunteers: A Look at the Italian Central Adriatic" Land 12, no. 11: 2023. https://doi.org/10.3390/land12112023
APA StyleCompagnone, F., Varricchione, M., Innangi, M., Di Febbraro, M., Loy, A., Stanisci, A., de Francesco, M. C., Matteucci, G., & Carranza, M. L. (2023). Coastal Biodiversity Assessment Aided by Citizen Science Volunteers: A Look at the Italian Central Adriatic. Land, 12(11), 2023. https://doi.org/10.3390/land12112023











