Mitigating Soil Erosion through Biomass-Derived Biochar: Exploring the Influence of Feedstock Types and Pyrolysis Temperature
Abstract
:1. Introduction
2. Materials and Methods
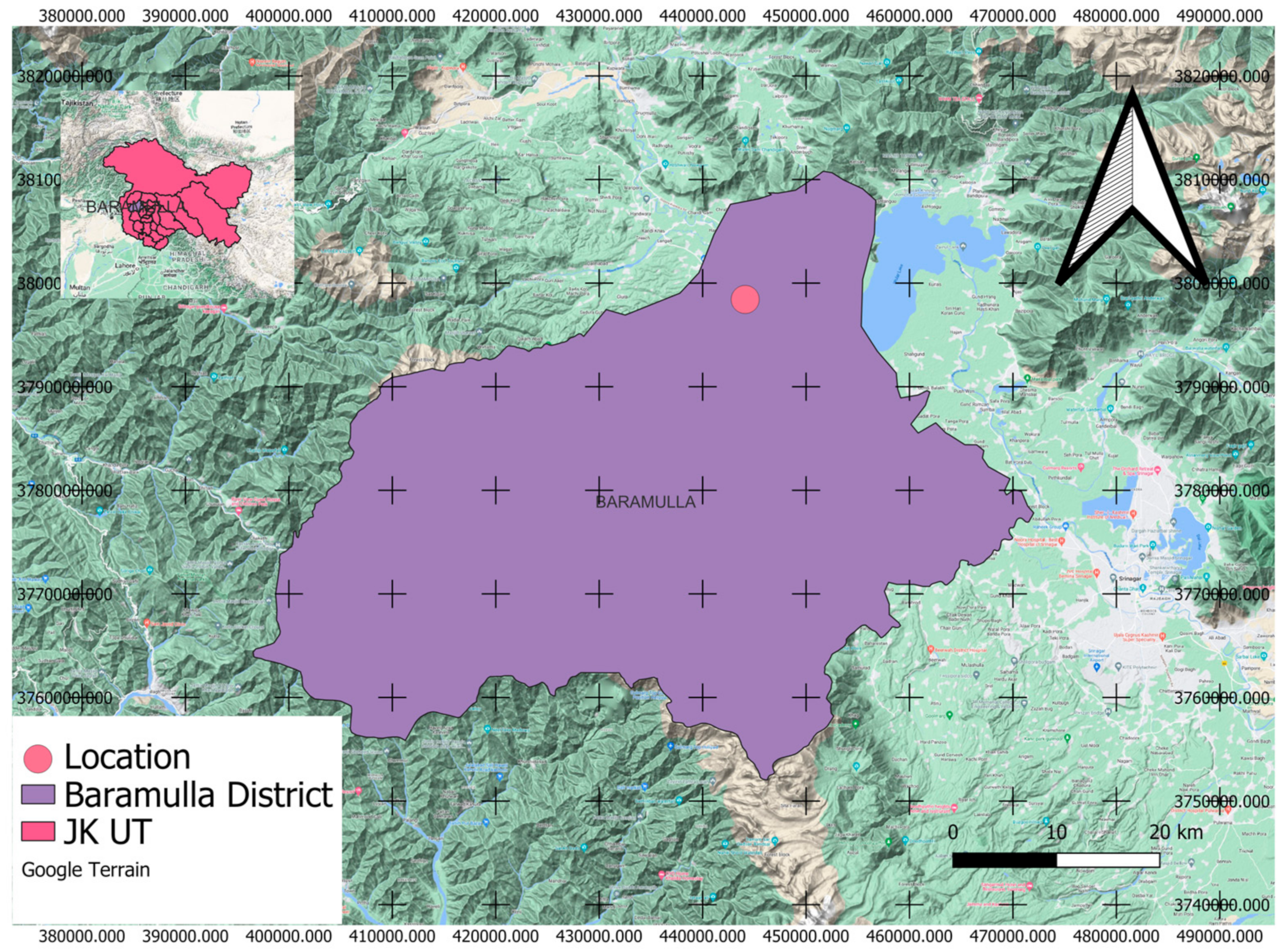
2.1. Study Area
2.2. Experimental Design and Sampling
2.3. Proximate Analysis
Estimation of Moisture Content, Volatile Matter, Fixed C, and Yield Content in Biochar
2.4. Erodibility Indices, Aggregate Stability and Total Organic Carbon
2.5. Statistical Analysis
3. Results and Discussion
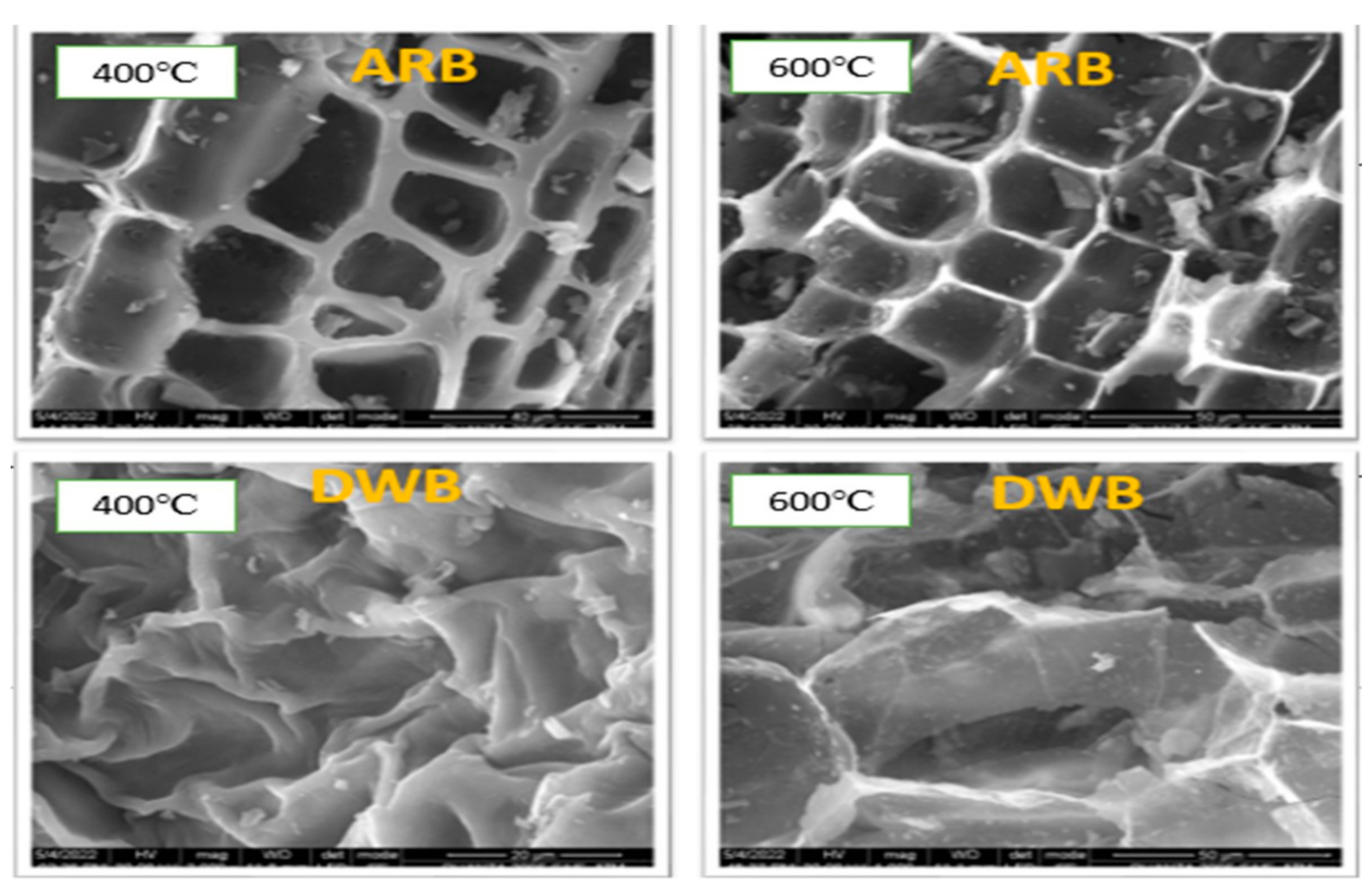
3.1. Effect of Feedstock and Pyrolysis Temperature on Proximate Analysis
3.2. Effect of the Amendment (Pyrolyzed and Unpyrolyzed), Rate, and Fertilizer on Soil Erodibility, Aggregate Stability and Total Organic Carbon
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Adimassu, Z.; Mekonnen, K.; Yirga, C.; Kessler, A. Effect of soil bunds on runoff, soil and nutrient losses, and crop yield in the central highlands of Ethiopia. Land Degrad. Dev. 2014, 25, 554–564. [Google Scholar]
- Borrelli, P.; Robinson, D.A.; Panagos, P.; Lugato, E.; Yang, J.E.; Alewell, C.; Wuepper, D.; Montanarella, L.; Ballabio, C. Land use and climate change impacts on global soil erosion by water (2015–2070). Proc. Natl. Acad. Sci. USA 2020, 117, 21994–22001. [Google Scholar]
- Zhide, Z.; Yuling, T. Sediment in Rivers-Origin and Challenges. Stockh. Water Front. 2010, 4, 8–10. [Google Scholar]
- Zdruli, P.; Lal, R.; Cherlet, M.; Kapur, S. New world atlas of desertification and issues of carbon sequestration, organic carbon stocks, nutrient depletion and implications for food security. In Carbon Management, Technologies, and Trends in Mediterranean Ecosystems; Springer: Berlin/Heidelberg, Germany, 2017; pp. 13–25. [Google Scholar]
- Saha, D.; Sahu, S.; Chandra, P.C. Arsenic-safe alternate aquifers and their hydraulic characteristics in contaminated areas of Middle Ganga Plain, Eastern India. Environ. Monit. Assess. 2011, 175, 331–348. [Google Scholar] [PubMed]
- Lal, R.; Smith, P.; Jungkunst, H.F.; Mitsch, W.J.; Lehmann, J.; Nair, P.R.; McBratney, A.B.; Sá, J.C.d.M.; Schneider, J.; Zinn, Y.L.; et al. The carbon sequestration potential of terrestrial ecosystems. J. Soil Water Conserv. 2018, 73, 145A–152A. [Google Scholar] [CrossRef]
- O’Laughlin, J.; McElligott, K. Biochar for Environmental Management: Science and Technology; Lehmann, J., Joseph, S.M., Eds.; Earthscan: London, UK, 2009; 448p. [Google Scholar]
- Shackley, S.; Carter, S.; Knowles, T.; Middelink, E.; Haefele, S.; Sohi, S.; Cross, A.; Haszeldine, S. Sustainable gasification–biochar systems? A case-study of rice-husk gasification in Cambodia, Part I: Context, chemical properties, environmental and health and safety issues. Energy Policy 2012, 42, 49–58. [Google Scholar]
- Sohi, S.P.; Krull, E.; Lopez-Capel, E.; Bol, R. A review of biochar and its use and function in soil. Adv. Agron. 2010, 105, 47–82. [Google Scholar]
- Liu, Y.; Yang, M.; Wu, Y.; Wang, H.; Chen, Y.; Wu, W. Reducing CH4 and CO2 emissions from waterlogged paddy soil with biochar. J. Soils Sediments 2011, 11, 930–939. [Google Scholar]
- Wang, J.; Wang, S. Preparation, modification and environmental application of biochar: A review. J. Clean. Prod. 2019, 227, 1002–1022. [Google Scholar]
- Waqas, M.; Asam, Z.; Rehan, M.; Anwar, M.N.; Khattak, R.A.; Ismail, I.M.I.; Tabatabaei, M.; Nizami, A.S. Development of biomass-derived biochar for agronomic and environmental remediation applications. Biomass Convers. Biorefin. 2021, 11, 339–361. [Google Scholar]
- Saha, R.; Galagedara, L.; Thomas, R.; Nadeem, M.; Hawboldt, K. Investigating the influence of biochar amendment on the physicochemical properties of podzolic soil. Agriculture 2020, 10, 471. [Google Scholar] [CrossRef]
- Omondi, M.O.; Xia, X.; Nahayo, A.; Liu, X.; Korai, P.K.; Pan, G. Quantification of biochar effects on soil hydrological properties using meta-analysis of literature data. Geoderma 2016, 274, 28–34. [Google Scholar] [CrossRef]
- Herath, H.; Camps-Arbestain, M.; Hedley, M. Effect of biochar on soil physical properties in two contrasting soils: An Alfisol and an Andisol. Geoderma 2013, 209, 188–197. [Google Scholar] [CrossRef]
- Githinji, L. Effect of biochar application rate on soil physical and hydraulic properties of a sandy loam. Arch. Agron. Soil Sci. 2014, 60, 457–470. [Google Scholar] [CrossRef]
- Edeh, I.G.; Mašek, O.; Buss, W. A meta-analysis on biochar’s effects on soil water properties–New insights and future research challenges. Sci. Total Environ. 2020, 714, 136857. [Google Scholar] [PubMed]
- Yang, F.; Xu, Z.; Huang, Y.; Tsang, D.C.; Ok, Y.S.; Zhao, L.; Qiu, H.; Xu, X.; Cao, X. Stabilization of dissolvable biochar by soil minerals: Release reduction and organo-mineral complexes formation. J. Hazard. Mater. 2021, 412, 125213. [Google Scholar] [CrossRef]
- Wang, D.; Fonte, S.J.; Parikh, S.J.; Six, J.; Scow, K.M. Biochar additions can enhance soil structure and the physical stabilization of C in aggregates. Geoderma 2017, 303, 110–117. [Google Scholar] [CrossRef]
- Jien, S.H.; Wang, C.S. Effects of biochar on soil properties and erosion potential in a highly weathered soil. Catena 2013, 110, 225–233. [Google Scholar] [CrossRef]
- Sun, F.; Lu, S. Biochars improve aggregate stability, water retention, and pore-space properties of clayey soil. J. Plant Nutr. Soil Sci. 2014, 177, 26–33. [Google Scholar] [CrossRef]
- Castellini, M.; Giglio, L.; Niedda, M.; Palumbo, A.; Ventrella, D. Impact of biochar addition on the physical and hydraulic properties of a clay soil. Soil Tillage Res. 2015, 154, 1–13. [Google Scholar] [CrossRef]
- Basso, A.S.; Miguez, F.E.; Laird, D.A.; Horton, R.; Westgate, M. Assessing potential of biochar for increasing water-holding capacity of sandy soils. Gcb Bioenergy 2013, 5, 132–143. [Google Scholar] [CrossRef]
- Liu, Z.; Chen, X.; Jing, Y.; Li, Q.; Zhang, J.; Huang, Q. Effects of biochar amendment on rapeseed and sweet potato yields and water stable aggregate in upland red soil. Catena 2014, 123, 45–51. [Google Scholar] [CrossRef]
- Song, J.; Wang, Y.; Zhang, S.; Song, Y.; Xue, S.; Liu, L.; Lvy, X.; Wang, X.; Yang, G. Coupling biochar with anaerobic digestion in a circular economy perspective: A promising way to promote sustainable energy, environment and agriculture development in China. Renew. Sustain. Energy Rev. 2021, 144, 110973. [Google Scholar] [CrossRef]
- Navarro, S.; León, M.; Roca-Pérez, L.; Boluda, R.; García-Ferriz, L.; Pérez-Bermúdez, P.; Gavidia, I. Characterisation of Bobal and Crujidera grape cultivars, in comparison with Tempranillo and Cabernet Sauvignon: Evolution of leaf macronutrients and berry composition during grape ripening. Food Chem. 2008, 108, 182–190. [Google Scholar] [CrossRef]
- Calcan, S.I.; Pârvulescu, O.C.; Ion, V.A.; Răducanu, C.E.; Bădulescu, L.; Madjar, R.; Dobre, T.; Egri, D.; Moț, A.; Iliescu, L.M.; et al. Effects of biochar on soil properties and tomato growth. Agronomy 2022, 12, 1824. [Google Scholar] [CrossRef]
- Yuan, P.; Wang, J.; Pan, Y.; Shen, B.; Wu, C. Review of biochar for the management of contaminated soil: Preparation, application and prospect. Sci. Total Environ. 2019, 659, 473–490. [Google Scholar] [CrossRef]
- Murtaza, G.; Ahmed, Z.; Usman, M.; Tariq, W.; Ullah, Z.; Shareef, M.; Iqbal, H.; Waqas, M.; Tariq, A.; Wu, Y.; et al. Biochar induced modifications in soil properties and its impacts on crop growth and production. J. Plant Nutr. 2021, 44, 1677–1691. [Google Scholar] [CrossRef]
- Schmidt, H.P.; Kammann, C.; Hagemann, N.; Leifeld, J.; Bucheli, T.D.; Sánchez Monedero, M.A.; Cayuela, M.L. Biochar in agriculture–A systematic review of 26 global meta-analyses. GCB Bioenergy 2021, 13, 1708–1730. [Google Scholar] [CrossRef]
- Calcan, S.I.; Pârvulescu, O.C.; Ion, V.A.; Răducanu, C.E.; Bădulescu, L.; Dobre, T.; Egri, D.; Moț, A.; Popa, V.; Crăciun, M.E. Valorization of vine prunings by slow pyrolysis in a fixed-bed reactor. Processes 2021, 10, 37. [Google Scholar] [CrossRef]
- Morgan, H.; Sohi, S.; Shackley, S. Biochar: An Emerging Carbon Abatement and Soil Management Strategy. In Oxford Research Encyclopedia of Environmental Science; Oxford University Press: Oxford, UK, 2020. [Google Scholar]
- Liao, W.; Zhang, X.; Shao, J.; Yang, H.; Zhang, S.; Chen, H. Simultaneous removal of cadmium and lead by biochar modified with layered double hydroxide. Fuel Process. Technol. 2022, 235, 107389. [Google Scholar] [CrossRef]
- Aborisade, M.A.; Oba, B.T.; Kumar, A.; Liu, J.; Chen, D.; Okimiji, O.P.; Zhao, L. Remediation of metal toxicity and alleviation of toxic metals-induced oxidative stress in Brassica chinensis L. using biochar-iron nanocomposites. In Plant and Soil; Springer: Berlin/Heidelberg, Germany, 2023; pp. 1–17. [Google Scholar]
- Yuan, X.; Wang, Q.; Wang, Z.; Wu, S.; Zhai, Y.; Zhang, H.; Zhou, L.; Lu, B.; Chen, K.; Wang, X. Optimization of Mixed-Based Biochar Preparation Process and Adsorption Performance of Lead and Cadmium. Sustainability 2023, 15, 11579. [Google Scholar] [CrossRef]
- Stokes, A.; Douglas, G.B.; Fourcaud, T.; Giadrossich, F.; Gillies, C.; Hubble, T.; Kim, J.H.; Loades, K.W.; Mao, Z.; McIvor, I.R.; et al. Ecological mitigation of hillslope instability: Ten key issues facing researchers and practitioners. Plant Soil 2014, 377, 1–23. [Google Scholar] [CrossRef]
- Jiang, H.; Cai, Y.; Liu, J. Engineering properties of soils reinforced by short discrete polypropylene fiber. J. Mater. Civ. Eng. 2010, 22, 1315–1322. [Google Scholar] [CrossRef]
- Peng, X.; Zhu, Q.; Xie, Z.; Darboux, F.; Holden, N. The impact of manure, straw and biochar amendments on aggregation and erosion in a hillslope Ultisol. Catena 2016, 138, 30–37. [Google Scholar] [CrossRef]
- Kusre, B.C.; Ghosh, P.; Nath, K. Prioritization of soil conservation measures using erodibility indices as criteria in Sikkim (India). J. Earth Syst. Sci. 2018, 127, 81. [Google Scholar] [CrossRef]
- Madari, B.E.; Maia, C.M.B.d.F.; Novotny, E.H. Context and importance of biochar research. Pesqui. Agropecuária Bras. 2012, 47, i–ii. [Google Scholar] [CrossRef]
- Ronsse, F.; Van Hecke, S.; Dickinson, D.; Prins, W. Production and characterization of slow pyrolysis biochar: Influence of feedstock type and pyrolysis conditions. Gcb Bioenergy 2013, 5, 104–115. [Google Scholar] [CrossRef]
- Carneiro, J.d.S.; Nogueira, R.M.; Martins, M.A.; Valladão, D.M.d.S.; Pires, E.M. The oven-drying method for determination of water content in Brazil nut. Biosci. J. 2018, 34, 595–602. [Google Scholar] [CrossRef]
- Sahoo, S.S.; Vijay, V.K.; Chandra, R.; Kumar, H. Production and characterization of biochar produced from slow pyrolysis of pigeon pea stalk and bamboo. Clean. Eng. Technol. 2021, 3, 100101. [Google Scholar] [CrossRef]
- Smíšek, M.; Černý, S. Active Carbon: Manufacture, Properties and Applications; Elsevier: Amsterdam, The Netherlands, 1970. [Google Scholar]
- SNI. Standar Karbon Aktif. 1995; No. 06–3730–1995. [Google Scholar]
- Gaskin, J.W.; Steiner, C.; Harris, K.; Das, K.C.; Bibens, B. Effect of low-temperature pyrolysis conditions on biochar for agricultural use. Trans. ASABE 2008, 51, 2061–2069. [Google Scholar] [CrossRef]
- Bouyoucos, G.J. Clay ratio as a criterion of susceptibility of soils to erosion. J. Am. Soc. Agron. 1935, 27, 738–741. [Google Scholar] [CrossRef]
- Gee, G.W.; Or, D. 2.4 Particle-size analysis. Methods Soil Anal. Part 4 Phys. Methods 2002, 5, 255–293. [Google Scholar]
- Logan, T.; Harrison, B. Physical Characteristics of Alkaline Stabilized Sewage Sludge (N-Viro Soil) and Their Effects on Soil Physical Properties; Wiley Online Library: Hoboken, NJ, USA, 1995. [Google Scholar]
- Halliwell, D.J.; Barlow, K.M.; Nash, D.M. A review of the effects of wastewater sodium on soil physical properties and their implications for irrigation systems. Soil Res. 2001, 39, 1259–1267. [Google Scholar] [CrossRef]
- Slater, C.S.; Byers, H.G. A Laboratory Study of the Field Percolation Rates of Soils; US Department of Agriculture: Washington, DC, USA, 1931.
- Balci, A. Soil Conservation. In IU Forestry Faculty Publication Number: 439; Istanbul University Press: Fatih, Istanbul, Turkey, 1996. [Google Scholar]
- Van Bavel, C. Report of the Committee on Physical Analyses 1951–1953, Soil Science Society of America. Soil Sci. Soc. Am. J. 1953, 17, 416–418. [Google Scholar] [CrossRef]
- Yeomans, J.C.; Bremner, J.M. A rapid and precise method for routine determination of organic carbon in soil. Commun. Soil Sci. Plant Anal. 1988, 19, 1467–1476. [Google Scholar] [CrossRef]
- Gomez, K.A.; Gomez, A.A. Statistical Procedures for Agricultural Research; John Wiley & Sons: Hoboken, NJ, USA, 1984. [Google Scholar]
- Cui, X.; Wang, J.; Wang, X.; Khan, M.B.; Lu, M.; Khan, K.Y.; Chen, G. Biochar from constructed wetland biomass waste: A review of its potential and challenges. Chemosphere 2022, 287, 132259. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.; Tong, C.; Gao, J.; Xiao, R. Effects of wetland plant biochars on heavy metal immobilization and enzyme activity in soils from the Yellow River estuary. Environ. Sci. Pollut. Res. 2022, 29, 40796–40811. [Google Scholar] [CrossRef]
- Mahdi, Z.; Hanandeh, A.E.; Yu, Q. Influence of pyrolysis conditions on surface characteristics and methylene blue adsorption of biochar derived from date seed biomass. Waste Biomass Valorization 2017, 8, 2061–2073. [Google Scholar] [CrossRef]
- Almutairi, A.A.; Ahmad, M.; Rafique, M.I.; Al-Wabel, M.I. Variations in composition and stability of biochars derived from different feedstock types at varying pyrolysis temperature. J. Saudi Soc. Agric. Sci. 2022, 22, 25–34. [Google Scholar] [CrossRef]
- Noor, N.M.; Shariff, A.; Abdullah, N.; Aziz, N.S.M. Temperature effect on biochar properties from slow pyrolysis of coconut flesh waste. Malays. J. Fundam. Appl. Sci. 2019, 15, 153–158. [Google Scholar] [CrossRef]
- Xu, D.; Li, Z.; Wang, P.; Bai, W.; Wang, H. Aquatic plant-derived biochars produced in different pyrolytic conditions: Spectroscopic studies and adsorption behavior of diclofenac sodium in water media. Sustain. Chem. Pharm. 2020, 17, 100275. [Google Scholar] [CrossRef]
- Mansoor, S.; Kour, N.; Manhas, S.; Zahid, S.; Wani, O.A.; Sharma, V.; Wijaya, L.; Alyemeni, M.N.; Alsahli, A.A.; El-Serehy, H.A.; et al. Biochar as a tool for effective management of drought and heavy metal toxicity. Chemosphere 2021, 271, 129458. [Google Scholar] [CrossRef] [PubMed]
- Hmid, A.; Mondelli, D.; Fiore, S.; Fanizzi, F.P.; Al Chami, Z.; Dumontet, S. Production and characterization of biochar from three-phase olive mill waste through slow pyrolysis. Biomass Bioenergy 2014, 71, 330–339. [Google Scholar] [CrossRef]
- Wani, O.A.; Kumar, S.S.; Hussain, N.; Wani, A.I.A.; Babu, S.; Alam, P.; Rashid, M.; Popescu, S.M.; Mansoor, S. Multi-scale processes influencing global carbon storage and land-carbon-climate nexus: A critical review. Pedosphere 2022, 33, 250–267. [Google Scholar] [CrossRef]
- Nguyen, L.X.; Do, P.T.M.; Nguyen, C.H.; Kose, R.; Okayama, T.; Pham, T.N.; Miyanishi, T. Properties of Biochars prepared from local biomass in the Mekong Delta, Vietnam. BioResources 2018, 13, 7325–7344. [Google Scholar] [CrossRef]
- Rahman, R.; Sofi, J.A.; Kirmani, N.; Dar, I.H.; Chesti, M.; Bangroo, S.A.; Mir, S.; Wani, O.A. Impact of biochar and pyrolytic temperature on nutrient content of different feed stocks. J. Indian Soc. Soil Sci. 2021, 69, 463–467. [Google Scholar] [CrossRef]
- Kumar, S.S.; Mir, S.A.; Wani, O.A.; Babu, S.; Yeasin, M.; Bhat, M.A.; Hussain, N.; Wani, A.I.A.; Kumar, R.; Yadav, D.; et al. Land-use systems regulate carbon geochemistry in the temperate Himalayas, India. J. Environ. Manag. 2022, 320, 115811. [Google Scholar] [CrossRef] [PubMed]
- Zahed, Z.; Mufti, S.; Kumar, S.S.; Wani, O.A.; Mushtaq, F.; Rasool, R.; Babu, S.; Abidi, I.; Gaber, A.; Hossain, A. Organic and inorganic mulches combination improves the productivity, quality and profitability of rainfed potato in the temperate himalayan region. Gesunde Pflanz. 2022, 74, 1109–1122. [Google Scholar] [CrossRef]
- Chen, T.; Zhang, Y.X.; Wang, H.T.; Lu, W.J.; Zhou, Z.Y.; Zhang, Y.C.; Ren, L.L. Influence of pyrolysis temperature on characteristics and heavy metal adsorptive performance of biochar derived from municipal sewage sludge. Bioresour. Technol. 2014, 164, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.-X.; Ta, N.; Wang, X.-D. Effect of temperature on the structural and physicochemical properties of biochar with apple tree branches as feedstock material. Energies 2017, 10, 1293. [Google Scholar] [CrossRef]
- Bashir, S.S.; Hussain, A.; Hussain, S.J.; Wani, O.A.; Nabi, S.Z.; Dar, N.A.; Baloch, F.S.; Mansoor, S. Plant drought stress tolerance: Understanding its physiological, biochemical and molecular mechanisms. Biotechnol. Biotechnol. Equip. 2021, 35, 1912–1925. [Google Scholar] [CrossRef]
- Mansoor, S.; Wani, O.A.; Lone, J.K.; Manhas, S.; Kour, N.; Alam, P.; Ahmad, A.; Ahmad, P. Reactive Oxygen Species in plants: From source to sink. Antioxidants 2022, 11, 225. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.-K.; Shim, T.; Kim, Y.-S.; Hyun, S.; Ryu, C.; Park, Y.-K.; Jung, J. Characterization of cadmium removal from aqueous solution by biochar produced from a giant Miscanthus at different pyrolytic temperatures. Bioresour. Technol. 2013, 138, 266–270. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Zhang, A.; Kang, Y.; Han, J.; Yang, B.; Hussain, Q.; Khan, M.A. Biochar promotes soil organic carbon sequestration and reduces net global warming potential in apple orchard: A two-year study in the Loess Plateau of China. Sci. Total Environ. 2022, 803, 150035. [Google Scholar] [CrossRef] [PubMed]
- Lataf, A.; Jozefczak, M.; Vandecasteele, B.; Viaene, J.; Schreurs, S.; Carleer, R.; Vandamme, D. The effect of pyrolysis temperature and feedstock on biochar agronomic properties. J. Anal. Appl. Pyrolysis 2022, 168, 105728. [Google Scholar] [CrossRef]
- Tran, H.N.; You, S.-J.; Chao, H.-P. Effect of pyrolysis temperatures and times on the adsorption of cadmium onto orange peel derived biochar. Waste Manag. Res. 2016, 34, 129–138. [Google Scholar] [CrossRef]
- Mollinedo, J.; Schumacher, T.E.; Chintala, R. Influence of feedstocks and pyrolysis on biochar’s capacity to modify soil water retention characteristics. J. Anal. Appl. Pyrolysis 2015, 114, 100–108. [Google Scholar] [CrossRef]
- Ippolito, J.A.; Cui, L.; Kammann, C.; Wrage-Mönnig, N.; Estavillo, J.M.; Fuertes-Mendizabal, T.; Cayuela, M.L.; Sigua, G.; Novak, J.; Spokas, K.; et al. Feedstock choice, pyrolysis temperature and type influence biochar characteristics: A comprehensive meta-data analysis review. Biochar 2020, 2, 421–438. [Google Scholar] [CrossRef]
- Aller, M.F. Biochar properties: Transport, fate, and impact. Crit. Rev. Environ. Sci. Technol. 2016, 46, 1183–1296. [Google Scholar] [CrossRef]
- Windeatt, J.H.; Ross, A.B.; Williams, P.T.; Forster, P.M.; Nahil, M.A.; Singh, S. Characteristics of biochars from crop residues: Potential for carbon sequestration and soil amendment. J. Environ. Manag. 2014, 146, 189–197. [Google Scholar] [CrossRef]
- Enders, A.; Hanley, K.; Whitman, T.; Joseph, S.; Lehmann, J. Characterization of biochars to evaluate recalcitrance and agronomic performance. Bioresour. Technol. 2012, 114, 644–653. [Google Scholar] [CrossRef]
- Viswanathan, S.P.; Njazhakunnathu, G.V.; Neelamury, S.P.; Padmakumar, B.; Ambatt, T.P. The efficiency of aquatic weed–derived biochar in enhanced removal of cationic dyes from aqueous medium. In Biomass Conversion and Biorefinery; Springer: Berlin/Heidelberg, Germany, 2022; pp. 1–16. [Google Scholar]
- Luo, L.; Xu, C.; Chen, Z.; Zhang, S. Properties of biomass-derived biochars: Combined effects of operating conditions and biomass types. Bioresour. Technol. 2015, 192, 83–89. [Google Scholar] [CrossRef]
- Li, Z.; Liu, C.; Dong, Y.; Chang, X.; Nie, X.; Liu, L.; Xiao, H.; Lu, Y.; Zeng, G. Response of soil organic carbon and nitrogen stocks to soil erosion and land use types in the Loess hilly–gully region of China. Soil Tillage Res. 2017, 166, 1–9. [Google Scholar] [CrossRef]
- Song, W.; Guo, M. Quality variations of poultry litter biochar generated at different pyrolysis temperatures. J. Anal. Appl. Pyrolysis 2012, 94, 138–145. [Google Scholar] [CrossRef]
- Mohammad, I.; Abakr, Y.; Kabir, F.; Yusuf, S.; Alshareef, I.; Chin, S. Pyrolysis of Napier grass in a fixed bed reactor: Effect of operating conditions on product yields and characteristics. BioResources 2015, 10, 6457–6478. [Google Scholar] [CrossRef]
- Lal, R. Soil erosion by wind and water: Problems and prospects. In Soil Erosion Research Methods; Routledge: Oxford, UK, 2017; pp. 1–10. [Google Scholar]
- Zahed, M.A.; Salehi, S.; Madadi, R.; Hejabi, F. Biochar as a sustainable product for remediation of petroleum contaminated soil. Curr. Res. Green Sustain. Chem. 2021, 4, 100055. [Google Scholar] [CrossRef]
- Baiamonte, G.; Crescimanno, G.; Parrino, F.; De Pasquale, C. Effect of biochar on the physical and structural properties of a sandy soil. Catena 2019, 175, 294–303. [Google Scholar] [CrossRef]
- Itami, K.; Kyuma, K. Dispersion behavior of soils from reclaimed lands with poor soil physical properties and their characteristics with special reference to clay mineralogy. Soil Sci. Plant Nutr. 1995, 41, 45–54. [Google Scholar] [CrossRef]
- Hammam, A.A.; Mohamed, E.S.; El-Namas, A.E.; Abd-Elmabod, S.K.; Badr Eldin, R.M. Impacted Application of Water-Hyacinth-Derived Biochar and Organic Manures on Soil Properties and Barley Growth. Sustainability 2022, 14, 13096. [Google Scholar] [CrossRef]
- Adekiya, A.O.; Agbede, T.M.; Olayanju, A.; Ejue, W.S.; Adekanye, T.A.; Adenusi, T.T.; Ayeni, J.F. Effect of biochar on soil properties, soil loss, and cocoyam yield on a tropical sandy loam Alfisol. Sci. World J. 2020, 2020, 9391630. [Google Scholar] [CrossRef]
- Adekiya, A.O.; Ojeniyi, S.O.; Owonifari, O.E. Effect of cow dung on soil physical properties, growth and yield of maize (Zea mays) in a tropical Alfisol. Sci. Agric. 2016, 15, 374–379. [Google Scholar]
- Amoah-Antwi, C.; Kwiatkowska-Malina, J.; Thornton, S.F.; Fenton, O.; Malina, G.; Szara, E. Restoration of soil quality using biochar and brown coal waste: A review. Sci. Total Environ. 2020, 722, 137852. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Bhattacharya, T.; Mukherjee, S.; Sarkar, B. A perspective on biochar for repairing damages in the soil–plant system caused by climate change-driven extreme weather events. Biochar 2022, 4, 22. [Google Scholar] [CrossRef]
- Obi, M. Soil Physics: A Compendium of Lectures; Atlanto, Publishers: Nsukka, Nigeria, 2000; 148p. [Google Scholar]
- Glaser, B.; Lehmann, J.; Zech, W. Ameliorating physical and chemical properties of highly weathered soils in the tropics with charcoal—A review. Biol. Fertil. Soils 2002, 35, 219–230. [Google Scholar] [CrossRef]
- Nwite, J.; Orji, J.; Okolo, C. Effect of different land use systems on soil carbon storage and structural indices in Abakaliki, Nigeria. Indian J. Ecol. 2018, 45, 522–527. [Google Scholar]
- El-Azeim, A.; Salah, Z.M.; Hammam, A.A. Assessment of water hyacinth biochar as a soil amendment for sandy soils. J. Soil Sci. Agric. Eng. 2021, 12, 431–444. [Google Scholar] [CrossRef]
- Kumari, K.; Swain, A.A.; Kumar, M.; Bauddh, K. Utilization of Eichhornia crassipes biomass for production of biochar and its feasibility in agroecosystems: A review. Environ. Sustain. 2021, 4, 285–297. [Google Scholar] [CrossRef]
- Singh, P.; Sharma, S.; Dhanorkar, M. Aquatic plant biomass-derived porous carbon: Biomaterials for sustainable waste management and climate change mitigation. Int. J. Environ. Sci. Technol. 2022, 11, 1–16. [Google Scholar] [CrossRef]
- Soinne, H.; Hovi, J.; Tammeorg, P.; Turtola, E. Effect of biochar on phosphorus sorption and clay soil aggregate stability. Geoderma 2014, 219, 162–167. [Google Scholar] [CrossRef]
- Amoakwah, E.; Frimpong, K.A.; Okae-Anti, D.; Arthur, E. Soil water retention, air flow and pore structure characteristics after corn cob biochar application to a tropical sandy loam. Geoderma 2017, 307, 189–197. [Google Scholar] [CrossRef]
- Ghorbani, M.; Neugschwandtner, R.W.; Konvalina, P.; Asadi, H.; Kopecký, M.; Amirahmadi, E. Comparative effects of biochar and compost applications on water holding capacity and crop yield of rice under evaporation stress: A two-years field study. Paddy Water Environ. 2023, 21, 47–58. [Google Scholar] [CrossRef]
- Schneider, E. The Effects of Biochar Age and Concentration on Soil Retention of Phosphorus and Infiltration Rate. Bachelor’s Thesis, California Polytechnic State University, San Luis Obispo, CA, USA, 2012. [Google Scholar]
- Doan, T.T.; Henry-des-Tureaux, T.; Rumpel, C.; Janeau, J.L.; Jouquet, P. Impact of compost, vermicompost and biochar on soil fertility, maize yield and soil erosion in Northern Vietnam: A three year mesocosm experiment. Sci. Total Environ. 2015, 514, 147–154. [Google Scholar] [CrossRef] [PubMed]
- Major, J.; Rondon, M.; Molina, D.; Riha, S.J.; Lehmann, J. Nutrient leaching in a Colombian savanna Oxisol amended with biochar. J. Environ. Qual. 2012, 41, 1076–1086. [Google Scholar] [CrossRef]
- Cheng, K.; Xu, X.; Cui, L.; Li, Y.; Zheng, J.; Wu, W.; Sun, J.; Pan, G. The role of soils in regulation of freshwater and coastal water quality. Philos. Trans. R. Soc. B 2021, 376, 20200176. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, R.; Gao, J.; Gao, Z.; Khan, A.; Ali, I.; Fahad, S. Influence of biochar on soil nutrients and associated Rhizobacterial communities of mountainous apple trees in northern loess plateau China. Microorganisms 2022, 10, 2078. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, F.; Yang, M.; Zhang, J.; Xie, Y. Impacts of biochar application rates and particle sizes on runoff and soil loss in small cultivated loess plots under simulated rainfall. Sci. Total Environ. 2019, 649, 1403–1413. [Google Scholar] [CrossRef]
- Sadeghi, S.H.; Hazbavi, Z.; Harchegani, M.K. Controllability of runoff and soil loss from small plots treated by vinasse-produced biochar. Sci. Total Environ. 2016, 541, 483–490. [Google Scholar] [CrossRef] [PubMed]
- Hseu, Z.Y.; Jien, S.H.; Chien, W.H.; Liou, R.C. Impacts of biochar on physical properties and erosion potential of a mudstone slopeland soil. Sci. World J. 2014, 2014, 602197. [Google Scholar] [CrossRef]
- Thomaz, E.; Luiz, J. Soil loss, soil degradation and rehabilitation in a degraded land area in Guarapuava (Brazil). Land Degrad. Dev. 2012, 23, 72–81. [Google Scholar] [CrossRef]
- Nugraha, S.S.; Sartohadi, J.; Nurudin, M. Field-Based Biochar, Pumice, and Mycorrhizae Application on Dryland Agriculture in Reducing Soil Erosion. Appl. Environ. Soil Sci. 2022, 2022, 1775330. [Google Scholar] [CrossRef]
- Joseph, S.D.; Camps-Arbestain, M.; Lin, Y.; Munroe, P.; Chia, C.H.; Hook, J.; van Zwieten, L.; Kimber, S.; Cowie, A.; Singh, B.P.; et al. An investigation into the reactions of biochar in soil. Aust. J. Soil Res. 2010, 48, 501–515. [Google Scholar] [CrossRef]
- Brodowski, S.; John, B.; Flessa, H.; Amelung, W. Aggregate-occluded black carbon in soil. Eur. J. Soil Sci. 2006, 57, 539–546. [Google Scholar] [CrossRef]
- Bashagaluke, J.B.; Logah, V.; Opoku, A.; Sarkodie-Addo, J.; Quansah, C. Soil nutrient loss through erosion: Impact of different cropping systems and soil amendments in Ghana. PLoS ONE 2018, 13, e0208250. [Google Scholar] [CrossRef] [PubMed]
- Zougmoré, R.; Zida, Z.; Kambou, N. Role of nutrient amendments in the success of half-moon soil and water conservation practice in semiarid Burkina Faso. Soil Tillage Res. 2003, 71, 143–149. [Google Scholar] [CrossRef]
- Olaniya, M.; Bora, P.K.; Das, S.; Chanu, P.H. Soil erodibility indices under different land uses in Ri-Bhoi district of Meghalaya (India). Sci. Rep. 2020, 10, 14986. [Google Scholar] [CrossRef]
- Ruxton, B.P. Measures of the degree of chemical weathering of rocks. J. Geol. 1968, 76, 518–527. [Google Scholar] [CrossRef]
- Yang, C.D.; Lu, S.G. Effects of five different biochars on aggregation, water retention and mechanical properties of paddy soil: A field experiment of three-season crops. Soil Tillage Res. 2021, 205, 104798. [Google Scholar] [CrossRef]
- Joseph, U.E.; Toluwase, A.O.; Kehinde, E.O.; Omasan, E.E.; Tolulope, A.Y.; George, O.O.; Zhao, C.; Hongyan, W. Effect of biochar on soil structure and storage of soil organic carbon and nitrogen in the aggregate fractions of an Albic soil. Arch. Agron. Soil Sci. 2020, 66, 1–12. [Google Scholar] [CrossRef]
- Six, J.; Bossuyt, H.; Degryze, S.; Denef, K. A history of research on the link between (micro) aggregates, soil biota, and soil organic matter dynamics. Soil Tillage Res. 2004, 79, 7–31. [Google Scholar] [CrossRef]
- Rizehbandi, E.; Khademalrasoul, A.; Zahedkolaei, M.T. Effects of recombinant synthetic organic and mineral mulches on physicomechanical properties of erodible soils using wind tunnel. Aeolian Res. 2021, 49, 100659. [Google Scholar] [CrossRef]
- Sheng, M.H.; Ai, X.Y.; Huang, B.C.; Zhu, M.K.; Liu, Z.Y.; Ai, Y.W. Effects of biochar additions on the mechanical stability of soil aggregates and their role in the dynamic renewal of aggregates in slope ecological restoration. Sci. Total Environ. 2023, 898, 165478. [Google Scholar] [CrossRef]
- Hua, L.; Wu, C.; Zhang, H.; Cao, L.; Wei, T.; Guo, J. Biochar-induced changes in soil microbial affect species of antimony in contaminated soils. Chemosphere 2021, 263, 127795. [Google Scholar] [CrossRef]
- Lei, O.; Zhang, R. Effects of biochars derived from different feedstocks and pyrolysis temperatures on soil physical and hydraulic properties. J. Soils Sediments 2013, 13, 1561–1572. [Google Scholar] [CrossRef]
- Lin, Y.; Munroe, P.; Joseph, S.; Henderson, R.; Ziolkowski, A. Water extractable organic carbon in untreated and chemical treated biochars. Chemosphere 2012, 87, 151–157. [Google Scholar] [CrossRef]
- Jindo, K.; Mizumoto, H.; Sawada, Y.; Sanchez-Monedero, M.A.; Sonoki, T. Physical and chemical characterization of biochars derived from different agricultural residues. Biogeosciences 2014, 11, 6613–6621. [Google Scholar] [CrossRef]
- Yadav, V.; Karak, T.; Singh, S.; Singh, A.K.; Khare, P. Benefits of biochar over other organic amendments: Responses for plant productivity (Pelargonium graveolens L.) and nitrogen and phosphorus losses. Ind. Crops Prod. 2019, 131, 96–105. [Google Scholar] [CrossRef]

| Pyrolysis Temperature | Proximate Analysis | Apple Biochar | Dal Weed Biochar |
|---|---|---|---|
| 400 °C | MC | 2.00 ± 0.10 d | 4.14 ± 0.36 d |
| AC | 7.75 ± 0.45 c | 39.64 ± 1.87 b | |
| VM | 32.02 ± 1.61 b | 21.64 ± 1.86 c | |
| FC | 60.73 ± 2.73 a | 39.47 ± 3.06 b | |
| YC | 36.80 ± 2.36 b | 47.34 ± 1.73 a | |
| 600 °C | MC | 2.77 ± 0.16 d | 4.44 ± 0.46 d |
| AC | 9.64 ± 0.56 c | 42.74 ± 2.25 a | |
| VM | 15.33 ± 1.03 b | 14.50 ± 2.00 c | |
| FC | 74.58 ± 3.76 a | 43.44 ± 2.31 a | |
| YC | 17.37 ± 1.06 b | 37.00 ± 2.00 b |
| Factor | Erodibility Indices and Soil Aggregate Stability | ||||||
|---|---|---|---|---|---|---|---|
| Amendment | DR | CMR | PR | ER | CR | MWD | TOC |
| NB | 0.37 ± 0.02 a | 0.68 ± 0.03 a | 24.49 ± 2.03 b | 0.55 ± 0.03 ab | 4.26 ± 0.91 a | 1.25 ± 0.01 c | 12.30 ± 0 d |
| AB400 | 0.35 ± 0.01 a | 0.67 ± 0.02 a | 23.51 ± 1.04 a | 0.53 ± 0.02 a | 4.12 ± 0.81 a | 1.45 ± 0.01 a | 28.83 ± 1.45 b |
| AB600 | 0.51 ± 0.03 b | 0.68 ± 0.03 a | 33.75 ± 3.03 d | 0.75 ± 0.03 d | 4.01 ± 0.62 a | 1.46 ± 0.01 a | 34.74 ± 1.5 a |
| RAC | 0.44 ± 0.02 a | 0.68 ± 0.08 a | 29.12 ± 0.97 b | 0.65 ± 0.07 ab | 4.40 ± 0.43 a | 1.32 ± 0.02 b | 25.94 ± 1.47 b |
| DB400 | 0.51 ± 0.03 a | 0.69 ± 0.09 a | 33.27 ± 4.07 b | 0.74 ± 0.09 b | 4.11 ± 0.89 a | 1.32 ± 0.01 b | 25.50 ± 1.48 bc |
| DW600 | 0.47 ± 0.04 a | 0.67 ± 0.04 a | 31.57 ± 3.03 b | 0.71 ± 0.05 ab | 4.32 ± 0.95 a | 1.31 ± 0.01 b | 29.69 ± 1.49 ab |
| RDW | 0.91 ± 0.01 c | 0.70 ± 0.01 a | 58.50 ± 1.03 c | 1.30 ± 0.06 c | 5.22 ± 0.79 a | 0.86 ± 0.02 d | 20.05 ± 1.47 c |
| LSD (0.05) | 1.89 | 0.34 | 0.44 | 2.10 | 1.98 | 1.89 | 1.89 |
| SEm± | 0.24 | 0.03 | 0.05 | 0.89 | 0.85 | 0.24 | 0.24 |
| Application rate | |||||||
| H | 0.41 ± 0.01 a | 0.69 ± 0.09 a | 26.74 ± 2.97 a | 0.60 ± 0.03 a | 4.12 ± 0.96 a | 1.01 ± 0.33 b | 19.73 ± 0.85 c |
| M | 0.48 ± 0.03 a | 0.68 ± 0.04 a | 31.77 ± 4.04 a | 0.71 ± 0.07 a | 4.88 ± 0.75 a | 1.26 ± 0.37 ab | 24.36 ± 1.05 b |
| L | 0.61 ± 0.02 b | 0.78 ± 0.07 a | 35.20 ± 3.02 a | 0.79 ± 0.09 b | 5.10 ± 0.66 a | 1.19 ± 0.33 a | 31.80 ± 1.42 a |
| LSD (0.05) | 1.89 | 0.34 | 0.44 | 2.10 | 1.98 | 1.77 | 1.89 |
| SEm± | 0.24 | 0.03 | 0.05 | 0.89 | 0.85 | 0.18 | 0.24 |
| Fertilizer | |||||||
| N | 0.49 ± 0.04 a | 0.68 ± 0.02 a | 32.43 ± 6.03 a | 0.73 ± 0.06 a | 4.34 ± 0.65 a | 1.29 ± 0.03 a | 24.45 ± 1.1 a |
| F | 0.38 ± 0.07 a | 0.73 ± 0.03 a | 23.43 ± 3.04 a | 0.53 ± 0.09 a | 4.50 ± 0.33 a | 1.27 ± 0.03 a | 26.14 ± 1.13 a |
| LSD (0.05) | 1.89 | 0.34 | 0.44 | 2.10 | 1.98 | 1.77 | 1.89 |
| SEm± | 0.24 | 0.03 | 0.05 | 0.89 | 0.85 | 0.18 | 0.24 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wani, O.A.; Akhter, F.; Kumar, S.S.; Babu, S.; Kanth, R.H.; Mir, S.A.; Mahdi, S.S.; Malik, A.R.; Bangroo, S.; Gaafar, A.-R.Z.; et al. Mitigating Soil Erosion through Biomass-Derived Biochar: Exploring the Influence of Feedstock Types and Pyrolysis Temperature. Land 2023, 12, 2111. https://doi.org/10.3390/land12122111
Wani OA, Akhter F, Kumar SS, Babu S, Kanth RH, Mir SA, Mahdi SS, Malik AR, Bangroo S, Gaafar A-RZ, et al. Mitigating Soil Erosion through Biomass-Derived Biochar: Exploring the Influence of Feedstock Types and Pyrolysis Temperature. Land. 2023; 12(12):2111. https://doi.org/10.3390/land12122111
Chicago/Turabian StyleWani, Owais Ali, Farida Akhter, Shamal Shasang Kumar, Subhash Babu, Raihana Habib Kanth, Shakeel Ahmad Mir, Syed Sheraz Mahdi, Abdul Raouf Malik, Shabir Bangroo, Abdel-Rhman Z. Gaafar, and et al. 2023. "Mitigating Soil Erosion through Biomass-Derived Biochar: Exploring the Influence of Feedstock Types and Pyrolysis Temperature" Land 12, no. 12: 2111. https://doi.org/10.3390/land12122111
APA StyleWani, O. A., Akhter, F., Kumar, S. S., Babu, S., Kanth, R. H., Mir, S. A., Mahdi, S. S., Malik, A. R., Bangroo, S., Gaafar, A.-R. Z., Popescu, S. M., & Rathore, S. S. (2023). Mitigating Soil Erosion through Biomass-Derived Biochar: Exploring the Influence of Feedstock Types and Pyrolysis Temperature. Land, 12(12), 2111. https://doi.org/10.3390/land12122111







