Performance of Rice Genotypes under Temporally Variable Wetland Salinity Conditions of a Semiarid Sub-Saharan Climatic Environment
Abstract
:1. Introduction
2. Materials and Methods
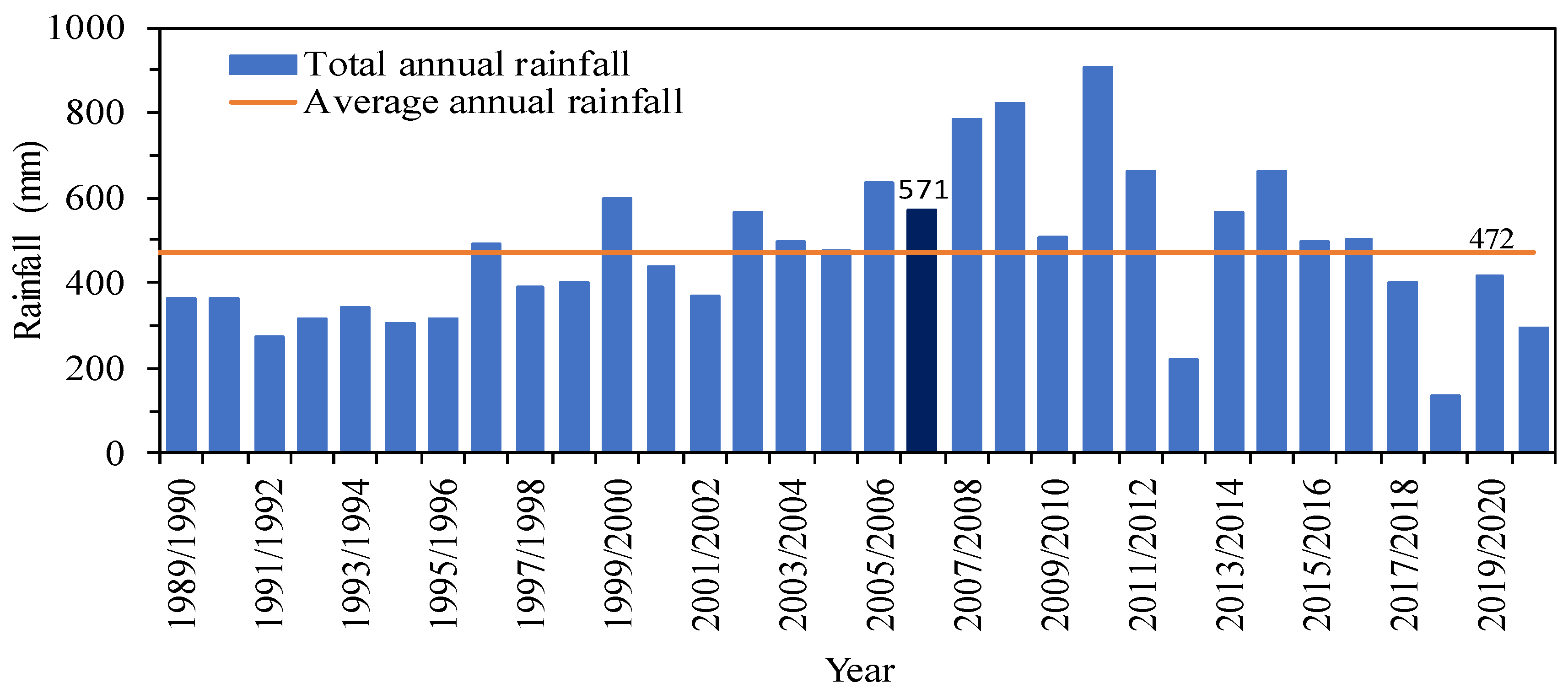
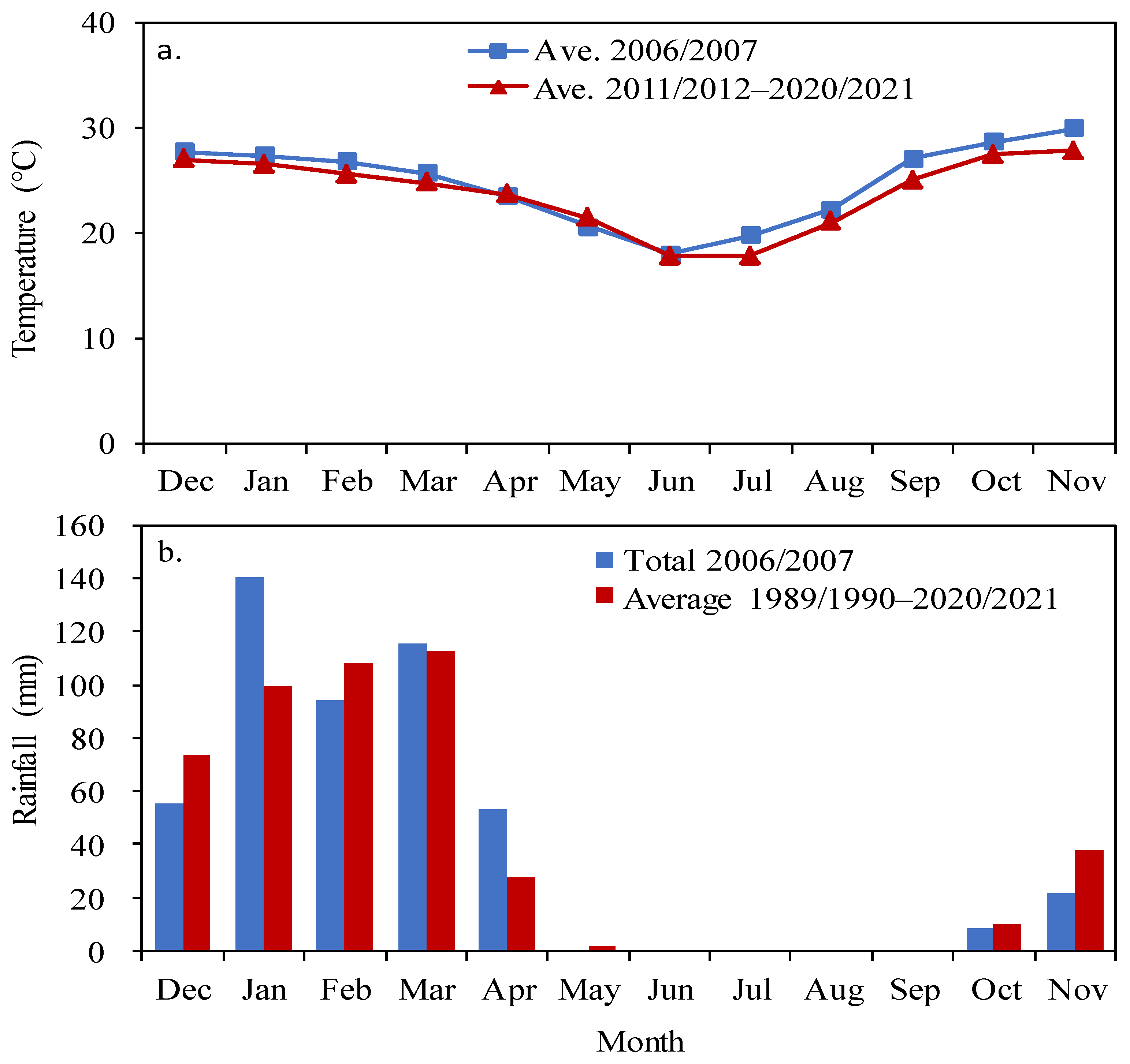
2.1. Study Site and Environmental Conditions
2.2. Plant Materials
2.3. Rainy-Season Experiment
2.4. Dry-Season Experiment
2.5. Rice Growth in Alternate-Months Saline Soils
2.6. Statistical Analysis
3. Results
3.1. Rainy-Season Grain Production Study (Exp. 1)
3.1.1. Genotype Comparative Performance in the Saline and Control Wetlands
3.1.2. Genotypic Absolute and Relative Performance
3.1.3. Correlations among Yield Characters
3.2. Dry-Season Plant Survival Rate and Growth in the Saline Wetland (Exp. 2)
3.3. Plant Growth in Soils Collected during the Rainy and Dry Seasons (Exp. 3)
4. Discussion
4.1. Environmental Conditions
4.2. Crop Growth in the Saline Wetland
4.3. Rice Paddy Production in the Saline Wetland
4.4. Genotypic Salinity Tolerance
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Panagos, P.; Borrelli, P.; Robinson, D. FAO Calls for Actions to Reduce Global Soil Erosion. Mitig. Adapt. Strateg. Glob. Chang. 2020, 25, 789–790. [Google Scholar] [CrossRef] [Green Version]
- Zhao, G.Q.; Ma, B.L.; Ren, C.Z. Growth, Gas Exchange, Chlorophyll Fluorescence, and Ion Content of Naked Oat in Response to Salinity. Crop Sci. 2007, 47, 123–131. [Google Scholar] [CrossRef]
- Shahid, S.A.; Zaman, M.; Heng, L. Soil Salinity: Historical Perspectives and a World Overview of the Problem. In Guideline for Salinity Assessment, Mitigation and Adaptation Using Nuclear and Related Techniques; Springer International Publishing: Cham, Switzerland, 2018; pp. 43–53. ISBN 9783319961903. [Google Scholar]
- Awala, S.K.; Yamane, K.; Izumi, Y.; Fujioka, Y.; Watanabe, Y.; Wada, K.C.; Kawato, Y.; Mwandemele, O.D.; Iijima, M. Field Evaluation of Mixed-Seedlings with Rice to Alleviate Flood Stress for Semi-Arid Cereals. Eur. J. Agron. 2016, 80, 105–112. [Google Scholar] [CrossRef] [Green Version]
- Porter, J.R.; Xie, L.; Challinor, A.J.; Cochrane, K.; Howden, S.M.; Iqbal, M.M.; Lobell, D.B.; Travasso, M.I. Food Security and Food Production Systems. In Climate Change 2014: Impacts, Adaptation, and Vulnerability. Part A: Global and Sectoral Aspects. Contribution of Working Group II to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Cambridge University Press: Cambridge, UK, 2014; pp. 485–533. [Google Scholar]
- Chaudhry, S.; Sidhu, G.P.S. Climate Change Regulated Abiotic Stress Mechanisms in Plants: A Comprehensive Review. Plant Cell Rep. 2021, 41, 1–31. [Google Scholar] [CrossRef]
- Parihar, P.; Singh, S.; Singh, R.; Singh, V.P.; Prasad, S.M. Effect of Salinity Stress on Plants and Its Tolerance Strategies: A Review. Environ. Sci. Pollut. Res. 2015, 22, 4056–4075. [Google Scholar] [CrossRef]
- Kaveh, H.; Nemati, H.; Farsi, M.; Jartoodeh, S.V. How Salinity Affect Germination and Emergence of Tomato Lines. J. Biol. Environ. Sci. 2011, 5, 159–163. [Google Scholar]
- Bybordi, A. The Influence of Salt Stress on Seed Germination, Growth and Yield of Canola Cultivars. Not. Bot. Horti Agrobot. Cluj-Napoca 2010, 38, 128–133. [Google Scholar]
- Khodahmpour, Z. Effects of NaCl Salinity on Maize (Zea mays L.) at Germination and Early Seedling Stage. Afr. J. Biotechnol. 2011, 11, 298–303. [Google Scholar] [CrossRef] [Green Version]
- Jēkabsone, A.; Andersone-Ozola, U.; Karlsons, A.; Romanovs, M.; Ievinsh, G. Effect of Salinity on Growth, Ion Accumulation and Mineral Nutrition of Different Accessions of a Crop Wild Relative Legume Species, Trifolium Fragiferum. Plants 2022, 11, 797. [Google Scholar] [CrossRef]
- Koyro, H.-W. Effect of Salinity on Growth, Photosynthesis, Water Relations and Solute Composition of the Potential Cash Crop Halophyte Plantago coronopus (L.). Environ. Exp. Bot. 2006, 56, 136–146. [Google Scholar] [CrossRef]
- Patel, D.; Jayswal, S.; Solanki, H.; Maitreya, B. Effect of Salinity on Different Vegetable Crops—A Review. Int. J. Recent Sci. 2020, 11, 37418–37422. [Google Scholar] [CrossRef]
- FAO; IFAD; UNICEF; WFP; WHO. The State of Food Security and Nutrition in the World 2019: Safeguarding against Economic Slowdowns and Downturns; FAO: Rome, Italy, 2019; ISBN 9789251315705. [Google Scholar]
- Heyns, P. Guidelines for the Utilisationof Water Resources and Protection of Wetlands in Namibia. Madoqua 1991, 1991, 249–251. [Google Scholar]
- Namibia Statistics Agency. Namibia 2011 Population & Housing Census—Main Report; Windhoek. 2013. Available online: https://cms.my.na/assets/documents/p19dmn58guram30ttun89rdrp1.pdf (accessed on 19 September 2021).
- Awala, S.K.; Hove, K.; Wanga, M.A.; Valombola, J.S.; Mwandemele, O.D. Rainfall Trend and Variability in Semi-Arid Northern Namibia: Implications for Smallholder Agricultural Production. Welwitschia Int. J. Agric. Sci. 2019, 1, 1–25. [Google Scholar]
- Gregorio, G.B.; Senadhira, D.; Mendoza, R.D.; Manigbas, N.L.; Roxas, J.P.; Guerta, C.Q. Progress in Breeding for Salinity Tolerance and Associated Abiotic Stresses in Rice. Field Crops Res. 2002, 76, 91–101. [Google Scholar] [CrossRef]
- Hirooka, Y.; Awala, S.K.; Hove, K.; Nanhapo, P.I.; Iijima, M. Effects of Cultivation Management on Pearl Millet Yield and Growth Differed with Rainfall Conditions in a Seasonal Wetland of Sub-Saharan Africa. Agronomy 2021, 11, 1767. [Google Scholar] [CrossRef]
- Mendelsohn, J.; Jarvis, A.; Robertson, T. A Profile and Atlas of the Cuvelai-Etosha Basin; Raison: Windhoek, Namibia, 2013; ISBN 9991678077. [Google Scholar]
- Mendelsohn, J.; Jarvis, A.; Roberts, C.; Robertson, T. Atlas of Namibia: A Portrait of the Land and Its People; David Philip Publishers: Cape Town, South Africa, 2002; ISBN 0-86486-516-3. [Google Scholar]
- Awala, S.K.; Hove, K.; Shivute, V.; Valombola, J.S.; Nanhapo, P.I.; Hirooka, Y.; Mwandemele, O.D.; Iijima, M. Growth and Productivity Assessment of Short-Duration Rice (Oryza sativa L. and Upland NERICA) Genotypes in Semiarid North-Central Namibia. Adv. Agric. 2021, 2021, 6676081. [Google Scholar] [CrossRef]
- Hirooka, Y.; Masuda, T.; Watanabe, Y.; Izumi, Y.; Inai, H.; Awala, S.K.; Iijima, M. Agronomic and Socio-Economic Assessment of the Introduction of a Rice-Based Mixed Cropping System to Cuvelai Seasonal Wetland System in Northern Namibia. Agrekon 2021, 60, 145–156. [Google Scholar] [CrossRef]
- Iijima, M.; Awala, S.K.; Watanabe, Y.; Kawato, Y.; Fujioka, Y.; Yamane, K.; Wada, K.C. Mixed Cropping Has the Potential to Enhance Flood Tolerance of Drought-Adapted Grain Crops. J. Plant Physiol. 2016, 192, 21–25. [Google Scholar] [CrossRef] [Green Version]
- Kotani, A.; Hiyama, T.; Ohta, T.; Hanamura, M.; Kambatuku, J.R.; Awala, S.K.; Iijima, M. Impact of Rice Cultivation on Evapotranspiration in Small Seasonal Wetlands of North-Central Namibia. Hydrol. Res. Lett. 2017, 11, 134–140. [Google Scholar] [CrossRef] [Green Version]
- Suzuki, T.; Ohta, T.; Hiyama, T.; Izumi, Y.; Mwandemele, O.; Iijima, M. Effects of the Introduction of Rice on Evapotranspiration in Seasonal Wetlands. Hydrol. Process. 2014, 28, 4780–4794. [Google Scholar] [CrossRef]
- Watanabe, Y.; Itanna, F.; Fujioka, Y.; Ruben, S.; Iijima, M. Soil Fertility Status of Seasonally Closed Wetland Ecosystem (Ondombe) in North-Central Namibia. Afr. J. Agric. Res. 2017, 12, 1538–1546. [Google Scholar] [CrossRef] [Green Version]
- Mendelsohn, J. Farming Systems in Namibia; Raison: Windhoek, Namibia, 2006; ISBN 9991678042. [Google Scholar]
- Lindeque, M.; Archibald, T.J. Seasonal Wetlands in Ovambo and the Etosha National Park. Madoqua 1991, 1991, 129–133. [Google Scholar]
- Khan, M.R.; Rahman, M.H.; Hasan, M.; Sarker, R.R.; Ali, M.M. Nutrient Management for Rice-Fallow-Rice Cropping Pattern Grown under Costal Saline Area of Satkhira, Bangladesh. Int. J. Biosci. 2018, 12, 310–316. [Google Scholar]
- Herrero, J.; Castañeda, C. Temporal Changes in Soil Salinity at Four Saline Wetlands in NE Spain. Catena 2015, 133, 145–156. [Google Scholar] [CrossRef] [Green Version]
- Shabbir, G.; Hussain, N.; Bhatti, M.K.; Ahmad, A.; Javed, M.A.; Shakir, M.A. Salt Tolerance Potential of Some Selected Fine Rice Cultivars. Online J. Biol. Sci. 2001, 1, 1175–1177. [Google Scholar] [CrossRef]
- Zeng, L. Exploration of Relationships between Physiological Parameters and Growth Performance of Rice (Oryza sativa L.) Seedlings under Salinity Stress Using Multivariate Analysis. Plant Soil 2005, 268, 51–59. [Google Scholar] [CrossRef]
- Zeng, L.; Shannon, M.C. Effects of Salinity on Grain Yield and Yield Components of Rice at Different Seeding Densities. Agron. J. 2000, 92, 418–423. [Google Scholar] [CrossRef]
- Awala, S.K.; Nanhapo, I.; Sakagami, J.-I.; Kanyomeka, L.; Iijima, M. Differential Salinity Tolerance among Oryza Glaberrima, Oryza Sativa and Their Interspecies Including NERICA. Plant Prod. Sci. 2010, 13, 3–10. [Google Scholar] [CrossRef] [Green Version]
- Erfandi, D.; Rachman, A. Identification of Soil Salinity Due to Seawater Intrusion on Rice Field in the Northern Coast of Indramayu, West Java. J. Trop. Soils 2013, 16, 115–121. [Google Scholar] [CrossRef]
- Zeng, L.; Shannon, M.C. Salinity Effects on Seedling Growth and Yield Components of Rice. Crop Sci. 2000, 40, 996–1003. [Google Scholar] [CrossRef] [Green Version]
- Hussain, S.; Cao, X.; Zhong, C.; Zhu, L.; Khaskheli, M.A.; Fiaz, S.; Zhang, J.; Jin, Q. Sodium Chloride Stress during Early Growth Stages Altered Physiological and Growth Characteristics of Rice. Chil. J. Agric. Res. 2018, 78, 183–197. [Google Scholar] [CrossRef]
- Saleethong, P.; Sanitchon, J.; Kong-ngern, K.; Theerakulpisut, P. Effects of Exogenous Spermidine (Spd) on Yield, Yield-Related Parameters and Mineral Composition of Rice (Oryza sativa L. ssp. Indica) Grains under Salt Stress. Aust. J. Crop Sci. 2013, 7, 1293–1301. [Google Scholar]
- Jagadish, S.V.K.; Craufurd, P.Q.; Wheeler, T.R. Phenotyping Parents of Mapping Populations of Rice for Heat Tolerance during Anthesis. Crop Sci. 2008, 48, 1140–1146. [Google Scholar] [CrossRef]
- Sahrawat, K.L. Reducing Iron Toxicity in Lowland Rice with Tolerant Genotypes and Plant Nutrition. Plant Stress 2010, 4, 70–75. [Google Scholar]
- Cabasan, M.T.N.; Kumar, A.; De Waele, D. Effects of Initial Nematode Population Density and Water Regime on Resistance and Tolerance to the Rice Root-Knot Nematode Meloidogyne Graminicola in African and Asian Rice Genotypes. Int. J. Pest Manag. 2018, 64, 252–261. [Google Scholar] [CrossRef]
- Touré, A.; Rodenburg, J.; Saito, K.; Oikeh, S.; Futakuchi, K.; Gumedzoe, D.; Huat, J. Cultivar and Weeding Effects on Weeds and Rice Yields in a Degraded Upland Environment of the Coastal Savanna. Weed Technol. 2011, 25, 322–329. [Google Scholar] [CrossRef]
- Fukuta, Y.; Konisho, K.; Senoo-Namai, S.; Yanagihara, S.; Tsunematsu, H.; Fukuo, A.; Kumashiro, T. Genetic Characterization of Rainfed Upland New Rice for Africa (NERICA) Varieties. Breed. Sci. 2012, 62, 27–37. [Google Scholar] [CrossRef] [Green Version]
- Jones, M.P.; Dingkuhn, M.; Aluko, G.K.; Semon, M. Interspecific Oryza sativa L. x O. glaberrima Steud. Progenies in Upland Rice Improvement. Euphytica 1997, 94, 237–246. [Google Scholar] [CrossRef]
- Ndjiondjop, M.N.; Seek, P.A.; Lorieux, M.; Futakuchi, K.; Yao, K.N.; Djedatin, G.; Sow, M.E.; Bocco, R.; Cisse, F.; Fatondji, B. Effect of Drought on Oryza Glaberrima Rice Accessions and Oryza Glaberrima Derived-Lines. Asian J. Agric. Res. 2012, 6, 144–157. [Google Scholar] [CrossRef]
- Korres, N.E.; Loka, D.A.; Gitsopoulos, T.K.; Varanasi, V.K.; Chachalis, D.; Price, A.; Slaton, N.A. Salinity Effects on Rice, Rice Weeds, and Strategies to Secure Crop Productivity and Effective Weed Control. A Review. Agron. Sustain. Dev. 2022, 42, 58. [Google Scholar] [CrossRef]
- Shahzad, B.; Yun, P.; Shabala, L.; Zhou, M.; Sellamuthu, G.; Venkataraman, G.; Chen, Z.H.; Shabala, S. Unravelling the Physiological Basis of Salinity Stress Tolerance in Cultivated and Wild Rice Species. Funct. Plant Biol. 2022, 49, 351–364. [Google Scholar] [CrossRef] [PubMed]
- Farooq, M.; Park, J.-R.; Jang, Y.-H.; Kim, E.-G.; Kim, K.-M. Rice Cultivars under Salt Stress Show Differential Expression of Genes Related to the Regulation of Na+/K+ Balance. Front. Plant Sci. 2021, 12, 680131. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.A.; Thomson, M.J.; Shah-E-Alam, M.; De Ocampo, M.; Egdane, J.; Ismail, A.M. Exploring Novel Genetic Sources of Salinity Tolerance in Rice through Molecular and Physiological Characterization. Ann. Bot. 2016, 117, 1083–1097. [Google Scholar] [CrossRef] [PubMed]

| Season | Sampling | EC * | SAR # | pH | HCO3−/CO32− | Cl | Na |
|---|---|---|---|---|---|---|---|
| Month | (dS m−1) | (meq/100 g) | (meq/100 g) | (meq/100 g) | |||
| Rainy | Control | ||||||
| Nov. 2006 | 0.3 | 0.1 | 6.3 | 0.04 | 0.04 | 0.07 | |
| Saline | |||||||
| Nov. 2006 | 7.3 | 69.3 | 5.7 | 0.00 | 55.56 | 14.50 | |
| Jan. 2007 | 2.9 | 38.2 | 6.3 | 0.07 | 3.68 | 5.25 | |
| Mar. | 2.8 | 16.0 | 5.5 | 0.55 | 0.13 | 0.68 | |
| May | 4.1 | 20.6 | 6.2 | 0.26 | 0.21 | 0.70 | |
| Dry | Jul. | 18.25 | 126.5 | 5.3 | 0.32 | 16.73 | 42.88 |
| Sept. | 34.25 | 205.3 | 5.0 | 0.11 | 45.71 | 62.39 |
| No. | Cultivar Name | Species # | Origin | Ecotype † | Experiment | ||
|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | |||||
| 1. | C0440 | G | Guinea | D | × | ||
| 2. | CG14 | G | Senegal | RL/U | × | × | × |
| 3. | Loubi tetera | G | L | × | |||
| 4. | Mala Noir Ⅳ | G | Niger | D | × | × | |
| 5. | Mala Noir V | G | Niger | D | × | ||
| 6. | Tataro | G | Mali | RL | × | ||
| 7. | NERICA 3 | I | Cote d’Ivoire | U | × | × | |
| 8. | NERICA 4 | I | Cote d’Ivoire | U | × | × | |
| 9. | WAB1159-2-12-11-2-10 | I | Cote d’Ivoire | L | × | ||
| 10. | WAB1159-4-10-15-1-3 | I | Cote d’Ivoire | L | × | ||
| 11. | WAS122-IDSA-10-WAS-1-1-FKR-1 | I | L | × | × | ||
| 12. | WAS161-B-6-B-3-1B | I | Senegal | L | × | × | |
| 13. | WAS173-B-2-1 | I | Senegal | L | × | ||
| 14. | EL Boutika | S | Mali | D | × | ||
| 15. | ITA230 (FARO50) | S | Nigeria | L | × | × | |
| 16. | LK1484-5 | S | Guinea | L | × | ||
| 17. | Nona Bokra | S | India | × | |||
| 18. | Pokkali | S | India | L | × | × | × |
| 19. | WAB56-104 | S | Cote d’Ivoire | U | × | × | |
| Variety | Plant Density (No. m−2) | Panicles (No. m−2) | Paddy Yield (g m−2) | |||
|---|---|---|---|---|---|---|
| Mean Diff. ± SE (C-S) | p-Value | Mean Diff. ± SE (C-S) | p-Value | Mean Diff. ± SE (C-S) | p-Value | |
| Pokkali | 3.7 ± 0.7 | 0.032 * | 20.7 ± 4.7 | 0.047 * | 95.4 ± 26.2 | 0.068 ns |
| WAB56-104 | 10.3 ± 1.7 | 0.025 * | 37.6 ± 4.3 | 0.013 * | 197.4 ± 25.3 | 0.016 * |
| ITA230 (FARO50) | 8.3 ± 1.9 | 0.046 * | 77.9 ± 17.2 | 0.046 * | 134.7 ± 22.7 | 0.027 * |
| NERICA 3 | 4.7 ± 1.2 | 0.060 ns | 31.3 ± 4.7 | 0.022 * | 101.5 ± 14.6 | 0.020 * |
| NERICA 4 | 5.0 ± 0.6 | 0.013 * | 28.8 ± 6.2 | 0.044 * | 204.2 ± 21.1 | 0.010 * |
| WAS161-B-6-B-3-1B | 4.0 ± 1.0 | 0.057 ns | 39.3 ± 12.5 | 0.088 ns | 138.0 ± 24.6 | 0.030 * |
| WAS122-IDSA-10-WAS-1-1-FKR-1 | 4.7 ± 0.7 | 0.020 * | 59.9 ± 12.6 | 0.041 * | 183.8 ± 24.6 | 0.018 * |
| CG14 | 5.3 ± 1.5 | 0.067 ns | 55.1 ± 27.2 | 0.180 ns | 122.1 ± 32.7 | 0.065 ns |
| C0440 | 6.0 ± 1.2 | 0.035 * | 66.7 ± 14.8 | 0.046 * | 140.9 ± 32.3 | 0.049 * |
| Mala Noir IV | 7.3 ± 1.9 | 0.058 ns | 52.6 ± 14.6 | 0.069 ns | 184.5 ± 14.1 | 0.006 ** |
| Mala Noir V | 10.0 ± 1.6 | 0.013 * | 38.8 ± 12.6 | 0.091 ns | 204.0 ± 28.0 | 0.018 * |
| Variety | Survival Rate (%) | Panicles. (No. m−2) | Relative Panicle No. (% Control) | Paddy Yield (g m−2) | Relative Paddy Yield (% Control) | ||
|---|---|---|---|---|---|---|---|
| Saline | Control | Saline | Control | Saline | |||
| Pokkali | 80.0a | 75.2ef | 54.5bcd | 72.9a | 331.1a | 235.7a | 71.9a |
| WAB56-104 | 42.6c | 57.3f | 19.7e | 34.4a | 232.5def | 35.2g | 15.3d |
| ITA230(FARO50) | 53.7bc | 130.8b | 52.9bcd | 41.3a | 208.6ef | 74.0ef | 36.0bc |
| NERICA 3 | 74.1ab | 63.2f | 31.9de | 50.6a | 154.7g | 53.2fg | 34.8bc |
| NERICA 4 | 72.2ab | 64.1f | 35.3de | 55.3a | 256.4bcd | 52.2fg | 20.6cd |
| WAS161-B-6-B-3-1B | 77.8a | 120.5bc | 81.2b | 68.4a | 273.0bc | 135.0b | 50.2b |
| WAS122-IDSA-10-WAS-1-1-FKR-1 | 74.1ab | 129.9b | 70.0bc | 54.9a | 292.8ab | 109.0bcd | 37.6bc |
| CG14 | 70.4ab | 182.9a | 127.8a | 70.5a | 244.9cde | 122.9bc | 51.6b |
| C0440 | 66.7ab | 127.4bc | 60.7bcd | 48.4a | 202.4f | 61.5fg | 32.4cd |
| Mala Noir IV | 59.3abc | 107.7cd | 55.1bcd | 52.2a | 281.0bc | 96.5cde | 34.2bc |
| Mala Noir V | 44.4c | 88.9de | 50.1cde | 57.2a | 279.1bc | 75.2def | 27.5cd |
| Genotype | The % Survival Rate in Saline Field | Absolute Shoot Dry Weight * | Relative Shoot Dry Weight (% Control) | |
|---|---|---|---|---|
| (g m−2) | ||||
| Control | Saline | |||
| Pokkali | 13 | 43.6abcd | 0.047b | 0.11 |
| WAB56-104 | 3 | 29.6def | 0.002b | 0.01 |
| ITA230 (FARO50) | 6 | 43.6abcd | 0.011b | 0.03 |
| NERICA 3 | 4 | 23.3f | 0.006b | 0.03 |
| NERICA 4 | 14 | 26.1ef | 0.094ab | 0.36 |
| WAS161-B-6-B-3-1B | 6 | 38.6abcdef | 0.019b | 0.05 |
| WAS122-IDSA-10-WAS-1-1-FKR-1 | 2 | 34.4bcdef | 0.004b | 0.01 |
| CG14 | 6 | 36.9bcdef | 0.009b | 0.02 |
| Mala Noir IV | 9 | 30.9cdef | 0.051b | 0.17 |
| WAS173-B-2-1 | 6 | 34.2bcdef | 0.023b | 0.07 |
| Loubi tetera # | 0 | 39.0abcdef | – | – |
| LK1484-5 | 11 | 50.1ab | 0.151ab | 0.30 |
| Tataro | 14 | 40.4abcde | 0.253a | 0.63 |
| WAB1159-2-12-11-2-10 | 2 | 46.4abc | 0.004b | 0.01 |
| EL Boutika | 3 | 36.2bcdef | 0.009b | 0.02 |
| WAB1159-4-10-15-1-3 # | 0 | 53.1a | – | – |
| Sampling Month | Soil Electrical Conductivity (dS m−1) | CG14 | Pokkali | ||
|---|---|---|---|---|---|
| Absolute Shoot Dry Weight (g pot−1) | Relative Shoot Dry Weight (% Control) | Absolute Shoot Dry Weight (g pot−1) | Relative Shoot Dry Weight (% Control) | ||
| Control | 0.3 | 6.2 ± 0.7 a | − | 8.0 ± 0.8 a | − |
| Mar-07 | 2.8 | 4.3 ± 1.0 b | 69 | 8.6 ± 0.5 a | 108 |
| May | 4.1 | 2.8 ± 0.3 c | 45 | 7.3 ± 1.2 b | 91 |
| Jul # | 18.25 | – | – | – | – |
| Sept # | 34.25 | – | – | – | – |
| p-value | – | 0.037 | – | 0.007 | – |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Awala, S.K.; Hove, K.; Simasiku, E.K.; Izumi, Y.; Mwandemele, O.D.; Iijima, M. Performance of Rice Genotypes under Temporally Variable Wetland Salinity Conditions of a Semiarid Sub-Saharan Climatic Environment. Land 2023, 12, 888. https://doi.org/10.3390/land12040888
Awala SK, Hove K, Simasiku EK, Izumi Y, Mwandemele OD, Iijima M. Performance of Rice Genotypes under Temporally Variable Wetland Salinity Conditions of a Semiarid Sub-Saharan Climatic Environment. Land. 2023; 12(4):888. https://doi.org/10.3390/land12040888
Chicago/Turabian StyleAwala, Simon Kamwele, Kudakwashe Hove, Evans Kamwi Simasiku, Yasuhiro Izumi, Osmund Damian Mwandemele, and Morio Iijima. 2023. "Performance of Rice Genotypes under Temporally Variable Wetland Salinity Conditions of a Semiarid Sub-Saharan Climatic Environment" Land 12, no. 4: 888. https://doi.org/10.3390/land12040888






