Biochar Aged for Five Years Altered Carbon Fractions and Enzyme Activities of Sandy Soil
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Site
2.2. Experimental Design and Soil Sampling
2.3. Soil Biophysiochemical Properties Analysis
2.4. Statistical Analysis
3. Results
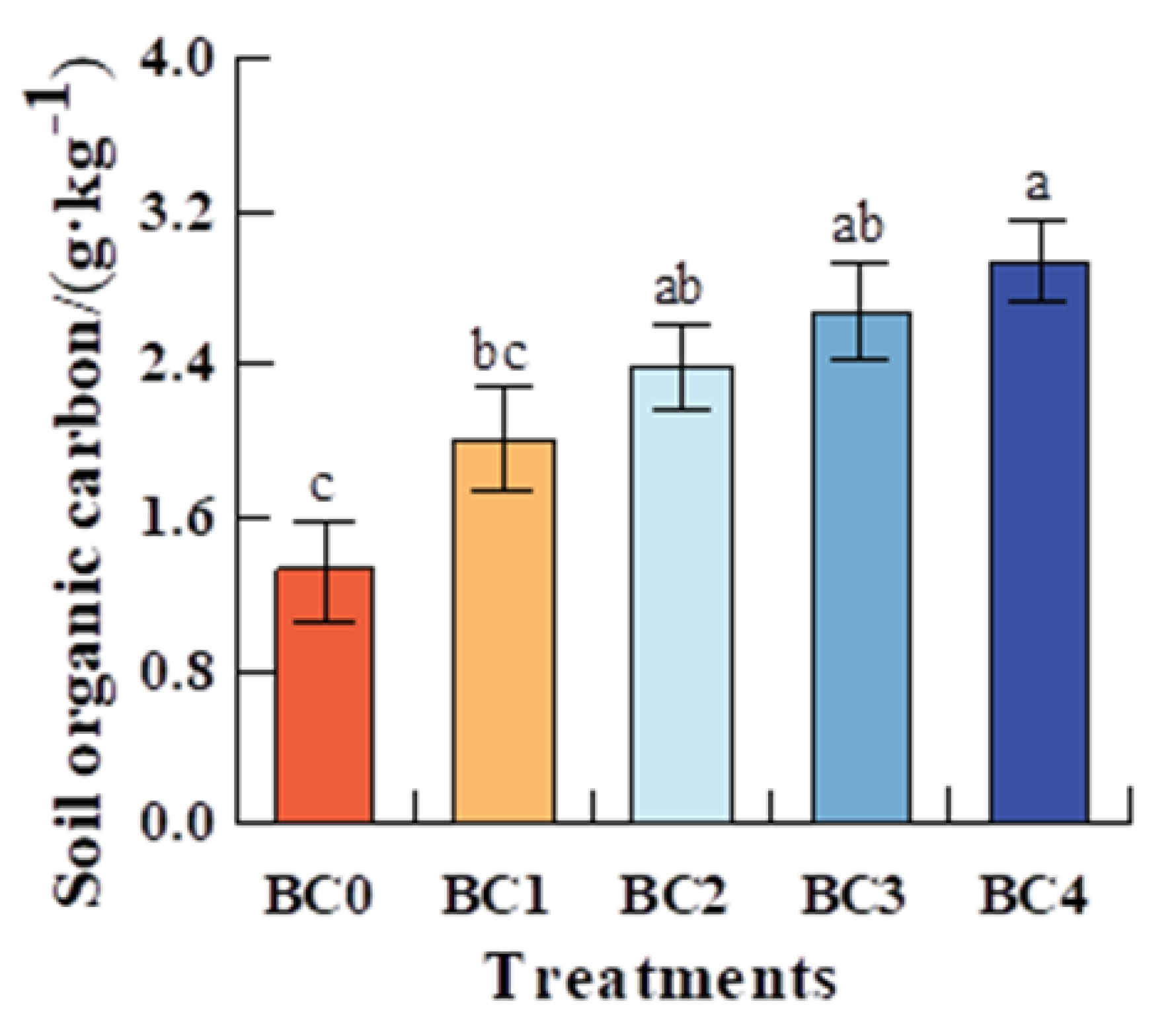
3.1. The Response of Soil Organic C and Soil Labile C to Biochar
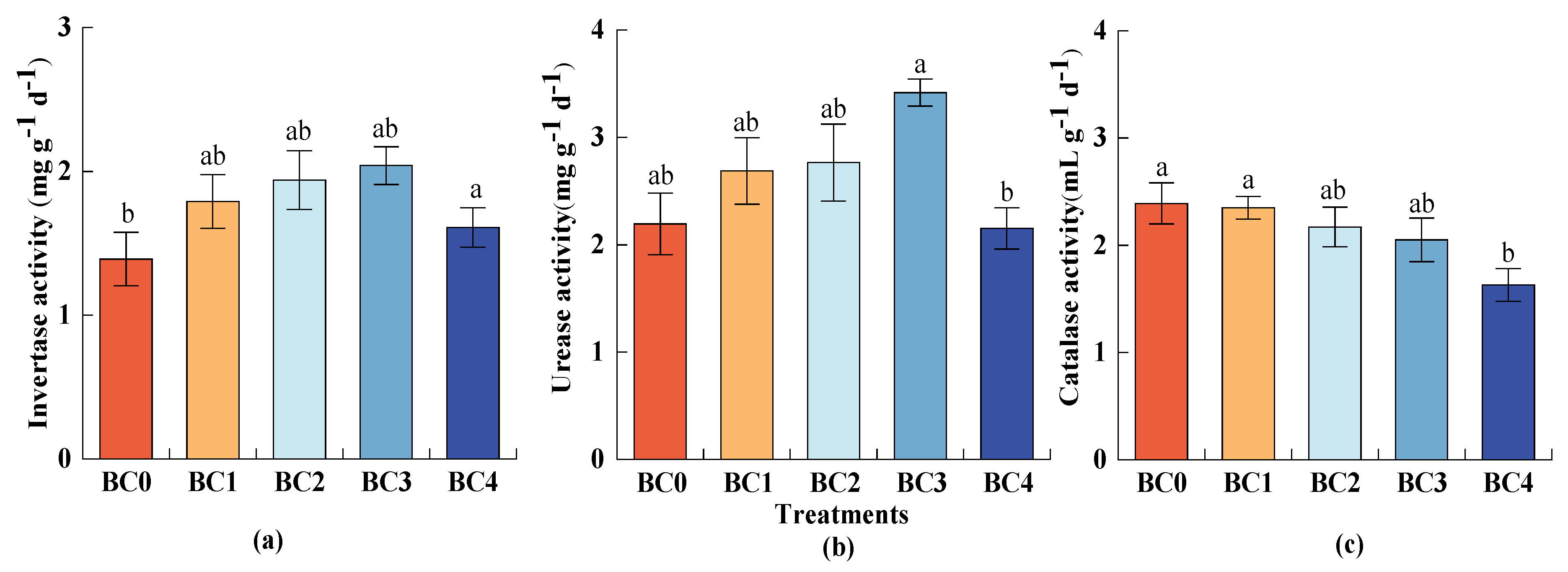
3.2. The Response of Soil Enzyme Activities to Biochar
3.3. Relationship between Organic Carbon and Enzyme Activities
4. Discussion
4.1. Biochar Effects on Carbon Fractions
4.2. Biochar Effects on Enzyme Activities
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhao, H.L.; Zhou, R.L.; Zhang, T.H.; Zhao, X.Y. Effects of Desertification on Soil and Crop Growth Properties in Horqin Sandy Cropland of Inner Mongolia, North China. Soil Tillage Res. 2006, 87, 175–185. [Google Scholar] [CrossRef]
- Khalifa, N.; Yousef, L.F. A Short Report on Changes of Quality Indicators for a Sandy Textured Soil after Treatment with Biochar Produced from Fronds of Date Palm. Energy Procedia 2015, 74, 960–965. [Google Scholar] [CrossRef]
- Brassard, P.; Godbout, S.; Raghavan, V. Soil Biochar Amendment as a Climate Change Mitigation Tool: Key Parameters and Mechanisms Involved. J. Env. Manag. 2016, 181, 484–497. [Google Scholar] [CrossRef] [PubMed]
- Dai, Z.; Zhang, X.; Tang, C.; Muhammad, N.; Wu, J.; Brookes, P.C.; Xu, J. Potential Role of Biochars in Decreasing Soil Acidification—A Critical Review. Sci. Total Env. 2017, 581–582, 601–611. [Google Scholar] [CrossRef] [PubMed]
- Sohi, S.P.; Krull, E.; Lopez-Capel, E.; Bol, R. A Review of Biochar and Its Use and Function in Soil. Adv. Agron. 2010, 105, 47–82. [Google Scholar]
- Lehmann, J.; Gaunt, J.; Rondon, M. Bio-Char Sequestration in Terrestrial Ecosystems—A Review. Mitig. Adapt. Strateg. Glob. Change 2006, 11, 403–427. [Google Scholar] [CrossRef]
- Weber, K.; Quicker, P. Properties of Biochar. Fuel 2018, 217, 240–261. [Google Scholar] [CrossRef]
- Lehmann, J.; Joseph, S. Biochar for Environmental Management: Science, Technology and Implementation; Earthscan: Oxford, UK, 2015; Volume 25, pp. 15801–15811. [Google Scholar]
- Mahmud, A.; Camps-Arbestain, M.; Hedley, M. Investigating the Influence of Biochar Particle Size and Depth of Placement on Nitrous Oxide (N2O) Emissions from Simulated Urine Patches. Agriculture 2018, 8, 175. [Google Scholar] [CrossRef]
- Partey, S.T.; Preziosi, R.F.; Robson, G.D. Short-Term Interactive Effects of Biochar, Green Manure, and Inorganic Fertilizer on Soil Properties and Agronomic Characteristics of Maize. Agric. Res. 2014, 3, 128–136. [Google Scholar] [CrossRef]
- Jaafar, N.M.; Clode, P.L.; Abbott, L.K. Biochar-Soil Interactions in Four Agricultural Soils. Pedosphere 2015, 25, 729–736. [Google Scholar] [CrossRef]
- Pho, K.-H.; Sarshad, M.; Alizadeh, P.; Mahmoudi, M.R. Soil Carbon Pool Changes Following Semi-Arid Lands Planting Programs. CATENA 2020, 191, 104563. [Google Scholar] [CrossRef]
- Baldock, J.A.; Sanderman, J.; Macdonald, L.M.; Puccini, A.; Hawke, B.; Szarvas, S.; McGowan, J. Quantifying the Allocation of Soil Organic Carbon to Biologically Significant Fractions. Soil Res. 2013, 51, 561–576. [Google Scholar] [CrossRef]
- Li, Y.; Li, Y.; Chang, S.X.; Liang, X.; Qin, H.; Chen, J.; Xu, Q. Linking Soil Fungal Community Structure and Function to Soil Organic Carbon Chemical Composition in Intensively Managed Subtropical Bamboo Forests. Soil Biol. Biochem. 2017, 107, 19–31. [Google Scholar] [CrossRef]
- Naik, S.K.; Maurya, S.; Bhatt, B.P. Soil Organic Carbon Stocks and Fractions in Different Orchards of Eastern Plateau and Hill Region of India. Agrofor. Syst. 2016, 91, 541–552. [Google Scholar] [CrossRef]
- Qi, R.; Li, J.; Lin, Z.; Li, Z.; Li, Y.; Yang, X.; Zhang, J.; Zhao, B. Temperature Effects on Soil Organic Carbon, Soil Labile Organic Carbon Fractions, and Soil Enzyme Activities under Long-Term Fertilization Regimes. Appl. Soil Ecol. 2016, 102, 36–45. [Google Scholar] [CrossRef]
- Han, L.; Sun, K.; Yang, Y.; Xia, X.; Li, F.; Yang, Z.; Xing, B. Biochar’s Stability and Effect on the Content, Composition and Turnover of Soil Organic Carbon. Geoderma 2020, 364, 114184. [Google Scholar] [CrossRef]
- Hurni, H.; Giger, M.; Liniger, H.; Mekdaschi Studer, R.; Messerli, P.; Portner, B.; Schwilch, G.; Wolfgramm, B.; Breu, T. Soils, Agriculture and Food Security: The Interplay between Ecosystem Functioning and Human Well-Being. Curr. Opin. Environ. Sustain. 2015, 15, 25–34. [Google Scholar] [CrossRef]
- Gong, W.; Yan, X.; Wang, J.; Hu, T.; Gong, Y. Long-Term Manure and Fertilizer Effects on Soil Organic Matter Fractions and Microbes under a Wheat–Maize Cropping System in Northern China. Geoderma 2009, 149, 318–324. [Google Scholar] [CrossRef]
- Demisie, W.; Liu, Z.; Zhang, M. Effect of Biochar on Carbon Fractions and Enzyme Activity of Red Soil. CATENA 2014, 121, 214–221. [Google Scholar] [CrossRef]
- Lin, Y.; Munroe, P.; Joseph, S.; Henderson, R.; Ziolkowski, A. Water Extractable Organic Carbon in Untreated and Chemical Treated Biochars. Chemosphere 2012, 87, 151–157. [Google Scholar] [CrossRef] [PubMed]
- Garbuz, S.; Camps-Arbestain, M.; Mackay, A.; DeVantier, B.; Minor, M. The Interactions between Biochar and Earthworms, and Their Influence on Soil Properties and Clover Growth: A 6-Month Mesocosm Experiment. Appl. Soil Ecol. 2020, 147, 103402. [Google Scholar] [CrossRef]
- Zhu, X.; Chen, B.; Zhu, L.; Xing, B. Effects and Mechanisms of Biochar-Microbe Interactions in Soil Improvement and Pollution Remediation: A Review. Environ. Pollut. 2017, 227, 98–115. [Google Scholar] [CrossRef]
- Thies, J.E.; Rillig, M.C. Characteristics of biochar: Biological properties. In Biochar for Environmental Management; Routledge: Hoboken, NJ, USA, 2012; pp. 117–138. [Google Scholar]
- Taylor, J.P.; Wilson, B.; Mills, M.S.; Burns, R. G Comparison of Microbial Numbers and Enzymatic Activities in Surface Soils and Subsoils Using Various Techniques. Soil Biol. Biochem. 2002, 34, 387–401. [Google Scholar] [CrossRef]
- Li, Y.-T.; Rouland, C.; Benedetti, M.; Li, F.-b.; Pando, A.; Lavelle, P.; Dai, J. Microbial Biomass, Enzyme and Mineralization Activity in Relation to Soil Organic C, N and P Turnover Influenced by Acid Metal Stress. Soil Biol. Biochem. 2009, 41, 969–977. [Google Scholar] [CrossRef]
- Bailey, V.L.; Fansler, S.J.; Smith, J.L.; Bolton, H. Reconciling Apparent Variability in Effects of Biochar Amendment on Soil Enzyme Activities by Assay Optimization. Soil Biol. Biochem. 2011, 43, 296–301. [Google Scholar] [CrossRef]
- Lehmann, J.; Rillig, M.C.; Thies, J.; Masiello, C.A.; Hockaday, W.C.; Crowley, D. Biochar Effects on Soil Biota—A Review. Soil Biol. Biochem. 2011, 43, 1812–1836. [Google Scholar] [CrossRef]
- Liu, X.; Jiang, N.; Jing, G.; Wang, S.; Chen, Z.; Zhang, Y. Amending Biochar Affected Enzyme Activities and Nitrogen Turnover in Phaeozem and Luvisol. GCB Bioenergy 2023, 15, 954–968. [Google Scholar] [CrossRef]
- Walkley, A.; Black, A.I. An Examination of the Degtjareff Method for Determining Soil Organic Matter, and a Proposed Modification of the Chromic Acid Titration Method. Soil Sci. 1934, 37, 29–38. [Google Scholar] [CrossRef]
- Blair, G.; Lefroy, R.D.B.; Lisle, L. Soil Carbon Fractions Based on Their Degree of Oxidation, and the Development of a Carbon Management Index for Agricultural Systems. Aust. J. Agric. Res. 1995, 46, 1459–1466. [Google Scholar] [CrossRef]
- Jones, D.L.; Willett, V.B. Experimental Evaluation of Methods to Quantify Dissolved Organic Nitrogen (Don) and Dissolved Organic Carbon (Doc) in Soil. Soil Biol. Biochem. 2006, 38, 991–999. [Google Scholar] [CrossRef]
- Bartlett, R.J.; Ross, D.S. Colorimetric Determination of Oxidizable Carbon in Acid Soil Solutions. Soil Sci. Soc. Am. J. 1988, 52, 1191–1192. [Google Scholar] [CrossRef]
- Wu, J.J.; Joergensen, R.G.; Pommerening, B.; Chaussod, R.; Brookes, P.C. Measurement of Soil Microbial Biomass C by Fumigation-Extraction-an Automated Procedure. Soil Biol. Biochem. 1990, 22, 1167–1169. [Google Scholar] [CrossRef]
- Johnson, J.L.; Temple, K.L. Some Variables Affecting the Measurement of “Catalase Activity” in Soil. Soil Sci. Soc. Am. J. 1964, 28, 207–209. [Google Scholar] [CrossRef]
- Kandeler, E.; Gerber, H. Short-Term Assay of Soil Urease Activity Using Colorimetric Determination of Ammonium. Biol. Fertil. Soils 1988, 6, 68–72. [Google Scholar] [CrossRef]
- Gopal, M.; Gupta, A.; Arunachalam, V.; Magu, S.P. Impact of Azadirachtin, an Insecticidal Allelochemical from Neem on Soil Microflora, Enzyme and Respiratory Activities. Bioresour. Technol. 2007, 98, 3154–3158. [Google Scholar] [CrossRef]
- Yu, P.; Liu, S.; Han, K.; Guan, S.; Zhou, D. Conversion of Cropland to Forage Land and Grassland Increases Soil Labile Carbon and Enzyme Activities in Northeastern China. Agric. Ecosyst. Environ. 2017, 245, 83–91. [Google Scholar] [CrossRef]
- Feng, J.; Yu, D.; Sinsabaugh, R.L.; Moorhead, D.L.; Andersen, M.N.; Smith, P.; Song, Y.; Li, X.; Huang, Q.; Liu, Y.R.; et al. Trade-Offs in Carbon-Degrading Enzyme Activities Limit Long-Term Soil Carbon Sequestration with Biochar Addition. Biol. Rev. 2023, 98, 1184–1199. [Google Scholar] [CrossRef]
- Burrell, L.D.; Zehetner, F.; Rampazzo, N.; Wimmer, B.; Soja, G. Long-Term Effects of Biochar on Soil Physical Properties. Geoderma 2016, 282, 96–102. [Google Scholar] [CrossRef]
- Keith, A.; Singh, B.; Dijkstra, F.A. Biochar Reduces the Rhizosphere Priming Effect on Soil Organic Carbon. Soil Biol. Biochem. 2015, 88, 372–379. [Google Scholar] [CrossRef]
- Lu, W.; Ding, W.; Zhang, J.; Li, Y.; Luo, J.; Bolan, N.; Xie, Z. Biochar Suppressed the Decomposition of Organic Carbon in a Cultivated Sandy Loam Soil: A Negative Priming Effect. Soil Biol. Biochem. 2014, 76, 12–21. [Google Scholar] [CrossRef]
- Luo, Y.; Durenkamp, M.; De Nobili, M.; Lin, Q.; Brookes, P.C. Short Term Soil Priming Effects and the Mineralisation of Biochar Following Its Incorporation to Soils of Different Ph. Soil Biol. Biochem. 2011, 43, 2304–2314. [Google Scholar] [CrossRef]
- Qi, L.; Pokharel, P.; Chang, S.X.; Zhou, P.; Niu, H.; He, X.; Wang, Z.; Gao, M. Biochar Application Increased Methane Emission, Soil Carbon Storage and Net Ecosystem Carbon Budget in a 2-Year Vegetable–Rice Rotation. Agric. Ecosyst. Environ. 2020, 292, 106831. [Google Scholar] [CrossRef]
- Yang, S.; Chen, X.; Jiang, Z.; Ding, J.; Sun, X.; Xu, J. Effects of Biochar Application on Soil Organic Carbon Composition and Enzyme Activity in Paddy Soil under Water-Saving Irrigation. Int. J. Environ. Res. Public Health 2020, 17, 333. [Google Scholar] [CrossRef] [PubMed]
- Singh, B.P.; Cowie, A.L. Long-Term Influence of Biochar on Native Organic Carbon Mineralisation in a Low-Carbon Clayey Soil. Sci. Rep. Adv. Agron. 2014, 4, 3687. [Google Scholar] [CrossRef] [PubMed]
- Rahmanian, M.; Khadem, A. The Effects of Biochar on Soil Extra and Intracellular Enzymes Activity. Biomass Convers. Biorefin. 2023. [Google Scholar] [CrossRef]
- Li, S.; Zhang, S.; Pu, Y.; Li, T.; Xu, X.; Jia, Y.; Deng, O.; Gong, G. Dynamics of Soil Labile Organic Carbon Fractions and C-Cycle Enzyme Activities under Straw Mulch in Chengdu Plain. Soil Tillage Res. 2016, 155, 289–297. [Google Scholar] [CrossRef]
- Fang, Y.; Singh, B.P.; Collins, D.; Armstrong, R.; Van Zwieten, L.; Tavakkoli, E. Nutrient Stoichiometry and Labile Carbon Content of Organic Amendments Control Microbial Biomass and Carbon-Use Efficiency in a Poorly Structured Sodic-Subsoil. Biol. Fertil. Soils 2019, 56, 219–233. [Google Scholar] [CrossRef]
- Prommer, J.; Walker, T.W.N.; Wanek, W.; Braun, J.; Zezula, D.; Hu, Y.; Hofhansl, F.; Richter, A. Increased Microbial Growth, Biomass, and Turnover Drive Soil Organic Carbon Accumulation at Higher Plant Diversity. Glob. Chang. Biol. 2020, 26, 669–681. [Google Scholar] [CrossRef]
- Kolb, S.E.; Fermanich, K.J.; Dornbush, M.E. Effect of Charcoal Quantity on Microbial Biomass and Activity in Temperate Soils. Soil Sci. Soc. Am. J. 2009, 73, 1173–1181. [Google Scholar] [CrossRef]
- Khalid, A.R.; Shah, T.; Asad, M.; Ali, A.; Samee, E.; Adnan, F.; Bhatti, M.F.; Marhan, S.; Kammann, C.I.; Haider, G. Biochar Alleviated the Toxic Effects of PVC Microplastic in a Soil-Plant System by Upregulating Soil Enzyme Activities and Microbial Abundance. Environ. Pollut. 2023, 332, 121810. [Google Scholar] [CrossRef] [PubMed]
- Wei, L.; Razavi, B.S.; Wang, W.; Zhu, Z.; Liu, S.; Wu, J.; Kuzyakov, Y.; Ge, T. Labile Carbon Matters More Than Temperature for Enzyme Activity in Paddy Soil. Soil Biol. Biochem. 2019, 135, 134–143. [Google Scholar] [CrossRef]
- Yerli, C. Influences of Farmyard Manure and Its Biochars Prepared at Different Temperatures on Soil Properties and Soil Enzymes and CO2 Emission from Soil under İrrigation Conditions with Treated Wastewater. Water Air Soil Pollut. 2023, 234, 59. [Google Scholar] [CrossRef]
- He, L.; Bi, Y.; Zhao, J.; Pittelkow, C.M.; Zhao, X.; Wang, S.; Xing, G. Population and Community Structure Shifts of Ammonia Oxidizers after Four-Year Successive Biochar Application to Agricultural Acidic and Alkaline Soils. Sci. Total Environ. 2018, 619–620, 1105–1115. [Google Scholar] [CrossRef]
- Czimczik, C.I.; Masiello, C.A. Controls on Black Carbon Storage in Soils. Glob. Biogeochem. Cycles 2007, 21, GB3005. [Google Scholar] [CrossRef]
- Fissore, C.; Giardina, C.P.; Kolka, R.K. Reduced Substrate Supply Limits the Temperature Response of Soil Organic Carbon Decomposition. Soil Biol. Biochem. 2013, 67, 306–311. [Google Scholar] [CrossRef]
- Zhang, L.; Chen, X.; Xu, Y.; Jin, M.; Ye, X.; Gao, H.; Chu, W.; Mao, J.; Thompson, M.L. Soil Labile Organic Carbon Fractions and Soil Enzyme Activities after 10 Years of Continuous Fertilization and Wheat Residue Incorporation. Sci. Rep. 2020, 10, 11318. [Google Scholar] [CrossRef]


| Items | Soil | Biochar |
|---|---|---|
| Sand (>0.05 mm), % | 53.2 | - |
| Silt (0.05–0.002 mm), % | 27.2 | - |
| Clay (<0.002 mm), % | 19.6 | - |
| EC | 66.13 | - |
| pH | 8.51 | 9.90 |
| OC, g·kg−1 | 1.38 | 670 |
| TN, g·kg−1 | 0.76 | - |
| AP, mg·kg−1 | 4.6 | 82.2 |
| AK, mg·kg−1 | 97 | 1590 |
| Soil Labile C | Treatments | Content of Labile C (g·kg−1soil) |
|---|---|---|
| ROC | BC0 | 0.944 ± 0.61 b |
| BC1 | 1.044 ± 0.60 ab | |
| BC2 | 1.197 ± 0.75 ab | |
| BC3 | 1.331 ± 0.26 ab | |
| BC4 | 1.452 ± 0.12 a | |
| DOC | BC0 | 0.137 ± 0.03 a |
| BC1 | 0.138 ± 0.13 a | |
| BC2 | 0.141 ± 0.01 a | |
| BC3 | 0.140 ± 0.01 a | |
| BC4 | 0.139 ± 0.01 a | |
| MBC | BC0 | 0.019 ± 1.2 b |
| BC1 | 0.079 ± 34.05 b | |
| BC2 | 0.101 ± 31.04 ab | |
| BC3 | 0.190 ± 34.07 a | |
| BC4 | 0.088 ± 36.12 b |
| SOC | ROC | DOC | MBC | CAT | INV | URE | |
|---|---|---|---|---|---|---|---|
| SOC | 1 | ||||||
| ROC | 0.969 ** | 1 | |||||
| DOC | −0.121 | −0.330 | 1 | ||||
| MBC | 0.713 | 0.640 | 0.024 | 1 | |||
| CAT | −0.863 | −0.949 * | 0.399 | 0.360 | 1 | ||
| INV | 0.576 | 0.410 | 0.251 | 0.879 * | −0.095 | 1 | |
| URE | 0.321 | 0.200 | 0.177 | 0.879 * | 0.129 | 0.895 * | 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Ma, W.; Sun, X.; Jiang, J.; Li, D.; Tang, G.; Xu, W.; Jia, H. Biochar Aged for Five Years Altered Carbon Fractions and Enzyme Activities of Sandy Soil. Land 2023, 12, 1645. https://doi.org/10.3390/land12081645
Zhang Y, Ma W, Sun X, Jiang J, Li D, Tang G, Xu W, Jia H. Biochar Aged for Five Years Altered Carbon Fractions and Enzyme Activities of Sandy Soil. Land. 2023; 12(8):1645. https://doi.org/10.3390/land12081645
Chicago/Turabian StyleZhang, Yuxin, Wenqi Ma, Xia Sun, Jingbailun Jiang, Dianpeng Li, Guangmu Tang, Wanli Xu, and Hongtao Jia. 2023. "Biochar Aged for Five Years Altered Carbon Fractions and Enzyme Activities of Sandy Soil" Land 12, no. 8: 1645. https://doi.org/10.3390/land12081645
APA StyleZhang, Y., Ma, W., Sun, X., Jiang, J., Li, D., Tang, G., Xu, W., & Jia, H. (2023). Biochar Aged for Five Years Altered Carbon Fractions and Enzyme Activities of Sandy Soil. Land, 12(8), 1645. https://doi.org/10.3390/land12081645






