Compact vs. Linear: Effects of Forest Structure, Patch Shape and Landscape Configuration on Black Alder Macromoth Communities
Abstract
:1. Introduction
2. Material and Methods
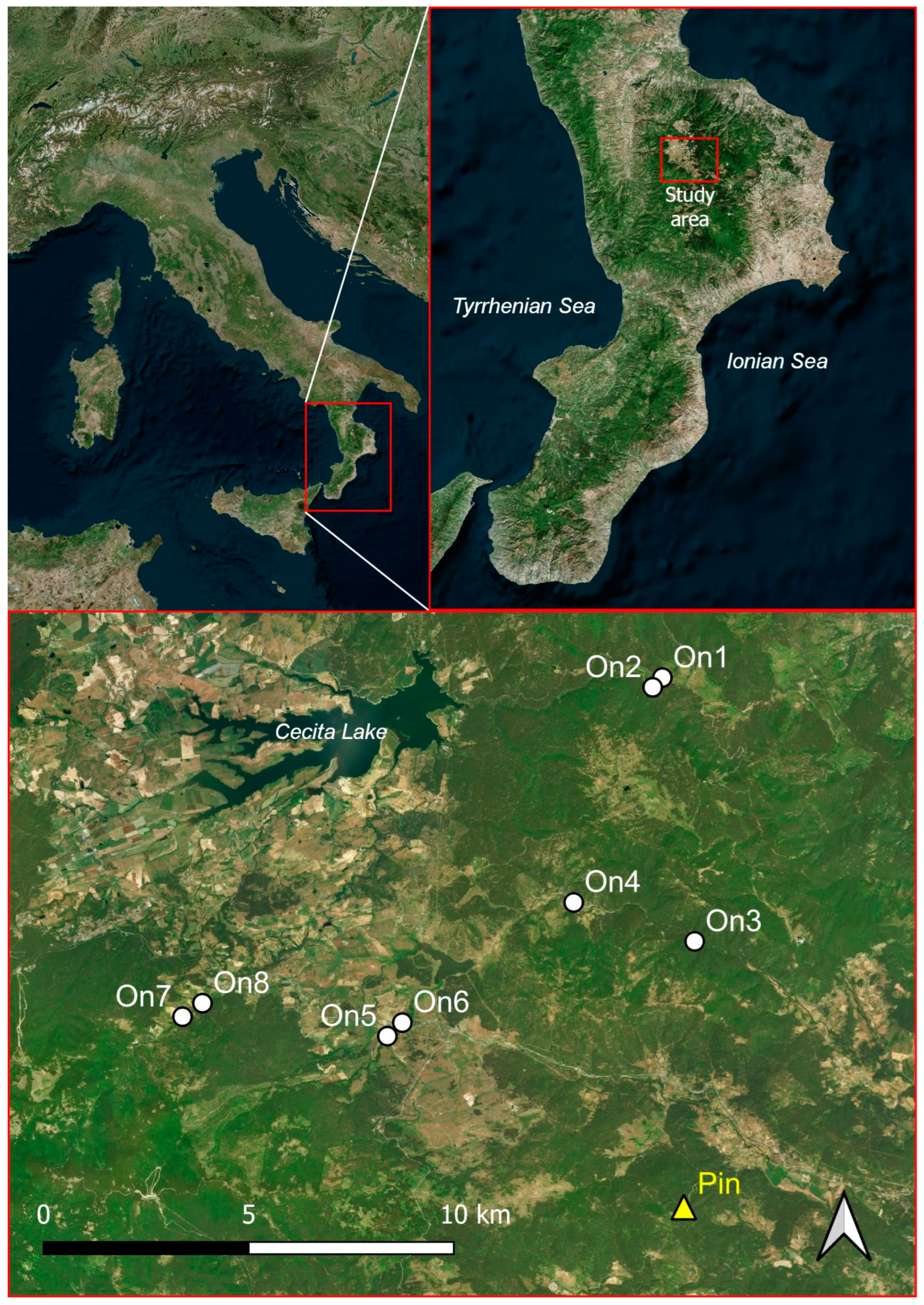
2.1. Study Area
2.2. Moth Sampling
2.3. Forest Structure
2.4. Landscape Configuration
2.5. Moth Analysis
3. Results
3.1. Moth
3.2. Forest Structure
3.3. Landscape Configuration
3.4. Moth–Forest Relationship
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Steffen, W.; Broadgate, W.; Deutsch, L.; Gaffney, O.; Ludwig, C. The trajectory of the Anthropocene: The great acceleration. Anthr. Rev. 2015, 2, 81–98. [Google Scholar]
- Curtis, P.G.; Slay, C.M.; Harris, N.L.; Tyukavina, A.; Hansen, M.C. Classifying drivers of global forest loss. Science 2018, 361, 1108–1111. [Google Scholar] [PubMed]
- Sala, O.E.; Stuart Chapin, F.I.I.I.; Armesto, J.J.; Berlow, E.; Bloomfield, J.; Dirzo, R.; Huber-Sanwald, E.; Huenneke, L.F.; Jackson, R.B.; Kinzig, A.; et al. Global biodiversity scenarios for the year 2100. Science 2000, 287, 1770–1774. [Google Scholar]
- Davies, K.F.; Margules, C.R.; Lawrence, J.F. Which traits of species predict population declines in experimental forest fragments? Ecology 2000, 81, 1450–1461. [Google Scholar]
- Sanderson, E.W.; Jaiteh, M.; Levy, M.A.; Redford, K.H.; Wannebo, A.V.; Woolmer, G. The human footprint and the last of the wild: The human footprint is a global map of human influence on the land surface, which suggests that human beings are stewards of nature, whether we like it or not. BioScience 2002, 52, 891–904. [Google Scholar]
- Fahrig, L. Effects of habitat fragmentation on biodiversity. Annu. Rev. Ecol. Syst. 2003, 34, 487–515. [Google Scholar]
- Foley, J.A.; DeFries, R.; Asner, G.P.; Barford, C.; Bonan, G.; Carpenter, S.R.; Chapin, F.S.; Coe, M.T.; Daily, G.C.; Gibbs, H.K.; et al. Global consequences of land use. Science 2005, 309, 570–574. [Google Scholar]
- Andren, H. Effects of habitat fragmentation on birds and mammals in landscapes with different proportions of suitable habitat: A review. Oikos 1994, 71, 355–366. [Google Scholar]
- Didham, R.K.; Ghazoul, J.; Stork, N.E.; Davis, A.J. Insects in fragmented forests: A functional approach. Trends Ecol. Evol. 1996, 11, 255–260. [Google Scholar]
- Recher, H. The state of Australia’s avifauna: A personal opinion and prediction for the new millennium. Aust. Zool. 1999, 31, 11–27. [Google Scholar]
- Fletcher, R.J., Jr.; Ries, L.; Battin, J.; Chalfoun, A.D. The role of habitat area and edge in fragmented landscapes: Definitively distinct or inevitably intertwined? Can. J. Zool. 2007, 85, 1017–1030. [Google Scholar]
- Yamaura, Y.; Kawahara, T.; Iida, S.; Ozaki, K. Relative importance of the area and shape of patches to the diversity of multiple taxa. Conserv. Biol. 2008, 22, 1513–1522. [Google Scholar]
- Fuentes-Montemayor, E.; Goulson, D.; Cavin, L.; Wallace, J.M.; Park, K.J. Factors influencing moth assemblages in woodland fragments on farmland: Implications for woodland management and creation schemes. Biol. Conserv. 2012, 153, 265–275. [Google Scholar]
- Murcia, C. Edge effects in fragmented forests: Implications for conservation. Trends Ecol. Evol. 1995, 10, 58–62. [Google Scholar]
- Tscharntke, T.; Steffan-Dewenter, I.; Kruess, A.; Thies, C. Characteristics of insect populations on habitat fragments: A mini review. Ecol. Res. 2002, 17, 229–239. [Google Scholar]
- Prugh, L.R.; Hodges, K.E.; Sinclair, A.R.; Brashares, J.S. Effect of habitat area and isolation on fragmented animal populations. Proc. Natl. Acad. Sci. USA 2008, 105, 20770–20775. [Google Scholar]
- Prevedello, J.A.; Vieira, M.V. Does the type of matrix matter? A quantitative review of the evidence. Biodivers. Conserv. 2010, 19, 1205–1223. [Google Scholar]
- Börschig, C.; Klein, A.M.; von Wehrden, H.; Krauss, J. Traits of butterfly communities change from specialist to generalist characteristics with increasing land-use intensity. Basic Appl. Ecol. 2013, 14, 547–554. [Google Scholar]
- Perović, D.; Gámez-Virués, S.; Börschig, C.; Klein, A.M.; Krauss, J.; Steckel, J.; Rothenwöhrer, C.; Erasmi, S.; Tscharntke, T.; Westphal, C. Configurational landscape heterogeneity shapes functional community composition of grassland butterflies. J. Appl. Ecol. 2015, 52, 505–513. [Google Scholar]
- Noss, R.F.; Beier, P. Arguing over little things: Response to Haddad et al. Conserv. Biol. 2000, 14, 1546–1548. [Google Scholar]
- Haila, Y. A conceptual genealogy of fragmentation research: From island biogeography to landscape ecology. Ecol. Appl. 2002, 12, 321–334. [Google Scholar]
- Levey, D.J.; Bolker, B.M.; Tewksbury, J.J.; Sargent, S.; Haddad, N.M. Effects of landscape corridors on seed dispersal by birds. Science 2005, 309, 146–148. [Google Scholar] [CrossRef] [PubMed]
- Gray, R.E.; Slade, E.M.; Chung, A.Y.; Lewis, O.T. Movement of moths through riparian reserves within oil palm plantations. Fron. For. Glob. Chang. 2019, 2, 68. [Google Scholar] [CrossRef]
- Usher, M.B.; Keiller, S.W. The macrolepidoptera of farm woodlands: Determinants of diversity and community structure. Biodivers. Conserv. 1998, 7, 725–748. [Google Scholar] [CrossRef]
- Blasi, C.; Marchetti, M.; Chiavetta, U.; Aleffi, M.; Audisio, P.; Azzella, M.M.; Brunialti, G.; Capotorti, G.; Del Vico, E.; Lattanzi, E.; et al. Multi-taxon and forest structure sampling for identification of indicators and monitoring of old-growth forest. Plant Biosyst. 2010, 144, 160–170. [Google Scholar] [CrossRef]
- Moning, C.; Müller, J. Critical Forest age thresholds for the diversity of lichens, molluscs and birds in beech (Fagus sylvatica L.) dominated forests. Ecol. Indic. 2009, 9, 922–932. [Google Scholar] [CrossRef]
- Ostaff, D.P.; Quiring, D.T. Population trends of a specialist herbivore, the spruce bud moth, in young white spruce stands. Can. Entomol. 2000, 132, 825–842. [Google Scholar] [CrossRef]
- Cherkaoui, I.; Selmi, S.; Boukhriss, J.; Hamid, R.I.; Mohammed, D. Factors affecting bird richness in a fragmented cork oak forest in Morocco. Acta Oecol. 2009, 35, 197–205. [Google Scholar] [CrossRef]
- Ienco, A.; Dapporto, L.; Greco, S.; Infusino, M.; Scalercio, S. β-diversity partitioning of moth communities within and between different forest types. Sci. Nat. 2020, 107, 8. [Google Scholar] [CrossRef]
- Sagar, R.; Singh, J.S. Tree density, basal area and species diversity in a disturbed dry tropical forest of northern India: Implications for conservation. Environ. Conserv. 2006, 33, 256–262. [Google Scholar] [CrossRef]
- Allan, J.D.; Flecker, A.S. Biodiversity conservation in running waters. BioScience 1993, 43, 32–43. [Google Scholar] [CrossRef]
- Tewksbury, J.J.; Black, A.E.; Nur, N.A.D.A.V.; Saab, V.A.; Logan, B.D.; Dobkin, D.S. Effects of anthropogenic fragmentation and livestock grazing on western riparian bird communities. Stud. Avian Biol. 2002, 25, 158–202. [Google Scholar]
- Jansson, R.; Nilsson, C.; Renöfält, B. Fragmentation of riparian floras in rivers with multiple dams. Ecology 2000, 81, 899–903. [Google Scholar] [CrossRef]
- Collinge, S.K.; Holyoak, M.; Barr, C.B.; Marty, J.T. Riparian habitat fragmentation and population persistence of the threatened valley elderberry longhorn beetle in central California. Biol. Conserv. 2001, 100, 103–113. [Google Scholar] [CrossRef]
- Lionello, P.; Abrantes, F.; Gacic, M.; Planton, S.; Trigo, R.; Ulbrich, U. The climate of the Mediterranean region: Research progress and climate change impacts. Reg. Environ. Chang. 2014, 14, 1679–1684. [Google Scholar] [CrossRef]
- Gibbons, J.W.; Scott, D.E.; Ryan, T.J.; Buhlmann, K.A.; Tuberville, T.D.; Metts, B.S.; Greene, L.J.; Mills, T.; Leiden, Y.; Poppy, S.; et al. The Global Decline of Reptiles, Déjà Vu Amphibians: Reptile species are declining on a global scale. Six significant threats to reptile populations are habitat loss and degradation, introduced invasive species, environmental pollution, disease, unsustainable use, and global climate change. BioScience 2000, 50, 653–666. [Google Scholar]
- Stuart, S.N.; Chanson, J.S.; Cox, N.A.; Young, B.E.; Rodrigues, A.S.; Fischman, D.L.; Waller, R.W. Status and trends of amphibian declines and extinctions worldwide. Science 2004, 306, 1783–1786. [Google Scholar] [CrossRef]
- Kitching, R.L.; Orr, A.G.; Thalib, L.; Mitchell, H.; Hopkins, M.S.; Graham, A.W. Moth assemblages as indicators of environmental quality in remnants of upland Australian rain forest. J. Appl. Ecol. 2000, 37, 284–297. [Google Scholar] [CrossRef]
- Jennings, N.; Pocock, M.J. Relationships between sensitivity to agricultural intensification and ecological traits of insectivorous mammals and arthropods. Conserv. Biol. 2009, 23, 1195–1203. [Google Scholar] [CrossRef]
- An, J.S.; Choi, S.W. Forest moth assemblages as indicators of biodiversity and environmental quality in a temperate deciduous forest. Eur. J. Entomol. 2013, 110, 509. [Google Scholar] [CrossRef]
- Greco, S.; Infusino, M.; Ienco, A.; Scalercio, S. How different management regimes of chestnut forests affect diversity and abundance of moth communities. Ann. Silvic. Res. 2018, 42, 59–67. [Google Scholar]
- Waring, P. Moth Conservation Project News Bulletin 2; Nature Conservancy Council: Peterborough, ON, Canada, 1989. [Google Scholar]
- Truxa, C.; Fiedler, K. Down in the flood? How moth communities are shaped in temperate floodplain forests. Insect Conserv. Diver. 2012, 5, 389–397. [Google Scholar] [CrossRef]
- Parenzan, P. Macrolepidotterofauna della Riserva Naturale WWF “Lago di Pignola”. Basilicata, Potenza. Contributi alla conoscenza della Lepidotterofauna dell’Italia meridionale. XXII. Phytophaga 2002, 12, 43–116. [Google Scholar]
- Scalercio, S.; Infusino, M. I Macrolepidotteri di fosso Scuotrapiti, lago dell’Angitola (Calabria, Italia meridionale) (Lepidoptera). Phytophaga 2003, 13, 25–52. [Google Scholar]
- Dapporto, L.; Fabiano, F.; Balderi, F. I macrolepidotteri della Val di Farma (Toscana). Aldrovandia 2005, 1, 37–54. [Google Scholar]
- Leonetti, F.L.; Greco, S.; Ienco, A.; Scalercio, S. Lepidopterological fauna of Alnus glutinosa (L.) Gaertn., forests in the Sila Massif (southern Italy) (Insecta: Lepidoptera). SHILAP-Rev. Lepidopt 2019, 47, 535–556. [Google Scholar]
- Scalercio, S. Nocturnal macrolepidoptera associated to cork oak woodlands and neighbour forest remnants in a fragmented Mediterranean landscape (Lepidoptera). Redia 2022, 105, 131–139. [Google Scholar] [CrossRef]
- Scalercio, S.; Russo, M.; Dapporto, L. Wetlands are refuge areas that delay global warming-induced range shift of Lepidoptera. In Handbook of Nature Conservation: Global, Environmental and Economic Issues; Aronoff, J.B., Ed.; Nova Publishers: New York, NY, USA, 2009; pp. 393–406. [Google Scholar]
- Scarciglia, F.; Marsico, A.; Capolongo, D. Weathering processes and rates of scarp retreat in a Mediterranean mountain environment: A multi-analytical approach from terrestrial laser scanning to microscale surveys. Geomorphology 2022, 413, 108356. [Google Scholar] [CrossRef]
- Moser, D.; Di Pasquale, G.; Scarciglia, F.; Nelle, O. Holocene mountain forest changes in central Mediterranean: Soil charcoal data from the Sila Massif (Calabria, southern Italy). Quatern. Int. 2017, 457, 113–130. [Google Scholar] [CrossRef]
- Govi, G.; Fiumi, G.; Barbut, J.; Scalercio, S.; Hausmann, A. An unexpected species complex unveiled in southern European populations of Phragmatiphila nexa (Hübner,[1808]) (Lepidoptera, Noctuidae, Noctuinae, Apameini). Zootaxa 2022, 5128, 355–383. [Google Scholar] [CrossRef]
- Huemer, P.; Haxaire, J.; Lee, K.M.; Mutanen, M.; Pekarsky, O.; Scalercio, S.; Ronkay, L. Revision of the genus Hoplodrina Boursin, 1937 (Lepidoptera, Noctuidae, Xyleninae). I. Hoplodrina octogenaria (Goeze, 1781) and its sister species H. alsinides (Costantini, 1922) sp. rev. in Europe. ZooKeys 2020, 927, 75. [Google Scholar]
- Lachat, T.; Wermelinger, B.; Gossner, M.M.; Bussler, H.; Isacsson, G.; Müller, J. Saproxylic beetles as indicator species for dead-wood amount and temperature in European beech forests. Ecol. Indic. 2012, 23, 323–331. [Google Scholar] [CrossRef]
- De Zan, L.R.; Bellotti, F.; D’Amato, D.; Carpaneto, G.M. Saproxylic beetles in three relict beech forests of central Italy: Analysis of environmental parameters and implications for forest management. Forest Ecol. Manag. 2014, 328, 229–244. [Google Scholar] [CrossRef]
- Castellani, C. Tavole stereometriche e alsometriche costruite per i boschi italiani. Ann. Ist. Sper. Per L’assestamento For. E L’alpicoltura 1970, 1, 1–750. [Google Scholar]
- Merckx, T.; Slade, E.M. Macro-moth families differ in their attraction to light: Implications for light-trap monitoring programmes. Insect Conserv. Diver. 2014, 7, 453–461. [Google Scholar] [CrossRef]
- Slade, E.M.; Merckx, T.; Riutta, T.; Bebber, D.P.; Redhead, D.; Riordan, P.; Macdonald, D.W. Life-history traits and landscape characteristics predict macro-moth responses to forest fragmentation. Ecology 2013, 94, 1519–1530. [Google Scholar] [CrossRef]
- McGarigal, K.; Cushman, S.A.; Neel, M.C.; Ene, E. Spatial Pattern Analysis Program for Categorical Maps. 2002. Available online: www.umass.edu/landeco/research/fragstats/fragstats.html. (accessed on 27 July 2023).
- Hammer, Ø.; Harper, D.A. Past: Paleontological statistics software package for educaton and data anlysis. Palaeontol. Electron. 2001, 4, 1. [Google Scholar]
- Almeida-Neto, M.; Guimaraes, P.; Guimaraes, P.R., Jr.; Loyola, R.D.; Ulrich, W.A. Consistent metric for nestedness analysis in ecological systems: Reconciling concept and measurement. Oikos 2008, 117, 1227–1239. [Google Scholar] [CrossRef]
- Strona, G.; Galli, P.; Seveso, D.; Montano, S.; Fattorini, S. Nestedness for Dummies (NeD): A user-friendly web interface for exploratory nestedness analysis. J. Stat. Softw. 2014, 59, 1–9. [Google Scholar] [CrossRef]
- Ienco, A.; Greco, S.; Scalercio, S. Is the “best season” the best choice? Temporal variations of β-diversity patterns in moth communities of Calabrian black pine forests. Ecol. Indic. 2020, 112, 106134. [Google Scholar] [CrossRef]
- Littlewood, N.A. Grazing impacts on moth diversity and abundance on a Scottish upland estate. Insect Conserv. Diver. 2008, 1, 151–160. [Google Scholar] [CrossRef]
- Enkhtur, K.; Brehm, G.; Boldgiv, B.; Pfeiffer, M. Effects of grazing on macro-moth assemblages in two different biomes in Mongolia. Ecol. Indic. 2021, 133, 108421. [Google Scholar] [CrossRef]
- Scalercio, S. On top of a Mediterranean Massif: Climate change and conservation of orophilous moths at the southern boundary of their range (Lepidoptera: Macroheterocera). Eur. J. Entomol. 2009, 106, 231. [Google Scholar] [CrossRef]
- Mönkkönen, M.; Mutanen, M. Occurrence of moths in boreal forest corridors. Conserv. Biol. 2003, 17, 468–475. [Google Scholar] [CrossRef]
- Woodroffe, R.; Ginsberg, J.R. Edge effects and the extinction of populations inside protected areas. Science 1998, 280, 2126–2128. [Google Scholar] [CrossRef]
- Ries, L.; Fletcher, R.J.; Battin, J.; Sisk, T.D. Ecological responses to habitat edges: Mechanisms, models, and variability explained. Annu. Rev. Ecol. Evol. Syst. 2004, 35, 491–522. [Google Scholar] [CrossRef]
- Lassauce, A.; Larrieu, L.; Paillet, Y.; Lieutier, F.; Bouget, C. The effects of forest age on saproxylic beetle biodiversity: Implications of shortened and extended rotation lengths in a French oak high forest. Insect. Conserv. Diver. 2013, 6, 396–410. [Google Scholar] [CrossRef]
- Bongers, F.J.; Schmid, B.; Bruelheide, H.; Bongers, F.; Li, S.; von Oheimb, G.; Li, Y.; Cheng, A.; Ma, K.; Liu, X. Functional diversity effects on productivity increase with age in a forest biodiversity experiment. Nat. Ecol. Evol. 2021, 5, 1594–1603. [Google Scholar] [CrossRef]
- Wirth, C.; Messier, C.; Bergeron, Y.; Frank, D.; Fankhänel, A. Old-growth forest definitions: A pragmatic view. In Old-Growth Forests: Function, Fate and Value; Wirth, C., Gleixner, G., Heimann, M., Eds.; Springer: Berlin/Heidelberg, Germany, 2009; pp. 11–33. [Google Scholar]
- De la Montana, E.; Rey-Benayas, J.M.; Carrascal, L.M. Response of bird communities to silvicultural thinning of Mediterranean maquis. J. Appl. Ecol. 2006, 43, 651–659. [Google Scholar] [CrossRef]
- Taki, H.; Inoue, T.; Tanaka, H.; Makihara, H.; Sueyoshi, M.; Isono, M.; Okabe, K. Responses of community structure, diversity, and abundance of understory plants and insect assemblages to thinning in plantations. Forest Ecol. Manag. 2010, 259, 607–613. [Google Scholar] [CrossRef]
- Fuentes-Montemayor, E.; Watts, K.; Sansum, P.; Scott, W.; Park, K.J. Moth community responses to woodland creation: The influence of woodland age, patch characteristics and landscape attributes. Divers. Distrib. 2022, 28, 1993–2007. [Google Scholar] [CrossRef]
- Mӧlder, A.; Bernhardt-Rӧmermann, M.; Schmidt, W. Herb-layer diversity in deciduous forests: Raised by tree richness or beaten by beech? Forest Ecol. Manag. 2008, 256, 272–281. [Google Scholar] [CrossRef]
- Wulf, M.; Naaf, T. Herb layer response to broadleaf tree species with different leaf litter quality and canopy structure in temperate forests. J. Veg. Sci. 2009, 20, 517–526. [Google Scholar] [CrossRef]
- Sercu, B.K.; Baeten, L.; van Coillie, F.; Martel, A.; Lens, L.; Verheyen, K.; Bonte, D. How tree species identity and diversity affect light transmittance to the understory in mature temperate forests. Ecol Evol. 2017, 7, 10861–10870. [Google Scholar] [CrossRef] [PubMed]
- Jung, T.S.; Thompson, I.D.; Titman, R.D.; Applejohn, A.P. Habitat selection by forest bats in relation to mixed-wood stand types and structure in central Ontario. J. Wildlife Manag. 1999, 63, 1306–1319. [Google Scholar] [CrossRef]
- Kalcounis, M.C.; Hobson, K.A.; Brigham, R.M.; Hecker, K.R. Bat activity in the boreal forest: Importance of stand type and vertical strata. J. Mammal. 1999, 80, 673–682. [Google Scholar] [CrossRef]
- Blakey, R.V.; Law, B.S.; Kingsford, R.T.; Stoklosa, J. Terrestrial laser scanning reveals below-canopy bat trait relationships with forest structure. Remote Sens. Environ. 2017, 198, 40–51. [Google Scholar] [CrossRef]
- Archaux, F.; Lorel, C.; Villemey, A. Landscape drivers of butterfly and burnet moth diversity in lowland rural areas. Landscape Ecol. 2018, 33, 1725–1739. [Google Scholar] [CrossRef]
- Bergman, K.O.; Dániel-Ferreira, J.; Milberg, P.; Öckinger, E.; Westerberg, L. Butterflies in Swedish grasslands benefit from forest and respond to landscape composition at different spatial scales. Landscape Ecol. 2018, 33, 2189–2204. [Google Scholar] [CrossRef]
- Scalercio, S.; Brandmayr, P.; Iannotta, N.; Petacchi, R.; Boccaccio, L. Correlations between landscape attributes and ecological traits of Lepidoptera communities in olive groves. Eur. J. Entomol. 2012, 109, 207–216. [Google Scholar] [CrossRef]
- Claessens, H.; Oosterbaan, A.; Savill, P.; Rondeux, J. A review of the characteristics of black alder (Alnus glutinosa (L.) Gaertn.) and their implications for silvicultural practices. Forestry 2010, 83, 163–175. [Google Scholar] [CrossRef]
- Kuglerová, L.; Jyväsjärvi, J.; Ruffing, C.; Muotka, T.; Jonsson, A.; Andersson, E.; Richardson, J.S. Cutting edge: A comparison of contemporary practices of riparian buffer retention around small streams in Canada, Finland, and Sweden. Water Resour. Res. 2020, 56, e2019WR026381. [Google Scholar] [CrossRef]


| S | N | H | J | α | |
|---|---|---|---|---|---|
| Linear woodlots | |||||
| On1 | 131 | 663 | 4.07 | 0.83 | 48.92 |
| On4 | 86 | 359 | 3.62 | 0.81 | 35.84 |
| On6 | 137 | 635 | 4.35 | 0.88 | 53.69 |
| On8 | 131 | 669 | 4.16 | 0.85 | 48.69 |
| Compact woodlots | |||||
| On2 | 157 | 1179 | 4.17 | 0.82 | 48.63 |
| On3 | 151 | 1140 | 4.05 | 0.81 | 46.66 |
| On5 | 151 | 849 | 4.23 | 0.84 | 53.41 |
| On7 | 147 | 704 | 4.36 | 0.87 | 56.57 |
| Alder Woodlots Linear vs. Compact | NODF | Nested | |
|---|---|---|---|
| On1 vs. On2 | 49.344 | NO | p > 0.05 |
| On4 vs. On3 | 45.554 | YES | p < 0.001 |
| On6 vs. On5 | 50.064 | NO | p > 0.05 |
| On8 vs. On7 | 50.177 | NO | p > 0.05 |
| Species | Linear Communities (N = 5) | Compact Communities (N = 3) | |||
|---|---|---|---|---|---|
| N | Rank | N | Rank | ||
| Non-characteristic species | |||||
| Orthosia (Orthosia) incerta | 229 | 1 | 103 | 4 | |
| Hoplodrina cfr. octogenaria | 166 | 2 | 62 | 9 | |
| Peribatodes rhomboidaria | 67 | 8 | 52 | 13 | |
| Epirrhoe alternata | 65 | 9 | 23 | 34 | |
| Pachetra sagittigera | 61 | 10 | 25 | 32 | |
| Lycia hirtaria | 48 | 14 | 145 | 3 | |
| Characteristic species for linear woodlots | |||||
| Agrotis cinerea | 145 | 3 | 21 | 36 | |
| Luperina testacea | 105 | 4 | 14 | 49 | |
| Luperina dumerilii | 82 | 5 | 4 | 105 | |
| Mythimna (Mythimna) impura | 79 | 6 | 16 | 44 | |
| Agrotis exclamationis | 76 | 7 | 8 | 43 | |
| Characteristic species for compact woodlots | |||||
| Eilema lurideola | 19 | 44 | 289 | 1 | |
| Eilema complana | 25 | 31 | 221 | 2 | |
| Lithosia quadra | 5 | 106 | 96 | 5 | |
| Xestia (Megasema) triangulum | 14 | 59 | 81 | 6 | |
| Diarsia mendica | 20 | 43 | 64 | 7 | |
| Dysstroma truncata | 2 | 155 | 64 | 8 | |
| Alcis repandata | 10 | 73 | 60 | 10 | |
| Shape of Alder Woodlots | Linear | Compact | ||||||
|---|---|---|---|---|---|---|---|---|
| Woodlot | On1 | On4 | On6 | On8 | On2 | On3 | On5 | On7 |
| All tree species | ||||||||
| Heightmean (m) | 35.9 ± 3.9 | 19.6 ± 1.9 | 18.9 ± 3.2 | 14.1 ± 4.7 | 20.6 ± 1.7 | 21.8 ± 3.6 | 16.2 ± 3.7 | 23.1 ± 2.6 |
| Estimated age (y) | 46 | 45 | 31 | 35 | 26 | 33 | 23 | 34 |
| Density (n/ha) | 589 | 287 | 597 | 342 | 835 | 916 | 1989 | 995 |
| DBHmean (cm) | 41.0 ± 8.9 | 41.2 ± 14.5 | 25.1 ± 12.1 | 32.3 ± 16.6 | 21.8 ± 11.2 | 27.2 ± 10.0 | 17.6 ± 6.4 | 28.0 ± 7.5 |
| BAtot (m2/ha) | 77.7 | 36.8 | 29.6 | 28.0 | 31.2 | 53.3 | 48.3 | 61.3 |
| Vtot (m3/ha) | 745.3 | 369.0 | 268.6 | 269.1 | 270.6 | 479.8 | 378.6 | 558.2 |
| Alders total | ||||||||
| Density (n/ha) | 589 | 279 | 597 | 326 | 716 | 836 | 1989 | 995 |
| DBHmean (cm) | 41.0 ± 8.9 | 40.2 ± 13.7 | 25.1 ± 12.1 | 29.9 ± 13.7 | 20.4 ± 10.6 | 27.9 ± 8.9 | 17.6 ± 6.4 | 28.0 ± 7.5 |
| BAtot (m2/ha) | 77.7 | 35.3 | 29.6 | 23.0 | 23.4 | 51.0 | 48.3 | 61.3 |
| Vtot (m3/ha) | 745.3 | 338.1 | 268.6 | 214.3 | 196.5 | 455.2 | 378.6 | 558.2 |
| Alive alders | ||||||||
| Density (n/ha) | 557 | 279 | 597 | 326 | 716 | 820 | 1870 | 971 |
| DBHmean (cm) | 41.5 ± 8.6 | 40.2 ± 13.7 | 25.1 ± 12.1 | 29.9 ± 13.7 | 20.4 ± 10.6 | 28.0 ± 8.9 | 17.9 ± 6.5 | 28.1 ± 7.4 |
| BAtot (m2/ha) | 75.2 | 35.3 | 29.6 | 23.0 | 23.4 | 50.6 | 47.0 | 60.2 |
| Vtot (m3/ha) | 720.7 | 338.1 | 268.5 | 214.3 | 196.5 | 450.9 | 371.2 | 549.1 |
| Dead alders | ||||||||
| Density (n/ha) | 32 | 0 | 0 | 0 | 0 | 16 | 119 | 24 |
| DBHmean (cm) | 31.5 ± 8.9 | 0 | 0 | 0 | 0 | 19.0 ± 6.4 | 11.6 ± 2.7 | 24.1 ± 11.0 |
| BAtot (m2/ha) | 2.47 | 0 | 0 | 0 | 0 | 0.45 | 1.27 | 1.09 |
| Vtot (m3/ha) | 24.6 | 0 | 0 | 0 | 0 | 4.3 | 7.4 | 9.1 |
| Other trees | ||||||||
| Density (n/ha) | 0 | 8 | 0 | 16 | 119 | 80 | 0 | 0 |
| DBHmean (cm) | 0 | 69.0 ± 0.0 | 0 | 63.5 ± 5.4 | 28.9 ± 12.7 | 19.2 ± 14.1 | 0 | 0 |
| BAtot (m2/ha) | 0 | 1.6 | 0 | 5.0 | 7.9 | 2.3 | 0 | 0 |
| Vtot (m3/ha) | 0 | 30.9 | 0 | 54.8 | 74.2 | 19.6 | 0 | 0 |
| Shape of Alder Woodlots | Linear | Compact | ||||||
|---|---|---|---|---|---|---|---|---|
| Woodlot | On1 | On4 | On6 | On8 | On2 | On3 | On5 | On7 |
| Landscape composition | ||||||||
| Grassland cover (%) | ||||||||
| buffer_25 | 14 | 46 | 46 | 59 | 0 | 0 | 3 | 0 |
| buffer_50 | 50 | 68 | 68 | 77 | 0 | 0 | 40 | 0 |
| buffer_200 | 62 | 62 | 92 | 58 | 9 | 1 | 82 | 29 |
| buffer_500 | 40 | 36 | 92 | 53 | 19 | 0 | 92 | 51 |
| buffer_1000 | 24 | 27 | 92 | 40 | 18 | 1 | 86 | 32 |
| Alder forest cover (%) | ||||||||
| buffer_25 | 86 | 54 | 54 | 41 | 100 | 100 | 97 | 100 |
| buffer_50 | 50 | 31 | 32 | 23 | 100 | 84 | 60 | 74 |
| buffer_200 | 7 | 6 | 8 | 9 | 32 | 22 | 18 | 7 |
| buffer_500 | 6 | 4 | 6 | 3 | 7 | 7 | 7 | 3 |
| buffer_1000 | 2 | 1 | 3 | 2 | 2 | 3 | 5 | 1 |
| Total forest cover (%) | ||||||||
| buffer_200 | 38 | 38 | 8 | 42 | 91 | 99 | 18 | 71 |
| buffer_500 | 60 | 64 | 8 | 47 | 80 | 100 | 8 | 49 |
| buffer_1000 | 76 | 73 | 8 | 60 | 82 | 99 | 14 | 68 |
| Ecotone forests/grasslands (m) | ||||||||
| buffer_50 | 205 | 210 | 230 | 242 | 0 | 0 | 196 | 0 |
| buffer_200 | 1490 | 1584 | 739 | 1453 | 915 | 225 | 1647 | 1292 |
| buffer_500 | 5697 | 7731 | 4267 | 6097 | 3496 | 375 | 4236 | 7176 |
| buffer_1000 | 1365 | 17,436 | 13,220 | 22,135 | 10,833 | 1524 | 22,642 | 17,279 |
| CA Axis 2 | S | N | a | |
|---|---|---|---|---|
| Forest total | ||||
| Estimated age (y) | N.S. | −0.754 * | N.S. | N.S. |
| Density (n/ha) | −0.765 * | N.S. | N.S. | N.S. |
| DBHmean (cm) | N.S. | −0.784 * | N.S. | N.S. |
| Alders total | ||||
| DBHmean (cm) | N.S. | −0.761 * | N.S. | N.S. |
| Alive alders | ||||
| DBHmean (cm) | N.S. | −0.754 * | N.S. | N.S. |
| Other trees | ||||
| Density (n/ha) | −0.821 * | N.S. | 0.825 * | N.S. |
| DBHmean (cm) | N.S. | N.S. | N.S. | −0.774 * |
| CA Axis 2 | N | J | |
|---|---|---|---|
| Grasslands cover (%) | |||
| buffer_25 | 0.719 * | N.S. | N.S. |
| buffer_50 | 0.898 ** | −0.760 * | N.S. |
| buffer_200 | 0.932 *** | N.S. | N.S. |
| buffer_500 | 0.786 * | N.S. | 0.732 * |
| Alder forest cover (%) | |||
| buffer_25 | −0.719 * | N.S. | N.S. |
| buffer_50 | −0.893 ** | 0.847 ** | N.S. |
| buffer_200 | N.S. | 0.910 ** | N.S. |
| Forest cover (%) | |||
| buffer_200 | −0.932 *** | N.S. | N.S. |
| buffer_500 | −0.784 * | N.S. | −0.732 * |
| Ecotone forests/grasslands (m) | |||
| buffer_50 | 0.928 *** | −0.709 * | N.S. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
La Cava, S.; Lombardo, M.; Bernardini, V.; Fumo, F.; Rijllo, G.; Turco, R.; Bevacqua, L.; Zucco, G.; Scalercio, S. Compact vs. Linear: Effects of Forest Structure, Patch Shape and Landscape Configuration on Black Alder Macromoth Communities. Land 2023, 12, 1670. https://doi.org/10.3390/land12091670
La Cava S, Lombardo M, Bernardini V, Fumo F, Rijllo G, Turco R, Bevacqua L, Zucco G, Scalercio S. Compact vs. Linear: Effects of Forest Structure, Patch Shape and Landscape Configuration on Black Alder Macromoth Communities. Land. 2023; 12(9):1670. https://doi.org/10.3390/land12091670
Chicago/Turabian StyleLa Cava, Sara, Margherita Lombardo, Vincenzo Bernardini, Federica Fumo, Giuseppe Rijllo, Rosario Turco, Laura Bevacqua, Giada Zucco, and Stefano Scalercio. 2023. "Compact vs. Linear: Effects of Forest Structure, Patch Shape and Landscape Configuration on Black Alder Macromoth Communities" Land 12, no. 9: 1670. https://doi.org/10.3390/land12091670






