Striped Field Mouse Invading Human-Modified Environments of Lithuania during Last Five Decades
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Site and Habitats
2.2. Small Mammal Trapping
2.3. Study Periods and Sample Size
2.4. Case Study Sites
2.4.1. Site 1: Žagarė Forest and Žagarė Regional Park
2.4.2. Site 2: Vicinity of Biržų Giria Forest
2.4.3. Site 3: Dzūkija National Park
2.4.4. Site 4: Ignalina Region
2.4.5. Site 5: Žemaitija National Park
2.4.6. Site 6: Lipliūnai Environs
2.4.7. Site 7: Zarasai Region
2.4.8. Site 8: Rusnė Environs
2.5. Data Analyses
3. Results
3.1. Temporal Changes in Striped Field Mouse Population
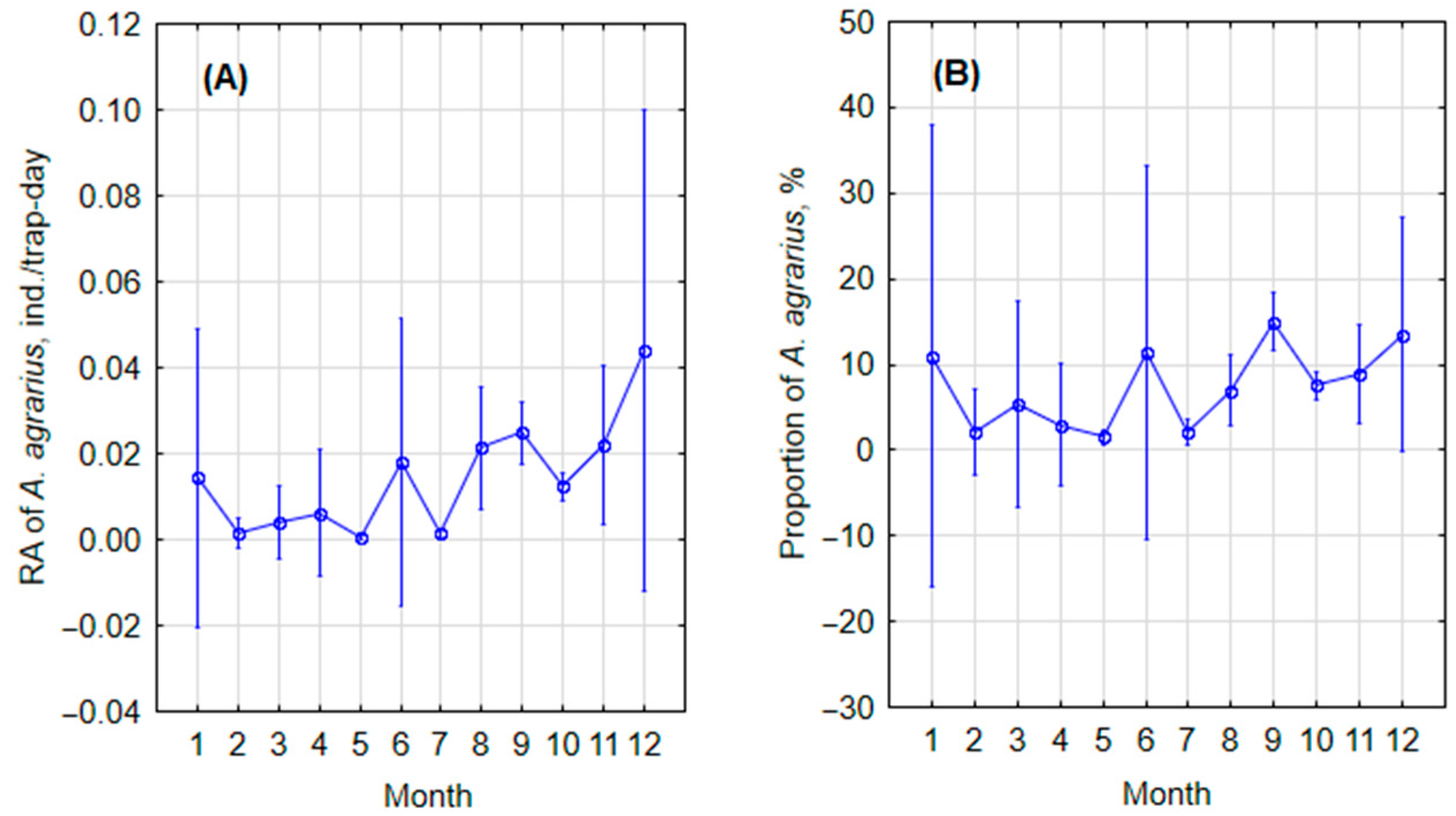
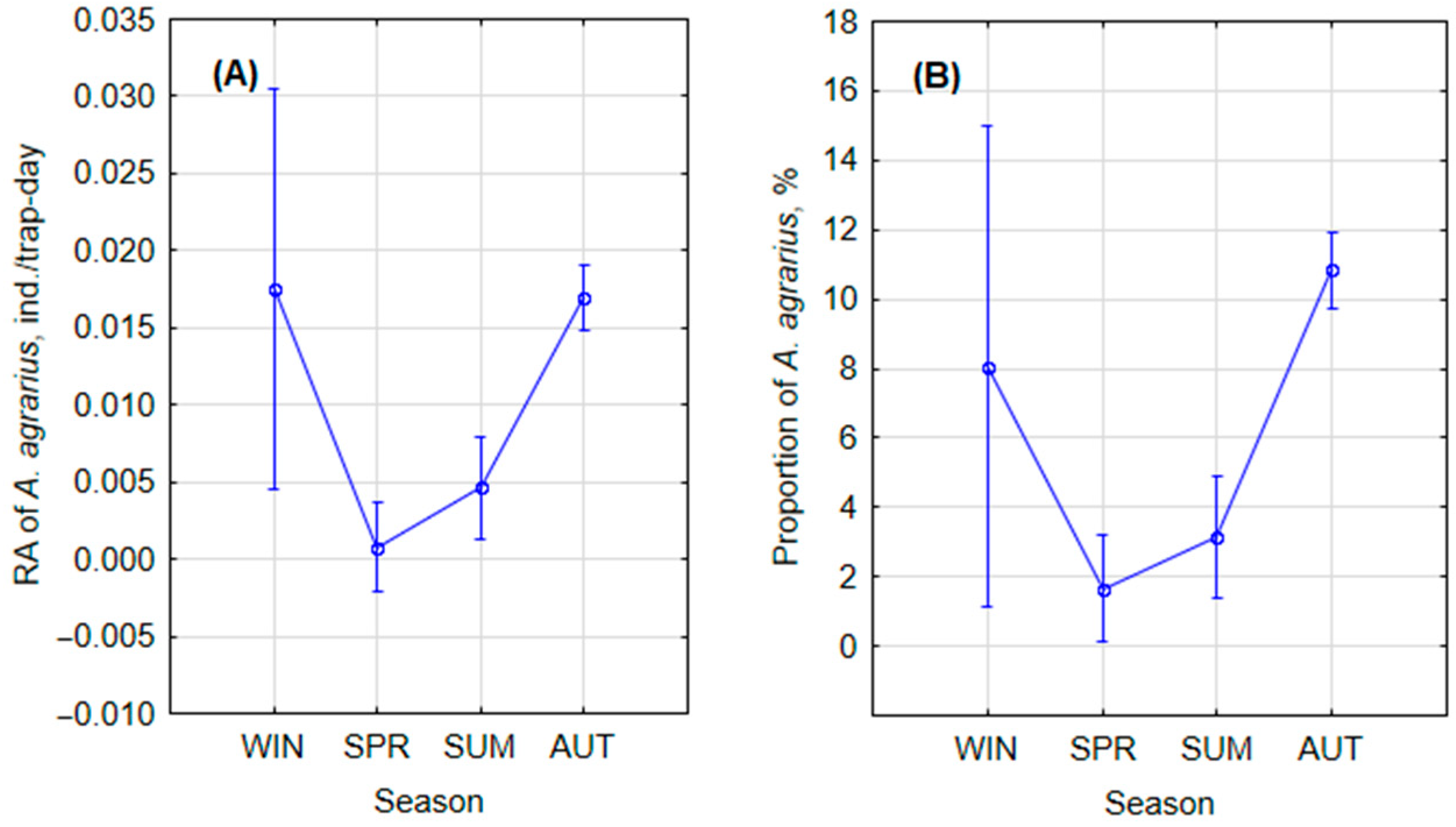
3.1.1. Monthly and Seasonal Trends
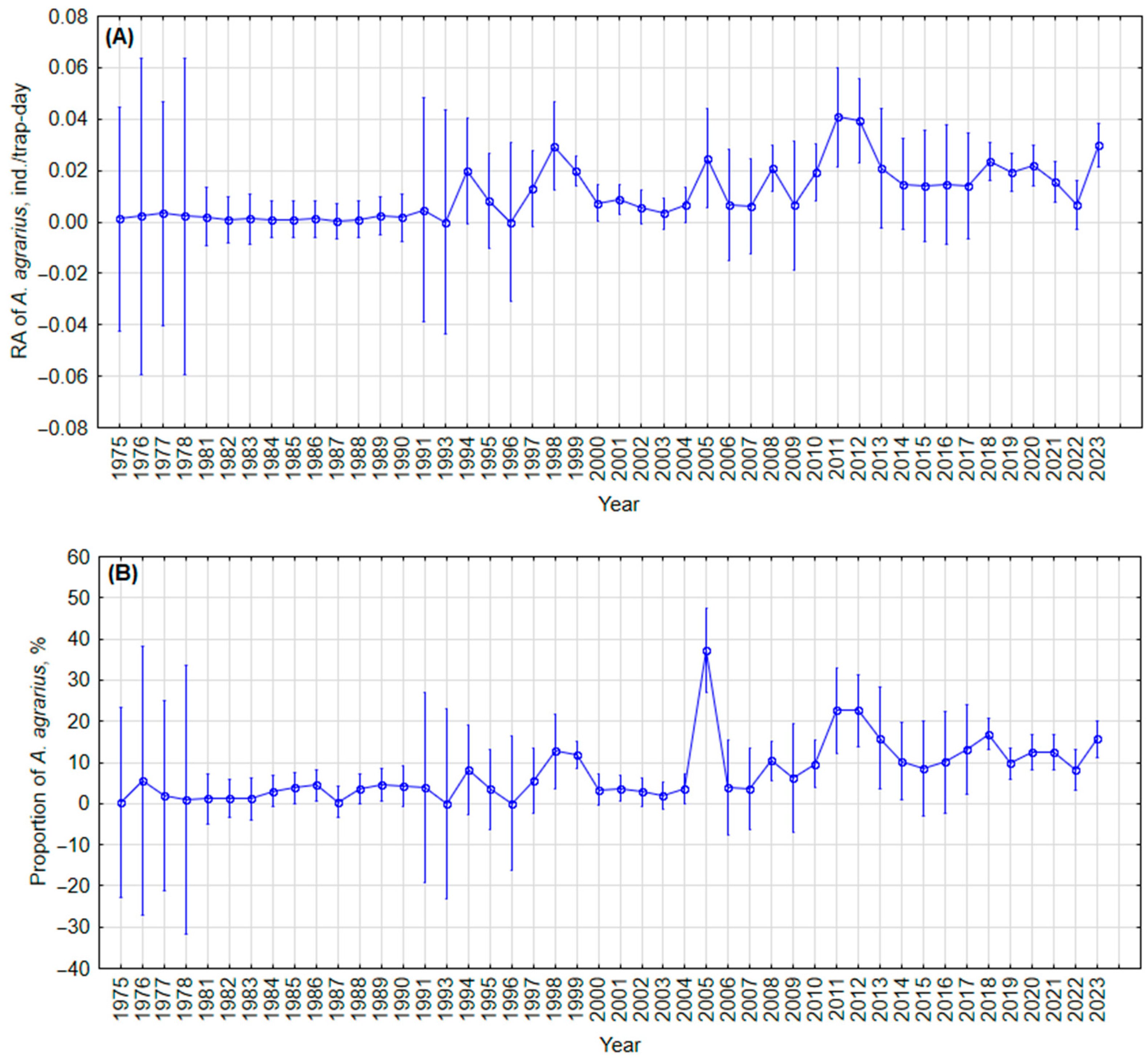
3.1.2. Annual Fluctuations
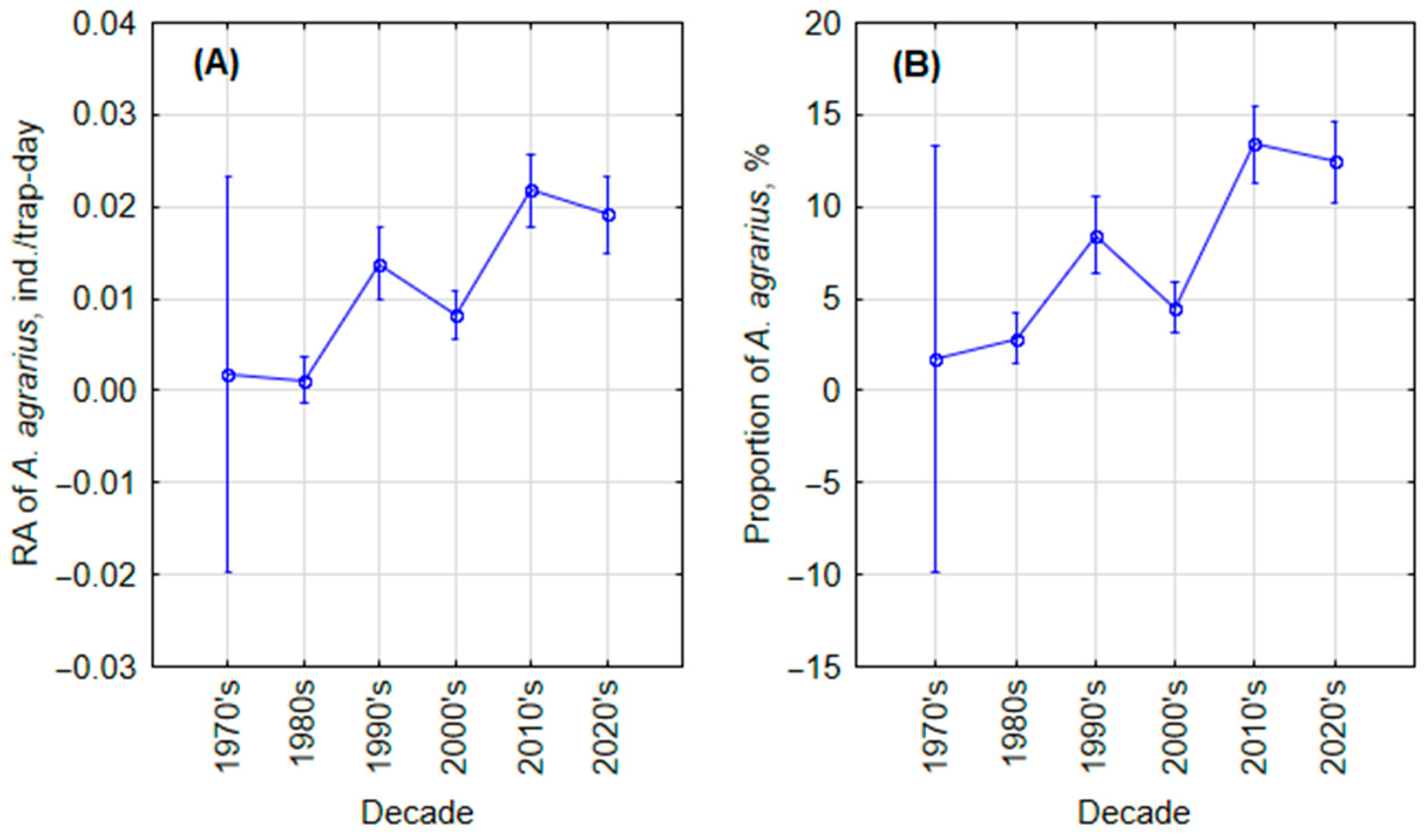
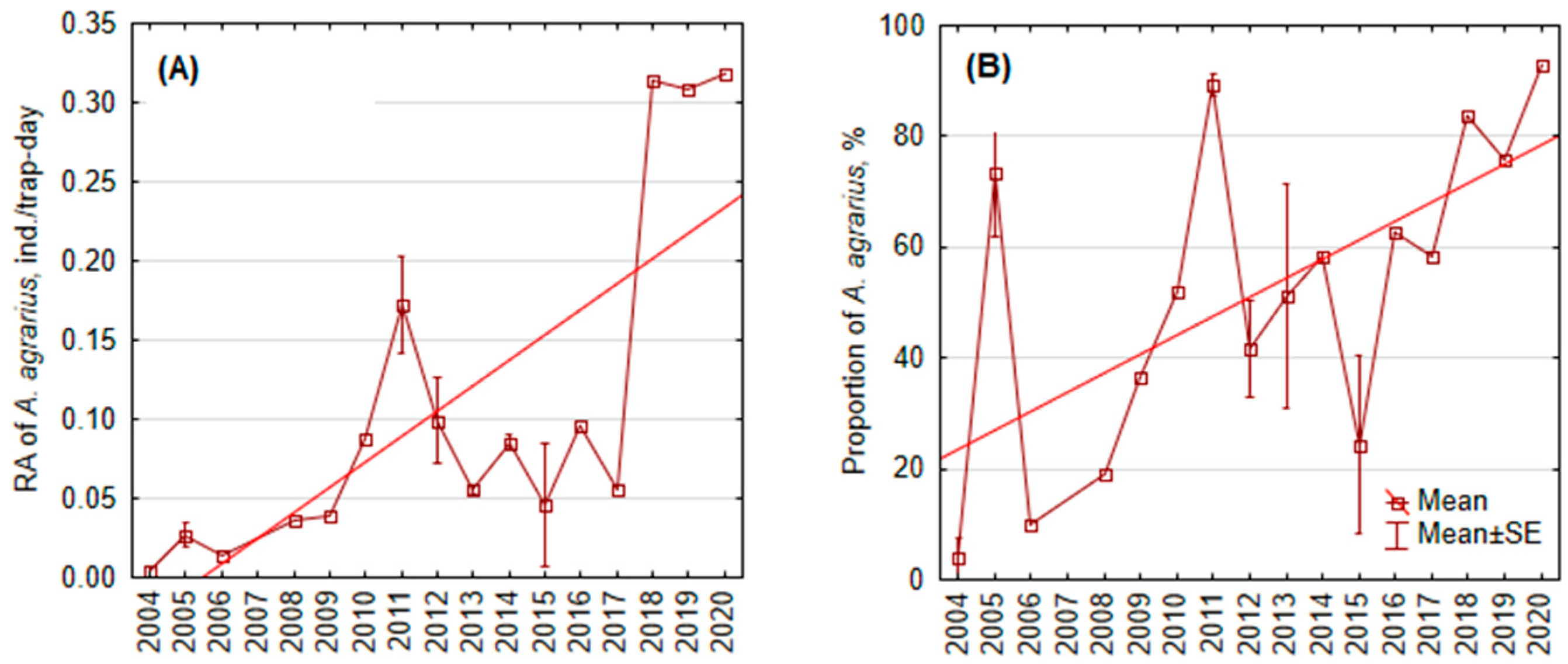
3.1.3. Long-Term Changes
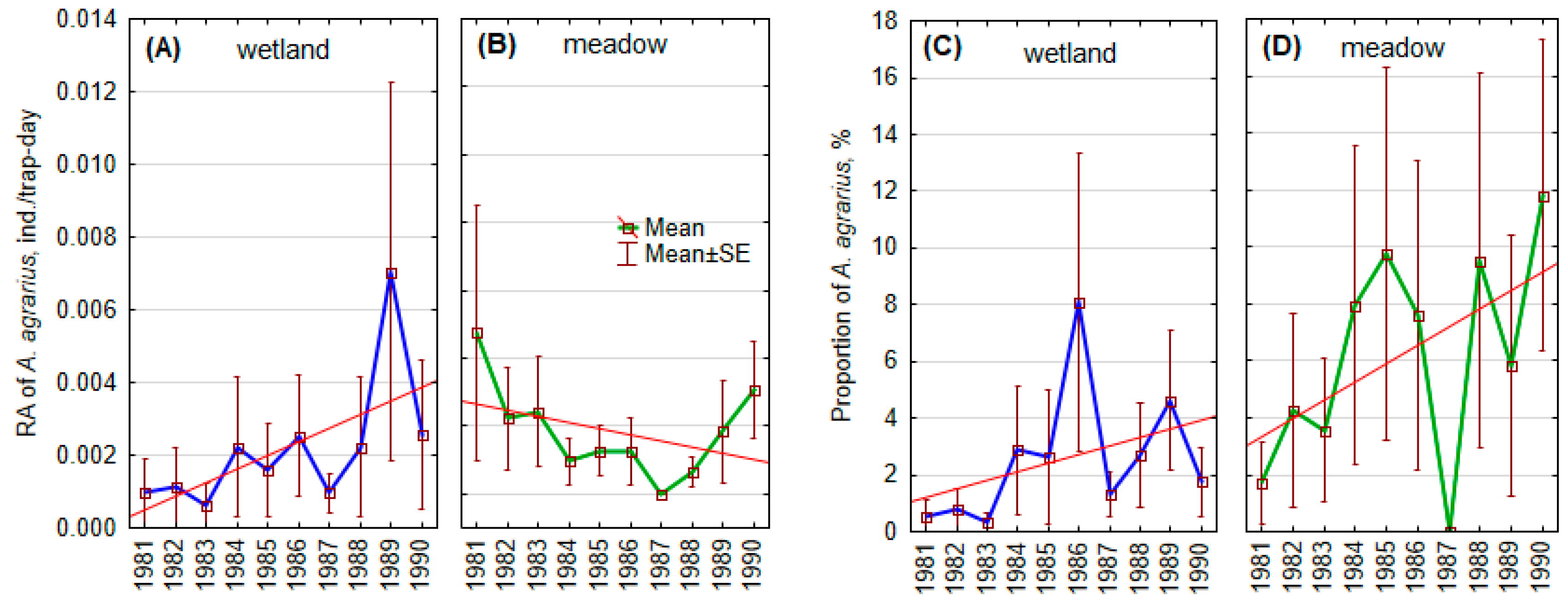
3.2. Local Changes in Striped Field Mouse Populations
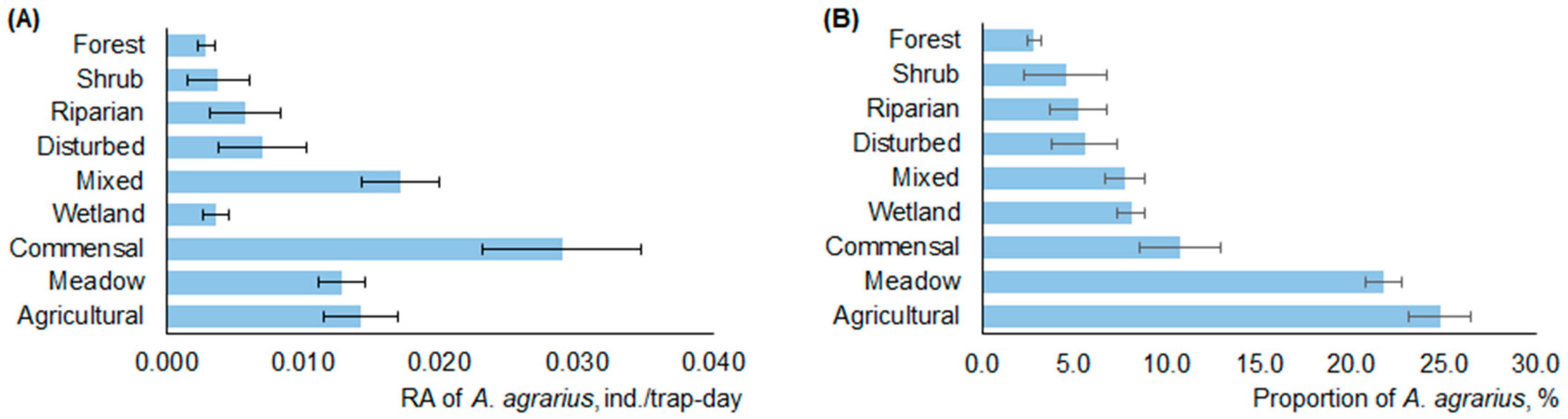
3.3. Habitats of Striped Field Mouse in Lithuania
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Van der Putten, W.H.; Macel, M.; Visser, M.E. Predicting species distribution and abundance responses to climate change: Why it is essential to include biotic interactions across trophic levels. Philos. Trans. R. Soc. B 2010, 365, 2025–2034. [Google Scholar] [CrossRef] [PubMed]
- Williams, J.J.; Newbold, T. Local climatic changes affect biodiversity responses to land use: A review. Divers. Distrib. 2020, 26, 76–92. [Google Scholar] [CrossRef]
- Spitzenberger, F.; Engelberg, S. A new look at the dynamic western distribution border of Apodemus agrarius in Central Europe (Rodentia: Muridae). Lynx 2014, 45, 69–79. [Google Scholar]
- Tulis, F.; Ambros, M.; Baláž, I.; Žiak, D.; Sládkovičová, V.H.; Miklós, P.; Dudich, A.; Stollmann, A.; Klimant, P.; Somogyi, B.; et al. Expansion of the Striped field mouse (Apodemus agrarius) in the south-western Slovakia during 2010–2015. Folia Oecologica 2016, 43, 64–73. [Google Scholar]
- Tulis, F.; Ševčík, M.; Jánošíková, R.; Baláž, I.; Ambros, M.; Zvaríková, L.; Horváth, G. The impact of the striped field mouse’s range expansion on communities of native small mammals. Sci. Rep. 2023, 13, 753. [Google Scholar] [CrossRef]
- Khlyap, L.A.; Dinets, V.; Warshavsky, A.A.; Osipov, F.A.; Dergunova, N.N.; Petrosyan, V.G. Aggregated occurrence records of the invasive alien striped field mouse (Apodemus agrarius Pall.) in the former USSR. Biodivers. Data J. 2021, 9, e69159. [Google Scholar] [CrossRef]
- Herzig-Straschil, B.; Bihari, Z.; Spitzenberger, F. Recent changes in the distribution of the field mouse (Apodemus agrarius) in the western part of the Carpathian basin. Ann. Naturhistorischen Mus. Wien. Ser. B Für Bot. Und Zool. 2003, 105, 421–428. [Google Scholar]
- Global Invasive Species Database. Available online: https://www.iucngisd.org/gisd/ (accessed on 26 August 2024).
- Petrosyan, V.; Osipov, F.; Feniova, I.; Dergunova, N.; Warshavsky, A.; Khlyap, L.; Dzialowski, A. The TOP-100 most dangerous invasive alien species in Northern Eurasia: Invasion trends and species distribution modelling. NeoBiota 2023, 82, 23–56. [Google Scholar] [CrossRef]
- Latinne, A.; Navascués, M.; Pavlenko, M.; Kartavtseva, I.; Ulrich, R.G.; Tiouchichine, M.L.; Catteau, G.; Sakka, H.; Quéré, J.P.; Chelomina, G.; et al. Phylogeography of the striped field mouse, Apodemus agrarius (Rodentia: Muridae), throughout its distribution range in the Palaearctic region. Mamm. Biol. 2020, 100, 19–31. [Google Scholar] [CrossRef]
- Kaneko, Y.; Kryštufek, B.; Zagarondnyuk, I.; Vohralík, V.; Batsaikhan, N.; Avirmed, D.; Sukhchuluun, G. Apodemus agrarius (errata version published in 2017). In The IUCN Red List of Threatened Species 2016; IUCN: Gland, Switzerland, 2016; p. e.T1888A115057408. [Google Scholar] [CrossRef]
- Mitchell-Jones, A.J.; Mitchell, J.; Amori, G.; Bogdanowicz, W.; Kryštufek, B.; Reijnders, P.H.; Spitzenberger, F.; Stubbe, M.; Thissen, J.; Vohralík, V.; et al. (Eds.) The Atlas of European Mammals; Academic Press: London, UK, 1999; pp. 1–484. [Google Scholar]
- Petrosyan, V.; Dinets, V.; Osipov, F.; Dergunova, N.; Khlyap, L. Range Dynamics of Striped Field Mouse (Apodemus agrarius) in Northern Eurasia under Global Climate Change Based on Ensemble Species Distribution Models. Biology 2023, 12, 1034. [Google Scholar] [CrossRef]
- Yalkovskaya, L.; Sibiryakov, P.; Borodin, A. Phylogeography of the striped field mouse (Apodemus agrarius Pallas, 1771) in light of new data from central part of Northern Eurasia. PLoS ONE 2022, 17, e0276466. [Google Scholar] [CrossRef] [PubMed]
- Nikitina, N.A. Features of the territory using by striped field mice (Apodemus agrarius Pall.). Zool. Zhurnal 1958, 37, 1387–1408. [Google Scholar]
- Andrzejewski, R.; Babińska-Werka, J.; Gliwicz, J.; Goszczyński, J. Synurbization processes in population of Apodemus agrarius. I. Characteristics of populations in an urbanization gradient. Acta Theriol. 1978, 23, 341–358. [Google Scholar] [CrossRef]
- Babińska-Werka, J.; Gliwicz, J.; Goszczyński, J. Synurbization processes in a population of Apodemus agrarius. II. Habitats of the striped field mouse in town. Acta Theriol. 1979, 24, 405–415. [Google Scholar] [CrossRef]
- Karaseva, E.V.; Tikhonova, G.N.; Bogomolov, P.L. Distribution of the Striped field mouse (Apodemus agrarius) and peculiarities of its ecology in different parts of its range. Zool. Zhurnal. 1992, 71, 106–115. [Google Scholar]
- Dammhahn, M.; Mazza, V.; Schirmer, A.; Göttsche, C.; Eccard, J.A. Of city and village mice: Behavioural adjustments of striped field mice to urban environments. Sci. Rep. 2020, 10, 13056. [Google Scholar] [CrossRef]
- Łopucki, R.; Klich, D.; Ścibior, A.; Gołębiowska, D. Hormonal adjustments to urban conditions: Stress hormone levels in urban and rural populations of Apodemus agrarius. Urban Ecosyst. 2019, 22, 435–442. [Google Scholar] [CrossRef]
- Gortat, T.; Rutkowski, R.; Gryczynska-Siemiatkowska, A.; Kozakiewicz, A.; Kozakiewicz, M. Genetic structure in urban and rural populations of Apodemus agrarius in Poland. Mamm. Biol. 2013, 78, 171–177. [Google Scholar] [CrossRef]
- Gortat, T.; Rutkowski, R.; Gryczyńska, A.; Pieniążek, A.; Kozakiewicz, A.; Kozakiewicz, M. Anthropopressure gradients and the population genetic structure of Apodemus agrarius. Conserv. Genet. 2015, 16, 649–659. [Google Scholar] [CrossRef]
- Abt, K.F.; Bock, W.F. Seasonal variations of diet composition in farmland field mice Apodemus spp. and bank voles Clethrionomys glareolus. Acta Theriol. 1998, 43, 379–389. [Google Scholar] [CrossRef]
- Butet, A.; Delettre, Y.R. Diet differentiation between European arvicoline and murine rodents. Acta Theriol. 2011, 56, 297. [Google Scholar] [CrossRef]
- Holišová, V. The food of Apodemus agrarius (Pall.). Zool. Listy 1967, 16, 1–14. [Google Scholar]
- Pineda-Munoz, S.; Alroy, J. Dietary characterization of terrestrial mammals. Proc. R. Soc. B-Biol. Sci. 2014, 281, 20141173. [Google Scholar] [CrossRef] [PubMed]
- Nemirov, K.; Vapalahti, O.; Lundkvist, Å.; Vasilenko, V.; Golovljova, I.; Plyusnina, A.; Niemimaa, J.; Laakkonen, J.; Henttonen, H.; Vaheri, A.; et al. Isolation and characterization of Dobrava hantavirus carried by the striped field mouse (Apodemus agrarius) in Estonia. J. Gen. Virol. 1999, 80, 371–379. [Google Scholar] [CrossRef] [PubMed]
- Klempa, B.; Avsic-Zupanc, T.; Clement, J.; Dzagurova, T.K.; Henttonen, H.; Heyman, P.; Jakab, F.; Kruger, D.H.; Maes, P.; Papa, A.; et al. Complex evolution and epidemiology of Dobrava-Belgrade hantavirus: Definition of genotypes and their characteristics. Arch. Virol. 2013, 158, 521–529. [Google Scholar] [CrossRef] [PubMed]
- Fischer, S.; Mayer-Scholl, A.; Imholt, C.; Spierling, N.G.; Heuser, E.; Schmidt, S.; Reil, D.; Rosenfeld, U.M.; Jacob, J.; Nöckler, K.; et al. Leptospira genomospecies and sequence type prevalence in small mammal populations in Germany. Vector-Borne Zoonotic Dis. 2018, 18, 188–199. [Google Scholar] [CrossRef]
- Jeske, K.; Herzig-Straschil, B.; Răileanu, C.; Kunec, D.; Tauchmann, O.; Emirhar, D.; Schmidt, S.; Trimpert, J.; Silaghi, C.; Heckel, G.; et al. Zoonotic pathogen screening of striped field mice (Apodemus agrarius) from Austria. Transbound. Emerg. Dis. 2022, 69, 886–890. [Google Scholar] [CrossRef] [PubMed]
- Andrzejewski, R.; Wrocławek, H. Mass occurrence of Apodemus agrarius (Pallas, 1771) and variations in the number of associated Muridae. Acta Theriol. 1961, 5, 173–184. [Google Scholar] [CrossRef]
- Chełkowska, H.; Walkowa, W.; Adamczyk, K. Spatial relationships in sympatric populations of the rodents: Clethrionomys glareolus, Microtus agrestis and Apodemus agrarius. Acta Theriol. 1985, 30, 51–78. [Google Scholar] [CrossRef]
- Zub, K.; Jędrzejewska, B.; Jędrzejewski, W.; Bartoń, K.A. Cyclic voles and shrews and non-cyclic mice in a marginal grassland within European temperate forest. Acta Theriol. 2012, 57, 205–216. [Google Scholar] [CrossRef]
- Simeonovska-Nikolova, D.M. Interspecific social interactions and behavioral responses of Apodemus agrarius and Apodemus flavicollis to conspecific and heterospecific odors. J. Ethol. 2007, 25, 41–48. [Google Scholar] [CrossRef]
- Vukićević-Radić, O.; Matić, R.; Kataranovski, D.; Stamenković, S. Spatial organization and home range of Apodemus flavicollis and A. agrarius on Mt. Avala, Serbia. Acta Zool. Acad. Sci. H. 2006, 52, 81–96. [Google Scholar]
- Likevičienė, N. Lietuvos TSR Peliniai Graužikai. Ph.D. Thesis, Vilnius University, Vilnius, Lithuania, 1959. [Google Scholar]
- Prūsaitė, J. Fauna of Lithuania. Mammals; Mokslas: Vilnius, Lithuania, 1988; pp. 121–123. [Google Scholar]
- Balčiauskas, L.; Balčiauskienė, L. Small Mammal Diversity Changes in a Baltic Country, 1975–2021: A Review. Life 2022, 12, 1887. [Google Scholar] [CrossRef] [PubMed]
- Balčiauskas, L.; Balčiauskienė, L. Habitat and Body Condition of Small Mammals in a Country at Mid-Latitude. Land 2024, 13, 1214. [Google Scholar] [CrossRef]
- Maldžiūnaitė, S. Small mammals in Žagarė reserve. In Žagarės Forest; Lekavičius, A., Jankevičienė, R., Tučienė, A., Eds.; Mokslas: Vilnius, Lithuania, 1980; pp. 48–50. [Google Scholar]
- Balčiauskas, L.; Alejūnas, P. Small mammal species diversity and abundance in Žagarė Regional Park. Acta Zool. Litu. 2011, 21, 163–172. [Google Scholar] [CrossRef]
- Čepkelių Rezervato Žinduolių bei Roplių Tyrimas ir jų Apsaugos Priemonių Nustatymas; Ataskaita; Zoologijos ir Parazitologijos Institutas: Vilnius, Lithuania, 1980.
- Mažeikytė, R.; Balčiauskas, L.; Baranauskas, K.; Ulevičius, A. Smulkiųjų žinduolių rūšių įvairovė ir gausumas integruoto monitoringo teritorijose ir agrostacionaruose. In Aplinkos Monitoringas, 1993–1995; Stoškus, L., Ed.; Lietuvos aplinkos apsaugos ministerija: Vilnius, Lithuania, 1996; pp. 73–79. [Google Scholar]
- Balčiauskas, L.; Juškaitis, R. Diversity of small mammal communities in Lithuania (1. A review). Acta Zool. Litu. 1997, 7, 29–45. [Google Scholar] [CrossRef]
- Smulkiųjų Žinduolių Monitoringas. Available online: https://aaa.lrv.lt/lt/veiklos-sritys/aplinkos-monitoringas/gyvosios-gamtos-monitoringas/smulkiuju-zinduoliu-monitoringas/ (accessed on 20 August 2024).
- Ulevičius, A.; Juškaitis, R. Dzūkijos nacionalinio parko žinduoliai (išskyrus šikšnosparnius). Theriol. Litu. 2003, 3, 11–29. [Google Scholar]
- Balčiauskas, L. Results of the long-term monitoring of small mammal communities in the Ignalina Nuclear Power Plant Region (Drūkšiai LTER site). Acta Zool. Litu. 2005, 15, 79–84. [Google Scholar] [CrossRef]
- Ulevičius, A.; Juškaitis, R.; Pauža, D.; Balčiauskas, L.; Ostasevičius, V. Žemaitijos nacionalinio parko žinduoliai. Theriol. Litu. 2002, 2, 1–20. [Google Scholar]
- Šinkūnas, R.; Balčiauskas, L. Small mammal communities in the fragmented landscape in Lithuania. Acta Zool. Litu. 2006, 16, 130–136. [Google Scholar] [CrossRef]
- Balčiauskas, L.; Gudaitė, A. Diversity of Small Mammals in Winter Season in North East Lithuania. Acta Zool. Litu. 2006, 16, 137–142. [Google Scholar] [CrossRef]
- Čepukienė, A. Small Mammal Community Changes during Early Forest Succession Stages. Ph.D. Dissertation, Vilnius University, Vilnius, Lithuania, 2014. [Google Scholar]
- Balčiauskas, L.; Balčiauskienė, L.; Janonytė, A. The influence of spring floods on small mammal communities in the Nemunas River Delta, Lithuania. Biologia 2012, 67, 1220–1229. [Google Scholar] [CrossRef]
- Balčiauskas, L.; Balčiauskienė, L. Long-term changes in a small mammal community in a temperate zone meadow subject to seasonal floods and habitat transformation. Integr. Zool. 2022, 17, 443–455. [Google Scholar] [CrossRef]
- Past 4—The Past of the Future. Available online: https://www.nhm.uio.no/english/research/resources/past/ (accessed on 1 January 2024).
- TIBCO Software Inc. Data Science Textbook. Available online: https://docs.tibco.com/data-science/textbook (accessed on 15 January 2024).
- Kozakiewicz, M.; Gortat, T.; Kozakiewicz, A.; Barkowska, M. Effects of habitat fragmentation on four rodent species in a Polish farm landscape. Landsc. Ecol. 1999, 14, 391–400. [Google Scholar] [CrossRef]
- Panzacchi, M.; Linnell, J.D.; Melis, C.; Odden, M.; Odden, J.; Gorini, L.; Andersen, R. Effect of land-use on small mammal abundance and diversity in a forest–farmland mosaic landscape in south-eastern Norway. For. Ecol. Manag. 2010, 259, 1536–1545. [Google Scholar] [CrossRef]
- Heroldová, M.; Bryja, J.; Zejda, J.; Tkadlec, E. Structure and diversity of small mammal communities in agriculture landscape. Agric. Ecosyst. Environ. 2007, 120, 206–210. [Google Scholar] [CrossRef]
- Gentili, S.; Sigura, M.; Bonesi, L. Decreased small mammals species diversity and increased population abundance along a gradient of agricultural intensification. Hystrix 2014, 25, 39–44. [Google Scholar] [CrossRef]
- Balestrieri, A.; Gazzola, A.; Formenton, G.; Canova, L. Long-term impact of agricultural practices on the diversity of small mammal communities: A case study based on owl pellets. Environ. Monit. Assess. 2019, 191, 725. [Google Scholar] [CrossRef]
- Paniccia, C.; Carranza, M.L.; Frate, L.; Di Febbraro, M.; Rocchini, D.; Loy, A. Distribution and functional traits of small mammals across the Mediterranean area: Landscape composition and structure definitively matter. Ecol. Indic. 2022, 135, 108550. [Google Scholar] [CrossRef]
- Barão, I.; Queirós, J.; Vale-Gonçalves, H.; Paupério, J.; Pita, R. Landscape characteristics affecting small Mammal occurrence in heterogeneous Olive Grove Agro-ecosystems. Conservation 2022, 2, 51–67. [Google Scholar] [CrossRef]
- Love, R.A.; Webon, C.; Glue, D.E.; Harris, S.; Harris, S. Changes in the food of British Barn Owls (Tyto alba) between 1974 and 1997. Mammal Rev. 2000, 30, 107–129. [Google Scholar] [CrossRef]
- Milana, G.; Luiselli, L.; Amori, G. Forty years of dietary studies on barn owl (Tyto alba) reveal long term trends in diversity metrics of small mammal prey. Anim. Biol. 2018, 68, 129–146. [Google Scholar] [CrossRef]
- Obuch, J.; Danko, Š.; Noga, M. Recent and subrecent diet of the barn owl (Tyto alba) in Slovakia. Raptor J. 2016, 10, 1–50. [Google Scholar] [CrossRef]
- Drebet, M. Monitoring of the mammal fauna by studying owl pellets: A case of small mammals in protected areas of Podillia. Theriol. Ukr. 2022, 24, 16–27. [Google Scholar] [CrossRef]
- Szép, D.; Krčmar, S.; Purger, J.J. Possible causes of temporal changes in the diet composition of Common Barn-owls Tyto alba (Scopoli, 1769) (Strigiformes: Tytonidae) in Baranja, Croatia. Acta Zool. Bulgar. 2021, 73, 87–94. [Google Scholar]
- Stefke, K.; Landler, L. Long-term monitoring of rodent and shrew communities in a biodiversity hot-spot in Austria using barn owl (Tyto alba) pellets. Acta Oecol. 2020, 109, 103660. [Google Scholar] [CrossRef]
- Grzędzicka, E.; Kus, K.; Nabielec, J. The effect of urbanization on the diet composition of the tawny owl (Strix aluco L.). Pol. J. Ecol. 2013, 61, 391–400. [Google Scholar]
- Lesiński, G.; Gryz, J.; Krauze-Gryz, D.; Stolarz, P. Population increase and synurbization of the yellow-necked mouse Apodemus flavicollis in some wooded areas of Warsaw agglomeration, Poland, in the years 1983–2018. Urban Ecosyst. 2021, 24, 481–489. [Google Scholar] [CrossRef]
- Romanowski, J.; Lesiński, G.; Bardzińska, M. Small mammals of the suburban areas of Warsaw in the diet of the tawny owl Strix aluco. Stud. Ecol. Bioethicae 2020, 18, 349–354. [Google Scholar] [CrossRef]
- Heisler, L.M.; Somers, C.M.; Poulin, R.G. Owl pellets: A more effective alternative to conventional trapping for broad-scale studies of small mammal communities. Methods in Ecol. Evol. 2016, 7, 96–103. [Google Scholar] [CrossRef]
- Goutner, V.; Alivizatos, H. Diet of the Barn Owl (Tyto alba) and Little Owl (Athene noctua) in wetlands of northeastern Greece. Belg.J. Zool. 2003, 133, 15–22. [Google Scholar]
- Kozakiewicz, M.; Kozakiewicz, A. Long-term dynamics and biodiversity changes in small mammal communities in a mosaic of agricultural and forest habitats. Ann. Zool. Fenn. 2008, 45, 263–269. [Google Scholar] [CrossRef]
- Pupila, A.; Bergmanis, U. Species diversity, abundance and dynamics of small mammals in the Eastern Latvia. Acta Univ. Latv. 2006, 710, 93–101. [Google Scholar]
- Avotins, A.; Avotins, A., Sr.; Ķerus, V.; Aunins, A. Numerical Response of Owls to the Dampening of Small Mammal Population Cycles in Latvia. Life 2023, 13, 572. [Google Scholar] [CrossRef]
- Neronov, V.M.; Khlyap, L.A.; Tupikova, N.V.; Warshavskii, A.A. Formation of rodent communities in arable lands of Northern Eurasia. Russ. J. Ecol. 2001, 32, 326–333. [Google Scholar] [CrossRef]
- Jeske, K.; Schulz, J.; Tekemen, D.; Balčiauskas, L.; Balčiauskienė, L.; Hiltbrunner, M.; Drewes, S.; Mayer-Scholl, A.; Heckel, G.; Ulrich, R.G. Cocirculation of Leptospira spp. and multiple orthohantaviruses in rodents, Lithuania, Northern Europe. Transbound. Emerg. Dis. 2022, 69, e3196–e3201. [Google Scholar] [CrossRef]
- Prakas, P.; Stirkė, V.; Šneideris, D.; Rakauskaitė, P.; Butkauskas, D.; Balčiauskas, L. Protozoan Parasites of Sarcocystis spp. in Rodents from Commercial Orchards. Animals 2023, 13, 2087. [Google Scholar] [CrossRef] [PubMed]
- Kitrytė, N. Diversity of Small Mammal Parasites and Factors Shaping Their Communities. Ph.D. Thesis, Vilnius University, Vilnius, Lithuania, 2022. [Google Scholar]
- Nackley, L.L.; West, A.G.; Skowno, A.L.; Bond, W.J. The nebulous ecology of native invasions. Trends Ecol. Evol. 2017, 32, 814–824. [Google Scholar] [CrossRef]
- Wang, D.; Anderson, D.P.; Li, K.; Guo, Y.; Yang, Z.; Pech, R.P. Predicted population dynamics of an indigenous rodent, Apodemus agrarius, in an agricultural system. Crop Prot. 2021, 147, 105683. [Google Scholar] [CrossRef]
- Fischer, C.; Thies, C.; Tscharntke, T. Small mammals in agricultural landscapes: Opposing responses to farming practices and landscape complexity. Biol. Conserv. 2011, 144, 1130–1136. [Google Scholar] [CrossRef]
- Hermoso, V.; Carvalho, S.B.; Giakoumi, S.; Goldsborough, D.; Katsanevakis, S.; Leontiou, S.; Markantonatou, V.; Rumes, B.; Vogiatzakis, I.N.; Yates, K.L. The EU Biodiversity Strategy for 2030: Opportunities and challenges on the path towards biodiversity recovery. Environ. Sci. Policy 2022, 127, 263–271. [Google Scholar] [CrossRef]
- Török, P.; Dembicz, I.; Dajic-Stevanovic, Z.; Kuzemko, A. Grasslands of eastern Europe. Encycl. World’s Biomes. 2020, 3, 703–713. [Google Scholar]
- Schils, R.L.M.; Bufe, C.; Rhymer, C.M.; Francksen, R.M.; Klaus, V.H.; Abdalla, M.; Milazzo, F.; Lellei-Kovács, E.; Berge, H.; Bertora, C.; et al. Permanent grasslands in Europe: Land use change and intensification decrease their multifunctionality. Agric. Ecosyst. Environ. 2022, 330, 107891. [Google Scholar] [CrossRef]
- Lomba, A.; Da Costa, J.F.; Ramil-Rego, P.; Corbelle-Rico, E. Assessing the link between farming systems and biodiversity in agricultural landscapes: Insights from Galicia (Spain). J. Environ. Manag. 2022, 317, 115335. [Google Scholar] [CrossRef] [PubMed]
- Müller, A.; Schneider, U.A.; Jantke, K. Evaluating and expanding the European Union’s protected-area network toward potential post-2020 coverage targets. Conserv. Biol. 2020, 34, 654–665. [Google Scholar] [CrossRef]
- Viteri, M.C.; Hadly, E.A. Spatiotemporal impacts of the Anthropocene on small mammal communities, and the role of small biological preserves in maintaining biodiversity. Front. Ecol. Evol. 2022, 10, 916239. [Google Scholar] [CrossRef]


| Agr | Com | Dis | For | Mea | Mix | Rip | Shr | Wet | Total | |
|---|---|---|---|---|---|---|---|---|---|---|
| TE | 36,806 | 26,516 | 19,525 | 110,075 | 125,865 | 137,040 | 9069 | 4200 | 40,968 | 510,064 |
| N | 634 | 534 | 112 | 332 | 2493 | 1563 | 63 | 16 | 236 | 5983 |
| Month, Season | Trapping Effort, Trap Days | A. agrarius, Specimens |
|---|---|---|
| January | 1320 | 10 |
| February | 1310 | 1 |
| March | 1300 | 3 |
| April | 1460 | 5 |
| May | 69,945 | 31 |
| June | 900 | 9 |
| July | 28,960 | 43 |
| August | 30,903 | 288 |
| September | 112,101 | 1675 |
| October | 114,073 | 2353 |
| November | 5164 | 101 |
| December | 1120 | 22 |
| Winter * | 6795 | 36 |
| Spring | 73,145 | 54 |
| Summer | 79,463 | 361 |
| Autumn | 270,081 | 4803 |
| 1970s | 1980s | 1990s | 2000s | 2010s | 2020s | |
|---|---|---|---|---|---|---|
| TE | 27,930 | 90,690 | 126,335 | 143,256 | 83,993 | 37,860 |
| N | 36 | 116 | 1364 | 1589 | 2198 | 680 |
| Site | Time Period | TE | NSM | S | NAA |
|---|---|---|---|---|---|
| 1 | 1975; 2008–2012 | 16,778 | 3987 | 14 | 292 |
| 2 | 1977; 1997–2005 | 1850 | 529 | 13 | 30 |
| 3 | 1978–1980; 1991–1995; 1999–2004 | 15,550 | 1764 | 13 | 3 |
| 4 | 1980–1990 | 75,550 | 3413 | 16 | 102 |
| 5 | 1993–1995; 1997–2004 | 8300 | 793 | 11 | 4 |
| 6 | 1997–2004 | 7650 | 1144 | 12 | 214 |
| 7 | 2000–2005; 2007–2012 | 18,122 | 2776 | 13 | 95 |
| 8 | 2004–2020 | 22,643 | 3550 | 13 | 1627 |
| Site | 1970s | 1980s | 1990s | 2000s | 2010s | 2020s | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| RA | % | RA | % | RA | % | RA | % | RA | % | RA | % | |
| 1 | 0.002 | 0.41 | * | 0.021 | 7.22 | 0.023 | 9.93 | |||||
| 2 | 0.005 | 1.25 | 0.04 | 7.02 | 0 | 0 | 0.04 | 9.77 | ||||
| 3 | 0 | 0 | 0.000 | 0.20 | 0.001 | 0.43 | ||||||
| 4 | 0.001 | 4.29 | ||||||||||
| 5 | 0.004 | 5.84 | 0 | 0 | ||||||||
| 6 | 0.010 | 5.28 | 0.016 | 11.81 | ||||||||
| 7 | 0.003 | 1.86 | 0.011 | 13.85 | ||||||||
| 8 | 0.023 | 21.43 | 0.128 | 61.72 | ||||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Balčiauskas, L.; Balčiauskienė, L. Striped Field Mouse Invading Human-Modified Environments of Lithuania during Last Five Decades. Land 2024, 13, 1555. https://doi.org/10.3390/land13101555
Balčiauskas L, Balčiauskienė L. Striped Field Mouse Invading Human-Modified Environments of Lithuania during Last Five Decades. Land. 2024; 13(10):1555. https://doi.org/10.3390/land13101555
Chicago/Turabian StyleBalčiauskas, Linas, and Laima Balčiauskienė. 2024. "Striped Field Mouse Invading Human-Modified Environments of Lithuania during Last Five Decades" Land 13, no. 10: 1555. https://doi.org/10.3390/land13101555
APA StyleBalčiauskas, L., & Balčiauskienė, L. (2024). Striped Field Mouse Invading Human-Modified Environments of Lithuania during Last Five Decades. Land, 13(10), 1555. https://doi.org/10.3390/land13101555







