Landscape Agroecology: Methodologies and Applications for the Design of Sustainable Agroecosystems
Abstract
:1. Introduction
1.1. Landscape Diversity and Biological Pest Control
1.2. A Simple Methodology to Assess Beneficial Insect Habitat Suitability at the Agrolandscape Level (ABIHS)
2. The Main Agroecological Structure (MAS): Assessing Interactions Between the Matrix of Semi-Natural Vegetation with Agroecosystems
2.1. Applying MAS to an Agrarian Landscape in the Chilean Mediterranean
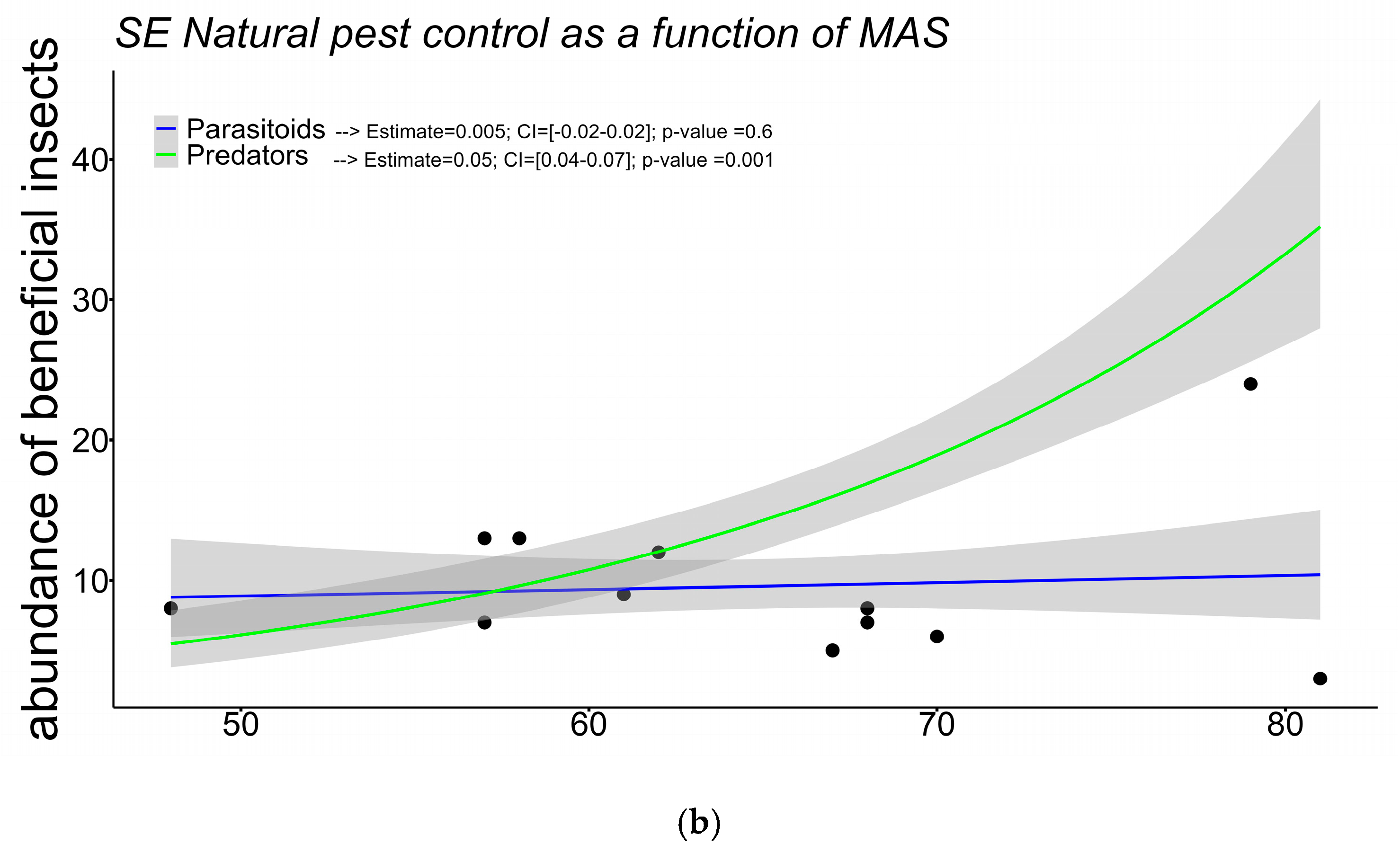
2.2. Landscape Structure, the Presence of Native Vegetation Patches, and the Response of Natural Enemies
3. A Metabolic Approach to Agricultural Landscapes: Assessing Energy, Material, and Information Exchange Between Agriculture and Its Socio-Ecological Environment

3.1. The Landscape as a Socio-Metabolic Footprint
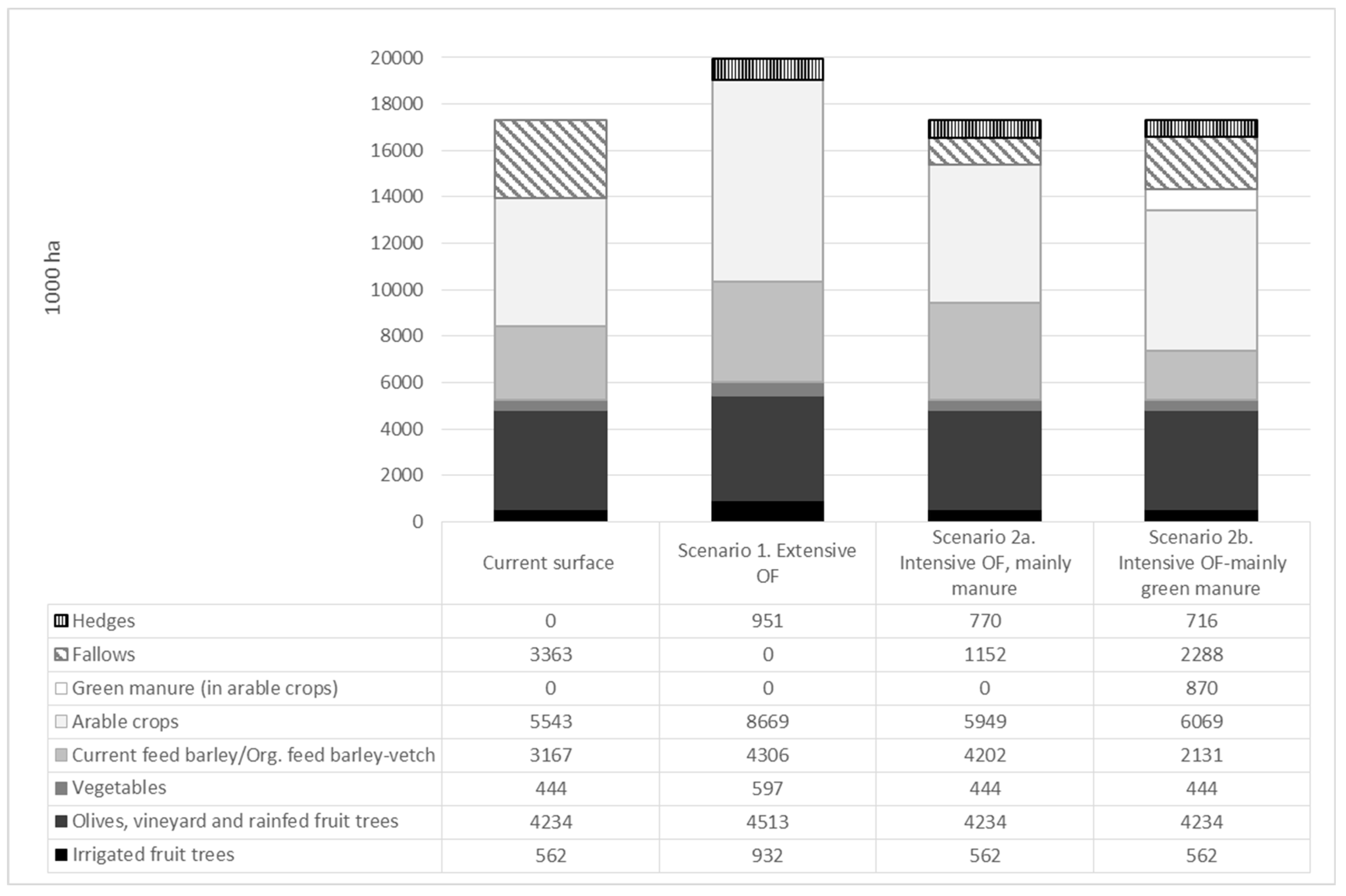
3.2. Restoring the Internal Loops in Spanish Agrolandscapes
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Batáry, P.; Báldi, A.; Ekroos, J.; Gallé, R.; Grass, I.; Tscharntke, T. Biologia Futura: Landscape perspectives on farmland biodiversity conservation. Biol. Futur. 2020, 71, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Sirami, C.; Gross, N.; Baillod, A.B.; Bertrand, C.; Carrié, R.; Hass, A.; Henckel, L.; Miguet, P.; Vuillot, C.; Alignier, A.; et al. Increasing crop heterogeneity enhances multitrophic diversity across agricultural regions. Proc. Natl. Acad. Sci. USA 2019, 116, 16442–16447. [Google Scholar] [CrossRef] [PubMed]
- Smith, F.P.; Prober, S.M.; House, A.P.; McIntyre, S. Maximizing retention of native biodiversity in Australian agricultural landscapes—The 10:20:40:30 guidelines. Agric. Ecosyst. Environ 2013, 166, 35–45. [Google Scholar] [CrossRef]
- Garibaldi, L.A.; Oddi, F.J.; Miguez, F.E.; Bartomeus, I.; Orr, M.C.; Jobbágy, E.G.; Kremen, C.; Schulte, L.A.; Hughes, A.C.; Bagnato, C.; et al. Working landscapes need at least 20% native habitat. Conserv. Lett. 2021, 14, e12773. [Google Scholar] [CrossRef]
- Altieri, M.A. The ecological role of biodiversity in agroecosystems. In Invertebrate Biodiversity as Bioindicators of Sustainable Landscapes; Elsevier: Amsterdam, The Netherlands, 1999; pp. 19–31. [Google Scholar]
- Perfecto, I.; Vandermeer, J. The agroecological matrix as alternative to the land-sparing/agriculture intensification model. Proc. Natl. Acad. Sci. USA 2010, 107, 5786–5791. [Google Scholar] [CrossRef] [PubMed]
- Priyadarshana, T.S.; Martin, E.A.; Sirami, C.; Woodcock, B.A.; Goodale, E.; Martínez-Núñez, C.; Lee, M.B.; Pagani-Núñez, E.; Raderschall, C.A.; Brotons, L.; et al. Crop and landscape heterogeneity increase biodiversity in agricultural landscapes: A global review and meta-analysis. Ecol. Lett. 2024, 27, e14412. [Google Scholar] [CrossRef]
- Scherr, S.J.; McNeely, J.A. Biodiversity conservation and agricultural sustainability: Towards a new paradigm of ‘ecoagriculture’ landscapes. Philos. Trans. R. Soc. B Biol. Sci. 2008, 363, 477–494. [Google Scholar] [CrossRef]
- Gomiero, T.; Pimentel, D.; Paoletti, M.G. Is there a need for a more sustainable agriculture? Crit. Rev. Plant Sci. 2011, 30, 6–23. [Google Scholar] [CrossRef]
- Altieri, M.; Nicholls, C. Biodiversity and Pest Management in Agroecosystems; CRC Press: Boca Raton, FL, USA, 2018. [Google Scholar]
- Clément, F.; Ruiz, J.; Rodríguez, M.A.; Blais, D.; Campeau, S. Landscape diversity and forest edge density regulate stream water quality in agricultural catchments. Ecol. Indic. 2017, 72, 627–639. [Google Scholar] [CrossRef]
- Kremen, C.; Merenlender, A.M. Landscapes that work for biodiversity and people. Science 2018, 362, eaau6020. [Google Scholar] [CrossRef]
- Landis, D.A.; Menalled, F.D.; Costamagna, A.C.; Wilkinson, T.K. Manipulating plant resources to enhance beneficial arthropods in agricultural landscapes. Weed Sci. 2005, 53, 902–908. [Google Scholar] [CrossRef]
- Quintero, I.; Daza-Cruz, Y.X.; León-Sicard, T. Main agro-ecological structure: An index for evaluating agro-biodiversity in agro-ecosystems. Sustainability 2022, 14, 13738. [Google Scholar] [CrossRef]
- Padró, R.; Tello, E.; Marco, I.; Olarieta, J.R.; Grasa, M.M.; Font, C. Modelling the scaling up of sustainable farming into Agroecology Territories: Potentials and bottlenecks at the landscape level in a Mediterranean case study. J. Clean. Prod. 2020, 275, 124043. [Google Scholar] [CrossRef]
- Tello, E.; González de Molina, M. Agrarian metabolism and socio-ecological transitions to agroecology landscapes. In The Barcelona School of Ecological Economics and Political Ecology: A Companion in Honour of Joan Martinez-Alier; Springer International Publishing: Cham, Switzerland, 2023; pp. 93–107. [Google Scholar]
- Andow, D.A. Vegetational diversity and arthropod population response. Annu. Rev. Entomol 1991, 36, 561–586. [Google Scholar] [CrossRef]
- Letourneau, D.K.; Armbrecht, I.; Rivera, B.S.; Lerma, J.M.; Carmona, E.J.; Daza, M.C.; Escobar, S.; Galindo, V.; Gutiérrez, C.; López, S.D.; et al. Does plant diversity benefit agroecosystems? A synthetic review. Ecol. Appl. 2011, 21, 9–21. [Google Scholar] [CrossRef]
- Zhao, J.; Yu, L.; Newbold, T.; Shen, X.; Liu, X.; Hua, F.; Kanniah, K.; Ma, K. Biodiversity responses to agricultural practices in cropland and natural habitats. Sci. Total Environ. 2024, 922, 171296. [Google Scholar] [CrossRef]
- Bianchi, F.J.; Booij, C.J.H.; Tscharntke, T. Sustainable pest regulation in agricultural landscapes: A review on landscape composition, biodiversity and natural pest control. Proc. R. Soc. B Biol. Sci. 2006, 273, 1715–1727. [Google Scholar] [CrossRef]
- Martin, E.A.; Seo, B.; Park, C.R.; Reineking, B.; Steffan-Dewenter, I. Scale-dependent effects of landscape composition and configuration on natural enemy diversity, crop herbivory, and yields. Ecol. Appl. 2016, 26, 448–462. [Google Scholar] [CrossRef]
- Franin, K.; Barić, B.; Kuštera, G. The role of ecological infrastructure on beneficial arthropods in vineyards. Span. J. Agric. Res. 2016, 14, 8. [Google Scholar] [CrossRef]
- Nicholls, C.I.; Parrella, M.; Altieri, M.A. The effects of a vegetational corridor on the abundance and dispersal of insect biodiversity within a northern California organic vineyard. Landsc. Ecol. 2001, 16, 133–146. [Google Scholar] [CrossRef]
- Redlich, S.; Martin, E.A.; Steffan-Dewenter, I. Landscape-level crop diversity benefits biological pest control. J. Appl. Ecol. 2018, 55, 2419–2428. [Google Scholar] [CrossRef]
- Chaplin-Kramer, R.; O’Rourke, M.E.; Blitzer, E.J.; Kremen, C. A meta-analysis of crop pest and natural enemy response to landscape complexity. Ecol. Lett. 2011, 14, 922–932. [Google Scholar] [CrossRef] [PubMed]
- Bosem Baillod, A.; Tscharntke, T.; Clough, Y.; Batáry, P. Landscape-scale interactions of spatial and temporal cropland heterogeneity drive biological control of cereal aphids. J. Appl. Ecol. 2017, 54, 1804–1813. [Google Scholar] [CrossRef]
- Chaplin-Kramer, R.; Kremen, C. Pest control experiments show benefits of complexity at landscape and local scales. Ecol. Appl. 2012, 22, 1936–1948. [Google Scholar] [CrossRef] [PubMed]
- Foltz, J.S.; Lee-Mader, E.; Hopwood, J.; Heidel-Baker, T.; Cruz, J.K.; Borders, B.; Gill, K.; Adamson, N.L.; Vaughan, M. Beneficial Insect Habitat Assessment Guide and Form: Farms and Agricultural Landscapes; The Xerces Society for Invertebrate Conservation: Portland, OR, USA, 2015; Available online: https://xerces.org/sites/default/files/2018-05/15-022_01_XercesSoc_HabitatAssessGuide_Beneficial-Insects_Farms%2BAg_web.pdf (accessed on 25 September 2024).
- León-Sicard, T. La Estructura Agroecológica Principal de los Agroecosistemas: Perspectivas Teórico-Prácticas; Universidad Nacional de Colombia, Instituto de Estudios Ambientales, IDEA: Bogotá, Colombia, 2021. [Google Scholar]
- Vanbergen, A.J.; Aizen, M.A.; Cordeau, S.; Garibaldi, L.A.; Garratt, M.P.; Kovács-Hostyánszki, A.; Lecuyer, L.; Ngo, H.T.; Potts, S.G.; Settele, J.; et al. Transformation of agricultural landscapes in the Anthropocene: Nature’s contributions to people, agriculture and food security. In Advances in Ecological Research; Academic Press: Cambridge, MA, USA, 2020; Volume 63, pp. 193–253. [Google Scholar]
- Marull, J.; Herrando, S.; Brotons, L.; Melero, Y.; Pino, J.; Cattaneo, C.; Pons, M.; Llobet, J.; Tello, E. Building on Margalef: Testing the links between landscape structure, energy and information flows driven by farming and biodiversity. Sci. Total Environ. 2019, 674, 603–614. [Google Scholar] [CrossRef]
- Cappelli, S.L.; Domeignoz-Horta, L.A.; Loaiza, V.; Laine, A.L. Plant biodiversity promotes sustainable agriculture directly and via belowground effects. Trends Plant Sci. 2022, 27, 674–687. [Google Scholar] [CrossRef]
- FAO. FAOSTAT. 2017. Available online: http://www.fao.org/faostat (accessed on 20 July 2020).
- INE. Censo Poblacional. Instituto Nacional de Estadística. 2017. Available online: https://www.bcn.cl (accessed on 20 September 2024).
- Kay, C. Chile’s neoliberal agrarian transformation and the peasantry. J. Agrar. Chang. 2002, 2, 464–501. [Google Scholar] [CrossRef]
- Armesto, J.J.; Manuschevich, D.; Mora, A.; Smith-Ramirez, C.; Rozzi, R.; Abarzúa, A.M.; Marquet, P.A. From the Holocene to the Anthropocene: A historical framework for land cover change in southwestern South America in the past 15,000 years. Land Use Policy 2010, 27, 148–160. [Google Scholar] [CrossRef]
- Nahuelhual, L.; Carmona, A.; Lara, A.; Echeverría, C.; González, M.E. Land-cover change to forest plantations: Proximate causes and implications for the landscape in south-central Chile. Landsc. Urban Plan. 2012, 107, 12–20. [Google Scholar] [CrossRef]
- Wratten, S.D.; Shields, M.W.; González-Chang, M. Posibilidades para la agricultura regenerativa en Chile. Agric. Sustain. 2019, 47, 1–6. [Google Scholar]
- Tamburini, G.; Bommarco, R.; Wanger, T.C.; Kremen, C.; Van Der Heijden, M.G.; Liebman, M.; Hallin, S. Agricultural diversification promotes multiple ecosystem services without compromising yield. Sci. Adv. 2020, 6, eaba1715. [Google Scholar] [CrossRef]
- ODEPA. Oficina de Estudios y Políticas Agrarias. Fichas Regionals. 2024. Disponible en Maule.pdf. Available online: http://www.odepa.gob.cl/ (accessed on 25 September 2024).
- Córdoba, C.; Triviño, C.; Toro Calderón, J. Agroecosystem resilience. A conceptual and methodological framework for evaluation. PLoS ONE 2020, 15, e0220349. [Google Scholar] [CrossRef] [PubMed]
- Wickham, H.; Chang, W.; Wickham, M.H. Package ‘ggplot2’. Create elegant data visualisations using the grammar of graphics. Version 2016, 2, 1–189. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing; R Core Team: Vienna, Austria, 2023; Available online: https://www.R-project.org/ (accessed on 12 August 2024).
- Liere, H.; Jha, S.; Philpott, S.M. Intersection between biodiversity conservation, agroecology, and ecosystem services. Agroecol. Sustain. Food Syst. 2017, 41, 723–760. [Google Scholar] [CrossRef]
- León-Sicard, T.E.; Toro Calderon, J.; Martínez-Bernal, L.F.; Cleves-Leguízamo, J.A. The main agroecological structure (MAS) of the agroecosystems: Concept, methodology and applications. Sustainability 2018, 10, 3131. [Google Scholar] [CrossRef]
- Van Geert, A.; Van Rossum, F.; Triest, L. Do linear landscape elements in farmland act as biological corridors for pollen dispersal? J. Ecol. 2010, 98, 178–187. [Google Scholar] [CrossRef]
- Salazar-Rojas, A.; Castro-Huerta, R.; Altieri, M. The main agroecological structure, a methodology for the collective analysis of the Mediterranean agroecological landscape of San Clemente, Region del Maule, Chile. Front. Sustain. Food Syst. 2023, 7, 1241648. [Google Scholar] [CrossRef]
- Jeanneret, P.; Aviron, S.; Alignier, A.; Lavigne, C.; Helfenstein, J.; Herzog, F.; Kay, S.; Petit, S. Agroecology landscapes. Landsc. Ecol 2021, 36, 2235–2257. [Google Scholar] [CrossRef]
- Fisher-Kowalski, M.; Haberl, H. Tons, Joules, and Money: Modes of Production and Their Sustainability Problems. Soc. Nat. Resour. 1997, 10, 61–85. [Google Scholar] [CrossRef]
- Eurostat. Economy-Wide Material Flow Accounts and Derived Indicators. A Methodological Guide; Eurostat, European Commission, Office for Official Publications of the European Communities: Luxembourg, 2001; pp. 1–92. [Google Scholar]
- González de Molina, M.; Soto Fernández, D.; Guzmán Casado, G.; Infante-Amate, J.; Aguilera Fernández, E.; Vila Traver, J.; García Ruiz, R. The Social Metabolism of Spanish Agriculture, 1900–2008: The Mediterranean Way Towards Industrialization. Springer Open Access. 2020. Available online: https://www.springer.com/gp/book/9783030208998 (accessed on 3 August 2024).
- Georgescu-Roegen, N. The Entropy Law and the Economic Process; Harvard University Press: Cambridge, MA, USA, 1971. [Google Scholar]
- Ho, M.W.; Ulanowicz, R. Sustainable systems as organisms? BioSystems 2005, 82, 39–51. [Google Scholar] [CrossRef]
- Ho, M.W. Circular Thermodynamics of Organisms and Sustainable Systems. Systems 2013, 1, 30–49. [Google Scholar] [CrossRef]
- Guzmán, G.; Aguilera, E.; Soto, D.; Cid, A.; Infante, J.; García Ruiz, R.; Herrera, A.; Villa, I.; González de Molina, M. Methodology and Conversion Factors to Estimate the Net Primary Productivity of Historical and Contemporary Agroecosystems. DT-SEHA n 1407. 2014. Available online: https://ideas.repec.org/p/seh/wpaper/1407.html (accessed on 2 April 2015).
- Guzmán, G.I.; González de Molina, M. Energy in Agroecosystems. A Tool for Assessing Sustainability, 1st ed.; CRC Press: Boca Raton, FL, USA, 2017; p. 399. [Google Scholar]
- Altieri, M.A. Agroecology: The Science of Sustainable Agriculture; Westview Press: Boulder, CO, USA, 1995. [Google Scholar]
- Guzmán GGonzález de Molina, M.; Sevilla Guzmán, E. Introducción a la Agroecología como Desarrollo Rural Sostenible; Mundi Prensa: Madrid, Spain, 2000. [Google Scholar]
- Ho, M.W. Are Sustainable Economic Systems Like Organisms. Evolution, Development and Economics; Springer: Berlin/Heidelberg, Germany, 1998. [Google Scholar]
- Guzmán, G.I.; González de Molina, M. Preindustrial agriculture versus organic agriculture. The land cost of sustainability. Land Use Policy 2009, 26, 502–510. [Google Scholar] [CrossRef]
- Guzmán, G.I.; González de Molina, M.; Alonso, A.M. The land cost of agrarian sustainability. An assessment. Land Use Policy 2011, 28, 825–835. [Google Scholar] [CrossRef]
- González de Molina, M.; Toledo, V. The Social Metabolism. A Socio-Ecological Theory of Historical Change, 2nd ed.; Springer: Berlin/Heidelberg, Germany, 2024. [Google Scholar]
- Agnoletti, M.; Emanueli, F. (Eds.) Biocultural Diversity in Europe; Springer: Berlin/Heidelberg, Germany, 2016. [Google Scholar]
- Soto, D.; Infante-Amate, J.; Guzmán, G.I.; Cid, A.; Aguilera, E.; García, R.; González de Molina, M. The social metabolism of biomass in Spain, 1900–2008: From food to feed-oriented changes in the agro-ecosystems. Ecol. Econ. 2016, 128, 130–138. [Google Scholar] [CrossRef]
- González de Molina, M.; Soto Fernández, D.; Infante-Amate, J.; Aguilera Fernández, E.; Vila Traver, J.; Guzmán Casado, G. Decoupling Food from Land: The Evolution of Spanish Agriculture from 1960 to 2010. Sustainability 2017, 9, 2348. [Google Scholar] [CrossRef]
- Thompson, R.M.; Brose, U.; Dunne, J.A.; Hall, R.O.; Hladyz, S.; Kitching, R.L.; Martinez, N.D.; Rantala, H.; Romanuk, T.N.; Stouffer, D.B.; et al. Food webs: Reconciling the structure and function of biodiversity. Trends Ecol. Evol. 2012, 27, 689–697. [Google Scholar] [CrossRef]
- Döring, T.F.; Kromp, B. Which carabid species benefit from organic agriculture? —A review of comparative studies in winter cereals from Germany and Switzerland. Agric. Ecosyst. Environ. 2003, 98, 153–161. [Google Scholar] [CrossRef]
- Rundlöf, M.; Nilsson, H.; Smith, H.G. Interacting effects of farming practice and landscape context on bumble bees. Biol. Conserv. 2008, 141, 417–426. [Google Scholar] [CrossRef]
- Gabriel, D.; Sait, S.M.; Kunin, W.E.; Benton, T.G. Food production versus biodiversity: Comparing organic and conventional agriculture. J. Appl. Ecol. 2013, 50, 355–364. [Google Scholar] [CrossRef]
- Guzmán, G.I.; González de Molina, M.; Soto Fernández, D.; Infante-Amate, J.; Aguilera, E. Spanish agriculture from 1900 to 2008: A long-term perspective on agroecosystem energy from an agroecological approach. Reg. Environ. Chang. 2018, 18, 995–1008. [Google Scholar] [CrossRef]
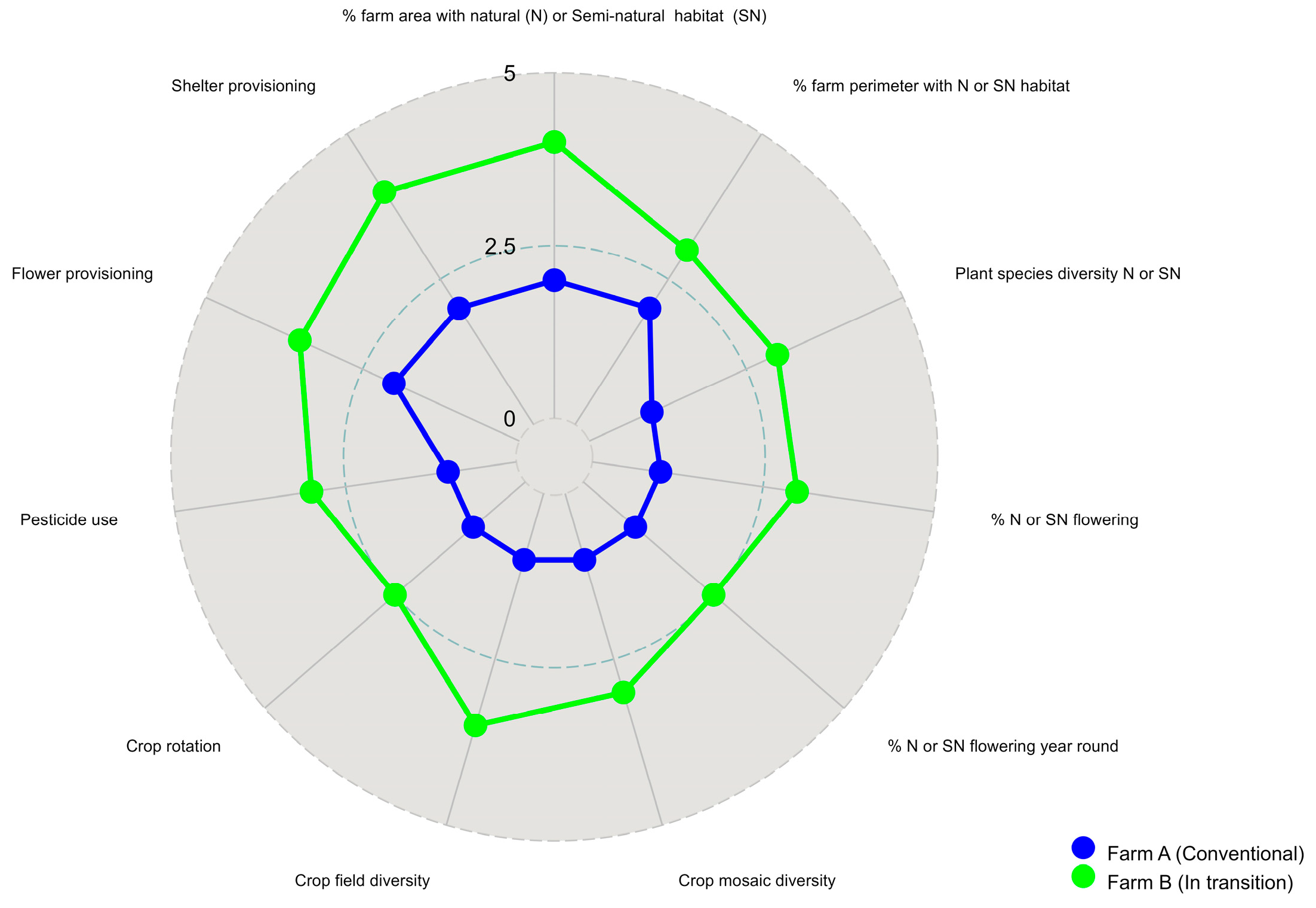

| Indicator | Value | Evaluation Criteria |
|---|---|---|
| Landscape Level | ||
| % of farm area with natural (N) or Semi-natural area (SN) | 1 | <10% |
| 2.5 | 10–30% | |
| 5 | >30% | |
| % of fam perimeter with N or SN habitat | 1 | 0–10% |
| 2.5 | 10–30% | |
| 5 | >30% | |
| Plant diversity composition of N and SN habitats | 1 | Hedgerow or weed patches composed of one or two species (naturalized or invasive) |
| 2.5 | Mix of 3 to 5 native or naturalized species | |
| 5 | >5 native and naturalized plant species | |
| % of vegetation composed of wildflowers and flowering shrubs and trees | 1 | <20% |
| 2.5 | 20–50% | |
| 5 | >50% | |
| % of plant species flowering in early, mid, and late crop season | 1 | <20% |
| 2.5 | 20–50% | |
| 5 | >50% | |
| Farm Level | ||
| # crops species deployed in the farm area in various fields or plots (crop mosaics) | 1 | One or two crops |
| 2.5 | 2–5 | |
| 5 | >5 | |
| Crop spatial diversity | 1 | Monoculture |
| 2.5 | 2–3 species intercropped | |
| 5 | >4 species intercropped | |
| Crop temporal diversity | 1 | No rotation, no vegetative fallow |
| 2.5 | One crop rotation per year, with or without fallow | |
| 5 | >2 rotations per year, including legume crop, with fallow | |
| Management Level | ||
| Pesticide use | 1 | Frequent use of chemical insecticides and herbicides |
| 2.5 | Use of microbial or botanical pesticides | |
| 5 | Reliance on practices that encourage biological control | |
| Provision of flowering resources | 1 | No flower provisioning |
| 2.5 | Provision of 1–2 flower species dispersed in the field | |
| 5 | Provision of 3 or > flower species along borders or strips within fields | |
| Practices to provide shelter | 1 | No practices |
| 2.5 | Use 1–2 practices (i.e rock piles, dispersed shrubs) | |
| 5 | >3 practices (i.e., fallows, rock piles, undisturbed ground, mulch, dead wood piles, etc.) | |
| Parameter | Description | Method |
|---|---|---|
| Connection with the main ecological landscape structure [CMELS] | Assesses the distance of the farm in relation to the nearby fragments of natural vegetation, mainly forest covers and bodies of water. | GIS/focus group |
| Extension of external connectors [EEC] | Evaluates the percentage of the linear extension of live fences located in the perimeter of the farms. | GIS/focus group |
| Extension of internal connectors [EIC] | Internally evaluates the percentage of the linear extension of the rows of vegetation. | GIS/focus group |
| Diversification of external connectors [DEC] | Evaluates the diversity of live fences or hedges located in the perimeter of the major agroecosystem. | GIS/focus group |
| Diversification of internal connectors [DIC] | Evaluates the diversification of internal live fences. | GIS/focus group |
| Soil Use and Conservation [USC] | This parameter evaluates the distribution percentage of different covers within the farm and the conservation of the soil (evidence of erosion). | GIS/ Interview/ focus group |
| Management of Weeds [MW] | Evaluates the management practices and systems of weeds. | Interview/focus group |
| Other Management Practices [OP] | Is an indicator that expresses the type of production system (ecological, conventional, or in transition) of each farm | Interview/focus group |
| Perception-Awareness [PA] | Evaluates the degree of conceptual clarity and awareness of producers regarding agrobiodiversity. | Interview/focus group |
| Level of Capacity for Action [CA] | Evaluates the capacities and possibilities of farmers to establish, maintain, or improve their MAS | Interview/focus group |
| Agroecosystems Assessment | |
|---|---|
| Area cultivated (ha) | 1.17 ± 1.1 |
| Parameters | |
| Area of influence (ha) | 48.98 ± 79 |
| Semi-natural habitat patches (%) | 5.5 ± 12.1 |
| CMELS | |
| 92.59 ± 73.88 |
| 102.8 ± 55.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Altieri, M.A.; Nicholls, C.I.; de Molina, M.G.; Rojas, A.S. Landscape Agroecology: Methodologies and Applications for the Design of Sustainable Agroecosystems. Land 2024, 13, 1746. https://doi.org/10.3390/land13111746
Altieri MA, Nicholls CI, de Molina MG, Rojas AS. Landscape Agroecology: Methodologies and Applications for the Design of Sustainable Agroecosystems. Land. 2024; 13(11):1746. https://doi.org/10.3390/land13111746
Chicago/Turabian StyleAltieri, Miguel A., Clara I. Nicholls, Manuel González de Molina, and Angel Salazar Rojas. 2024. "Landscape Agroecology: Methodologies and Applications for the Design of Sustainable Agroecosystems" Land 13, no. 11: 1746. https://doi.org/10.3390/land13111746
APA StyleAltieri, M. A., Nicholls, C. I., de Molina, M. G., & Rojas, A. S. (2024). Landscape Agroecology: Methodologies and Applications for the Design of Sustainable Agroecosystems. Land, 13(11), 1746. https://doi.org/10.3390/land13111746







