Impact of Cropland Management on Invertebrate Richness and Abundance in Agroforestry Systems in Bali, Indonesia
Abstract
:1. Introduction
2. Materials and Methods
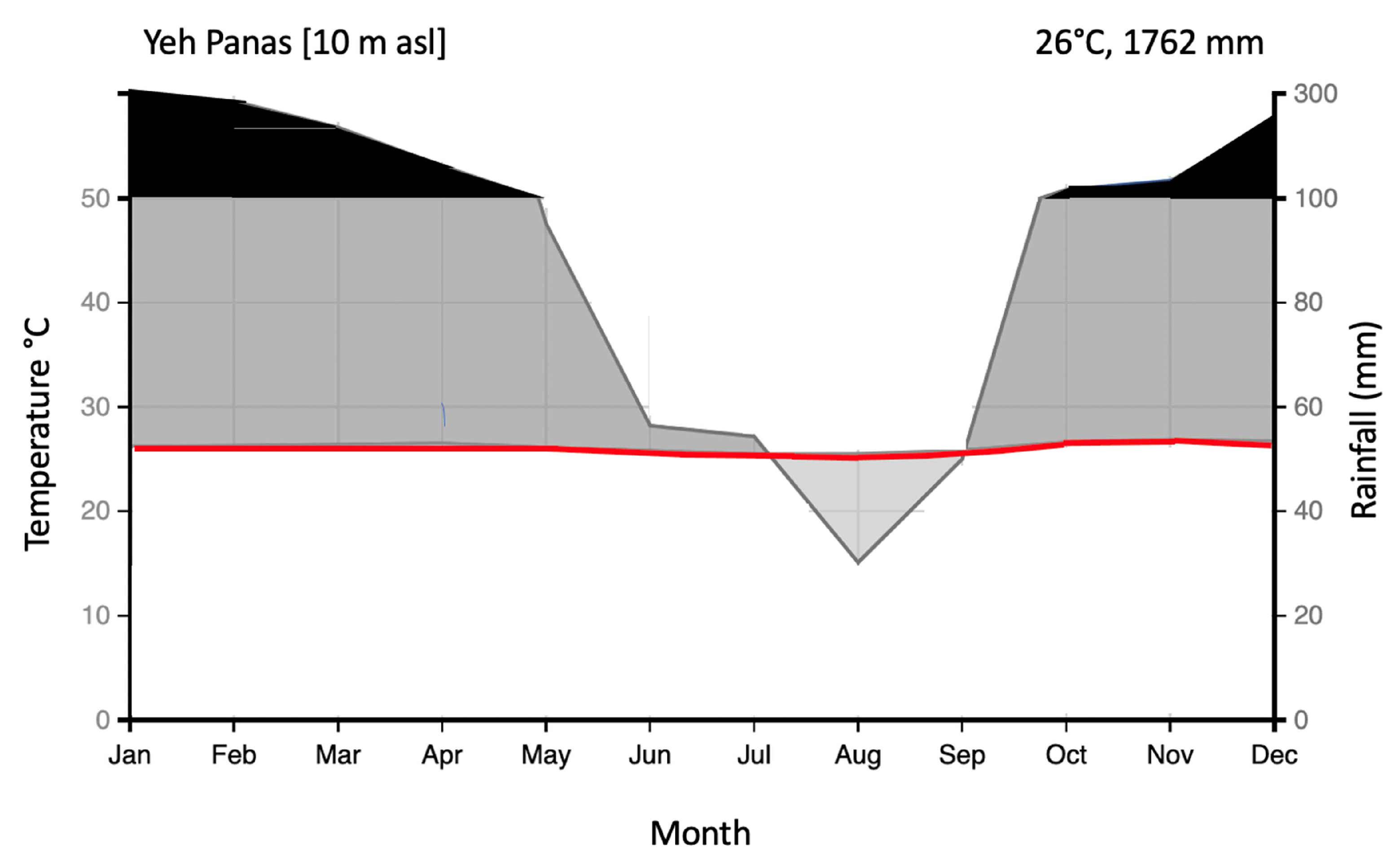
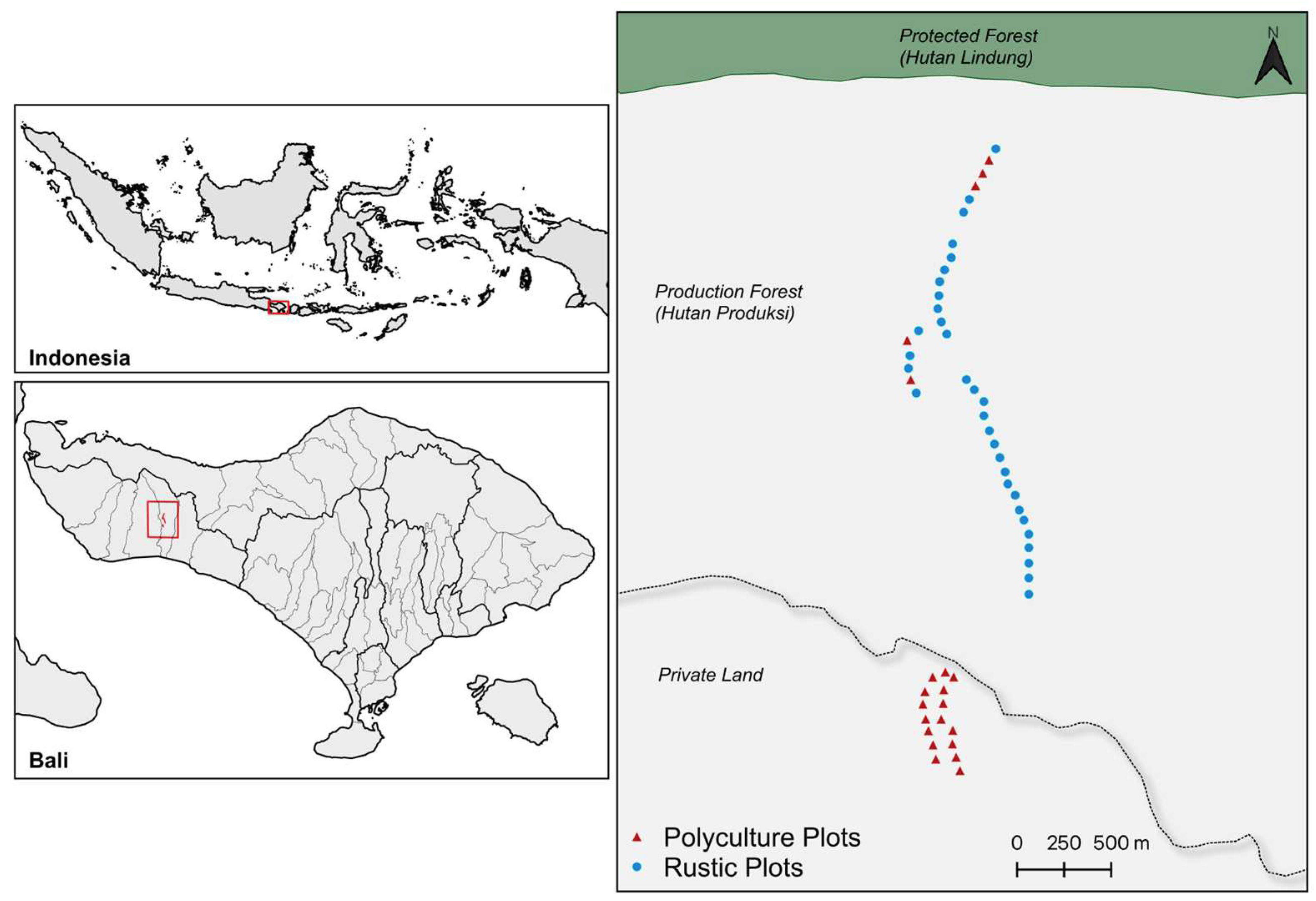
2.1. Study Area
2.2. Data Collection
2.3. Ethics and Permission
2.4. Data Analysis
3. Results
4. Discussion
4.1. The Effect of Cropland Management
4.2. Study Limitations
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Response | Predictor | Coefficient | Std Error | Z Value | p-Value |
|---|---|---|---|---|---|
| Araneae (abundance) | Intercept | 0.582 | 0.498 | 1.17 | 0.242 |
| Canopy cover | −0.005 | 0.007 | −0.71 | 0.478 | |
| Crop richness | 0.010 | 0.085 | 0.11 | 0.909 | |
| Habitat a | 0.361 | 0.397 | 0.91 | 0.363 | |
| Tree richness | 0.042 | 0.085 | 0.49 | 0.623 | |
| Yields (USD) | 4.3 × 10−4 | 3.3 × 10−4 | 1.29 | 0.196 | |
| Araneae (richness) | Intercept | 0.795 | 0.528 | 1.51 | 0.132 |
| Canopy cover | 0.000 | 0.007 | 0.01 | 0.989 | |
| Crop richness | −0.169 | 0.099 | −1.69 | 0.090 | |
| Habitat a | 0.000 | 0.419 | 0.00 | 0.999 | |
| Tree richness | −0.009 | 0.010 | −0.09 | 0.929 | |
| Yields (USD) | 4.7 × 10−4 | 3.0 × 10−4 | 1.56 | 0.120 | |
| Blattodea (abundance) | Intercept | −0.198 | 1.446 | −0.14 | 0.891 |
| Canopy cover | 0.004 | 0.015 | 0.26 | 0.794 | |
| Crop richness | −0.305 | 0.268 | −1.14 | 0.255 | |
| Habitat a | 0.989 | 0.915 | 1.08 | 0.279 | |
| Tree richness | −0.057 | 0.192 | −0.30 | 0.768 | |
| Yields (USD) | 6.5 × 10−4 | 4.3 × 10−4 | 1.53 | 0.125 | |
| Blattodea (richness) | Intercept | −1.149 | 1.296 | −0.89 | 0.375 |
| Canopy cover | 0.006 | 0.014 | 0.43 | 0.668 | |
| Crop richness | −0.234 | 0.235 | −1.00 | 0.319 | |
| Habitat a | 0.939 | 0.896 | 1.05 | 0.295 | |
| Tree richness | −0.004 | 0.177 | −0.02 | 0.983 | |
| Yields (USD) | 7.0 × 10−4 | 4.2 × 10−4 | 1.65 | 0.099 | |
| Coleoptera (abundance) | Intercept | −1.454 | 1.056 | −1.37 | 0.169 |
| Canopy cover | 0.027 | 0.013 | 2.07 * | 0.038 | |
| Crop richness | 0.006 | 0.161 | 0.04 | 0.971 | |
| Habitat a | −2.136 | 1.103 | −1.94 | 0.053 | |
| Tree richness | 0.415 | 0.210 | 1.98 * | 0.048 | |
| Yields (USD) | −5.8 × 10−4 | 9.9 × 10−4 | −0.58 | 0.562 | |
| Coleoptera (richness) | Intercept | −1.590 | 1.083 | −1.47 | 0.142 |
| Canopy cover | 0.029 | 0.014 | 2.02 * | 0.043 | |
| Crop richness | 0.019 | 0.165 | 0.12 | 0.907 | |
| Habitat a | −2.113 | 1.152 | −1.84 | 0.066 | |
| Tree richness | 0.358 | 0.227 | 1.58 | 0.114 | |
| Yields (USD) | −5.0 × 10−4 | 10.0 × 10−4 | −0.50 | 0.619 | |
| Dermaptera (abundance) | Intercept | −2.954 | 1.627 | −1.82 | 0.070 |
| Canopy cover | 0.028 | 0.020 | 1.42 | 0.154 | |
| Crop richness | 0.165 | 0.238 | 0.69 | 0.488 | |
| Habitat a | −0.090 | 1.246 | −0.07 | 0.942 | |
| Tree richness | 0.067 | 0.306 | −0.22 | 0.827 | |
| Yields (USD) | −0.1 × 10−4 | 10.8 × 10−4 | −0.01 | 0.990 | |
| Dermaptera (richness) | Intercept | −3.809 | 1.674 | −2.28 * | 0.023 |
| Canopy cover | 0.015 | 0.019 | 0.82 | 0.413 | |
| Crop richness | 0.308 | 0.237 | 1.30 | 0.193 | |
| Habitat a | −0.191 | 1.341 | −0.14 | 0.887 | |
| Tree richness | 0.131 | 0.273 | 0.48 | 0.631 | |
| Yields (USD) | −1.3 × 10−4 | 11.1 × 10−4 | −0.12 | 0.907 | |
| Diptera-pest (abundance) | Intercept | 1.162 | 0.593 | 1.96 | 0.050 |
| Canopy cover | −0.002 | 0.008 | −0.29 | 0.772 | |
| Crop richness | 0.039 | 0.102 | 0.39 | 0.700 | |
| Habitat a | 0.037 | 0.555 | 0.07 | 0.947 | |
| Tree richness | 0.025 | 0.118 | 0.21 | 0.830 | |
| Yields (USD) | −4.4 × 10−4 | 4.3 × 10−4 | −1.03 | 0.305 | |
| Diptera-pest (richness) | Intercept | 0.019 | 0.573 | 0.03 | 0.973 |
| Canopy cover | 0.001 | 0.007 | 0.10 | 0.923 | |
| Crop richness | 0.051 | 0.096 | 0.53 | 0.594 | |
| Habitat a | −0.163 | 0.484 | −0.34 | 0.737 | |
| Tree richness | 0.060 | 0.104 | 0.58 | 0.565 | |
| Yields (USD) | −4.1 × 10−4 | 4.3 × 10−4 | −0.94 | 0.349 | |
| Diptera-non pest (abundance) | Intercept | 1.974 | 0.811 | 2.43 * | 0.015 |
| Canopy cover | 0.020 | 0.009 | 2.30 * | 0.021 | |
| Crop richness | −0.160 | 0.143 | −1.11 | 0.265 | |
| Habitat a | −0.723 | 0.645 | −1.12 | 0.262 | |
| Tree richness | 0.042 | 0.142 | 0.30 | 0.767 | |
| Yields (USD) | −7.6 × 10−4 | 7.9 × 10−4 | −0.97 | 0.334 | |
| Diptera-non pest (richness) | Intercept | −0.256 | 0.672 | −0.38 | 0.704 |
| Canopy cover | 0.012 | 0.008 | 1.42 | 0.128 | |
| Crop richness | −0.011 | 0.111 | −0.10 | 0.922 | |
| Habitat a | −0.169 | 0.544 | −0.31 | 0.757 | |
| Tree richness | 0.040 | 0.116 | 0.35 | 0.729 | |
| Yields (USD) | −5.1 × 10−4 | 5.3 × 10−4 | −0.95 | 0.342 | |
| Hemiptera (abundance) | Intercept | −0.640 | 0.908 | −0.71 | 0.481 |
| Canopy cover | 0.008 | 0.011 | 0.69 | 0.491 | |
| Crop richness | 0.084 | 0.152 | 0.55 | 0.580 | |
| Habitat a | −1.312 | 0.752 | −1.75 | 0.081 | |
| Tree richness | 0.485 | 0.165 | 2.93 ** | 0.003 | |
| Yields (USD) | 10.5 × 10−4 | 5.5 × 10−4 | 1.92 | 0.055 | |
| Hemiptera (richness) | Intercept | −1.641 | 0.777 | −2.11 * | 0.035 |
| Canopy cover | 0.009 | 0.009 | 1.03 | 0.305 | |
| Crop richness | 0.160 | 0.115 | 1.39 | 0.165 | |
| Habitat a | −0.743 | 0.644 | −1.15 | 0.249 | |
| Tree richness | 0.329 | 0.126 | 2.62 ** | 0.009 | |
| Yields (USD) | 3.5 × 10−4 | 4.0 × 10−4 | 0.86 | 0.389 | |
| Hymenoptera-biological control (abundance) | Intercept | −0.593 | 0.910 | −0.65 | 0.515 |
| Canopy cover | −0.023 | 0.011 | −1.99 * | 0.046 | |
| Crop richness | 0.281 | 0.146 | 1.92 | 0.055 | |
| Habitat a | −1.376 | 0.793 | −1.74 | 0.083 | |
| Tree richness | 0.533 | 0.163 | 3.26 ** | 0.001 | |
| Yields (USD) | 8.7 × 10−4 | 5.1 × 10−4 | 1.69 | 0.091 | |
| Hymenoptera-biological control (richness) | Intercept | −0.028 | 0.662 | −0.04 | 0.966 |
| Canopy cover | −0.009 | 0.009 | −0.98 | 0.327 | |
| Crop richness | −0.016 | 0.117 | −0.14 | 0.892 | |
| Habitat a | −0.519 | 0.582 | −0.89 | 0.373 | |
| Tree richness | 0.159 | 0.127 | 1.25 | 0.213 | |
| Yields (USD) | 6.8 × 10−4 | 2.8 × 10−4 | 2.41 * | 0.016 | |
| Hymenoptera-Formicidae b (abundance) | Intercept | 1.354 | 0.606 | 2.23 * | 0.026 |
| Canopy cover | 0.017 | 0.008 | 2.13 * | 0.033 | |
| Crop richness | 0.070 | 0.109 | 0.64 | 0.519 | |
| Habitat a | 0.887 | 0.546 | 1.67 | 0.095 | |
| Tree richness | 0.074 | 0.118 | 0.63 | 0.530 | |
| Yields (USD) | 8.0 × 10−4 | 3.1 × 10−4 | 2.56 * | 0.011 | |
| Hymenoptera-Formicidae b (richness) | Intercept | 0.493 | 0.260 | 1.90 | 0.058 |
| Canopy cover | 0.008 | 0.003 | 2.37 * | 0.018 | |
| Crop richness | 0.011 | 0.043 | 0.26 | 0.792 | |
| Habitat a | −0.117 | 0.222 | −0.53 | 0.598 | |
| Tree richness | 0.071 | 0.050 | 1.43 | 0.153 | |
| Yields (USD) | 2.1 × 10−4 | 1.9 × 10−4 | 1.12 | 0.263 | |
| Isopoda (abundance) | Intercept | −1.286 | 1.931 | −0.67 | 0.506 |
| Canopy cover | 0.008 | 0.018 | 0.43 | 0.667 | |
| Crop richness | −0.217 | 0.312 | −0.69 | 0.487 | |
| Habitat a | 0.417 | 1.454 | 0.29 | 0.774 | |
| Tree richness | 0.233 | 0.232 | 1.00 | 0.317 | |
| Yields (USD) | −7.7 × 10−4 | 14.7 × 10−4 | −0.53 | 0.598 | |
| Isopoda (richness) | Intercept | −1.340 | 1.823 | −0.74 | 0.462 |
| Canopy cover | 0.010 | 0.019 | 0.56 | 0.578 | |
| Crop richness | −0.330 | 0.319 | −1.04 | 0.300 | |
| Habitat a | 0.271 | 1.483 | 0.18 | 0.855 | |
| Tree richness | 0.195 | 0.241 | 0.81 | 0.418 | |
| Yields (USD) | −9.4 × 10−4 | 16.7 × 10−4 | −0.56 | 0.575 | |
| Orthoptera (abundance) | Intercept | 0.862 | 0.525 | 1.64 | 0.101 |
| Canopy cover | −0.006 | 0.007 | −0.81 | 0.417 | |
| Crop richness | −0.003 | 0.090 | −0.03 | 0.974 | |
| Habitat a | −0.662 | 0.451 | −1.47 | 0.142 | |
| Tree richness | 0.208 | 0.094 | 2.22 * | 0.026 | |
| Yields (USD) | 1.4 × 10−4 | 4.2 × 10−4 | 0.03 | 0.973 | |
| Orthoptera (richness) | Intercept | 0.198 | 0.630 | 0.31 | 0.753 |
| Canopy cover | 0.008 | 0.008 | 1.04 | 0.298 | |
| Crop richness | −0.090 | 0.109 | −0.82 | 0.410 | |
| Habitat a | −0.745 | 0.549 | −1.36 | 0.175 | |
| Tree richness | 0.163 | 0.116 | 1.40 | 0.162 | |
| Yields (USD) | 1.2 × 10−4 | 3.9 × 10−4 | 0.31 | 0.755 | |
| Talitridae (abundance) | Intercept | −1.559 | 1.498 | −1.04 | 0.298 |
| Canopy cover | −0.011 | 0.016 | −0.71 | 0.478 | |
| Crop richness | 0.164 | 0.241 | 0.68 | 0.495 | |
| Habitat a | 1.911 | 0.880 | 2.17 | 0.030 | |
| Tree richness | −0.243 | 0.193 | −1.26 | 0.208 | |
| Yields (USD) | 8.7 × 10−4 | 4.0 × 10−4 | 2.17 * | 0.030 | |
| Talitridae (richness) | Intercept | −2.015 | 1.638 | −1.23 | 0.219 |
| Canopy cover | −0.017 | 0.020 | −0.85 | 0.397 | |
| Crop richness | 0.131 | 0.279 | 0.47 | 0.639 | |
| Habitat a | 2.415 | 1.221 | 1.98 * | 0.048 | |
| Tree richness | −0.386 | 0.282 | −1.37 | 0.171 | |
| Yields (USD) | 6.2 × 10−4 | 11.1 × 10−4 | 0.56 | 0.578 | |
| Zygentoma (abundance) | Intercept | −2.372 | 0.987 | −2.40 * | 0.016 |
| Canopy cover | 0.003 | 0.012 | 0.27 | 0.790 | |
| Crop richness | 0.306 | 0.152 | 2.02 * | 0.044 | |
| Habitat a | 0.267 | 0.791 | 0.34 | 0.736 | |
| Tree richness | 0.124 | 0.163 | 0.76 | 0.446 | |
| Yields (USD) | 1.8 × 10−4 | 7.1 × 10−4 | 0.26 | 0.799 | |
| Zygentoma (richness) | Intercept | −1.720 | 1.360 | −1.26 | 0.206 |
| Canopy cover | 0.000 | 0.017 | 0.00 | 0.998 | |
| Crop richness | 0.196 | 0.227 | 0.86 | 0.387 | |
| Habitat a | 0.227 | 1.057 | 0.21 | 0.830 | |
| Tree richness | 0.125 | 0.237 | 0.53 | 0.597 | |
| Yields (USD) | 5.0 × 10−4 | 10.3 × 10−4 | 0.48 | 0.629 | |
| Total abundance | Intercept | 2.682 | 0.409 | 6.56 ** | <0.001 |
| Canopy cover | 0.011 | 0.006 | 2.02 * | 0.044 | |
| Crop richness | 0.036 | 0.075 | 0.48 | 0.634 | |
| Habitat a | 0.334 | 0.387 | 0.86 | 0.389 | |
| Tree richness | 0.101 | 0.085 | 1.19 | 0.233 | |
| Yields (USD) | 6.3 × 10−4 | 2.2 × 10−4 | 2.80 ** | 0.005 | |
| Total richness | Intercept | 2.098 | 0.236 | 8.89 ** | <0.001 |
| Canopy cover | 0.004 | 0.003 | 1.48 | 0.139 | |
| Crop richness | 0.003 | 0.040 | 0.07 | 0.942 | |
| Habitat a | −0.221 | 0.192 | −1.16 | 0.248 | |
| Tree richness | 0.095 | 0.042 | 2.28 * | 0.023 | |
| Yields (USD) | 2.0 × 10−4 | 1.9 × 10−4 | 1.04 | 0.298 |
References
- Wagner, D.L. Insect declines in the Anthropocene. An. Rev. Entomol. 2020, 65, 457–480. [Google Scholar] [CrossRef] [PubMed]
- Wagner, D.L.; Grames, E.M.; Forister, M.L.; Berenbaum, M.R.; Stopak, D. Insect decline in the Anthropocene: Death by a thousand cuts. Proc. Natl. Acad. Sci. USA 2021, 118, e2023989118. [Google Scholar] [CrossRef] [PubMed]
- Philpott, S.M.; Arendt, W.J.; Armbrecht, I.; Bichier, P.; Diestch, T.V.; Gordon, C.; Greenberg, R.; Perfecto, I.; Reynoso-Santos, R.; Soto-Pinto, L.; et al. Biodiversity loss in Latin American coffee landscapes: Review of the evidence on ants, birds, and trees. Conserv. Biol. 2008, 22, 1093–1105. [Google Scholar] [CrossRef] [PubMed]
- Perfecto, I.; Mas, A.; Dietsch, T.; Vandermeer, J. Conservation of biodiversity in coffee agroecosystems: A tri-taxa comparison in southern Mexico. Biodivers. Conserv. 2003, 12, 1239–1252. [Google Scholar] [CrossRef]
- Raven, P.H.; Wagner, D.L. Agricultural intensification and climate change are rapidly decreasing insect biodiversity. Proc. Natl. Acad. Sci. USA 2021, 118, e2002548117. [Google Scholar] [CrossRef] [PubMed]
- Manson, S.; Nekaris, K.A.I.; Nijman, V.; Campera, M. Effect of shade on biodiversity within coffee farms: A meta-analysis. Sci. Total Environ. 2024, 914, 169882. [Google Scholar] [CrossRef] [PubMed]
- Philpott, S.M. Biodiversity and pest control services. In Encyclopedia of Biodiversity, 2nd ed.; Levin, S., Ed.; Academic Press: Waltham, MA, USA, 2013; pp. 373–385. [Google Scholar]
- Jose, S. Agroforestry for ecosystem services and environmental benefits: An overview. Agroforest Syst. 2009, 76, 1–10. [Google Scholar] [CrossRef]
- Bhagwat, S.A.; Willis, K.J.; Birks, H.J.B.; Whittaker, R.J. Agroforestry: A refuge for tropical biodiversity? Trends Ecol. Evol. 2008, 23, 261–267. [Google Scholar] [CrossRef] [PubMed]
- McNeely, J.A.; Schroth, G. Agroforestry and biodiversity conservation—Traditional practices, present dynamics, and lessons for the future. Biodivers. Conserv. 2006, 15, 549–554. [Google Scholar] [CrossRef]
- Staab, M.; Gossner, M.M.; Simons, N.K.; Achury, R.; Ambarlı, D.; Bae, S.; Schall, P.; Weisser, W.W.; Blüthgen, N. Insect decline in forests depends on species’ traits and may be mitigated by management. Commun. Biol. 2023, 6, 338. [Google Scholar] [CrossRef] [PubMed]
- Mace, G.M.; Norris, K.; Fitter, A.H. Biodiversity and ecosystem services: A multilayered relationship. Trends Ecol. Evol. 2012, 27, 19–26. [Google Scholar] [CrossRef]
- Campera, M.; Budiadi, B.; Bušina, T.; Fathoni, B.H.; Dermody, J.; Nijman, V.; Imron, M.A.; Nekaris, K.A.I. Abundance and richness of invertebrates in shade-grown versus sun-exposed coffee home gardens in Indonesia. Agroforest Syst. 2022, 96, 829–841. [Google Scholar] [CrossRef]
- Tscharntke, T.; Clough, Y.; Bhagwat, S.A.; Buchori, D.; Faust, H.; Hertel, D.; Hölscher, D.; Juhrbandt, J.; Kessler, M.; Perfecto, I.; et al. Multifunctional shade-tree management in tropical agroforestry landscapes—A review. J. Appl. Ecol. 2011, 48, 619–629. [Google Scholar] [CrossRef]
- Arenas-Clavijo, A.; Armbrecht, I. Soil ants (Hymenoptera: Formicidae) and ground beetles (Coleoptera: Carabidae) in a coffee agroforestry landscape during a severe-drought period. Agroforest Syst. 2019, 93, 1781–1792. [Google Scholar] [CrossRef]
- Iwasaki, J.M.; Hoogendorn, K. Non-insecticide pesticide impacts on bees: A review of methods and reported outcomes. Agric. Ecosyst. Environ. 2021, 314, 107423. [Google Scholar] [CrossRef]
- Manson, S.; Nekaris, K.A.I.; Hedger, K.; Balestri, M.; Ahmad, N.; Adinda, E.; Budiadi, B.; Imron, M.A.; Nijman, V.; Campera, M. Flower Visitation Time and Number of Visitor Species Are Reduced by the Use of Agrochemicals in Coffee Home Gardens. Agronomy 2022, 12, 509. [Google Scholar] [CrossRef]
- Klein, A.-M.; Vaissière, B.E.; Cane, J.H.; Steffan-Derwenter, I.; Cunningham, S.A.; Kremen, C.; Tscharntke, T. Importance of pollinators in changing landscapes for world crops. Proc. Royal Soc. B 2007, 274, 303–313. [Google Scholar] [CrossRef] [PubMed]
- Grames, E.M.; Montgomery, G.A.; Youngflesh, C.; Tingley, M.W.; Elphick, C.S. The effect of insect food availability on songbird reproductive success and chick body condition: Evidence from a systematic review and meta-analysis. Ecol. Lett. 2023, 26, 658–673. [Google Scholar] [CrossRef] [PubMed]
- Forister, M.L.; Black, S.H.; Elphick, C.S.; Grames, E.M.; Halsch, C.A.; Schultz, C.B.; Wagner, D.L. Missing the bigger picture: Why insect monitoring programs are limited in their ability to document the effects of habitat loss. Conserv. Lett. 2023, 16, e12951. [Google Scholar] [CrossRef]
- Cramer, M.J.; Willig, M.R. Habitat heterogeneity, species diversity and null models. Oikos 2005, 108, 209–218. [Google Scholar] [CrossRef]
- Astorga, A.; Death, R.; Death, F.; Paavola, R.; Chakraborty, M.; Muotka, T. Habitat heterogeneity drives the geographical distribution of beta diversity: The case of New Zealand stream invertebrates. Ecol. Evol. 2014, 4, 2693–2702. [Google Scholar] [CrossRef] [PubMed]
- Cole, L.J.; Pollock, M.L.; Robertson, D.; Holland, J.P.; McCracken, D.I.; Harrison, W. The influence of fine-scale habitat heterogeneity on invertebrate assemblage structure in upland semi-natural grassland. Agric. Ecosyst. Environ. 2010, 136, 69–80. [Google Scholar] [CrossRef]
- Holt, A.R.; Warren, P.H.; Gaston, K.J. The importance of habitat heterogeneity, biotic interactions and dispersal in abundance-occupancy relationships. J. An. Ecol. 2004, 73, 841–851. [Google Scholar] [CrossRef]
- Dennis, P.; Young, M.R.; Gordon, I.J. Distribution and abundance of small insects and arachnids in relation to structural heterogeneity of grazed, indigenous grasslands. Ecol. Entomol. 1998, 23, 253–264. [Google Scholar] [CrossRef]
- Santoro, A.; Piras, F.; Yu, Q. Spatial analysis of deforestation in Indonesia in the period 1950–2017 and the role of protected areas. Biodivers. Conserv. 2023. [Google Scholar] [CrossRef]
- Kartawinata, K. The classification and utilization of forests in Indonesia. In Assessing Tropical Forest Lands; Carpenter, R.A., Ed.; Tycooly International Publishing Ltd.: Dublin, Ireland, 1981; pp. 163–174. [Google Scholar]
- Cordeiro, A.A.C.; Coelho, S.D.; Ramos, N.C.; Meira-Neto, J.A.A. Agroforestry systems reduce invasive species richness and diversity in the surroundings of protected areas. Agroforest Syst. 2018, 92, 1495–1505. [Google Scholar] [CrossRef]
- Haggar, J.; Pons, S.; Saenz, L.; Vides, M. Contribution of agroforestry systems to sustaining biodiversity in fragmented forest landscapes. Agric. Ecosyst. Environ. 2019, 283, 106567. [Google Scholar] [CrossRef]
- Osadolor, N.; Isese, M.O.O. Crop-based agroforestry systems in the buffers of protected areas: Implications for tree species conservation in Okomu National Park, Nigeria. J. Res. For. Wildl. Environ. 2023, 15, 94–104. [Google Scholar]
- Manson, S.; Campera, M.; Hedger, K.; Ahmad, N.; Adinda, E.; Nijman, V.; Budiadi, B.; Imron, M.A.; Lukmandaru, G.; Nekaris, K.A.I. The effectiveness of a biopesticide in the reduction of coffee berry borers in coffee plants. Crop Prot. 2022, 161, 106075. [Google Scholar] [CrossRef]
- Larsen, T.H.; Forsyth, A. Trap spacing and transect design for dung beetle biodiversity studies. Biotropica 2005, 37, 322–325. [Google Scholar] [CrossRef]
- Oxbrough, A.; Irwin, S.; Kelly, T.C.; O’Halloran, J. Ground-dwelling invertebrates in reforested conifer plantations. For. Ecol. Manag. 2010, 259, 2111–2121. [Google Scholar] [CrossRef]
- Oliver, I.A.N.; Beattie, A.J. Designing a cost-effective invertebrate survey: A test of methods for rapid assessment of biodiversity. Ecol. Appl. 1996, 6, 594–607. [Google Scholar] [CrossRef]
- Patrignani, A.; Ochsner, T.E. Canopeo: A powerful new tool for measuring fractional green canopy cover. Agron. J. 2015, 107, 2312–2320. [Google Scholar] [CrossRef]
- Campera, M.; Balestri, M.; Manson, S.; Hedger, K.; Ahmad, N.; Adinda, E.; Nijman, V.; Budiadi, B.; Imron, M.A.; Nekaris, K.A.I. Shade trees and agrochemical use affect butterfly assemblages in coffee home gardens. Agric. Ecosyst. Environ. 2021, 319, 107547. [Google Scholar] [CrossRef]
- Heneberg, P.; Bogusch, P. To enrich or not to enrich? Are there any benefits of using multiple colors of pan traps when sampling aculeate Hymenoptera? J. Insect Conserv. 2014, 18, 1123–1136. [Google Scholar] [CrossRef]
- ASAB/ABS Guidelines for the ethical treatment of nonhuman animals in behavioural research and teaching. An. Behav. 2024, 207, 1–11.
- Folgarait, P.J. Ant biodiversity and its relationship to ecosystem functioning: A review. Biodiver. Conserv. 1998, 7, 1221–1244. [Google Scholar] [CrossRef]
- Brooks, M.E.; Kristensen, K.; Van Benthem, K.J.; Magnusson, A.; Berg, C.W.; Nielsen, A.; Skaug, H.J.; Machler, M.; Bolker, B.M. glmmTMB balances speed and flexibility among packages for zero-inflated generalized linear mixed modeling. R. J. 2017, 9, 378–400. [Google Scholar] [CrossRef]
- Hartig, F.; Hartig, M.F. Package ‘dharma’. R package. 2022. Available online: http://florianhartig.github.io/DHARMa/ (accessed on 20 February 2024).
- Geeraert, L.; Aerts, R.; Jordaens, K.; Dox, I.; Wellens, S.; Couri, M.; Berecha, G.; Honnay, O. Intensification of Ethiopian coffee agroforestry drives impoverishment of the Arabica coffee flower visiting bee and fly communities. Agrofores Syst. 2019, 93, 1729–1739. [Google Scholar] [CrossRef]
- Hafsah, H.; Iriawati, T.S.; Syamsudin, S. Flower visiting insects to Coffea arabica flower at different temperatures and the production of the fruit of arabica coffee. IOP Conf. Ser. Earth Environ. Sci. 2021, 948, 012046. [Google Scholar] [CrossRef]
- Krishnan, S.; Kushalappa, C.G.; Shaanker, R.U.; Ghazoul, J. Status of pollinators and their efficiency in coffee fruit set in a fragmented landscape mosaic in South India. Basic. Appl. Ecol. 2012, 13, 277–285. [Google Scholar] [CrossRef]
- Vandromme, M.; de Sande, E.V.; Pinceel, T.; Vanhove, W.; Trekels, H.; Vanschoenwinkel, B. Resolving the identity and breeding habitats of cryptic dipteran cacao flower visitors in a neotropical cacao agroforestry system. Basic. Appl. Ecol. 2023, 68, 35–45. [Google Scholar] [CrossRef]
- Udawatta, R.P.; Rankoth, L.; Jose, S. Agroforestry and Biodiversity. Sustainability 2019, 11, 2879. [Google Scholar] [CrossRef]
- Bujan, J.; Roeder, K.A.; de Beurs, K.; Weiser, M.D.; Kaspari, M. Thermal diversity of North American ant communities: Cold tolerance but not heat tolerance tracks ecosystem temperature. Global Ecol. Biogeogr. 2020, 29, 1486–1494. [Google Scholar] [CrossRef]
- Wenda, C.; Gaitán-Espitia, J.D.; Solano-Iguaran, J.J.; Nakamura, A.; Majcher, B.M.; Ashton, L.A. Heat tolerance variation reveals vulnerability of tropical herbivore-parasitoid interactions to climate change. Ecol. Lett. 2023, 26, 278–290. [Google Scholar] [CrossRef] [PubMed]
- Kaspari, M.; Weiser, M.D. Ant activity along moisture gradients in a neotropical forest. Biotropica 2000, 32, 703–711. [Google Scholar] [CrossRef]
- Clough, Y.; Barkmann, J.; Juhrbandt, J.; Kessler, M.; Wanger, T.C.; Anshary, A.; Buchori, D.; Cicuzza, D.; Darras, K.; Putra, D.D.; et al. Combining high biodiversity with high yields in tropical agroforests. Proc. Natl. Acad. Sci. USA 2011, 108, 8311–8316. [Google Scholar] [CrossRef]
- Piato, K.; Subía, C.; Lefort, F.; Pico, J.; Calderón, D.; Norgrove, L. No reduction in yield of young robusta coffee when grown under shade trees in Ecuadorian Amazonia. Life 2022, 12, 807. [Google Scholar] [CrossRef] [PubMed]
- Sperber, C.F.; Nakayama, K.; Valverde, M.J.; de Siqueira Neves, F. Tree species richness and density affect parasitoid diversity in cacao agroforestry. Basic. Appl. Ecol. 2004, 5, 241–251. [Google Scholar] [CrossRef]
- Pak, D.; Iverson, A.L.; Ennis, K.K.; Gonthier, D.J.; Vandermeer, J.H. Parasitoid wasps benefit from shade tree size and landscape complexity in Mexican coffee agroecosystems. Agric. Ecosyst. Environ. 2015, 206, 21–32. [Google Scholar] [CrossRef]
- Kök, S.; Tomanović, Z.; Karabacak, E.; Kasap, I. Do primary and secondary host plants affect aphid- parasitoid interactions in fruit orchards? Bull. Entomol. Res. 2022, 113, 326–334. [Google Scholar] [CrossRef] [PubMed]
- Ortis, G.; Triapitsyn, S.V.; Cavaletto, G.; Martinez-Sañudo, I.; Mazzon, L. Taxonomic identification and biological traits of Platystethynium triclavatum (Donev & Huber, 2002), comb. n. (Hymenoptera, Mymaridae), a newly recorded egg parasitoid of the Italian endemic pest Barbitistes vicetinus (Orthoptera, Tettigoniidae). PeerJ 2020, 8, e9667. [Google Scholar]
- Ward, S.E.; Hoffmann, A.A.; Van Helden, M.; Slavenko, A.; Umina, P.A. The effects of insecticide seed treatments on the parasitism and predation of Myzus persicae (Homoptera: Aphididae) in canola. J. Econ. Entomol. 2024, 117, 102–117. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Schmid, B.; Schmidt, A.; Li, S.; Wang, M.-Q.; Fornoff, F.; Staab, M.; Guo, P.-F.; Anttonen, P.; Chesters, D.; et al. Multitrophic arthropod diversity mediates tree diversity effects on primary productivity. Nat. Ecol. Evol. 2023, 7, 832–840. [Google Scholar] [CrossRef] [PubMed]
- Staton, T.; Walters, R.; Smith, J.; Breeze, T.; Girling, R. Management to promote flowering understoreys benefits natural enemy diversity, aphid suppression and income in an agroforestry system. Agronomy 2021, 11, 651. [Google Scholar] [CrossRef]
- Kurniawan, I.D.; Kinasih, I.; Akbar, R.T.M.; Chaidir, L.; Iqbal, S.; Pamungkas, B.; Imanudin, Z. Arthropod community structure indicating soil quality recovery in the organic agroecosystem of Mount Ciremai National Park’s buffer zone. J. Sustain. Agric. 2023, 38, 229–243. [Google Scholar] [CrossRef]
- Elmquist, D.C.; Kahl, K.B.; Johnson-Maynard, J.L.; Eigenbrode, S.D. Linking agricultural diversification practices, soil arthropod communities and soil health. J. Appl. Ecol. 2023, 60, 1952–1963. [Google Scholar] [CrossRef]
- Iheshiulo, E.M.-A.; Larney, F.J.; Hernandez-Ramirez, G.; St Luce, M.; Liu, K.; Chau, H.W. Do diversified crop rotations influence soil physical health? A meta-analysis. Soil. Tillage Res. 2023, 233, 105781. [Google Scholar] [CrossRef]
- Mwabvu, T.; Nxele, T.; Yekwayo, I. Does habitat type in no-tillage agroecosystems influence ground-dwelling macroarthropod community structure? A case study in KwaZulu-Natal, South Africa. Afr. J. Ecol. 2023, 61, 736–740. [Google Scholar] [CrossRef]
- Stenchly, K.; Clough, Y.; Tscharntke, T. Spider species richness in cocoa agroforesty systems, comparing vertical strata, local management and distance to forest. Agric. Ecosyst. Environ. 2012, 149, 189–194. [Google Scholar] [CrossRef]
- Philpott, S.M.; Birchier, P.; Rice, R.A.; Greenberg, R. Biodiversity conservation, yield, and alternative products in coffee agroecosystems in Sumatra, Indonesia. Biodivers. Conserv. 2008, 17, 1805–1820. [Google Scholar] [CrossRef]
- Satrya, I.D.G.; Kaihatu, T.S.; Budidharmanto, L.P.; Karya, D.F.; Rusadi, N.W.P. The role of ecotourism in preserving environmental awareness, cultural and natural attractiveness for promoting local communities in Bali, Indonesia. J. East. Eur. Cent. Asian Res. 2023, 10, 1063–1075. [Google Scholar] [CrossRef]
- Hairiah, K.; Widianto, W.; Suprayogo, D.; Van Noordwijk, M. Tree roots anchoring and binding soil: Reducing landslide risk in Indonesian agroforestry. Land 2020, 9, 256. [Google Scholar] [CrossRef]
- Verburg, R.; Rahn, E.; Verweij, P.; van Kuijk, M.; Ghazoul, J. An innovation perspective to climate change adaptation in coffee systems. Environ. Sci. Policy 2019, 97, 16–24. [Google Scholar] [CrossRef]
- Imron, M.A.; Campera, M.; Al Bihad, D.; Rachmawati, F.D.; Nugroho, F.E.; Budiadi, B.; Wianti, K.F.; Suprapto, E.; Nijman, V.; Nekaris, K.A.I. Bird Assemblages in Coffee Agroforestry Systems and Other Human Modified Habitats in Indonesia. Biology 2022, 11, 310. [Google Scholar] [CrossRef] [PubMed]
- Campera, M.; Hedger, K.; Birot, H.; Manson, S.; Balestri, M.; Budiadi, B.; Imron, M.A.; Nijman, V.; Nekaris, K.A.I. Does the presence of shade trees and distance to the forest affect detection rates of terrestrial vertebrates in coffee home gardens? Sustainability 2021, 13, 8540. [Google Scholar] [CrossRef]
- Popic, T.J.; Davila, Y.C.; Wardle, G.M. Evaluation of common methods for sampling invertebrate pollinator assemblages: Net sampling out-perform pan traps. PLoS ONE 2013, 8, e66665. [Google Scholar] [CrossRef] [PubMed]
- Jaques, S.A.; Jofré-Pérez, C.; Murúa, M.M.; Vieli, L.; Fontúrbel, F.E. Crop-Specific Effects on Pan-Trap Sampling of Potential Pollinators as Influenced by Trap Color and Location. Agronomy 2023, 13, 552. [Google Scholar] [CrossRef]
- Hohbein, R.R.; Conway, C.J. Pitfall traps: A review of methods for estimating arthropod abundance. Wildl. Soc. Bull. 2018, 42, 597–606. [Google Scholar] [CrossRef]

| Variable a | Rustic (N = 32) | Polyculture (N = 21) | Z Value | p-Value |
|---|---|---|---|---|
| Canopy cover (%) | 34.60 (2.98) | 21.10 (3.68) | 2.86 ** | 0.004 |
| Crop richness | 3.99 (0.21) | 5.49 (0.30) | −4.31 ** | <0.001 |
| Tree richness | 3.56 (0.32) | 0.00 (0.00) | NA | NA |
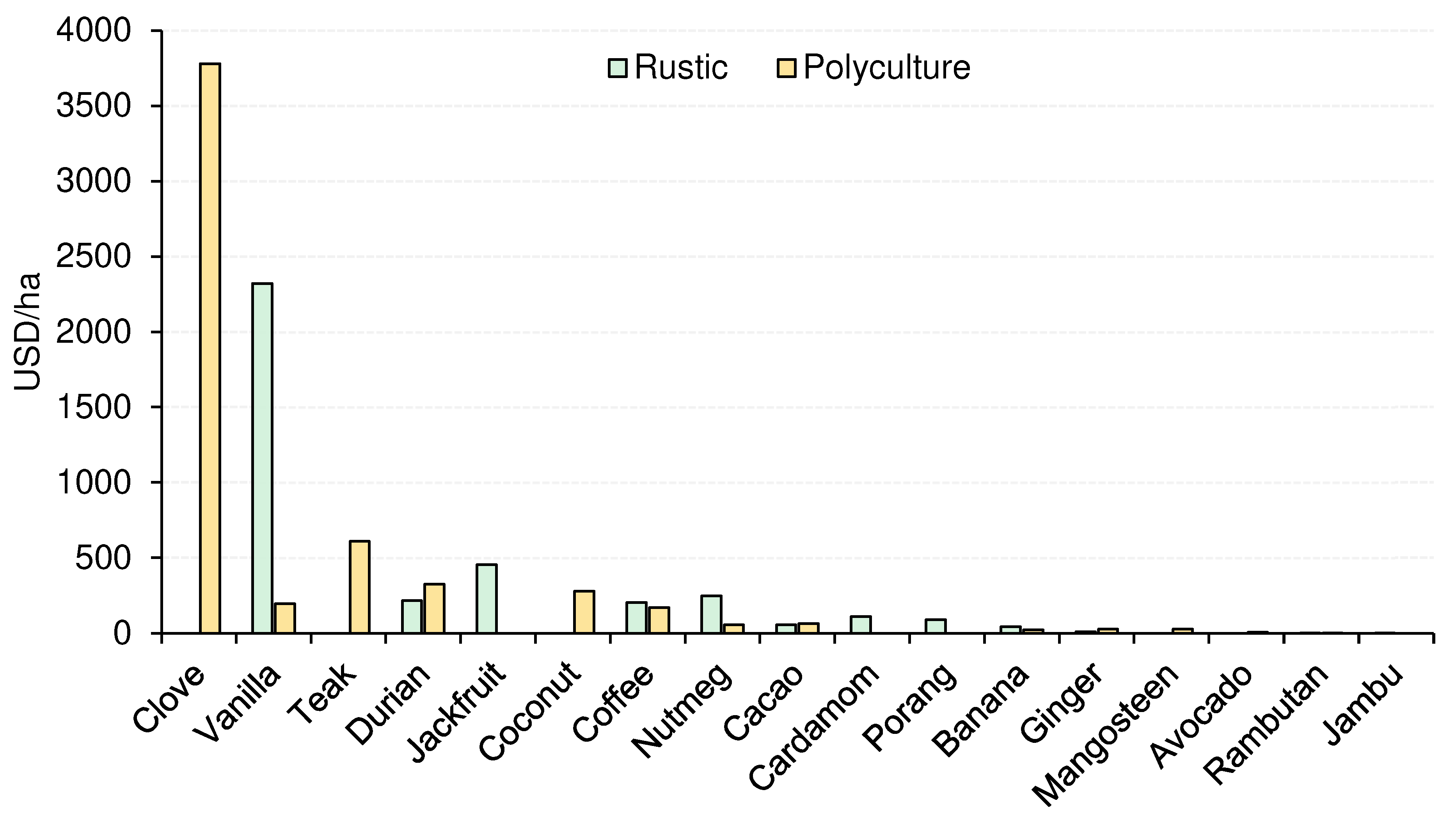
| Yields (USD) | 234.0 (38.3) | 347.0 (67.6) | 1.55 | 0.120 |
| Rustic (N = 32) | Polyculture (N = 21) | Total (N = 53) | ||||
|---|---|---|---|---|---|---|
| Taxa | Richness | Abundance | Richness | Abundance | Richness | Abundance |
| Araneae | 11 | 95 | 10 | 42 | 16 | 137 |
| Blattodea | 4 | 25 | 2 | 4 | 4 | 29 |
| Coleoptera | 10 | 13 | 7 | 8 | 15 | 21 |
| Dermaptera | 4 | 7 | 1 | 6 | 4 | 13 |
| Diptera (pest) | 8 | 113 | 8 | 71 | 10 | 184 |
| Diptera (other) | 8 | 128 | 3 | 79 | 8 | 207 |
| Hemiptera | 13 | 74 | 8 | 33 | 16 | 107 |
| Hymenoptera (biological control) | 15 | 82 | 11 | 46 | 20 | 128 |
| Hymenoptera (pollinator) | 2 | 4 | 5 | 5 | 7 | 9 |
| Hymenoptera (Formicidae) a | 17 | 1321 | 8 | 214 | 17 | 1535 |
| Isopoda | 2 | 86 | 1 | 50 | 2 | 136 |
| Lepidoptera | 4 | 4 | 2 | 2 | 6 | 6 |
| Opiliones | 1 | 1 | 0 | 0 | 1 | 1 |
| Orthoptera | 3 | 71 | 2 | 44 | 3 | 115 |
| Phasmatodea | 0 | 0 | 1 | 1 | 1 | 1 |
| Talitridae | 1 | 42 | 1 | 8 | 1 | 50 |
| Thysanoptera | 1 | 1 | 0 | 0 | 1 | 1 |
| Zygentoma | 1 | 26 | 1 | 14 | 1 | 40 |
| Total | 105 | 2027 | 71 | 576 | 133 | 2603 |
| Response | Predictor | Coefficient | Std Error | Z Value | p-Value |
|---|---|---|---|---|---|
| Araneae (richness) | Crop richness | −0.169 | 0.099 | −1.69 | 0.090 |
| Blattodea (richness) | Yields (USD) | 7.0 × 10−4 | 4.2 × 10−4 | 1.65 | 0.099 |
| Coleoptera (abundance) | Canopy cover | 0.027 | 0.013 | 2.07 * | 0.038 |
| Habitat a | −2.136 | 1.103 | −1.94 | 0.053 | |
| Tree richness | 0.415 | 0.210 | 1.98 * | 0.048 | |
| Coleoptera (richness) | Canopy cover | 0.029 | 0.014 | 2.02 * | 0.043 |
| Habitat a | −2.114 | 1.152 | −1.84 | 0.067 | |
| Diptera-non pest (abundance) | Canopy cover | 0.020 | 0.009 | 2.30 * | 0.021 |
| Hemiptera (abundance) | Habitat a | −1.312 | 0.752 | −1.75 | 0.081 |
| Tree richness | 0.485 | 0.165 | 2.93 ** | 0.003 | |
| Yields (USD) | 10.5 × 10−4 | 5.5 × 10−4 | 1.92 | 0.055 | |
| Hemiptera (richness) | Tree richness | 0.329 | 0.126 | 2.62 ** | 0.009 |
| Hymenoptera-biological control (abundance) | Canopy cover | −0.023 | 0.011 | −1.99 * | 0.046 |
| Crop richness | 0.281 | 0.146 | 1.92 | 0.055 | |
| Habitat a | −1.376 | 0.793 | −1.74 | 0.083 | |
| Tree richness | 0.533 | 0.163 | 3.26 ** | 0.001 | |
| Yields (USD) | 8.7 × 10−4 | 5.1 × 10−4 | 1.69 | 0.091 | |
| Hymenoptera-biological control (richness) | Yields (USD) | 6.8 × 10−4 | 2.8 × 10−4 | 2.41 * | 0.016 |
| Hymenoptera-Formicidae b (abundance) | Canopy cover | 0.017 | 0.008 | 2.13 * | 0.033 |
| Habitat a | 0.887 | 0.546 | 1.67 | 0.095 | |
| Yields (USD) | 8.0 × 10−4 | 3.1 × 10−4 | 2.56 * | 0.011 | |
| Hymenoptera-Formicidae b (richness) | Canopy cover | 0.008 | 0.003 | 2.37 * | 0.018 |
| Orthoptera (abundance) | Tree richness | 0.208 | 0.094 | 2.22 * | 0.026 |
| Talitridae (abundance) | Habitat a | 1.911 | 0.880 | 2.17 | 0.030 |
| Yields (USD) | 8.7 × 10−4 | 4.0 × 10−4 | 2.17 * | 0.030 | |
| Talitridae (richness) | Habitat a | 2.415 | 1.221 | 1.98 * | 0.048 |
| Zygentoma (abundance) | Crop richness | 0.306 | 0.152 | 2.02 * | 0.044 |
| Total abundance | Canopy cover | 0.011 | 0.006 | 2.02 * | 0.044 |
| Yields (USD) | 6.3 × 10−4 | 2.2 × 10−4 | 2.80 ** | 0.005 | |
| Total richness | Tree richness | 0.095 | 0.042 | 2.28 * | 0.023 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Campera, M.; Chavez, J.; Humber, C.; Jain, V.; Cioci, H.; Aulia, F.; Alua, K.A.; Prawerti, D.A.D.; Ali, S.R.R.; Swastika, I.W.; et al. Impact of Cropland Management on Invertebrate Richness and Abundance in Agroforestry Systems in Bali, Indonesia. Land 2024, 13, 493. https://doi.org/10.3390/land13040493
Campera M, Chavez J, Humber C, Jain V, Cioci H, Aulia F, Alua KA, Prawerti DAD, Ali SRR, Swastika IW, et al. Impact of Cropland Management on Invertebrate Richness and Abundance in Agroforestry Systems in Bali, Indonesia. Land. 2024; 13(4):493. https://doi.org/10.3390/land13040493
Chicago/Turabian StyleCampera, Marco, Jessica Chavez, Coral Humber, Vinni Jain, Hannah Cioci, Fadilla Aulia, Kristiana Aurel Alua, Desak Ayu Diah Prawerti, Sabarian Riskinto Ramadani Ali, I Wayan Swastika, and et al. 2024. "Impact of Cropland Management on Invertebrate Richness and Abundance in Agroforestry Systems in Bali, Indonesia" Land 13, no. 4: 493. https://doi.org/10.3390/land13040493
APA StyleCampera, M., Chavez, J., Humber, C., Jain, V., Cioci, H., Aulia, F., Alua, K. A., Prawerti, D. A. D., Ali, S. R. R., Swastika, I. W., Dusak, P. G. B. J., Priatama, I. P. A., Jones, A. K., Bulbert, M. W., Putra, N. G. M., Kuntayuni, Sukmadewi, D. K. T., Nijman, V., Setiawan, I. M., & Manson, S. (2024). Impact of Cropland Management on Invertebrate Richness and Abundance in Agroforestry Systems in Bali, Indonesia. Land, 13(4), 493. https://doi.org/10.3390/land13040493








