Distribution Characteristics and Main Influencing Factors of Organic Carbon in Sediments of Spartina Alterniflora Wetlands along the Northern Jiangsu Coast, China
Abstract
:1. Introduction
2. Materials and Methods
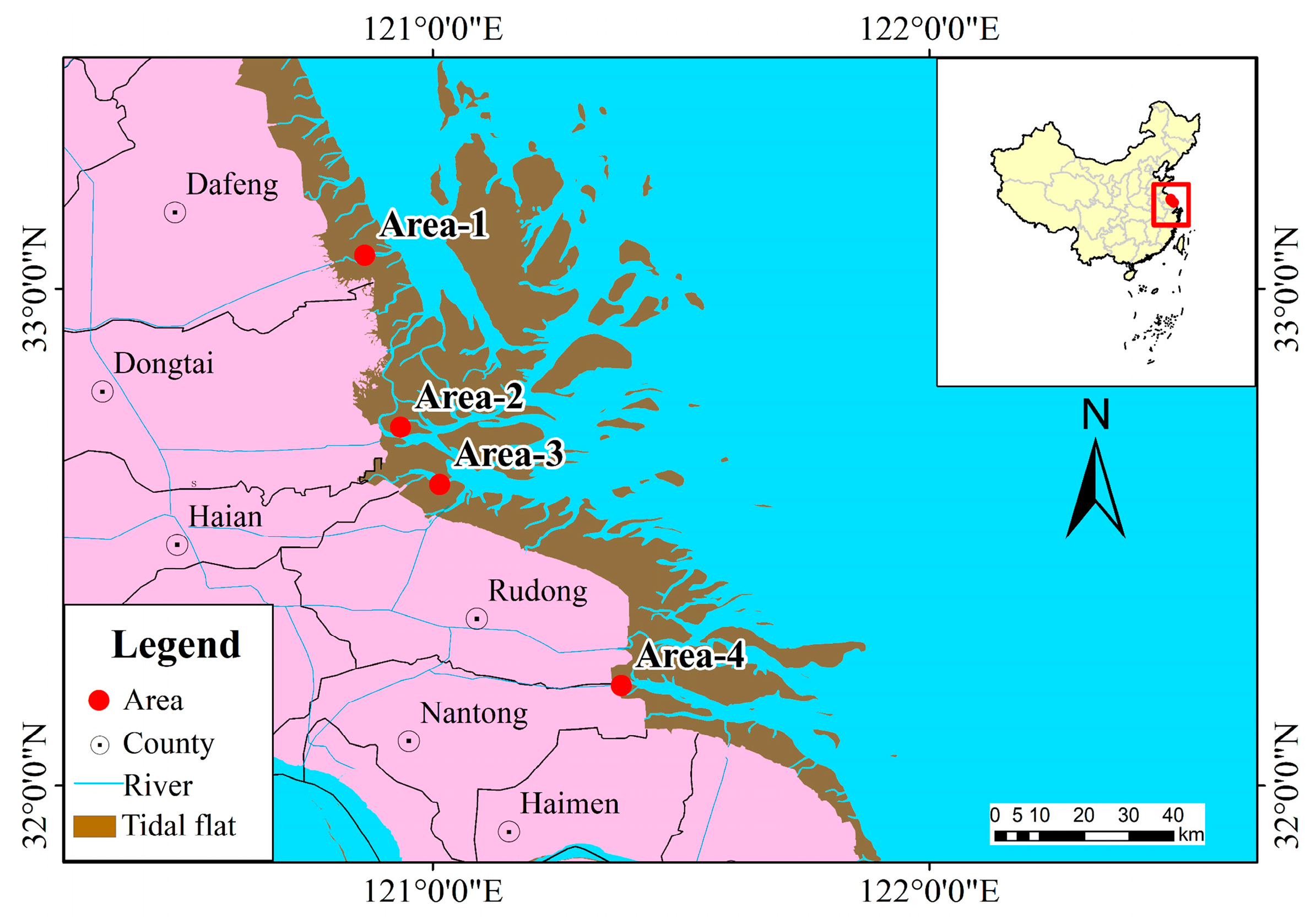
2.1. Study Area
2.2. Sample Collection and Laboratory Analyses
2.2.1. Total Carbon, Organic Carbon, and Total Nitrogen Analyses
2.2.2. Particle Size Analyses
2.2.3. Total Phosphorus and Total Sulfur Analyses
2.2.4. Bulk Density Analyses
2.2.5. Moisture Content Analyses
2.2.6. Salinity and pH Analyses
2.3. Impact Factors and Their Importance to Organic Carbon
2.4. Statistical Analyses
3. Results
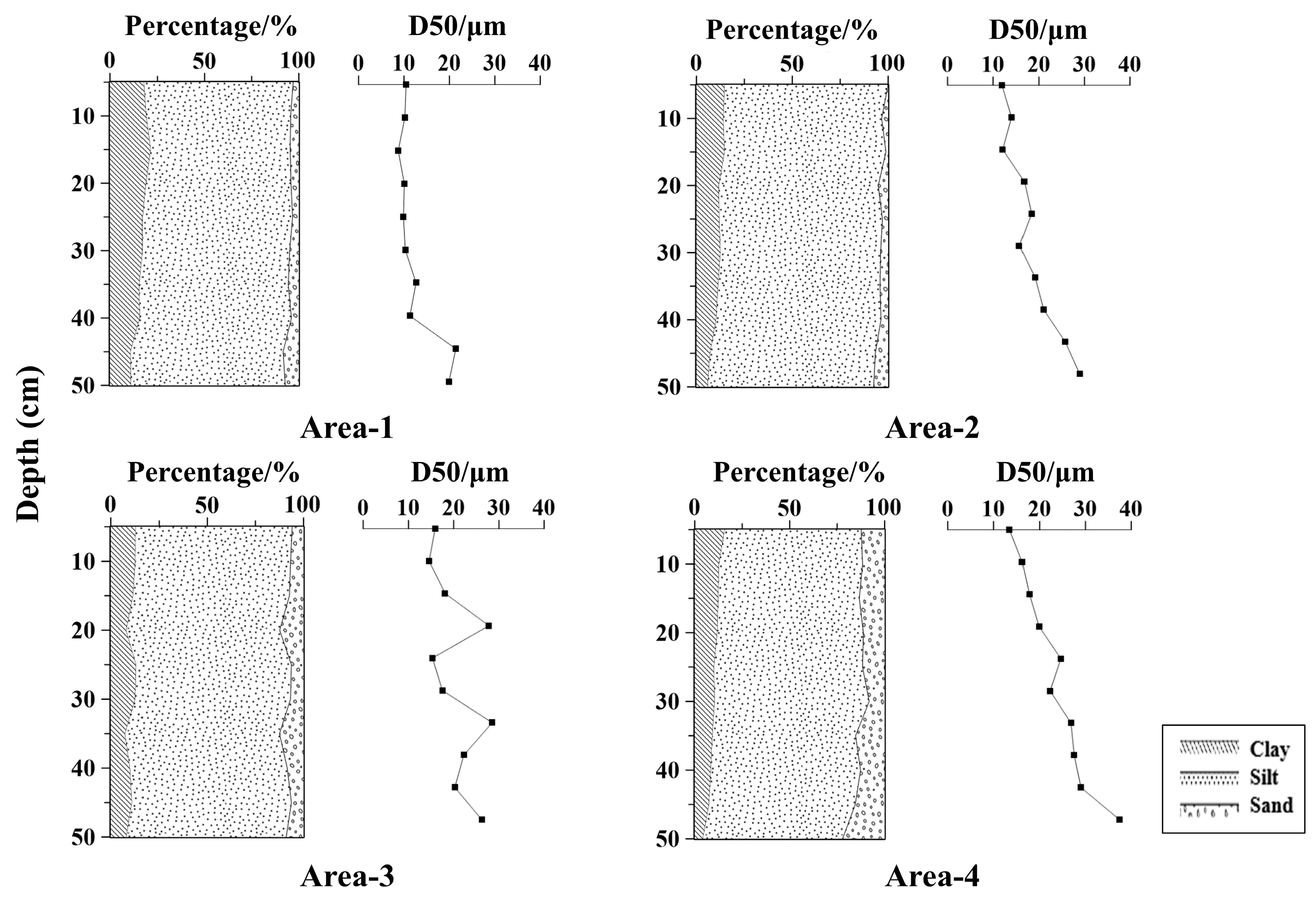
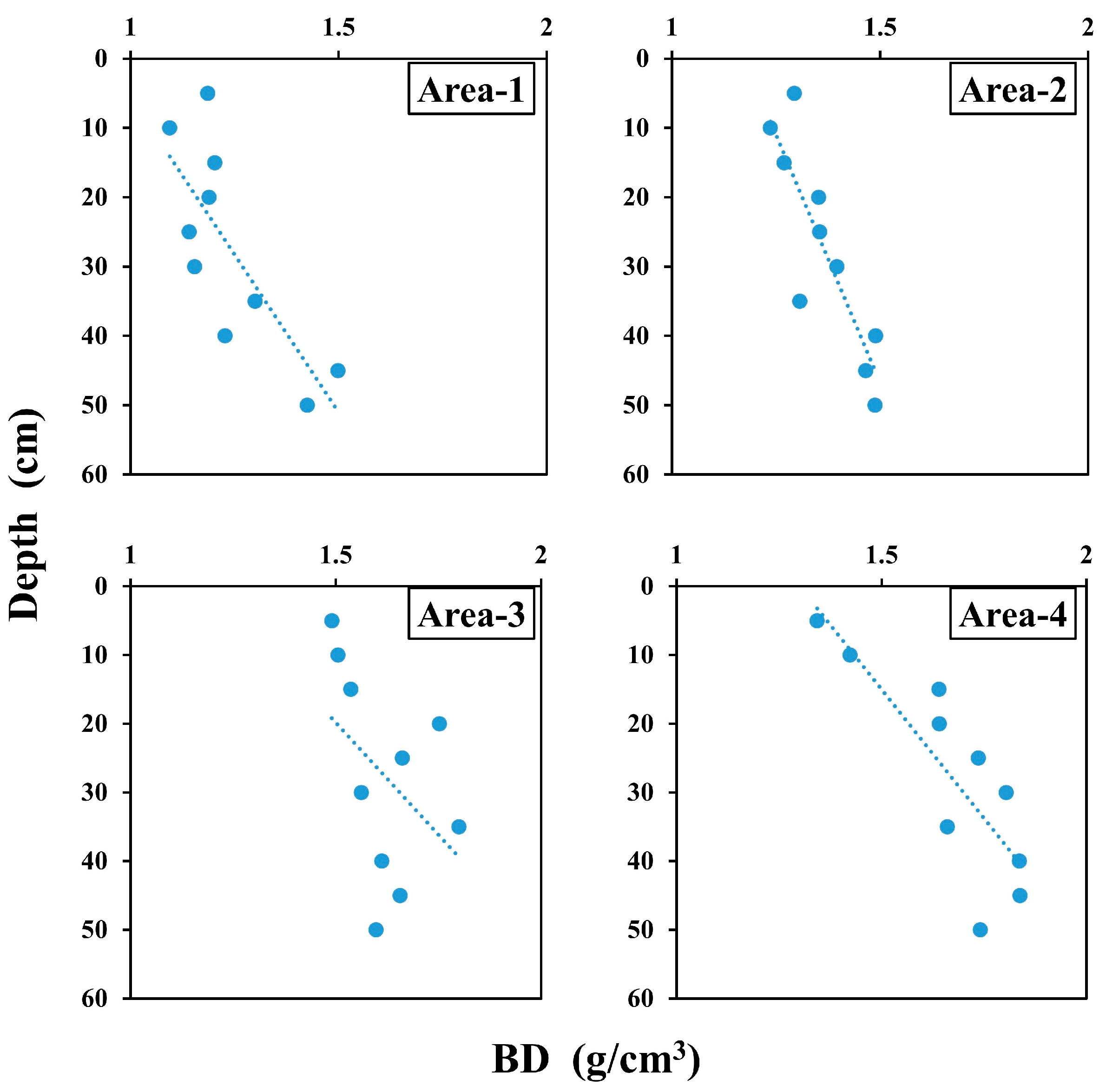
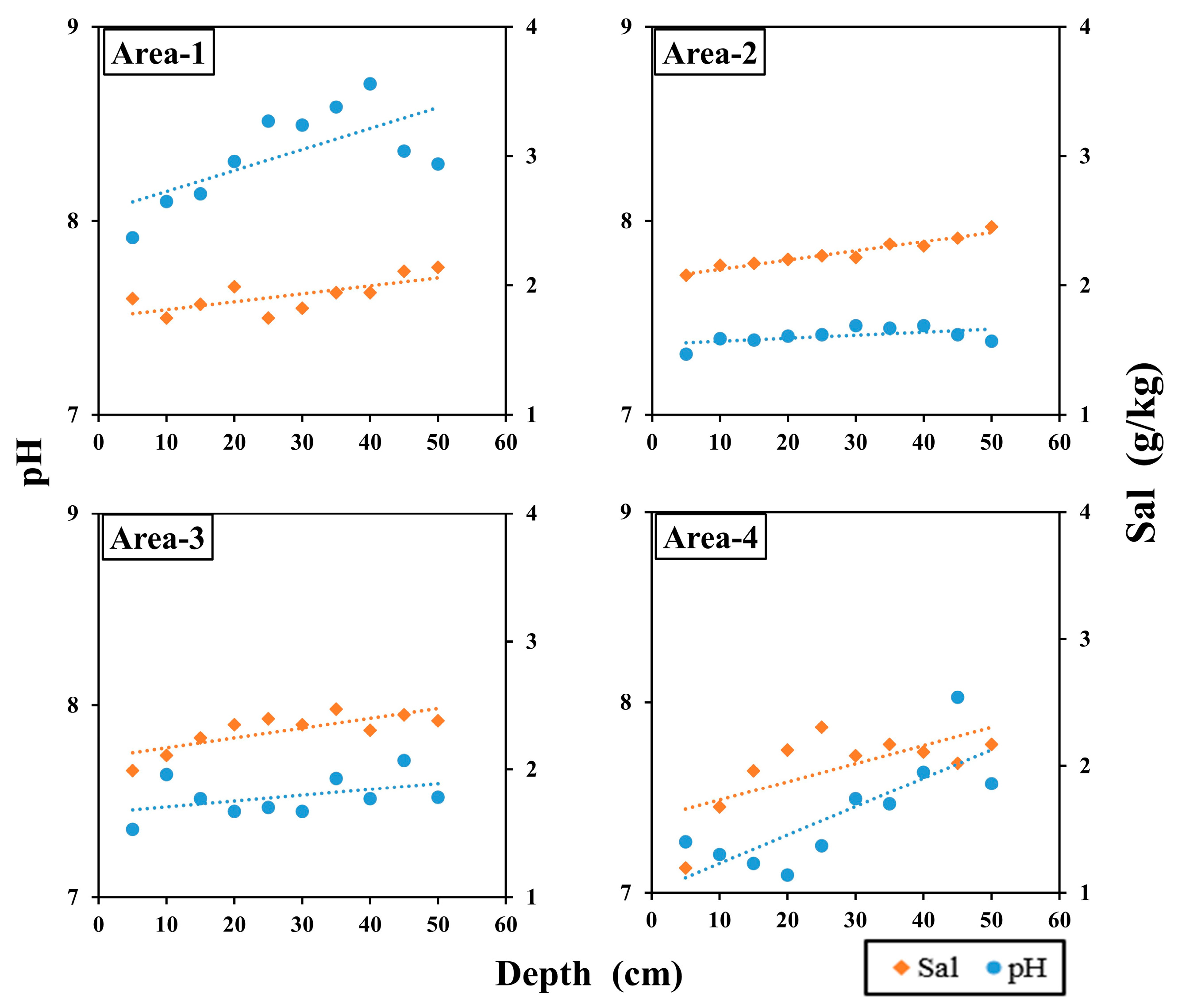
3.1. Distribution Characteristics of Physical and Chemical Parameters of Sediment
3.2. Distribution Characteristics of the Organic Carbon Content of Sediment
3.3. Analysis of Influencing Factors of Sediments’ Organic Carbon Content
3.3.1. Pearson’s Correlation Analysis of Sediments’ Organic Carbon and Physicochemical Indexes
3.3.2. Partial Least Squares Regression Model Analysis
4. Discussion
4.1. Driving Factors of Changes in Physicochemical Indexes of Sediments
4.2. Response Mechanism of Organic Carbon Content to Influencing Factors
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pörtner, H.O.; Scholes, R.J.; Agard, J.; Archer, E.; Arneth, A.; Bai, X.; Barnes, D.; Burrows, M.; Chan, L.; Cheung, W.; et al. Scientific Outcome of the IPBES-IPCC Cosponsored Workshop on Biodiversity and Climate Change (Version 5); IPBES Secretariat: Bonn, Germany, 2021. [Google Scholar]
- Ciais, P.; Dolman, A.J.; Bombelli, A.; Duren, R.; Peregon, A.; Rayner, P.J.; Miller, C.; Gobron, N.; Kinderman, G.; Marland, G.; et al. Current systematic carbon-cycle observations and the need for implementing a policy-relevant carbon observing system. Biogeosciences 2014, 11, 3547–3602. [Google Scholar] [CrossRef]
- Nellemann, C.; Corcoran, E.; Duarte, C.M.; Valdes, L.; Deyoung, C.; Fonseca, L.; Grimsditch, G. (Eds.) Blue Carbon. The Role of Healthy Oceans in Binding Carbon. A Rapid Response Assessment; United Nations Environment Programme, GRID-Arendal: Arendal, Norway, 2009. [Google Scholar]
- Frolking, S.; Roulet, N.T.; Moore, T.R.; Richard, P.J.H.; Lavoie, M.; Muller, S.D. Modeling northern peatland decomposition and peat accumulation. Ecosystems 2001, 4, 479–498. [Google Scholar] [CrossRef]
- Tian, P.; Cao, L.; Li, J.; Pu, R.; Liu, Y.; Zhang, H.; Wang, C. Ecosystem Stability Assessment of Yancheng Coastal Wetlands, a World Natural Heritage Site. Land 2022, 11, 564. [Google Scholar] [CrossRef]
- Jin, B.; Yan, H.; Zhang, L.; Zeng, C. Spartina-temporal variations and theirs influence factors of soil organic carbon under the Spartina alterniflora wetland in China. Ecol. Environ. Sci. 2016, 25, 2021–2027, (In Chinese with English Abstract). [Google Scholar]
- Xie, X.F.; Sun, X.M.; Wu, T.; Jiang, G.J.; Xiang, Q. Impacts of Spartina alterniflora invasion on coastal wetland ecosystem: Advances and prospects. J. Appl. Ecol. 2020, 31, 2119–2128. [Google Scholar]
- Tong, X.; Sun, Z.; Zeng, A.; Chen, B.; Wang, H. Effects of Spartina alterniflora invasion in a seaward direction on variations of inorganic sulfur forms in marsh soils of the Minjiang River estuary, China. Chin. J. Appl. Ecol. 2019, 30, 3518–3526, (In Chinese with English Abstract). [Google Scholar]
- Gao, S.; Du, Y.; Xie, W.; Gao, W.; Wang, D.; Wu, X. Environment-ecosystem dynamic processes of Spartina alterniflora salt-marshes along the eastern China coastlines. Sci. China Earth Sci. 2014, 57, 2567–2586. [Google Scholar] [CrossRef]
- Failon, C.M.; Wittyngham, S.S.; Johnson, D.S. Ecological Associations of Littoraria irrorata with Spartina cynosuroides and Spartina alterniflora. Wetlands 2020, 40, 1317–1325. [Google Scholar] [CrossRef]
- Yu, C.; Chen, P.; Liu, C.; Zhang, Y.; Han, M.; Zhou, S.; Lu, W. Research progress on carbon storage and carbon sink of Spartina alterniflora wetland. Ocean. Deve. Manag. 2014, 31, 85–89, (In Chinese with English Abstract). [Google Scholar]
- Li, B.; Liao, C.; Zhang, X.; Chen, H.; Wang, Q.; Chen, Z.; Gan, X.; Wu, J.; Zhao, B.; Ma, Z.; et al. Spartina alterniflora invasions in the Yangtze River estuary, China: An overview of current status and ecosystem effects. Ecol. Eng. 2009, 35, 511–520. [Google Scholar] [CrossRef]
- Yang, R.M. Interacting Effects of Plant Invasion, Climate, and Soils on Soil Organic Carbon Storage in Coastal Wetlands. J. Geophys. Res. Biogeosci. 2019, 124, 2554–2564. [Google Scholar] [CrossRef]
- Liu, J.; Deng, D.; Zou, C.; Han, R.; Xin, Y.; Shu, Z.; Zhang, L. Spartina alterniflora saltmarsh soil organic carbon properties and sources in coastal wetlands. J. Soils Sediments 2021, 21, 3342–3351. [Google Scholar] [CrossRef]
- Schuerch, M.; Spencer, T.; Temmerman, S.; Kirwan, M.L.; Wolff, C.; Lincke, D.; McOwen, C.J.; Pickering, M.D.; Reef, R.; Vafeidis, A.T.; et al. Future response of global coastal wetlands to sea-level rise. Nature 2018, 561, 231–234. [Google Scholar] [CrossRef]
- Hang, Z.; Wang, G.; Liu, J.; Wang, G.; Wang, H. Characterization of soil organic carbon fractions at Spartina alterniflora saltmarsh in North Jiangsu. Acta. Ecol. Sin. 2014, 34, 4175–4182, (In Chinese with English Abstract). [Google Scholar]
- Huang, X.; Liang, S.; Tao, Y.; Wang, X.; Duan, Y. Distribution characteristics of organic carbon stocks of Spartina alterniflora in Dafeng River Estuary, Beibu Gulf. Guihaia 2021, 41, 853–861, (In Chinese with English Abstract). [Google Scholar]
- Bu, N.; Yang, X.; Li, G.; Ma, X.; Song, Y.; Ma, F.; Li, B.; Fang, C.; Yan, Z. Effects of Spartina alterniflora invasion on soil carbon dynamics in wetlands of the Yangtze River estuary. China Environ. Sci. 2018, 38, 2671–2679, (In Chinese with English Abstract). [Google Scholar]
- Dan, S.; Wang, T.; Jin, Q. Geography of Jiangsu Province; Jiangsu Education Publishing House: Nanjing, China, 1986. [Google Scholar]
- Jiangsu Climate Compilation Group. Jiangsu Climate; China Meteorological Press: Beijing, China, 1991. [Google Scholar]
- Lu, W.; Yang, S.; Chen, L.; Wang, W.; Du, X.; Wang, C.; Ma, Y.; Lin, G. Changes in Carbon Pool and Stand Structure of a Native Subtropical Mangrove Forest after Inter-Planting with Exotic Species Sonneratia apetala. PLoS ONE 2014, 9, 91238. [Google Scholar] [CrossRef]
- Chen, Q.; Liu, Y.; Li, Q.; Huang, J. Use of stable isotopes to understand food webs in Macao wetlands. Wetlands Ecol. Manage 2017, 25, 59–66. [Google Scholar] [CrossRef]
- Yang, P.; Shu, Q.; Liu, Q.; Hu, Z.; Zhang, S.; Ma, Y. Distribution and factors influencing organic and inorganic carbon in surface sediments of tidal flats in northern Jiangsu, China. Ecol. Indic. 2021, 126, 107633. [Google Scholar] [CrossRef]
- Wold, H. Soft modelling, the basic design and some extensions. In Systems under Indirect Observation: Causality-Structure Prediction; Wold, H., Jöreskog, K.G., Eds.; North-Holland Publishing Company: Amsterdam, The Netherlands, 1982; Part II; pp. 1–54. [Google Scholar]
- Wold, S.; Sjöström, M.; Eriksson, L. PLS-regression: A basic tool of chemometrics. Chemom. Intell. Lab. Syst. 2001, 58, 109–130. [Google Scholar] [CrossRef]
- Fang, N.; Shi, Z.; Chen, F.; Wang, Y. Partial Least Squares Regression for Determining the Control Factors for Runoff and Suspended Sediment Yield during Rainfall Events. Water 2015, 7, 3925–3942. [Google Scholar] [CrossRef]
- Tong, L.; Fang, N.; Xiao, H.; Shi, Z. Sediment deposition changes the relationship between soil organic and inorganic carbon: Evidence from the Chinese Loess Plateau. Agric. Ecosyst. Environ. 2020, 302, 107076. [Google Scholar] [CrossRef]
- Yan, B.; Fang, N.; Zhang, P.; Shi, Z. Impacts of land use change on watershed streamflow and sediment yield: An assessment using hydrologic modelling and partial least squares regression. J. Hydrol. 2013, 484, 26–37. [Google Scholar] [CrossRef]
- Shi, Z.; Ai, L.; Li, X.; Huang, X.; Wu, G.; Liao, W. Partial least-squares regression for linking land-cover patterns to soil erosion and sediment yield in watersheds. J. Hydrol. 2013, 498, 165–176. [Google Scholar] [CrossRef]
- Carrascal, L.M.; Galván, I.; Gordo, O. Partial least squares regression as an alternative to current regression methods used in ecology. Oikos 2009, 118, 681–690. [Google Scholar] [CrossRef]
- Zhou, Y.; Xu, J.; Yin, W.; Ai, L.; Fang, N.; Tan, W.; Yan, F.; Shi, Z. Hydrological and environmental controls of the stream nitrate concentration and flux in a small agricultural watershed. J. Hydrol. 2017, 545, 355–366. [Google Scholar] [CrossRef]
- Onderka, M.; Wrede, S.; Rodný, M.; Pfister, L.; Hoffmann, L. Hydrogeologic and landscape controls of dissolved inorganic nitrogen (DIN) and dissolved silica (DSi) fluxes in heterogeneous catchments. J. Hydrol. 2012, 450–451, 36–47. [Google Scholar] [CrossRef]
- Chambers, R.M.; Mozdzer, T.J.; Ambrose, J.C. Effects of salinity and sulfide on the distribution of Phragmites australis and Spartina alterniflora in a tidal saltmarsh. Aquat. Bot. 1998, 62, 161–169. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, H.; He, C.; Liu, G.; Liang, X.; Chen, X. Spatiotemporal variability in soil sulfur storage is changed by exotic Spartina alterniflora in the Jiuduansha Wetland, China. Ecol. Eng. 2019, 133, 160–166. [Google Scholar] [CrossRef]
- Zhang, G.; Bai, J.; Jia, J.; Wang, X.; Wang, W.; Zhao, Q.; Zhang, S. Soil Organic Carbon Contents and Stocks in Coastal Salt Marshes with Spartina alterniflora Following an Invasion Chronosequence in the Yellow River Delta, China. Chin. Geogr. Sci. 2018, 28, 374–385. [Google Scholar] [CrossRef]
- Huang, M.; Ge, C.; Zuo, P.; Ji, Z.; Fang, W.; Zhou, M. The contribution of Spartina introduction on organic matter and its effects on carbon burial in tidal flat. J. Nanjing Univ. Nat. Sci. 2018, 54, 655–664, (In Chinese with English Abstract). [Google Scholar]
- Liu, J.; Duan, X.; Li, G.; Cai, Z.; Wei, S. Changes in Bacterial Communities and Their Effects on Soil Carbon Storage in Spartina alterniflora Invasion Areas, Coastal Wetland Bare Flats, and Sueada salsa Areas. Int. J. Environ. Res. Public Health 2023, 20, 4308. [Google Scholar] [CrossRef]
- Crnobrna, B.; Llanqui, I.B.; Cardenas, A.D.; Panduro Pisco, G. Relationships between Organic Matter and Bulk Density in Amazonian Peatland Soils. Sustainability 2022, 14, 12070. [Google Scholar] [CrossRef]
- Yao, X.; Yan, D.; Li, J.; Liu, Y.; Sheng, Y.; Xie, S.; Luan, Z. Spatial Distribution of Soil Organic Carbon and Total Nitrogen in a Ramsar Wetland, Dafeng Milu National Nature Reserve. Water 2022, 14, 197. [Google Scholar] [CrossRef]
- Huang, X.; Duan, Y.; Tao, Y.; Tao, Y.; Wang, X.; Long, H.; Luo, C.; Lai, Y. Effects of Spartina alterniflora Invasion on Soil Organic Carbon Storage in the Beihai Coastal Wetlands of China. Front. Mar. Sci. 2022, 9, 890811. [Google Scholar] [CrossRef]
- Qi, Q.; Zhang, D.; Zhang, M.; Tong, S.; Wang, W.; An, Y. Spatial distribution of soil organic carbon and total nitrogen in disturbed Carex tussock wetland. Ecol. Indic. 2021, 120, 106930. [Google Scholar] [CrossRef]
- Liu, J.; Han, R.; Su, H.; Wu, Y.; Zhang, L.; Richardson, C.; Wang, G. Effects of exotic Spartina alterniflora on vertical soil organic carbon distribution and storage amount in coastal salt marshes in Jiangsu, China. Ecol. Eng. 2017, 106, 132–139. [Google Scholar] [CrossRef]
- Alongi, D.M. Carbon cycling and storage in mangrove forests. Annu. Rev. Mar. Sci. 2014, 6, 195–219. [Google Scholar] [CrossRef]
- Barr, J.G.; Fuentes, J.D.; DeLonge, M.S.; O’Halloran, T.L.; Barr, D.; Zieman, J.C. Summertime influences of tidal energy advection on the surface energy balance in a mangrove forest. Biogeosciences 2013, 10, 501–511. [Google Scholar] [CrossRef]
- Li, S.; Chen, P.; Huang, J.; Hsueh, M.L.; Lee, C.L.; Lin, H. Factors regulating carbon sinks in mangrove ecosystems. Global Chang. Biol. 2018, 24, 4195–4210. [Google Scholar] [CrossRef]
- Xia, S.; Wang, W.; Song, Z.; Kuzyakov, Y.; Guo, L.; Van Zwieten, L.; Li, Q.; Hartley, L.P.; Yang, Y.; Wang, Y.; et al. Spartina alterniflora invasion controls organic carbon stocks in coastal marsh and mangrove soils across tropics and subtropics. Global Chang. Biol. 2021, 27, 1627–1644. [Google Scholar] [CrossRef] [PubMed]
- Donato, D.C.; Kauffman, J.B.; Murdiyarso, D.; Kurnianto, S.; Stidham, M.; Kanninen, M. Mangroves among the most carbon-rich forests in the tropics. Nat. Geosci. 2011, 4, 293–297. [Google Scholar] [CrossRef]
- Wu, L.; Li, L.; Yao, Y.; Qin, F.; Guo, Y.; Gao, Y.; Zhang, M. Spatial distribution of soil organic carbon and its influencing factors at different soil depths in a semiarid region of China. Environ. Earth Sci. 2017, 76, 654. [Google Scholar] [CrossRef]
- Gao, Y.; Zhou, J.; Wang, L.M.; Guo, J.; Feng, J.; Wu, H.; Lin, G. Distribution patterns and controlling factors for the soil organic carbon in four mangrove forests of China. Glob. Ecol. Conserv. 2019, 17, e00575. [Google Scholar] [CrossRef]
- Batjes, N.H. Carbon and nitrogen stocks in the soils of Central and Eastern Europe. Soil Use Manag. 2002, 18, 324–329. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, F.; Li, X.; Xie, X.; Zhou, X.; Li, Q.; Lei, J. Influence of exogenous N import on growth and leaf character of Spartina alterniflora. Ecol. Environ. Sci. 2010, 19, 2297–2301, (In Chinese with English Abstract). [Google Scholar]
- Stevenson, F.J. Organic form of soil nitrogen. In Nitrogen in Agricultural Soils; Stevenson, F.J., Ed.; American Society of Agronomy: Madison, WI, USA, 1982; pp. 101–104. [Google Scholar]
- Zhang, H.; Wu, P.; Fan, M.; Zheng, S.; Wu, J.; Yang, X.; Zhang, M.; Yin, A.; Gao, C. Dynamics and driving factors of the organic carbon fractions in agricultural land reclaimed from coastal wetlands in eastern China. Ecol. Indic. 2018, 89, 639–647. [Google Scholar] [CrossRef]
- Zhang, H.; Yin, A.; Yang, X.; Wu, P.; Fan, M.; Wu, J.; Zhang, M.; Gao, C. Changes in surface soil organic/inorganic carbon concentrations and their driving forces in reclaimed coastal tidal flats. Geoderma 2019, 352, 150–159. [Google Scholar] [CrossRef]
- Duan, L.; Li, Z.; Xie, H.; Yuan, H.; Li, Z.; Zhou, Q. Regional pattern of soil organic carbon density and its influence upon the plough layers of cropland. Land Degrad. Dev. 2020, 31, 2197–2515. [Google Scholar] [CrossRef]
- Zhang, S.; Wang, L.; Hu, J.; Zhang, W.; Fu, X.; Le, Y.; Jin, F. Organic carbon accumulation capability of two typical tidal wetland soils in Chongming Dongtan, China. J. Environ. Sci. 2011, 23, 87–94. [Google Scholar] [CrossRef]
- Wang, L.; Ying, R.; Shi, J.; Long, T.; Lin, Y. Research progress on the adsorption and fixation mechanism of soil minerals on organic matter. Acta Pedol. Sin. 2017, 54, 805–818, (In Chinese with English Abstract). [Google Scholar]
- Wan, S.; Liu, X.; Mou, X.; Zhao, Y. Comparison of Carbon, Nitrogen, and Sulfur in Coastal Wetlands Dominated by Native and Invasive Plants in the Yancheng National Nature Reserve, China. Chin. Geogr. Sci. 2020, 30, 202–216. [Google Scholar] [CrossRef]
- Wang, H.; Qian, H.; Gao, Y.; Li, Y. Classification and physical characteristics of bound water in loess and its main clay minerals. Engin. Geol. 2020, 265, 105394. [Google Scholar] [CrossRef]
- Gui, H.; Li, F.; Wei, Y.; Yamada, T. Adsorption characteristics of natural organic matter on activated carbons with different pore size distribution. Int. J. Environ. Sci. Technol. 2018, 15, 1619–1628. [Google Scholar] [CrossRef]
- Li, Z. Distribution Characteristics of Soil Organic Carbon in Mangrove Wetland in Hainan Island. Master’s Thesis, Hainan Normal University, Haikou, China, 2013. (In Chinese with English Abstract). [Google Scholar]
- Feng, D.; Gao, Q.; Liu, J.; Tang, J.; Hua, Z.; Sun, X. Categories of exogenous substances and their effect on alleviation of plant salt stress. Eur. J. Agron. 2023, 142, 126656. [Google Scholar] [CrossRef]
- Bai, J.; Yan, J.; He, D.; Cai, J.; Wang, R.; You, W.; Xiao, S.; Hou, D.; Li, W. Effects of spartina alterniflora invasion in eastern Fujian coastal wetland on the physicochemical properties and enzyme activities of mangrove soil. J. Beijing For. Univ. 2017, 39, 70–77, (In Chinese with English Abstract). [Google Scholar]

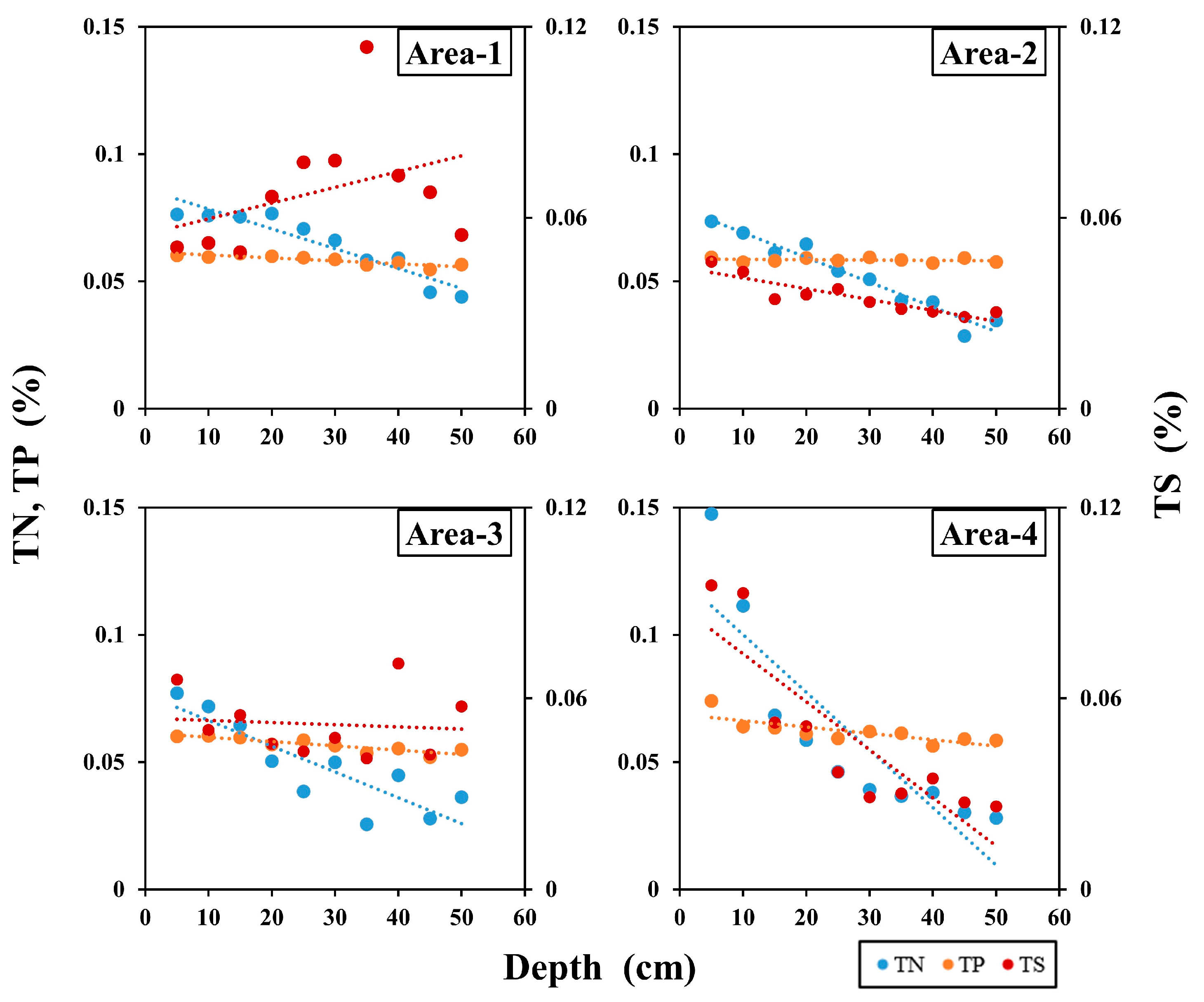
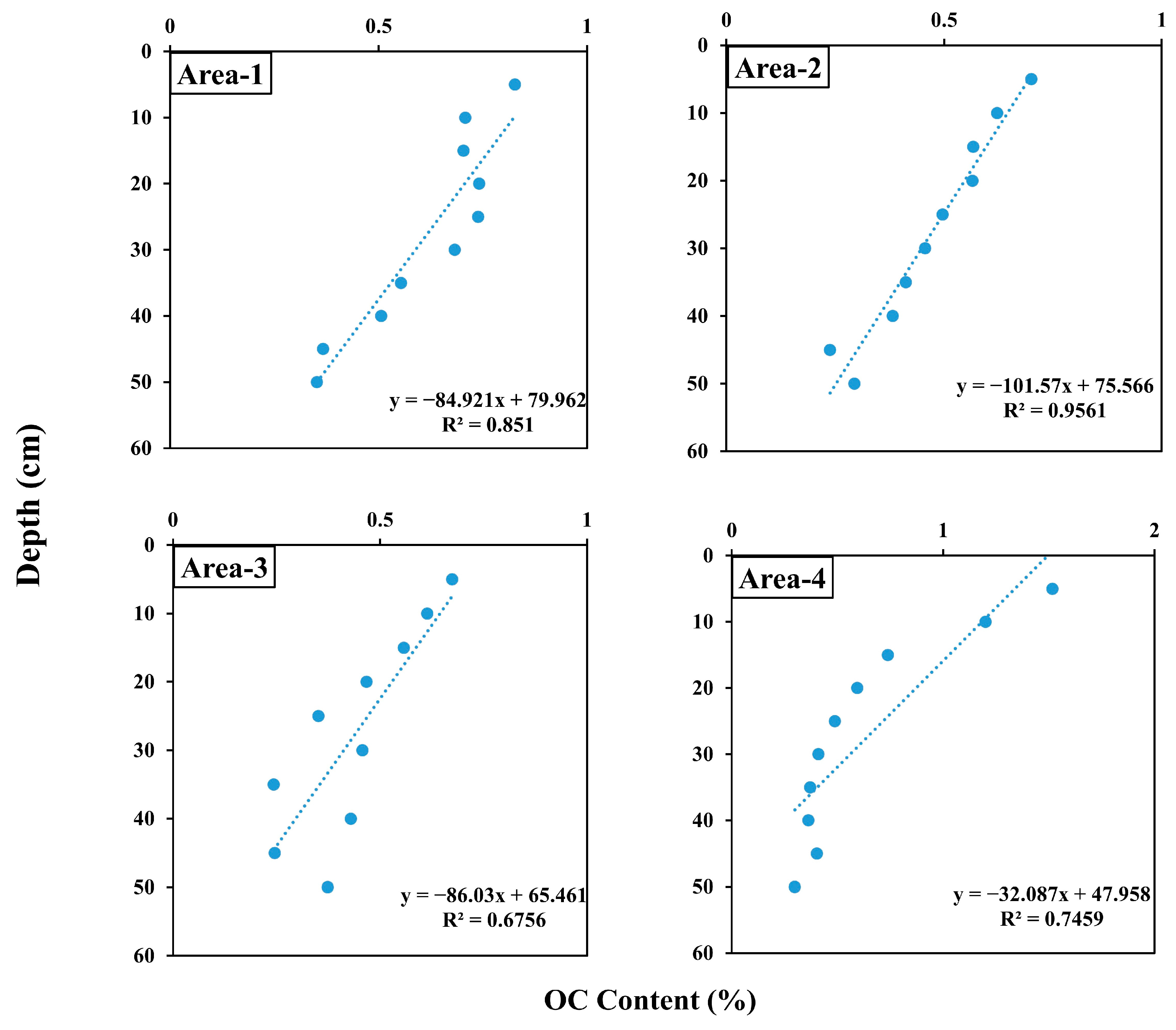

| Sampling Area | Area-1 | Area-2 | Area-3 | Area-4 | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Min | Max | Mean | CV | Min | Max | Mean | CV | Min | Max | Mean | CV | Min | Max | Mean | CV | |
| OC (%) | 0.35 | 0.83 | 0.62 | 0.27 | 0.24 | 0.70 | 0.47 | 0.31 | 0.24 | 0.67 | 0.44 | 0.33 | 0.30 | 1.52 | 0.64 | 0.64 |
| Sal (g/kg) | 2.37 | 3.56 | 3.01 | 0.12 | 1.47 | 1.69 | 1.61 | 0.04 | 1.53 | 2.07 | 1.79 | 0.09 | 1.08 | 2.06 | 1.39 | 0.23 |
| pH | 7.50 | 7.76 | 7.61 | 0.01 | 7.72 | 7.97 | 7.83 | 0.01 | 7.66 | 7.98 | 7.87 | 0.01 | 7.13 | 7.87 | 7.65 | 0.03 |
| BD (g/cm3) | 1.09 | 1.50 | 1.24 | 0.10 | 1.24 | 1.49 | 1.37 | 0.07 | 1.49 | 1.80 | 1.62 | 0.06 | 1.34 | 1.84 | 1.67 | 0.10 |
| MC (%) | 36.10 | 51.20 | 44.46 | 0.12 | 27.80 | 45.19 | 34.15 | 0.17 | 25.73 | 44.30 | 32.71 | 0.17 | 27.58 | 55.67 | 35.16 | 0.27 |
| TN (%) | 0.04 | 0.08 | 0.07 | 0.19 | 0.03 | 0.07 | 0.05 | 0.29 | 0.03 | 0.08 | 0.05 | 0.37 | 0.03 | 0.15 | 0.06 | 0.65 |
| TP (%) | 0.06 | 0.06 | 0.06 | 0.03 | 0.06 | 0.06 | 0.06 | 0.01 | 0.05 | 0.06 | 0.06 | 0.05 | 0.06 | 0.07 | 0.06 | 0.08 |
| TS (%) | 0.01 | 0.08 | 0.06 | 0.34 | 0.03 | 0.05 | 0.04 | 0.16 | 0.04 | 0.07 | 0.05 | 0.20 | 0.03 | 0.10 | 0.05 | 0.55 |
| Clay (%) | 10.93 | 21.49 | 16.54 | 0.20 | 5.89 | 14.74 | 11.44 | 0.26 | 7.43 | 13.17 | 10.77 | 0.21 | 4.30 | 15.85 | 10.32 | 0.31 |
| Silt (%) | 73.92 | 81.63 | 78.54 | 0.03 | 82.58 | 86.53 | 84.68 | 0.02 | 79.55 | 82.90 | 81.05 | 0.01 | 71.80 | 81.37 | 76.28 | 0.04 |
| Sand (%) | 2.98 | 8.35 | 4.93 | 0.34 | 0.00 | 7.58 | 3.89 | 0.59 | 5.91 | 12.50 | 8.18 | 0.30 | 8.15 | 22.18 | 13.41 | 0.28 |
| D50 (μm) | 8.73 | 21.40 | 12.48 | 0.36 | 12.17 | 26.42 | 18.79 | 0.27 | 14.57 | 28.52 | 20.64 | 0.33 | 13.48 | 37.45 | 23.51 | 0.30 |
| Area-1 | Area-2 | Area-3 | Area-4 | |
|---|---|---|---|---|
| OC (%) | OC (%) | OC (%) | OC (%) | |
| OC (%) | 1.00 *** | 1.00 *** | 1.00 *** | 1.00 *** |
| Depth | −0.92 *** | −0.98 *** | −0.82 *** | −0.86 *** |
| Sal (g/kg) | −0.46 | −0.52 | −0.58 * | −0.51 |
| pH | −0.77 *** | −0.94 *** | −0.93 *** | −0.93 *** |
| BD (g/cm3) | −0.88 *** | −0.83 *** | −0.77 *** | −0.94 *** |
| MC (%) | 0.78 *** | 0.94 *** | 0.86 *** | 0.99 *** |
| TN (%) | 0.97 *** | 0.99 *** | 0.99 *** | 1.00 *** |
| TP (%) | 0.90 *** | 0.42 | 0.88 *** | 0.89 *** |
| TS (%) | 0.29 | 0.91 *** | 0.54 * | 0.98 *** |
| Clay (%) | 0.87 *** | 0.91 *** | 0.72 *** | 0.84 *** |
| Silt (%) | −0.64 ** | −0.64 ** | −0.49 | −0.56 * |
| Sand (%) | −0.87 *** | −0.82 *** | −0.56 * | −0.30 |
| Variable | R2 | Q2 | Components | Explained Variation in Y (%) | Cum Explained Variation in Y (%) | Q2cum |
|---|---|---|---|---|---|---|
| OC | 0.9437 | 0.9180 | 1 | 89.62 | 89.82 | 0.8475 |
| 2 | 4.75 | 94.37 | 0.9180 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, A.; Lv, W.; Shu, Q.; Chen, Z.; Du, Y.; Ye, H.; Xu, L.; Liu, S. Distribution Characteristics and Main Influencing Factors of Organic Carbon in Sediments of Spartina Alterniflora Wetlands along the Northern Jiangsu Coast, China. Land 2024, 13, 741. https://doi.org/10.3390/land13060741
Zhang A, Lv W, Shu Q, Chen Z, Du Y, Ye H, Xu L, Liu S. Distribution Characteristics and Main Influencing Factors of Organic Carbon in Sediments of Spartina Alterniflora Wetlands along the Northern Jiangsu Coast, China. Land. 2024; 13(6):741. https://doi.org/10.3390/land13060741
Chicago/Turabian StyleZhang, Aijuan, Wenlong Lv, Qiang Shu, Zhiling Chen, Yifan Du, Hui Ye, Linlu Xu, and Shengzhi Liu. 2024. "Distribution Characteristics and Main Influencing Factors of Organic Carbon in Sediments of Spartina Alterniflora Wetlands along the Northern Jiangsu Coast, China" Land 13, no. 6: 741. https://doi.org/10.3390/land13060741




