Conservation Management Practices for Biodiversity Preservation in Urban Informal Green Spaces: Lessons from Central European City
Abstract
:1. Introduction
2. Materials and Methods
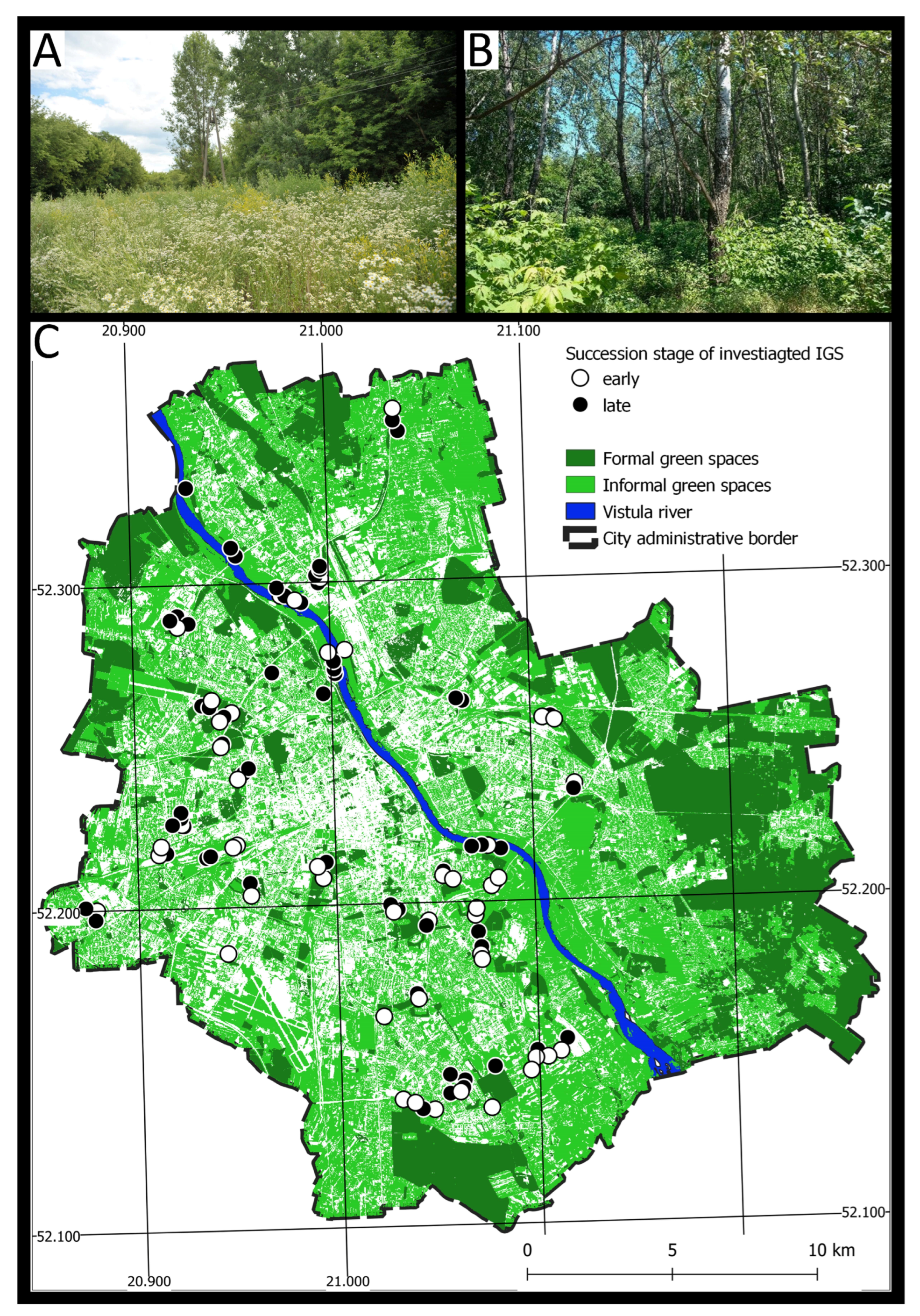
2.1. Study Area
2.2. Study Sites
2.3. Vegetation Quality of IGS
2.4. Factors Affecting IGS Vegetation
| Indicator | Description and Literature | |
|---|---|---|
| Trampling | Number of inhabitants in 500 m buffer zone [67] | Number of people living within 500 m according to the Central Statistical Office [49], based on the PESEL database. A greater number of people residing in the vicinity of IGS (buffer zone) results in increased pressure due to a higher number of users. This pressure mainly manifests through trampling, which, in turn, affects the vegetation composition [73]. |
| Soil quality | pH | Determined by potentiometry. |
| Cd, Ni, Cr, Cu, Zn, Pb | Indirect index of anthropopressure in habitat. Determined on the basis of the norm PN-ISO 11047: 2001 | |
| Habitat continuity | The period measured from the occurrence of permanent land use transformation resulting from human activities, such as the cessation of economic or agricultural activity or landscaping activities. Habitat continuity is considered a key factor in many biodiversity indicators. High biodiversity and many habitat specialist species are linked to long-term habitat continuity [74]. | |
| Neighborhood of study plot (100 m buffer) | low vegetation, high vegetation, without vegetation | Share of high and low vegetation in vicinity of study plot. Connectivity to other vegetation patches play important role in providing new species [75,76]. Forest habitats tend to be more reliable on connectivity to other forested patches, due to low seed dispersion of many species [77,78]. |
| Patch size [m2] | The surface area of each vegetation patch within which vegetation plots were located. The positive effects of habitat patch size on biodiversity manifest in the increased species richness, diversity, and overall ecological health within larger and more extensive habitat patches [79]. | |
| Former land use type | Arable lands Cultivated grasslands and pastures Built-up areas Waterbodies Gardens and orchards Other | Previous land use of IGS area. Previous land use has a key impact on soil structure and carbon, nitrogen, and phosphorus contents [80], which affects succession and vegetation composition [81]. |
2.5. Statistical Analysis
3. Results
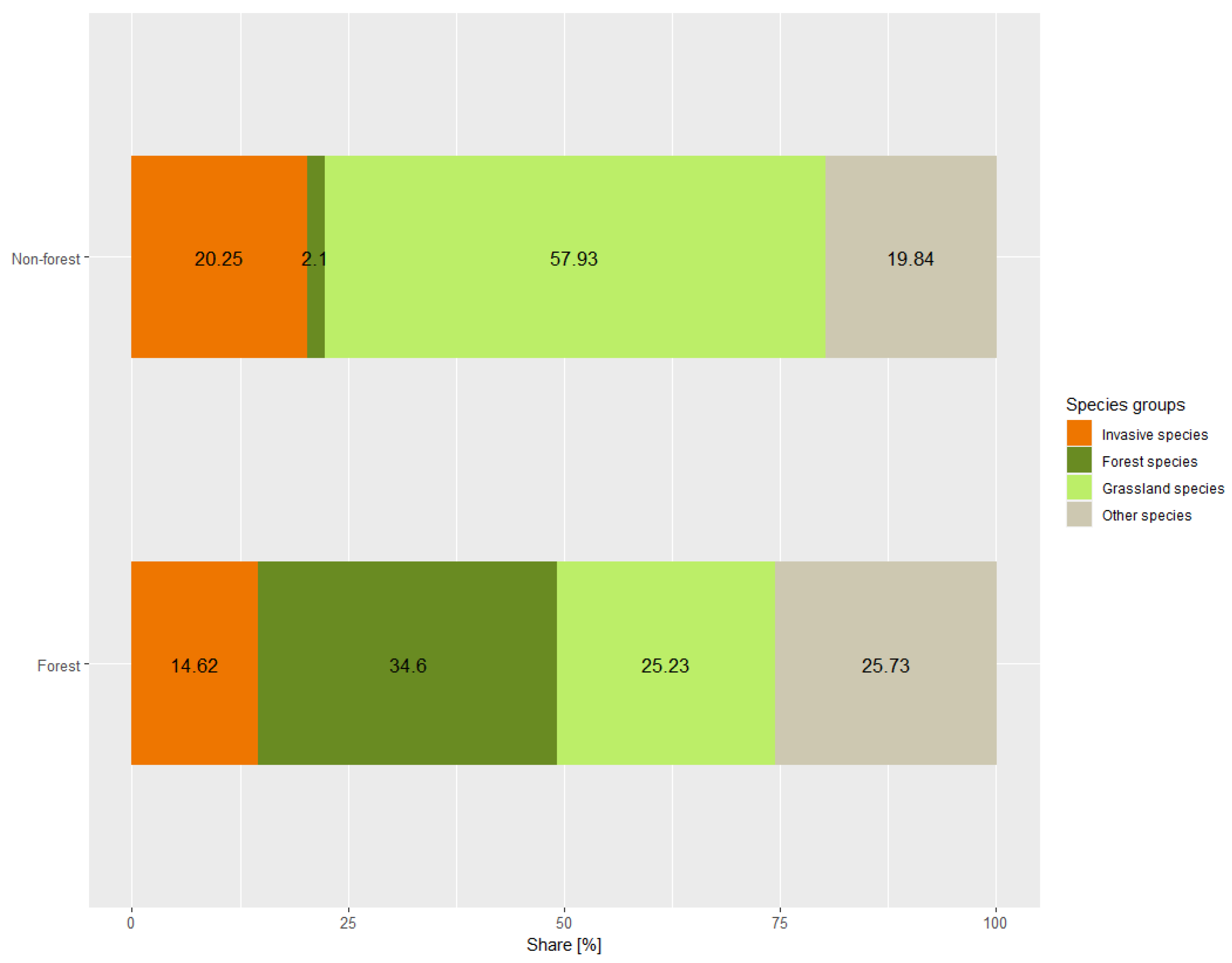
3.1. Vegetation of Non-Forest and Forest IGS
3.2. Factors Affecting Vegetation Structure and Biodiversity Indicators of Non-Forest and Forest IGS
4. Discussion
4.1. Factors Shaping Informal Green Spaces
4.2. Impact of Management Regimes on Non-Forest and Forest IGS
4.3. Limitations of the Study
4.4. Management Guidelines for IGS
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Kühn, I.; Klotz, S. Urbanization and Homogenization—Comparing the Floras of Urban and Rural Areas in Germany. Biol. Conserv. 2006, 127, 292–300. [Google Scholar] [CrossRef]
- Lososová, Z.; Chytrý, M.; Danihelka, J.; Tichý, L.; Ricotta, C. Biotic Homogenization of Urban Floras by Alien Species: The Role of Species Turnover and Richness Differences. J. Veg. Sci. 2016, 27, 452–459. [Google Scholar] [CrossRef]
- Pyšek, P.; Chocholousková, Z.; †Pyšek, A.; Jarošík, V.; Chytrý, M.; Tichý, L. Trends in Species Diversity and Composition of Urban Vegetation over Three Decades. J. Veg. Sci. 2004, 15, 781–788. [Google Scholar] [CrossRef]
- Simkin, R.D.; Seto, K.C.; McDonald, R.I.; Jetz, W. Biodiversity Impacts and Conservation Implications of Urban Land Expansion Projected to 2050. Proc. Natl. Acad. Sci. USA 2022, 119, e2117297119. [Google Scholar] [CrossRef] [PubMed]
- Croci, S.; Butet, A.; Georges, A.; Aguejdad, R.; Clergeau, P. Small Urban Woodlands as Biodiversity Conservation Hot-Spot: A Multi-Taxon Approach. Landsc. Ecol. 2008, 23, 1171–1186. [Google Scholar] [CrossRef]
- Myers, N.; Mittermeier, R.A.; Mittermeier, C.G.; da Fonseca, G.A.B.; Kent, J. Biodiversity Hotspots for Conservation Priorities. Nature 2000, 403, 853–858. [Google Scholar] [CrossRef] [PubMed]
- Nilon, C.H.; Aronson, M.F.J.; Cilliers, S.S.; Dobbs, C.; Frazee, L.J.; Goddard, M.A.; O’Neill, K.M.; Roberts, D.; Stander, E.K.; Werner, P.; et al. Planning for the Future of Urban Biodiversity: A Global Review of City-Scale Initiatives. BioScience 2017, 67, 332–342. [Google Scholar] [CrossRef]
- Sikorski, P.; Gawryszewska, B.; Sikorska, D.; Chormański, J.; Schwerk, A.; Jojczyk, A.; Ciężkowski, W.; Archiciński, P.; Łepkowski, M.; Dymitryszyn, I.; et al. The Value of Doing Nothing—How Informal Green Spaces Can Provide Comparable Ecosystem Services to Cultivated Urban Parks. Ecosyst. Serv. 2021, 50, 101339. [Google Scholar] [CrossRef]
- Rupprecht, C.D.D.; Byrne, J.A. Informal Urban Greenspace: A Typology and Trilingual Systematic Review of Its Role for Urban Residents and Trends in the Literature. Urban For. Urban Green. 2014, 13, 597–611. [Google Scholar] [CrossRef]
- Bonthoux, S.; Brun, M.; Di Pietro, F.; Greulich, S.; Bouché-Pillon, S. How Can Wastelands Promote Biodiversity in Cities? A Review. Landsc. Urban Plan. 2014, 132, 79–88. [Google Scholar] [CrossRef]
- Kowarik, I. Novel Urban Ecosystems, Biodiversity, and Conservation. Environ. Pollut. 2011, 159, 1974–1983. [Google Scholar] [CrossRef] [PubMed]
- Luo, S.; Patuano, A. Multiple Ecosystem Services of Informal Green Spaces: A Literature Review. Urban For. Urban Green. 2023, 81, 127849. [Google Scholar] [CrossRef]
- Planchuelo, G.; Kowarik, I.; von der Lippe, M. Endangered Plants in Novel Urban Ecosystems Are Filtered by Strategy Type and Dispersal Syndrome, Not by Spatial Dependence on Natural Remnants. Front. Ecol. Evol. 2020, 8, 18. [Google Scholar] [CrossRef]
- Włodarczyk-Marciniak, R.; Sikorska, D.; Krauze, K. Residents’ Awareness of the Role of Informal Green Spaces in a Post-Industrial City, with a Focus on Regulating Services and Urban Adaptation Potential. Sustain. Cities Soc. 2020, 59, 102236. [Google Scholar] [CrossRef] [PubMed]
- Rall, E.L.; Haase, D. Creative Intervention in a Dynamic City: A Sustainability Assessment of an Interim Use Strategy for Brownfields in Leipzig, Germany. Landsc. Urban Plan. 2011, 100, 189–201. [Google Scholar] [CrossRef]
- Köppler, M.-R.; Kowarik, I.; Kühn, N.; von der Lippe, M. Enhancing Wasteland Vegetation by Adding Ornamentals: Opportunities and Constraints for Establishing Steppe and Prairie Species on Urban Demolition Sites. Landsc. Urban Plan. 2014, 126, 1–9. [Google Scholar] [CrossRef]
- Schröder, R.; Kiehl, K. Ecological Restoration of an Urban Demolition Site through Introduction of Native Forb Species. Urban For. Urban Green. 2020, 47, 126509. [Google Scholar] [CrossRef]
- Przybysz, A.; Wińska-Krysiak, M.; Małecka-Przybysz, M.; Stankiewicz-Kosyl, M.; Skwara, M.; Kłos, A.; Kowalczyk, S.; Jarocka, K.; Sikorski, P. Urban Wastelands: On the Frontline between Air Pollution Sources and Residential Areas. Sci. Total Environ. 2020, 721, 137695. [Google Scholar] [CrossRef] [PubMed]
- Fischer, L.K.; Honold, J.; Cvejić, R.; Delshammar, T.; Hilbert, S.; Lafortezza, R.; Nastran, M.; Nielsen, A.B.; Pintar, M.; van der Jagt, A.P.N.; et al. Beyond Green: Broad Support for Biodiversity in Multicultural European Cities. Glob. Environ. Change 2018, 49, 35–45. [Google Scholar] [CrossRef]
- Planchuelo, G.; von Der Lippe, M.; Kowarik, I. Untangling the Role of Urban Ecosystems as Habitats for Endangered Plant Species. Landsc. Urban Plan. 2019, 189, 320–334. [Google Scholar] [CrossRef]
- Rupprecht, C.D.D.; Byrne, J.A.; Garden, J.G.; Hero, J.-M. Informal Urban Green Space: A Trilingual Systematic Review of Its Role for Biodiversity and Trends in the Literature. Urban For. Urban Green. 2015, 14, 883–908. [Google Scholar] [CrossRef]
- Brun, M.; Di Pietro, F.; Bonthoux, S. Residents’ Perceptions and Valuations of Urban Wastelands Are Influenced by Vegetation Structure. Urban For. Urban Green. 2018, 29, 393–403. [Google Scholar] [CrossRef]
- Hotta, K.; Ishii, H.; Sasaki, T.; Doi, N.; Azuma, W.; Oyake, Y.; Imanishi, J.; Yoshida, H. Twenty-One Years of Stand Dynamics in a 33-Year-Old Urban Forest Restoration Site at Kobe Municipal Sports Park, Japan. Urban For. Urban Green. 2015, 14, 309–314. [Google Scholar] [CrossRef]
- Kowarik, I.; Hiller, A.; Planchuelo, G.; Seitz, B.; von der Lippe, M.; Buchholz, S. Emerging Urban Forests: Opportunities for Promoting the Wild Side of the Urban Green Infrastructure. Sustainability 2019, 11, 6318. [Google Scholar] [CrossRef]
- Sasaki, T.; Ishii, H.; Morimoto, Y. Evaluating Restoration Success of a 40-Year-Old Urban Forest in Reference to Mature Natural Forest. Urban For. Urban Green. 2018, 32, 123–132. [Google Scholar] [CrossRef]
- Archiciński, P.; Sikorski, P.; Sikorska, D.; Przybysz, A. Roślinność wieloletnich nieużytków miejskich—Systematyka zbiorowisk, ich struktura i pełnione usługi ekosystemowe = Vegetation of perennial urban wastelands—Syntaxonomy, structure and ecosystem services. Prz. Geogr. 2021, 93, 341–363. [Google Scholar] [CrossRef]
- Kattwinkel, M.; Biedermann, R.; Kleyer, M. Temporary Conservation for Urban Biodiversity. Biol. Conserv. 2011, 144, 2335–2343. [Google Scholar] [CrossRef]
- Meffert, P.J.; Dziock, F. What Determines Occurrence of Threatened Bird Species on Urban Wastelands? Biol. Conserv. 2012, 153, 87–96. [Google Scholar] [CrossRef]
- Muratet, A.; Machon, N.; Jiguet, F.; Moret, J.; Porcher, E. The Role of Urban Structures in the Distribution of Wasteland Flora in the Greater Paris Area, France. Ecosystems 2007, 10, 661–671. [Google Scholar] [CrossRef]
- Rebele, F. Differential Succession towards Woodland along a Nutrient Gradient. Appl. Veg. Sci. 2013, 16, 365–378. [Google Scholar] [CrossRef]
- Yuan, X.; Guo, Z.; Wang, S.; Zhao, L.; Yuan, M.; Gao, Y.; Huang, L.; Liu, C.; Duan, C. Drivers and Mechanisms of Spontaneous Plant Community Succession in Abandoned PbZn Mining Areas in Yunnan, China. Sci. Total Environ. 2023, 904, 166871. [Google Scholar] [CrossRef] [PubMed]
- Dyderski, M.K.; Wrońska-Pilarek, D.; Jagodziński, A.M. Ecological Lands for Conservation of Vascular Plant Diversity in the Urban Environment. Urban Ecosyst. 2017, 20, 639–650. [Google Scholar] [CrossRef]
- Twerd, L.; Banaszak-Cibicka, W. Wastelands: Their Attractiveness and Importance for Preserving the Diversity of Wild Bees in Urban Areas. J. Insect Conserv. 2019, 23, 573–588. [Google Scholar] [CrossRef]
- Schebella, M.F.; Weber, D.; Schultz, L.; Weinstein, P. The Wellbeing Benefits Associated with Perceived and Measured Biodiversity in Australian Urban Green Spaces. Sustainability 2019, 11, 802. [Google Scholar] [CrossRef]
- Taylor, L.; Hochuli, D.F. Creating Better Cities: How Biodiversity and Ecosystem Functioning Enhance Urban Residents’ Wellbeing. Urban Ecosyst. 2015, 18, 747–762. [Google Scholar] [CrossRef]
- Hobbs, R.J.; Arico, S.; Aronson, J.; Baron, J.S.; Bridgewater, P.; Cramer, V.A.; Epstein, P.R.; Ewel, J.J.; Klink, C.A.; Lugo, A.E.; et al. Novel Ecosystems: Theoretical and Management Aspects of the New Ecological World Order. Glob. Ecol. Biogeogr. 2006, 15, 1–7. [Google Scholar] [CrossRef]
- Hobbs, R.J.; Higgs, E.S.; Hall, C.M. (Eds.) Novel Ecosystems: Intervening in the New Ecological World Order; John Wiley & Sons, Ltd.: Chichester, UK, 2013; ISBN 978-1-118-35418-6. [Google Scholar]
- Lososová, Z.; Horsák, M.; Chytrý, M.; Čejka, T.; Danihelka, J.; Fajmon, K.; Hájek, O.; Juřičková, L.; Kintrová, K.; Láníková, D.; et al. Diversity of Central European Urban Biota: Effects of Human-Made Habitat Types on Plants and Land Snails: Biodiversity of Central European Cities. J. Biogeogr. 2011, 38, 1152–1163. [Google Scholar] [CrossRef]
- Miao, X.; Pan, Y.; Chen, H.; Zhang, M.-J.; Hu, W.; Li, Y.; Wu, R.; Wang, P.; Fang, S.; Niu, K.; et al. Understanding Spontaneous Biodiversity in Informal Urban Green Spaces: A Local-Landscape Filtering Framework with a Test on Wall Plants. Urban For. Urban Green. 2023, 86, 127996. [Google Scholar] [CrossRef]
- Vega, K.A.; Küffer, C. Promoting Wildflower Biodiversity in Dense and Green Cities: The Important Role of Small Vegetation Patches. Urban For. Urban Green. 2021, 62, 127165. [Google Scholar] [CrossRef]
- Schadek, U.; Strauss, B.; Biedermann, R.; Kleyer, M. Plant Species Richness, Vegetation Structure and Soil Resources of Urban Brownfield Sites Linked to Successional Age. Urban Ecosyst. 2009, 12, 115–126. [Google Scholar] [CrossRef]
- Westermann, J.R.; von der Lippe, M.; Kowarik, I. Seed Traits, Landscape and Environmental Parameters as Predictors of Species Occurrence in Fragmented Urban Railway Habitats. Basic Appl. Ecol. 2011, 12, 29–37. [Google Scholar] [CrossRef]
- Small, E.C.; Sadler, J.P.; Telfer, M.G. Carabid Beetle Assemblages on Urban Derelict Sites in Birmingham, UK. J. Insect Conserv. 2002, 6, 233–246. [Google Scholar] [CrossRef]
- Kattwinkel, M.; Strauss, B.; Biedermann, R.; Kleyer, M. Modelling Multi-Species Response to Landscape Dynamics: Mosaic Cycles Support Urban Biodiversity. Landsc. Ecol. 2009, 24, 929–941. [Google Scholar] [CrossRef]
- Meffert, P.J.; Dziock, F. The Influence of Urbanisation on Diversity and Trait Composition of Birds. Landsc. Ecol. 2013, 28, 943–957. [Google Scholar] [CrossRef]
- Strauss, B.; Biedermann, R. Urban Brownfields as Temporary Habitats: Driving Forces for the Diversity of Phytophagous Insects. Ecography 2006, 29, 928–940. [Google Scholar]
- Klaus, V.H.; Kiehl, K. A Conceptual Framework for Urban Ecological Restoration and Rehabilitation. Basic Appl. Ecol. 2021, 52, 82–94. [Google Scholar] [CrossRef]
- Perring, M.P.; Audet, P.; Lamb, D. Novel Ecosystems in Ecological Restoration and Rehabilitation: Innovative Planning or Lowering the Bar? Ecol. Process. 2014, 3, 8. [Google Scholar] [CrossRef]
- GUS Powierzchnia i Ludność w Przekroju Terytorialnym w 2022 Roku. Available online: https://stat.gov.pl/obszary-tematyczne/ludnosc/ludnosc/powierzchnia-i-ludnosc-w-przekroju-terytorialnym-w-2022-roku,7,19.html (accessed on 24 April 2023).
- Sikorska, D.; Łaszkiewicz, E.; Krauze, K.; Sikorski, P. The Role of Informal Green Spaces in Reducing Inequalities in Urban Green Space Availability to Children and Seniors. Environ. Sci. Policy 2020, 108, 144–154. [Google Scholar] [CrossRef]
- Sikorska, D.; Ciężkowski, W.; Babańczyk, P.; Chormański, J.; Sikorski, P. Intended Wilderness as a Nature-Based Solution: Status, Identification and Management of Urban Spontaneous Vegetation in Cities. Urban For. Urban Green. 2021, 62, 127155. [Google Scholar] [CrossRef]
- Feltynowski, M.; Kronenberg, J.; Bergier, T.; Kabisch, N.; Łaszkiewicz, E.; Strohbach, M.W. Challenges of Urban Green Space Management in the Face of Using Inadequate Data. Urban For. Urban Green. 2018, 31, 56–66. [Google Scholar] [CrossRef]
- Pietrzyk-Kaszyńska, A.; Czepkiewicz, M.; Kronenberg, J. Eliciting Non-Monetary Values of Formal and Informal Urban Green Spaces Using Public Participation GIS. Landsc. Urban Plan. 2017, 160, 85–95. [Google Scholar] [CrossRef]
- Matuszkiewicz, W. Przewodnik do Oznaczania Zbiorowisk Roslinnych Polski; Wydawnictwo Naukowe PWN: Warszawa, Poland, 2017; ISBN 978-83-01-16707-3. [Google Scholar]
- Chytrý, M.; Otýpková, Z. Plot Sizes Used for Phytosociological Sampling of European Vegetation. J. Veg. Sci. 2003, 14, 563–570. [Google Scholar] [CrossRef]
- Inwazyjne Gatunki Obce Roślin i Zwierząt—Otwarte Dane. Available online: https://dane.gov.pl/pl/dataset/1760/resource/21068,inwazyjne-gatunki-obce-roslin-i-zwierzat/table?page=1&per_page=20&q=&sort= (accessed on 6 March 2024).
- Iannone, B.; Carnevale, S.; Main, M.; Hill, J.; McConnell, J.; Johnson, S.; Enloe, S.; Andreu, M.; Bell, E.; Cuda, J.; et al. Invasive Species Terminology: Standardizing for Stakeholder Education. J. Ext. 2020, 58, 27. [Google Scholar] [CrossRef]
- Mirek, Z. (Ed.) Flowering Plants and Pteridophytes of Poland: A Checklist; krytyczna Lista Roślin Naczyniowych Polski; Biodiversity of Poland; Szafer Institute of Botany, Polish Academy of Sciences: Krakow, Poland, 2002; ISBN 978-83-85444-83-1. [Google Scholar]
- Warren, C.R. Perspectives on the ‘alien’ versus ‘native’ Species Debate: A Critique of Concepts, Language and Practice. Prog. Hum. Geogr. 2007, 31, 427–446. [Google Scholar] [CrossRef]
- Magurran, A.E. Measuring Biological Diversity; Blackwell Pub: Malden, MA, USA, 2004; ISBN 978-0-632-05633-0. [Google Scholar]
- Dzwonko, Z.; Loster, S. Ancient Woodland Plant Species Indicators and Their Importance for Nature Conservation and Vegetation Mapping. Pr. Geogr. 2001, 178, 119–132. [Google Scholar]
- Sikorski, P.; Sudnik-Wójcikowska, B.; Zaniewska, E.; Zaniewski, P.; Kowalska, A.; Wrzosek, M. Charakterystyka Przestrzenna i Różnorodności Biologicznej ostoi Roślinnych oraz Oddziaływania na nie Rozwiązań Planistycznych Proponowanych w Projekcie Studium Uwarunkowań i kierunków Zagospodarowania Przestrzennego m.st. Warszawy 2020; Office of Planning and Architecture of the City of Warsaw: Warsaw, Poland, 2020. [Google Scholar]
- Sudnik-Wójcikowska, B. Iva Xanthiifolia Nutt. and Its Communities within Warsaw. Acta Soc. Bot. Pol. 1987, 56, 155. [Google Scholar] [CrossRef]
- Sukopp, H. Der Einfluss Des Menschen Auf Die Vegetation. Vegetatio 1969, 17, 360–371. [Google Scholar] [CrossRef]
- Kühn, I.; Durka, W.; Klotz, S. BiolFlor—A New Plant-Trait Database as a Tool for Plant Invasion Ecology: BiolFlor—A Plant-Trait Database. Divers. Distrib. 2004, 10, 363–365. [Google Scholar] [CrossRef]
- Klotz, S.; Kühn, I. Indikatoren Des Anthropogenen Einflusses Auf Die Vegetation. Schriftenreihe Veg. 2002, 38, 241–246. [Google Scholar]
- Roo, M.; Kuypers, V.H.M.; Lenzholzer, S. The Green City Guidelines: Techniques for a Healthy Liveable City; The Green City: Bend, OR, USA, 2011. [Google Scholar]
- Hofmeister, J.; Hošek, J.; Brabec, M.; Hermy, M.; Dvořák, D.; Fellner, R.; Malíček, J.; Palice, Z.; Tenčík, A.; Holá, E.; et al. Shared Affinity of Various Forest-Dwelling Taxa Point to the Continuity of Temperate Forests. Ecol. Indic. 2019, 101, 904–912. [Google Scholar] [CrossRef]
- Strauss, B.; Biedermann, R. Fit for Succession—Community Structure and Life Strategies of Leafhoppers in Urban Brownfields. Ecol. Entomol. 2008, 33, 107–118. [Google Scholar] [CrossRef]
- Chojnacki, J. Zróźnicowanie Przestrzenne Roślinności Warszawy; Wydawnictwo UW: Warszawa, Poland, 1991; ISBN 83-230-0600-8. [Google Scholar]
- Kobendza, R. Roślinność Ruderalna Na Gruzach Miast Polskich, In Sprawozdania z posiedzeń Wydziału IV Nauk Biologicznych; Towarzystwo Naukowe Warszawskie: Warsaw, Poland, 1952; pp. 49–60. [Google Scholar]
- Kozłowska, A. Mapa roślinności Warszawy w skali 1: 10 000–założenia teoretyczne, metoda wykonania i zastosowanie. Przegląd Geogr. 2001, 180, 107–119. [Google Scholar]
- Hamberg, L.; Lehvävirta, S.; Minna, M.-L.; Rita, H.; Kotze, D.J. The Effects of Habitat Edges and Trampling on Understorey Vegetation in Urban Forests in Helsinki, Finland. Appl. Veg. Sci. 2008, 11, 83–98. [Google Scholar] [CrossRef]
- Nordén, B.; Dahlberg, A.; Brandrud, T.E.; Fritz, Ö.; Ejrnaes, R.; Ovaskainen, O. Effects of Ecological Continuity on Species Richness and Composition in Forests and Woodlands: A Review. Écoscience 2014, 21, 34–45. [Google Scholar] [CrossRef]
- Bailey, S. Increasing Connectivity in Fragmented Landscapes: An Investigation of Evidence for Biodiversity Gain in Woodlands. For. Ecol. Manag. 2007, 238, 7–23. [Google Scholar] [CrossRef]
- Correa Ayram, C.A.; Mendoza, M.E.; Etter, A.; Salicrup, D.R.P. Habitat Connectivity in Biodiversity Conservation: A Review of Recent Studies and Applications. Prog. Phys. Geogr. Earth Environ. 2016, 40, 7–37. [Google Scholar] [CrossRef]
- Howe, H.F.; Smallwood, J. Ecology of Seed Dispersal. Annu. Rev. Ecol. Syst. 1982, 13, 201–228. [Google Scholar] [CrossRef]
- McEuen, A.B.; Curran, L.M. Seed Dispersal and Recruitment Limitation across Spatial Scales in Temperate Forest Fragments. Ecology 2004, 85, 507–518. [Google Scholar] [CrossRef]
- Beninde, J.; Veith, M.; Hochkirch, A. Biodiversity in Cities Needs Space: A Meta-Analysis of Factors Determining Intra-Urban Biodiversity Variation. Ecol. Lett. 2015, 18, 581–592. [Google Scholar] [CrossRef]
- Koerner, W.; Dupouey, J.L.; Dambrine, E.; Benoit, M. Influence of Past Land Use on the Vegetation and Soils of Present Day Forest in the Vosges Mountains, France. J. Ecol. 1997, 85, 351–358. [Google Scholar] [CrossRef]
- Alard, D.; Chabrerie, O.; Dutoit, T.; Roche, P.; Langlois, E. Patterns of Secondary Succession in Calcareous Grasslands: Can We Distinguish the Influence of Former Land Uses from Present Vegetation Data? Basic Appl. Ecol. 2005, 6, 161–173. [Google Scholar] [CrossRef]
- Fomby, T.B.; Hill, R.C.; Johnson, S.R. Advanced Econometric Methods; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2012. [Google Scholar]
- Hettmansperger, T.P.; McKean, J.W. Robust Nonparametric Statistical Methods; CRC Press: Boca Raton, FL, USA, 2010. [Google Scholar]
- Maindonald, J.; Braun, J. Data Analysis and Graphics Using R: An Example-Based Approach; Cambridge University Press: Cambridge, UK, 2006; ISBN 978-1-139-46053-8. [Google Scholar]
- Ferrari, S.; Cribari-Neto, F. Beta Regression for Modelling Rates and Proportions. J. Appl. Stat. 2004, 31, 799–815. [Google Scholar] [CrossRef]
- Box, G.E.P.; Cox, D.R. An Analysis of Transformations. J. R. Stat. Soc. Ser. B (Methodol.) 1964, 26, 211–243. [Google Scholar] [CrossRef]
- Moran, P.A. Notes on Continuous Stochastic Phenomena. Biometrika 1950, 37, 17–23. [Google Scholar] [CrossRef] [PubMed]
- Breusch, T.S.; Pagan, A.R. A Simple Test for Heteroscedasticity and Random Coefficient Variation. Econom. J. Econom. Soc. 1979, 47, 1287–1294. [Google Scholar] [CrossRef]
- Shaphiro, S.; Wilk, M.B.J.B. An Analysis of Variance Test for Normality. Biometrika 1965, 52, 591–611. [Google Scholar] [CrossRef]
- Efroymson, M.A. Multiple Regression Analysis. In Mathematical Methods for Digital Computers; John Wiley: New York, NY, USA, 1960; pp. 191–203. [Google Scholar]
- Venables, B.; Ripley, B.D. Modern Applied Statistics with S; Springer: New York, NY, USA, 2002; ISBN 0-387-95457-0. [Google Scholar]
- Hebbali, A. Olsrr: Tools for Building OLS Regression Models. R Package Version 0.5 2020, 3. Available online: https://cran.r-project.org/web/packages/olsrr/olsrr.pdf (accessed on 27 May 2024).
- Zeileis, A.; Hothorn, T. Diagnostic Checking in Regression Relationships. R News 2002, 2, 7–10. [Google Scholar]
- Croissant, Y.; Millo, G. Panel Data Econometrics in R: The Plm Package. J. Stat. Softw. 2008, 27, 1–43. [Google Scholar] [CrossRef]
- Cribari-Neto, F.; Zeileis, A. Beta Regression in R. J. Stat. Softw. 2010, 34, 1–24. [Google Scholar] [CrossRef]
- Stasinopoulos, D.M.; Rigby, R.A. Generalized Additive Models for Location Scale and Shape (GAMLSS) in R. J. Stat. Softw. 2008, 23, 1–46. [Google Scholar] [CrossRef]
- Sehrt, M.; Bossdorf, O.; Freitag, M.; Bucharova, A. Less Is More! Rapid Increase in Plant Species Richness after Reduced Mowing in Urban Grasslands. Basic Appl. Ecol. 2020, 42, 47–53. [Google Scholar] [CrossRef]
- Watson, C.J.; Carignan-Guillemette, L.; Turcotte, C.; Maire, V.; Proulx, R. Ecological and Economic Benefits of Low-intensity Urban Lawn Management. J. Appl. Ecol. 2020, 57, 436–446. [Google Scholar] [CrossRef]
- Wulf, M. Plant Species Richness of Afforestations with Different Former Use and Habitat Continuity. For. Ecol. Manag. 2004, 195, 191–204. [Google Scholar] [CrossRef]
- Raduła, M.W.; Szymura, T.H.; Szymura, M.; Swacha, G.; Kącki, Z. Effect of Environmental Gradients, Habitat Continuity and Spatial Structure on Vascular Plant Species Richness in Semi-Natural Grasslands. Agric. Ecosyst. Environ. 2020, 300, 106974. [Google Scholar] [CrossRef]
- Guilherme, F.; Vicente, J.R.; Carretero, M.A.; Farinha-Marques, P. Mapping Multigroup Responses to Land Cover Legacy for Urban Biodiversity Conservation. Biol. Conserv. 2024, 291, 110508. [Google Scholar] [CrossRef]
- Beveridge, R.; Kip, M.; Oevermann, H. From Wastelands to Waiting Lands: Retrieving Possibility from the Voids of Berlin. City 2022, 26, 281–303. [Google Scholar] [CrossRef]
- Culley, T.M.; Dreisilker, K.; Clair Ryan, M.; Schuler, J.A.; Cavallin, N.; Gettig, R.; Havens, K.; Landel, H.; Shultz, B. The Potential Role of Public Gardens as Sentinels of Plant Invasion. Biodivers. Conserv. 2022, 31, 1829–1844. [Google Scholar] [CrossRef]
- Lenda, M.; Skórka, P.; Knops, J.M.H.; Moroń, D.; Tworek, S.; Woyciechowski, M. Plant Establishment and Invasions: An Increase in a Seed Disperser Combined with Land Abandonment Causes an Invasion of the Non-Native Walnut in Europe. Proc. R. Soc. B Biol. Sci. 2011, 279, 1491–1497. [Google Scholar] [CrossRef]
- Mayer, K.; Haeuser, E.; Dawson, W.; Essl, F.; Kreft, H.; Pergl, J.; Pyšek, P.; Weigelt, P.; Winter, M.; Lenzner, B.; et al. Naturalization of Ornamental Plant Species in Public Green Spaces and Private Gardens. Biol. Invasions 2017, 19, 3613–3627. [Google Scholar] [CrossRef]
- Smith, R.G.; Maxwell, B.D.; Menalled, F.D.; Rew, L.J. Lessons from Agriculture May Improve the Management of Invasive Plants in Wildland Systems. Front. Ecol. Environ. 2006, 4, 428–434. [Google Scholar] [CrossRef]
- Bell, C.E.; Wilen, C.A.; Stanton, A.E. Invasive Plants of Horticultural Origin. Hortscience 2003, 38, 14–16. [Google Scholar] [CrossRef]
- Gioria, M.; Le Roux, J.J.; Hirsch, H.; Moravcová, L.; Pyšek, P. Characteristics of the Soil Seed Bank of Invasive and Non-Invasive Plants in Their Native and Alien Distribution Range. Biol. Invasions 2019, 21, 2313–2332. [Google Scholar] [CrossRef]
- Fontúrbel, F.E.; Candia, A.B.; Malebrán, J.; Salazar, D.A.; González-Browne, C.; Medel, R. Meta-Analysis of Anthropogenic Habitat Disturbance Effects on Animal-Mediated Seed Dispersal. Glob. Change Biol. 2015, 21, 3951–3960. [Google Scholar] [CrossRef] [PubMed]
- Kowarik, I. On the Role of Alien Species in Urban Flora and Vegetation. In Urban Ecology: An International Perspective on the Interaction between Humans and Nature; Springer: Boston, MA, USA, 2008; pp. 321–338. [Google Scholar]
- McKinney, M.L. Urbanization as a Major Cause of Biotic Homogenization. Biol. Conserv. 2006, 127, 247–260. [Google Scholar] [CrossRef]
- Alberti, M.; Correa, C.; Marzluff, J.M.; Hendry, A.P.; Palkovacs, E.P.; Gotanda, K.M.; Hunt, V.M.; Apgar, T.M.; Zhou, Y. Global Urban Signatures of Phenotypic Change in Animal and Plant Populations. Proc. Natl. Acad. Sci. USA 2017, 114, 8951–8956. [Google Scholar] [CrossRef] [PubMed]
- Hobbs, R.J.; Higgs, E.; Hall, C.M.; Bridgewater, P.; Chapin, F.S.; Ellis, E.C.; Ewel, J.J.; Hallett, L.M.; Harris, J.; Hulvey, K.B.; et al. Managing the Whole Landscape: Historical, Hybrid, and Novel Ecosystems. Front. Ecol. Environ. 2014, 12, 557–564. [Google Scholar] [CrossRef]
- Johnson, A.L.; Borowy, D.; Swan, C.M. Land Use History and Seed Dispersal Drive Divergent Plant Community Assembly Patterns in Urban Vacant Lots. J. Appl. Ecol. 2018, 55, 451–460. [Google Scholar] [CrossRef]
- Gelmi-Candusso, T.A.; Hämäläinen, A.M. Seeds and the City: The Interdependence of Zoochory and Ecosystem Dynamics in Urban Environments. Front. Ecol. Evol. 2019, 7, 41. [Google Scholar] [CrossRef]
- von der Lippe, M.; Bullock, J.M.; Kowarik, I.; Knopp, T.; Wichmann, M. Human-Mediated Dispersal of Seeds by the Airflow of Vehicles. PLoS ONE 2013, 8, e52733. [Google Scholar] [CrossRef]
- von der Lippe, M.; Kowarik, I. Do Cities Export Biodiversity? Traffic as Dispersal Vector across Urban-Rural Gradients: Traffic as Dispersal Vector across Urban-Rural Gradients. Divers. Distrib. 2008, 14, 18–25. [Google Scholar] [CrossRef]
- Tello-García, E.; Gamboa-Badilla, N.; Álvarez, E.; Fuentes, L.; Basnou, C.; Espelta, J.M.; Pino, J. Plant Species Surplus in Recent Peri-Urban Forests: The Role of Forest Connectivity, Species’ Habitat Requirements and Dispersal Types. Biodivers. Conserv. 2021, 30, 365–384. [Google Scholar] [CrossRef]
- Niu, H.; Rehling, F.; Chen, Z.; Yue, X.; Zhao, H.; Wang, X.; Zhang, H.; Schabo, D.G.; Farwig, N. Regeneration of Urban Forests as Influenced by Fragmentation, Seed Dispersal Mode and the Legacy Effect of Reforestation Interventions. Landsc. Urban Plan. 2023, 233, 104712. [Google Scholar] [CrossRef]
- Bornkamm, R. Spontaneous Development of Urban Woody Vegetation on Differing Soils. Flora-Morphol. Distrib. Funct. Ecol. Plants 2007, 202, 695–704. [Google Scholar] [CrossRef]
- Riley, C.B.; Perry, K.I.; Ard, K.; Gardiner, M.M. Asset or Liability? Ecological and Sociological Tradeoffs of Urban Spontaneous Vegetation on Vacant Land in Shrinking Cities. Sustainability 2018, 10, 2139. [Google Scholar] [CrossRef]
- Fischer, L.K.; von der Lippe, M.; Rillig, M.C.; Kowarik, I. Creating Novel Urban Grasslands by Reintroducing Native Species in Wasteland Vegetation. Biol. Conserv. 2013, 159, 119–126. [Google Scholar] [CrossRef]
- Lonati, M.; Probo, M.; Gorlier, A.; Pittarello, M.; Scariot, V.; Lombardi, G.; Ravetto Enri, S. Plant Diversity and Grassland Naturalness of Differently Managed Urban Areas of Torino (NW Italy). Acta Hortic. 2018, 1215, 247–254. [Google Scholar] [CrossRef]
- Perotti, E.; Probo, M.; Pittarello, M.; Lonati, M.; Lombardi, G. A 5-Year Rotational Grazing Changes the Botanical Composition of Sub-Alpine and Alpine Grasslands. Appl. Veg. Sci. 2018, 21, 647–657. [Google Scholar] [CrossRef]
- Lorenzo, P.; Pazos-Malvido, E.; Rubido-Bará, M.; Reigosa, M.J.; González, L. Invasion by the Leguminous Tree Acacia Dealbata (Mimosaceae) Reduces the Native Understorey Plant Species in Different Communities. Aust. J. Bot. 2012, 60, 669–675. [Google Scholar] [CrossRef]
- Maurel, N.; Salmon, S.; Ponge, J.-F.; Machon, N.; Moret, J.; Muratet, A. Does the Invasive Species Reynoutria Japonica Have an Impact on Soil and Flora in Urban Wastelands? Biol. Invasions 2010, 12, 1709–1719. [Google Scholar] [CrossRef]
- Weber, E.; Jakobs, G. Biological Flora of Central Europe: Solidago gigantea Aiton. Flora-Morphol. Distrib. Funct. Ecol. Plants 2005, 200, 109–118. [Google Scholar] [CrossRef]
- Lenda, M.; Witek, M.; Skórka, P.; Moroń, D.; Woyciechowski, M. Invasive Alien Plants Affect Grassland Ant Communities, Colony Size and Foraging Behaviour. Biol. Invasions 2013, 15, 2403–2414. [Google Scholar] [CrossRef]
- Brown, B.J.; Mitchell, R.J.; Graham, S.A. Competition for Pollination Between an Invasive Species (Purple Loosestrife) and a Native Congener. Ecology 2002, 83, 2328–2336. [Google Scholar] [CrossRef]
- Schuster, M.J.; Wragg, P.D.; Reich, P.B. Using Revegetation to Suppress Invasive Plants in Grasslands and Forests. J. Appl. Ecol. 2018, 55, 2362–2373. [Google Scholar] [CrossRef]
- Shea, K.; Chesson, P. Community Ecology Theory as a Framework for Biological Invasions. Trends Ecol. Evol. 2002, 17, 170–176. [Google Scholar] [CrossRef]
- Martinez, J.A.; Dornbush, M.E. Use of a Native Matrix Species to Facilitate Understory Restoration in an Overbrowsed, Invaded Woodland. Invasive Plant Sci. Manag. 2013, 6, 219–230. [Google Scholar] [CrossRef]
- Funk, J.L.; Cleland, E.E.; Suding, K.N.; Zavaleta, E.S. Restoration through Reassembly: Plant Traits and Invasion Resistance. Trends Ecol. Evol. 2008, 23, 695–703. [Google Scholar] [CrossRef] [PubMed]
- Swanson, M.E.; Franklin, J.F.; Beschta, R.L.; Crisafulli, C.M.; DellaSala, D.A.; Hutto, R.L.; Lindenmayer, D.B.; Swanson, F.J. The Forgotten Stage of Forest Succession: Early-Successional Ecosystems on Forest Sites. Front. Ecol. Environ. 2011, 9, 117–125. [Google Scholar] [CrossRef]
- Didham, R.K.; Tylianakis, J.M.; Hutchison, M.A.; Ewers, R.M.; Gemmell, N.J. Are Invasive Species the Drivers of Ecological Change? Trends Ecol. Evol. 2005, 20, 470–474. [Google Scholar] [CrossRef]
- Dillon, W.W.; Lieurance, D.; Hiatt, D.T.; Clay, K.; Flory, S.L. Native and Invasive Woody Species Differentially Respond to Forest Edges and Forest Successional Age. Forests 2018, 9, 381. [Google Scholar] [CrossRef]
- Flory, S.L.; Clay, K. Invasive Shrub Distribution Varies with Distance to Roads and Stand Age in Eastern Deciduous Forests in Indiana, USA. Plant Ecol. 2006, 184, 131–141. [Google Scholar] [CrossRef]
- Trzeciak, M.; Sikorska, D. Application of UAV and Ground Measurements for Urban Vegetation Cooling Benefits Assessment, Wilanów Palace Case Study. Sci. Rev. Eng. Environ. Sci. (SREES) 2024, 33, 53–68. [Google Scholar] [CrossRef]
- Dusza-Dobek, A. Badania geochemiczne gleb w wybranych parkach Warszawy. Biul. Państwowego Inst. Geol. 2012, 450, 35–46. [Google Scholar]
- Gancarczyk-Gola, M.; Palowski, B.D. of E. Heavy Metals and Acidity of Surface Soil Horizons in Surroundings of Industrial Centers and in Non-Contaminated Regions. Rocz. Glebozn. 2005, 56, 59–66. [Google Scholar]
- Belaire, J.A.; Whelan, C.J.; Minor, E.S. Having Our Yards and Sharing Them Too: The Collective Effects of Yards on Native Bird Species in an Urban Landscape. Ecol. Appl. 2014, 24, 2132–2143. [Google Scholar] [CrossRef] [PubMed]
- Sushinsky, J.R.; Rhodes, J.R.; Possingham, H.P.; Gill, T.K.; Fuller, R.A. How Should We Grow Cities to Minimize Their Biodiversity Impacts? Glob. Change Biol. 2013, 19, 401–410. [Google Scholar] [CrossRef] [PubMed]
- Rupprecht, C.D.D.; Byrne, J.A.; Ueda, H.; Lo, A.Y. ‘It’s Real, Not Fake like a Park’: Residents’ Perception and Use of Informal Urban Green-Space in Brisbane, Australia and Sapporo, Japan. Landsc. Urban Plan. 2015, 143, 205–218. [Google Scholar] [CrossRef]
- Sikorska, D.; Sikorski, P.; Archiciński, P.; Chormański, J.; Hopkins, R.J. You Can’t See the Woods for the Trees: Invasive Acer Negundo L. in Urban Riparian Forests Harms Biodiversity and Limits Recreation Activity. Sustainability 2019, 11, 5838. [Google Scholar] [CrossRef]
- Lange-Kabitz, C.; Reich, M.; Zoch, A. Extensively Managed or Abandoned Urban Green Spaces and Their Habitat Potential for Butterflies. Basic Appl. Ecol. 2021, 54, 85–97. [Google Scholar] [CrossRef]
- Doroski, D.A.; Felson, A.J.; Bradford, M.A.; Ashton, M.P.; Oldfield, E.E.; Hallett, R.A.; Kuebbing, S.E. Factors Driving Natural Regeneration beneath a Planted Urban Forest. Urban For. Urban Green. 2018, 29, 238–247. [Google Scholar] [CrossRef]
- Silvertown, J.; Buesching, C.D.; Jacobson, S.K.; Rebelo, T. Citizen Science and Nature Conservation. In Key Topics in Conservation Biology 2; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2013; pp. 127–142. ISBN 978-1-118-52017-8. [Google Scholar]
- Turrini, T.; Knop, E. A Landscape Ecology Approach Identifies Important Drivers of Urban Biodiversity. Glob. Change Biol. 2015, 21, 1652–1667. [Google Scholar] [CrossRef]
| Indicator | Description and Literature | |
|---|---|---|
| Vertical vegetation structure | Canopy cover [%] Shrub cover [%] Herbaceous vegetation [%] | Percentage cover of different vegetation layers. |
| Biodiversity indicators | Total number of species [no.] | Total number of vascular plant species, nomenclature according to Mirek [58]. |
| Shannon-Wiener index | Shannon-Wiener index after Magguran [60], which takes into account total species number and their percentage cover calculated for herbaceous vegetation layer. | |
| Vegetation composition | Forest species [%] Grassland species [%] Invasive plant species [%] | Percentage cover of species typical for different ecological groups [54]. Forest plant communities (species from Salicetea purpureae and Querco-Fagetea); Grassland plant communities (species from Molinio-Arrhenatheretea, Artemisietea vulgaris, Agropyretea intermedio-repentis and Epilobietea angustofolii classes); Invasive plant species (percentage cover of Acer negundo, Quercus rubra, Robinia pseudoacacia, Solidago canadensis and Solidago gigantea). |
| Ancient forest plant species [no.] | Number of ancient forest species, characterized by low dispersal ability which may indicate a long continuous history for the habitat, and may be indicative of more original forest conditions. List of species as by Dzownko and Loster [61]. | |
| Rare plant species [no.] | Number of rare species in the herbaceous layer for the area of Warsaw [62,63]. | |
| Invasive species | Invasive species in canopy [%] Invasive species in shrub layer [%] Invasive species in herbaceous layer [%] | Percentage cover of Acer negundo, Quercus rubra, Robinia pseudoacacia, Solidago canadensis, and Solidago gigantea in different vegetation layers. |
| Indicators of naturalness | Hemeroby | Index expressing the vegetation deviation from the potential natural state due to anthropopressure on a scale of 1–7 [64]. The average value of the index per plot calculated based on the BiolFlor database [65], on the basis of the species occurrence and coverage [66]. |
| Urbanity | The average value of the index that evaluates the attachment of plant species with urban-environments [66]. The average value of index per plot calculated on the basis of the BiolFlor database [65], on the basis of the list of species and their coverage. |
| Name | Description | Regression Model |
|---|---|---|
| Dependent variables | ||
| Vegetation quality indicators | ||
| Total number of species | Total number of vascular plant species | Linear model with Box-Cox transformation |
| Ancient forest plant species | Number of ancient forest plant species | quasi-Poisson model |
| Rare plant species | Number of rare species in herbaceous vegetation layer | quasi-Poisson model |
| Hemeroby | The average value of the index of response of vegetation to anthropopressure | Linear model with Box-Cox transformation |
| Urbanity | The average value of the index of tendency to occur in cities | Linear model with Box-Cox transformation |
| Vegetation composition | ||
| Forest species [%] | share of species characteristic for forests | beta regression model |
| Grassland species [%] | share of species characteristic for grasslands | beta regression model |
| Invasive plant species [%] | share of invasive plant species | beta regression model |
| Explanatory variables | ||
| Habitat continuity [years] | The period measured from the occurrence of permanent land use transformation resulting from human activities. | |
| Neighborhood of study plot (100 m buffer): | ||
| Low vegetation | A set of binary variables which take the value of 1 for a dominant type, within the 100 m buffer from study plot, and 0 otherwise. | |
| High vegetation | ||
| Without vegetation | ||
| Patch size [m2] | Surface area of each studied IGS | |
| Former land use type: | ||
| Arable land | A set of binary variables which take the value of 1 for previous land use, determined on the basis of historical orthophotomaps, and 0 otherwise. | |
| Cultivated grasslands and pastures | ||
| Built-up areas | ||
| Waterbodies | ||
| Gardens and orchards | ||
| Other |
| Non-Forest IGS n = 47 | Forest IGS n = 61 | p-Value | |
|---|---|---|---|
| Habitat continuity and vertical vegetation structure | |||
| Habitat continuity (years) | 39.30 | 43.80 | 0.789 |
| Canopy cover (%) | 7.05 | 78.54 | 0.000 |
| Shrub cover (%) | 10.23 | 33.49 | 0.001 |
| Herbaceous vegetation (%) | 97.34 | 66.14 | 0.001 |
| Biodiversity indicators | |||
| Total number of species (n) | 15.60 | 12.70 | 0.032 |
| Shannon-Wiener index | 1.82 | 1.68 | 0.852 |
| Vegetation composition | |||
| Ancient forest plant species (n) | 0.36 | 1.34 | 0.012 |
| Rare plant species (n) | 0.32 | 1.59 | 0.018 |
| Invasive species | |||
| Invasive species in canopy (%) | 3.38 | 32.79 | 0.000 |
| Invasive species in shrub layer (%) | 6.87 | 10.18 | 0.123 |
| Invasive species in herbaceous layer (%) | 21.62 | 10.26 | 0.004 |
| Indicators of naturalness | |||
| Hemeroby | 4.14 | 3.86 | 0.673 |
| Urbanity | 2.69 | 2.64 | 0.982 |
| Trampling | 2930 | 3009 | 0.957 |
| Soil quality | |||
| pH | 7.19 | 7.10 | 0.981 |
| Pb | 50.49 | 46.54 | 0.549 |
| Cd | 0.31 | 0.34 | 0.892 |
| Ni | 12.99 | 12.13 | 0.711 |
| Cr | 14.45 | 14.23 | 0.894 |
| Cu | 50.05 | 45.55 | 0.759 |
| Zn | 135.78 | 122.45 | 0.914 |
| Non-Forest IGS | Forest IGS | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Total Number of Species | Ancient Forest Plant Species | Rare Plant Species | Hemeroby | Urbanity | Total Number of Species | Ancient Forest Plant Species | Rare Plant Species | Hemeroby | Urbanity | |
| Habitat continuity | 0.039 *** | - | - | −0.064 * | - | 0.009 *** | 0.016 ** | 0.024 *** | −0.008 ** | −0.007 * |
| High vegetation in vicinity | −1.017 * | - | - | 3.320 * | - | - | - | - | - | - |
| Former built-up areas | 1.885 * | - | - | - | - | −1.285 *** | - | - | - | - |
| Non-Forest IGS | Forest IGS | |||||
|---|---|---|---|---|---|---|
| Forest Species | Grassland Species | Invasive Species | Forest Species | Grassland Species | Invasive Species | |
| Habitat continuity | - | - | - | 0.013 * | - | −0.013 * |
| High vegetation in vicinity | - | - | - | 1.342 ** | - | - |
| Former built-up areas | - | - | 1.761 ** | −1.500 * | - | - |
| Former gardens and orchards | - | - | 1.900 *** | - | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Archiciński, P.; Przybysz, A.; Sikorska, D.; Wińska-Krysiak, M.; Da Silva, A.R.; Sikorski, P. Conservation Management Practices for Biodiversity Preservation in Urban Informal Green Spaces: Lessons from Central European City. Land 2024, 13, 764. https://doi.org/10.3390/land13060764
Archiciński P, Przybysz A, Sikorska D, Wińska-Krysiak M, Da Silva AR, Sikorski P. Conservation Management Practices for Biodiversity Preservation in Urban Informal Green Spaces: Lessons from Central European City. Land. 2024; 13(6):764. https://doi.org/10.3390/land13060764
Chicago/Turabian StyleArchiciński, Piotr, Arkadiusz Przybysz, Daria Sikorska, Marzena Wińska-Krysiak, Anderson Rodrigo Da Silva, and Piotr Sikorski. 2024. "Conservation Management Practices for Biodiversity Preservation in Urban Informal Green Spaces: Lessons from Central European City" Land 13, no. 6: 764. https://doi.org/10.3390/land13060764






