Abstract
The aim of the present review is to compile and analyze information on biology of Rhinanthus species in the context of grassland biodiversity. Root hemiparasites have been relatively less studied in comparison to economically important holoparasitic weed species. Rhinanthus species appear to be genetically polymorphic, but also possess high phenotypic plasticity, and ecological factors are important determinants in evolution of specialization to most appropriate hosts. Rhinanthus individuals have a relatively short life span, and flowering is a photoperiod- or host plant-independent phenomenon. Both insect pollination and self-pollination can occur. Seeds do not form a persistent soil seed bank and have physiological dormancy broken by stratification. In general, Rhinanthus species have low host specificity, but there clearly are ‘preferred’ or ‘avoided’ hosts in natural conditions. In controlled conditions, interaction with most grass species result in more prominent parasite growth stimulation in comparison to that of legumes, and, especially, forbs, but there are significant gradations and exceptions. Ecological requirements of Rhinanthus species have been rarely studied, but it can be expected that significant tolerance against mineral nutrient heterogeneity and water shortage can be found. It seems that host plant characteristics are important determinants of the environmental resilience of Rhinanthus. Parasites not only obtain resources (water and minerals) from host plants but also negatively affect their physiological functions. The most intriguing and practically unexplored question is the exchange of chemical signals between the Rhinanthus parasite and the host plant. Extending this idea, it can be predicted that signals will also be exchanged between multiple host plants whose roots are connected through the parasite. It is highly possible that the exchange of small RNAs between plants could influence their environmental tolerance. Host selectivity forms the functional basis of changes in species diversity in grasslands, but the outcome seems to be highly dependent on other conditions, especially, soil edaphic factors. Development of new model systems is necessary to further expand our knowledge about the complex effects of parasitic plants on ecosystems.
1. Introduction
Among plant–plant interactions, which are mostly non-specific and based on different types of competition, the relationship between parasitic plants and their host plants clearly stands out. Parasitism has evolved several times in the plant world, and parasitic species are found mainly in the family Orobanchaceae, as well as in Santalaceae and Convolvulaceae. The total number of known parasitic plant species is around 4500 from about 28 families [1].
The most clearly visible morphological feature in such a relationship is the formation of a special organ, haustoria, on the parasite, with the help of which it “connects” to the host’s vascular system. Depending on attachment to either xylem or phloem, the parasite obtains resources from the host in the form of water, minerals and photosynthates. In the classification of parasitic plants, three groups of features are used, which describe the dependence on the interaction for the completion of the life cycle, the place of attachment to the host plant, and the ability to carry out photosynthesis. In contrast to holoparasites that lack chlorophyll and cannot fix CO2, hemiparasites retain photosynthetic function at least to a certain degree. Holoparasites are always obligate, but hemiparasites can be either obligate or facultative. All types of parasitic plants attach either to stem or root of a host plant. For general information on functional diversity and parasitic plants, readers are encouraged to consult several recent comprehensive reviews [2,3,4,5,6,7].
Plants of genus Rhinanthus (Orobanchaceae) are typical representatives of the Rhinanthoid group, consisting of 10 hemiparasitic and one holoparisitic genera [8]. In contrast to many parasitic plants that are economically important weeds (such as Striga, Orobanche, Phelipanche), Rhinanthus species have gained attention as positive players in improving grassland biodiversity. Perhaps for these practical differences, Rhinanthus species have been much less studied with respect to the mechanisms of parasitism relationship formation and host plant responses. Therefore, the aim of the present review was to compile and analyze information on the biology of Rhinanthus species in the context of grassland biodiversity with a special emphasis on open questions and identification of knowledge gaps in respect to ecologically and practically important aspects of Rhinanthus biology.
2. Taxonomic, Evolutionary, and Geographic Aspects
Among about 25 to 30 recognized Rhinanthus species, only a few are widely distributed throughout Europe. Among them, Rhinanthus angustifolius C.C.Gmel. and Rhinanthus minor L. are the most common species, with a high rate of co-occurrence in grasslands [9]. According to the recent taxonomy, R. angustifolius as well as Rhinanthus serotinus (Schönh) Oborny, are synonyms of Rhinanthus major L. [10]. In the present review, all species names are used as in the original publications. Rhinanthus alectorolophus (Scop.) Pollich is another species of the genus native to Central and Eastern Europe relatively frequently used as a model in studies with root hemiparasites.
Interspecific hybridization is common within the genus Rhinanthus [11], and several morphologically and ecologically similar but genetically different endemic taxa have been described [9,12]. It seems that ecotypes of Rhinanthus species evolve in a rather short time span, possibly due to local environmental and even anthropogenic factors, such as different grassland mowing regimes [13]. It is interesting that genetically determined differentiation in R. angustifolius is combined with relatively high phenotypic plasticity [14]. No geographical genetical structure has been established for R. angustifolius in Europe, which possibly reflects extensive hybridization or persistence of ancestral polymorphisms [15]. In contrast, R. minor s.s. populations showed a clear geographical structure of genetic similarity [9]. However, several populations initially identified as belonging to R. minor probably belonged to a previously unidentified taxon [9].
Traditional taxonomy previously used in local flora of Sweden distinguished broadly adapted R. angustifolius subsp. angustifolius (found in grasslands of road verges and sea coast) and meadow-specialized R. angustifolius subsp. grandiflorus (adapted to mowing practices). Moreover, a common garden experiment revealed a possibility that three groups of populations exist, representing grasslands of road verges, coastal sites, and mowed meadows [14]. When 17 R. angustifolius populations from South Sweden were analyzed, it appeared that interspecific genetic structure was clearly related to geographical distance [16]. Consequently, previously identified subspecies or population groups appear to be irrelevant in the context of the genetic structure of R. angustifolius, and a broader geographical range of populations needs to be considered for conservation purposes instead of focusing on specific habitats. Similarly, genetic factors most likely had no effect on specialization of R. serotinus to the most beneficial host plants, but ecological factors are more important in evolution of specialization in hemiparasitic plants [17].
3. Reproductive Biology of Rhinanthus
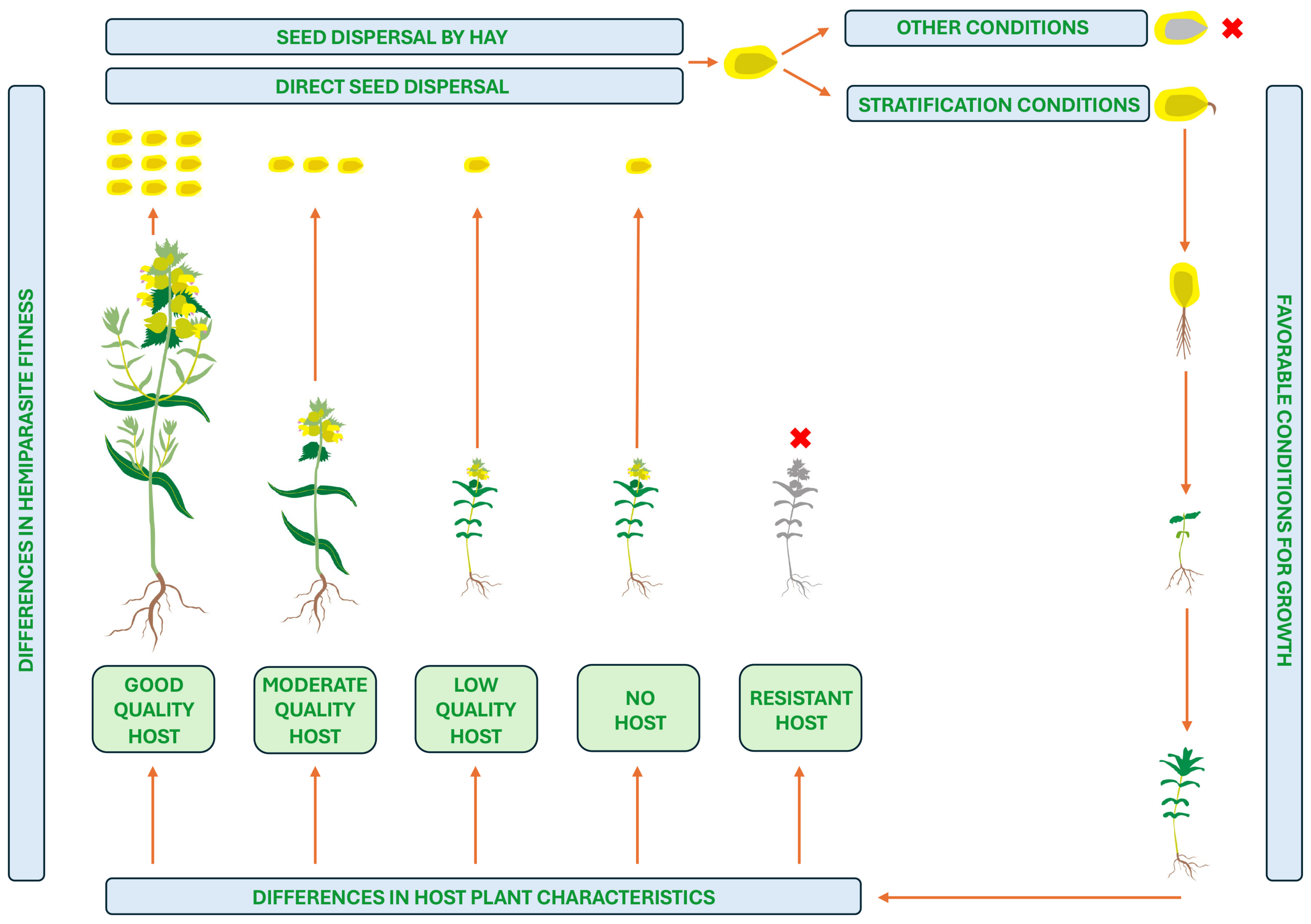
The life span of individual R. minor plants is only about 16 weeks. The life cycle of individuals of typical Rhinanthus species is shown in Figure 1, taking into account the formation of associations with different host plants. Flowering is induced at a stage with a certain node number independently from photoperiod or presence of host plant, but due to genetic differences, can occur from May to July or late July to September [18]. Hermaphroditic flowers of Rhinanthus species produce nectar, and are pollinated by several Hymenoptera species, mostly belonging to Bombus, but self-pollination also occurs [19]. After pollination, flowers develop into capsules, containing variable numbers of relatively large flat winged seeds. Capsules dehisce several weeks later, but seeds remain in capsules until mechanical disturbance leading to local dispersal.
Figure 1.
The life cycle of an individual of a typical Rhinanthus species showing dependence on host plant quality.
Unattached R. minor plants rarely produce more than two seed capsules per plant, but the number of capsules can exceed 20 with more than 170 seeds per plant in optimum conditions [19]. Seeds of Rhinanthus species lack special features for long term dispersal, and do not form a persistent seed bank in soil. At the time of dispersal, seeds of Rhinanthus plants have typical physiological dormancy that can be broken only by stratification. None of various seed treatments, including scarification, thermal induction, hormonal treatments (gibberellins and cytokinins), or treatment with host root extracts, can lead to an enhanced germination rate [20]. In addition, conditions of seed storage before stratification also affect germination success. However, requirements for breaking the dormancy period varies between the species within genus Rhinanthus, showing high species-specificity not depending on actual climatic conditions [21]. In particular, two distinct patterns can be seen within a group of five species. Species of the first group (R. minor, R. angustifolius) required a long stratification period (12 weeks and more), but species of the second group (R. alectorolophus, R. glacialis, R. mediterraneus, R. antiquus) required only up to 12 weeks of stratification.
Seeds can germinate in the absence of any host plants when necessary conditions are met. However, it appears that response to temperature conditions after full stratification is also a species-specific trait. For the first group of Rhinanthus species mentioned above, transfer of seeds from stratification temperatures to 20 °C accelerated germination but decreased final germination percentage [21]. Just seven warm days in a row was a sufficient duration to accelerate germination. For the second group of species, increase in temperature did not accelerate germination while decreasing germination percentage. In fact, any duration of warmer period (1 to 7 days) slowed down germination for these Rhinanthus species. It is believed that the noted differences in stratification and germination patterns reflect adaptation to temperate climates with possible temperature fluctuations in spring (R. minor, R. angustifolius) or to warmer climates with relatively stable temperature conditions in spring (R. alectorolophus, R. glacialis, R. mediterraneus, R. antiquus) [21].
Different aspects of seed quality affect seedling establishment and survival, as well as future productivity of Rhinanthus plants. Among them, seed vigor, characterized as the time period between seed imbibition and radicle emergence, is extremely important, as high seedling mortality as well as low rate of biomass accumulation are direct consequences of low seed vigor [22]. Consequently, in addition to particular environmental factors, hemiparasite seed quality can also affect the results of interaction between parasites and host plants.
An extremely important aspect of seedling establishment in R. alectrolophus in field conditions is associated with the positive effect of sowing density on rate of seedling establishment, which is in striking contrast to the majority of plant species, showing existence of a prominent self-thinning mechanism at high densities [23]. As the experiment was performed on the background of potential host species, resource sharing among parasite seedlings through haustorial connections was suggested as a possible mechanism for increased survival with increasing sowing density. In contrast, degree of plant survival was not affected by sowing density, but mean plant biomass linearly decreased along with increased density.
4. Host Range and Selectivity
Analysis of a host range for parasitic plants associated with roots includes problems of a methodological nature. Such studies are usually carried out in field conditions, establishing the existence of associations between the haustoria of the parasite and the plants of a particular species, which in itself is a technically very complex process. Therefore, it is not surprising that such studies are often carried out in places with sparse vegetation, such as coastal dunes for R. minor [24]. Further problems are related to the interpretation of the results, because ideally the absolute data of root contacts should be related to the frequency of occurrence of the host plant in the specific location. As an alternative to direct counting of root contacts, association analysis is used to determine which plant species usually grow together with the parasite species and which do not. It is clear that such a method cannot give an accurate picture of the hosts of the parasite, but can only be useful as a preliminary approach.
By comparing frequency of roots for a particular host species available in a soil sample with number of actual haustorial contacts with that species, it was established that R. minor plants show a certain selectivity towards host species, as the number of haustorial contacts with roots of some species was significantly lower or higher than would be expected by chance [24]. Thus, it was concluded that some potential host species are either ‘preferred’ or ‘avoided’, but everything was complicated by the fact that certain species appeared as preferred hosts at one study site and as avoided hosts at another. It was argued that the site-specific selectivity of Rhinanthus species might be related either to differences in environmental conditions, variation in relative abundance of potential host plants, or genetic structure of both parasite and host populations [20]. It has also been established that there is a positive relationship between biomass of R. minor and the number of haustorial contacts made for a particular parasite–host plant interaction [25].
From a total of 50 species identified as hosts of R. minor in Great Britain and Central Europe, 16 represented Poaceae and 11 Fabaceae [24]. However, not all species from these families were preferred by a parasite. It can also be assumed that grass species are more often parasitized, because they are more common in Rhinanthus habitats, in grasslands. Legume species are less frequent in these habitats, and preference for them could be related to the fact that they are supplying the soil (and parasite) with additional nitrogen as a result of bacterial symbiosis.
Until recently, only in relatively rare cases is a comparison of the suitability of several host plants for a given parasite carried out under controlled conditions [20]. Examples of various host plants with different qualities are shown in Table 1. Some recent studies have used a large number of individual species as hosts to Rhinanthus as well as mixtures of them. Thus, among 13 single hosts for R. alectrolophus (four grasses, five legumes, four forbs), the highest parasite growth stimulation was with grass species, followed by forbs and legumes [26]. However, one grass species (Anthoxanthum odoratum) and two legume species (Onobrychis viciifolia and Anhyllis vulneraria) were poor hosts and interaction with them did not result in any biomass increase of attached R. alectrolophus plants in comparison to unattached ones. Interestingly, degree of suppression of host growth was linearly related to parasite biomass.

Table 1.
Examples of putative differences in host plant quality for various Rhinanthus species.
In a recent study, performance of two related Rhinathus species (R. minor and R. alectorolophus) was compared using 25 host species in controlled conditions at two soil nutrient availability levels [27]. Most importantly, the two closely related parasite species had similar host specificity: most nonleguminous forbs were poorer hosts in comparison to legumes and, especially, grasses. However, in contrast to the previous study, the damage caused by parasites to the hosts was not related to the degree of benefit obtained for the parasites. Some good host species, including Trifolium repens, Trifolium pratense, and Medicago lupulina, were not negatively affected by the parasitic interaction.
An interesting question is the ability of one individual of a parasitic plant to associate with several individuals of the same host plant species or even individuals from different species. Evidence for multiple associations comes both from field observations as well as experiments in controlled conditions. In several coastal habitats in the UK, R. minor plants were connected with 34 different host species, and the number of host species connected with one individual parasitic plant ranged from one to seven [24]. The most frequent number of attached host species per individual parasite was four.
Simultaneous attachment to several host species in controlled conditions has been shown for a root hemiparasite Castilleja wightii, which has characteristics similar to those of Rhinanthus species [29]. It was evident that growth and reproductive performance of the parasite was significantly improved by parasitizing two different host species simultaneously. Other studies also showed an ability of Rhinanthus species to form multiple interspecies associations in controlled conditions [25]. Such a feature raises the question of whether signal exchange is possible not only between the parasite and the host plant, but also between two host plants through the parasite. These aspects are further discussed in Section 9.
In general, better suitability of legume species as hosts for parasites usually has been attributed to symbiotic nitrogen fixation and, consequently, better supply of nitrogen [30], and the suitability of grasses has been associated with low defense responses [31]. However, variation in host quality within these groups is usually relatively high, indicating that additional mechanisms are involved. Thus, among legumes, species from genera Anthyllis and Onobrychis were very poor hosts for Rhinanthus [26]. There is a possibility that active defense responses of low quality host plants can result in dieback of hemiparasitic plants. In a preliminary experiment, R. major plants established haustorial contacts with both Lotus maritimus and Anthyllis vulneraria subsp. maritima plants, but growth of the parasite was extremely poor in the case of Anthyllis (Figure 2). In contrast to unattached control plants of R. major, which remained alive longer, all parasites attached to Anthyllis plant roots died after several weeks.

Figure 2.
Different growth of Rhinanthus major plants attached to roots of two legume species, Lotus maritimus (on the left) and Anthyllis vulneraria subsp. maritima (on the right).
Thus, it seems that more physiological evidence is needed on mechanisms of host plant resistance to Rhinanthus parasites. Possible host-associated characteristics relevant to their ability to resist Rhinanthus infection have been proposed, including constitutive presence or induction of a physical barrier in roots, localized host cell death at the infection point, and production of defense-related compounds [28]. Available experimental evidence in this respect is limited, but induction of cellular fragmentation and lignification has been shown for R. minor interaction with two non-host species, Plantago lanceolata and Leucanthemum vulgare [28].
An intriguing question concerns possible local adaptation of parasitic plants to their sympatric (locally coexistent) host plants. This possibility was tested by using R. serotinus and Agrostis capillaris, and it appeared that, in a common garden experiment, parasite performance was similar on both sympatric and allopatric hosts, suggesting no local adaptation to sympatric hosts [32]. However, effect on host biomass was highly variable, indicating genetic variation in virulence between parasite populations.
5. Establishment of Parasitic Relationship
There is no reason to doubt that, as in other root parasites, compounds secreted by host plant roots act as signals for haustoria development in Rhinanthus species as well. The initial step of haustoria development, prehaustorium formation, is induced by host-derived chemical compounds, collectively known as haustorium-inducing factors (HIFs) [33]. For other hemiparasite species, different phenolic compounds with a methoxyphenol moiety with hydroxy or oxo groups at position 3 and methoxy groups at position 3 or 5 are common HIFs [6]. Most likely, these HIFs are derivates of lignin monomers produced through laccase activity. In particular, dimetoxy-p-benzoquinone is a potent HIF in respect to many Orobanchaceae species [33]. However, so far there is no information available on the chemical nature of potential HIFs in Rhinanthus species.
Recognition of HIFs in parasite tissues occurs through receptor kinase complexes located in the plasma membrane of root cells and involves Ca2+ intake [6]. Several hours after signal recognition, cell expansion and division starts, leading to differentiation of prehaustorium structures within a few days [33]. In contrast to obligate parasite species usually developing “terminal” haustoria on radicle tips, facultative parasites, including Rhinanthus, develop “lateral” haustoria formed as a lateral extension of roots [31]. The haustorium is a rather complex structure, and further functional details on this subject can be found elsewhere [34]. Most importantly, direct anatomical continuity in haustoria between xylem vessel elements of the host plant with those of the parasite is formed (lumen to lumen connection), allowing for mass flow-driven uptake of solutes [31]. A comprehensive review on functional diversity of haustoria in parasitic plants needs to be consulted for further detail [35].
It appears that host quality has not been determined during the early phases of interaction, including host recognition and haustoria formation, but is a result of further resource supply and/or competition between the partners. In a growth experiment using agar plates, it was possible to perform choice tests with R. alectorolophus and different pairs of putative host plants [36]. A good grass host was represented by Dactylis glomerata, and a poor grass host by Anthoxanthum odoratum, while a good legume host was Medicago sativa and a poor legume host was Anthyllis vulneraria. Lateral growth of the parasite roots was measured for indication of directed growth. Within three weeks, growth of R. alectorolophus roots showed no differences between good or poor hosts either in the case of grasses or legumes. However, when a combination of a grass and a legume was used, there was a preferred root growth towards the legume host. The number of haustoria formed was not affected by host type, and functional xylem connections were made even with poor hosts. In comparison to other related hemiparasitic species, such as Odontites rubra, connective R. serotinus plants had relatively high host dependence, expressed as 23-fold growth stimulation after attachment to Medicago sativa for R. serotinus in comparison to that of 14-fold for O. rubra [37].
Attachment to a host of a hemiparasitic plant has greater significance for further parasite development as compared to seedling emergence, as it coincides with a rapid elongation phase and production of true leaves [38]. During this phase, haustorium maturation occurs, allowing for full xylem connectivity between the partners. Xylem-transported cytokinins produced either by a host plant or a parasite appear to have a significant role in establishment of the parasitic relationship [39].
Growth response of Rhinanthus plants after attachment to a suitable host is relatively fast. Already a week after a transplantation together with Festuca cinerea, R. major plants showed significant differences in height (Figure 3A), which were more prominent 20 days later, when attached plants started flowering (Figure 3B).

Figure 3.
Differences between Rhinanthus major plants growing without attachment or attached to Festuca cinerea plants 1 week after transplantation (A) and 20 days later (B).
Host specificity of hemiparasitic Scrophulariaceae species is relatively low, but the suitability of hosts for the parasites (“host quality”) can vary considerably [18]. Some hosts can even have negative consequences for parasite growth, as in the case of Plantago lanceolata [40]. Variation in success of Rhinanthus plants on different hosts seems to be associated with differences in local responses in host plant roots following haustorial contact. In contrast to legumes (high quality hosts) showing only weak and local root lignification in a response to haustoria attachment, Plantago lanceolata plants (low quality hosts) exhibit full anatomical isolation of xylem from haustorium by means of fragmentation of haustorial surface [31]. In the case of graminoid hosts, lignification of cell walls at the interface was evident, and host root endodermis near the penetration site had suberized lamellar layer [31].
6. Mineral Nutrition of Rhinanthus
Mineral nutrition of Rhinanthus species has not been much studied. It has been proposed, though, that significant differences need to be evident between unattached and host-attached plants due to the underdeveloped root system of the parasite [41]. However, when R. minor plants not attached to hosts received additional N, K, or P fertilizers, only in the case of P did significant growth stimulation occur, together with significant increase in leaf P concentration and enhanced rate of photosynthesis [41]. For unattached R. serotinus plants, increase in nutrient (only NPK) levels resulted in increased biomass of both shoots and roots as well as leaf length and plant height [42].
The results of studies on mineral nutrition of attached parasitic plants are more difficult to interpret. A detailed study of R. minor and Lolium perenne interaction at two P levels (limiting and optimal) revealed that parasite biomass at optimal P availability decreased by 90% in comparison to that in P-limiting conditions [43]. At the same time, biomass of parasitized L. perenne plants at optimum P availability was 74% higher than that in limiting conditions. Thus, from the attached parasite’s point of view, it turns out that a low level of P is optimal for its growth, while a high level is inhibitory. However, it seems logical to propose that some indirect mechanism was responsible for suppression of parasite growth in high P conditions. As number of haustoria and rate of success of initial attachment drastically decreased at optimal P availability, it was evident that changes in some morphological characteristics of host roots were related to the negative effects of increased P on R. minor plants [43]. However, in a similar study, high P availability suppressed growth of R. angustifolius attached to L. perenne plants but stimulated it when attached to Deschampsia flexulosa plants [44]. Therefore, suppression of haustoria formation and lower nutrient flux to parasite can be related to increased sink strength of host species relative to that of the parasite, where fast-growing L. perenne plants had increased sink strength in comparison to that of slow-growing D. flexulosa.
Increase in soil N availability resulted in increased shoot growth together with reduced biomass allocation to roots of host plants, but such an effect on relative root biomass was not evident for the attached hemiparasitic R. alectorolophus, while shoot biomass of the parasite increased [45]. The negative effect of parasite on host biomass was not affected by N availability, and these results indicate that Rhinanthus species can have strong effects on host plants at different soil fertility levels.
In a study using balanced fertilizer composition at two rates (low- and high-nutrient treatments), interactive effects of nutrient availability with soil moisture on performance of R. alectrolophus plants was shown [46]. In particular, parasite growth was stimulated by increased soil water availability at low nutrient concentration, but it was inhibited by increased soil moisture at high nutrient concentration. Moreover, when Rhinanthus plants were cultivated at low soil moisture but high nutrient availability and produced the largest biomass, the proportion of host-derived carbon was the lowest, indicating decreased parasitism.
7. Environmental Resilience of Rhinanthus
Ecological requirements of Rhinanthus species have not been broadly generalized, but some detailed analysis has been performed for R. minor [19]. In particular, it was suggested that the species has high phenotypic plasticity. Similarly, high phenotypic plasticity together with local genetic adaptation was shown to determine environmental responses of R. angustifolius plants [14]. Ecological optimum curves have been determined for R. serotinus plants as a spatial distribution of individuals along a gradient of soil edaphic factors [47]. There was no dependence of plant occurrence on macronutrient (NPK) variability, but the distribution curve in respect to ground water was skewed, with low density of individuals in dry spots and highest density close to the humid side.
In field conditions, it is frequently observed that R. angustifolius plants prefer relatively wetter grasslands in comparison to R. minor plants [18]. When effects of soil water availability on the two species were compared in experimental conditions, it appeared that R. angustifolius plants had the highest number of flowers per plant in wet conditions, but R. minor plants showed the highest number of flowers in moist conditions [48]. Both species had relatively similar drought tolerance. It has been argued that high drought tolerance of Rhinanthus species is a direct consequence of their parasitic growth habit due to accumulation of abscisic acid and mannitol [49]. Therefore, plants can rapidly recover even after relatively severe wilting following transient water shortage. Considering the fact that root hemiparasites rely on transpiration-driven acquisition of host water and solutes, it is logical to assume that soil water availability could play a critical role in ensuring the growth success of the parasitic plant. Water shortage increased proportion of biomass in roots for both host species and hemiparasite R. alectorolophus [45]. When drought stress response was assessed in R. alectorolophus plants, it appeared that water shortage led both to reduced survival of seedlings as well as decreased biomass of adult plants [50]. Still, at low irrigation intensity, parasites had active transpiration and an increased rate of photosynthesis.
One of the environmental factors that has a multifaceted effect on Rhinanthus plants is the availability of light. One such aspect is the “distance dilemma”, which predicts that the reduced availability of light when situated closer to the host plant reduces the parasite’s survival abilities as a result of competition for light, but closer situated parasites have better parasitism efficiency. Distance from host indeed appears to play a crucial role in development of a Rhinanthus individual, but the effects are ambiguous [51]. Results from experiments in controlled conditions confirm that Rhinanthus plants located closer to the host received significantly less light. However, by analysis of direct root contacts in field conditions it has been established that the majority of attached R. minor plants were found within 10 cm of the host [24]. In addition, more sophisticated experiments in controlled conditions have revealed that the growth of already attached R. minor was not affected by reduced light availability [52]. In particular, when grass-attached R. minor plants were cultivated either in ambient irradiance intensity or in shaded conditions resulting in less than a half irradiance intensity at plant level for four weeks, there were no significant differences in parasite biomass, number of haustorial contacts per plant or whole-plant carbon and nitrogen concentration. Final height of R. minor plants in shaded conditions was even significantly higher in comparison to that in full light, suggesting that plants exhibited typical shade-avoidance syndrome [53]. Consequently, distance effect of parasite placement relative to host plant is not primarily associated with decreased light intensity.
Regarding global climate change, the question of a possible effect of increased atmospheric CO2 concentration is of particular importance for hemiparasitic interactions with Rhinanthus species and possible consequences for grassland diversity. There is only a limited number of studies dedicated to this topic. When an experiment was performed with R. minor and a high quality host species Poa pratensis in controlled conditions, elevated CO2 concentration increased both photosynthesis and biomass accumulation of the parasite, but transpiration rate and N concentration was unaffected [54]. At the same time, increased CO2 led to elevated photosynthesis of host plants in the presence of parasite, while transpiration and biomass was not significantly affected. However, the total biomass of the system significantly increased at high CO2, but not at the expense of host plant productivity. In contrast, in obligate hemiparasite species Striga hermonthica, photosynthesis of the parasite increased with no increase in biomass accumulation and no changes in negative effects on host plants [55].
The question of the tolerance of Rhinanthus species to various environmental factors cannot be separated from the question of the tolerance of potential host plants. There is reason to believe that the level of tolerance of the host plant to the given factor will have a significant effect on the degree of resistance of the parasite, but no experimental evidence is available to support this hypothesis. It would be necessary to test the responses of several various parasite–host plant combinations against a gradient of the several individual environmental factors (soil moisture, photon flux density of photosynthetically active radiation, temperature, substrate salinity) and their combinations in controlled conditions. As hemiparasitic plants, including Rhinanthus species, are commonly found in both relatively dry and wet coastal grasslands [56], it can be expected that they are at least partially salinity tolerant. Given the potential signal exchange between the interconnected parasites and multiple host plant species, it is possible that Rhinanthus parasitism affects the salt tolerance of these species, allowing less tolerant species to increase their efficiency under heterogeneous soil salinity conditions.
8. How Do Rhinanthus Plants Affect Host Plant Performance and Growth?
From the above analysis, it appears that the effect of Rhinanthus parasites on their hosts is species specific. From the perspective of the host plant, they can be more or less susceptible or even tolerant to parasite attachment. This peculiarity can lead to indirect positive effects of parasite presence in multi-species associations. Effects of Rhinanthus plants on their hosts can be either direct or indirect [57]. In contrast to phloem parasites that have access to host photosynthates, xylem-attached Rhinanthus plants exceptionally use host-derived water and minerals. It is also suggested that parasitic plants directly compete with their hosts in respect to resources from soil [57].
In fact, besides resource capture, parasitic plants can directly suppress crucial physiological functions of their host plants that makes them similar to pathogens because of use of certain chemical signals. Thus, host photosynthesis is mostly negatively affected by Rhinanthus attachment at the level of photochemical activity [57,58]. However, no effect of R. minor infection on photosynthetic oxygen production in Poa alpina plants was evident in earlier studies [59].
The question of the harmful effects of the parasite on the host plant is more complicated than it might seem at first. Rhinanthus plants, as typical hemiparasites, have their own organic carbon fixation through photosynthesis and therefore rely mostly on water and minerals as resources from their hosts. Consequently, it could be assumed that under conditions of optimal availability of these resources, the effect of the parasite should be minimal. However, as discussed in the next chapter, the proportion of heterotrophically acquired carbon in the biomass of parasites can be substantial.
It is logical to assume that in natural conditions, the intensity of negative effects of Rhinanthus parasites on host plants will depend on the density of parasitic plants. Experiments in controlled conditions have provided support for this relationship. Thus, growth of a perennial grass Agrostis capillaris was more negatively affected by two simultaneously attached R. serotinus individuals than by a single parasite, and this effect remained also in the second growing season [60]. In addition, number of panicles per plant progressively decreased with increasing rate of parasitism. In field conditions, it has been observed that an increase in the frequency of parasitic plants is associated with a greater negative effect on growth of host plants. The existence of such a phenomenon has also been confirmed in studies under partially controlled conditions [61].
One might ask, will the negative effect of one season’s annual parasite on a perennial plant have lasting effects in subsequent growing seasons? Indeed, it has been shown that Agrostis capillaris plants parasitized with R. serotinus for one season produced less biomass in the next season in comparison to control plants [60]. Such a feature could buffer the effect of the parasite against fluctuations in its numbers between seasons and ensure a continuous interseasonal effect.
In field conditions, total biomass of vascular plants in grasslands usually decreases due to the presence of Rhinanthus species. Indeed, while biomass of dominant species is decreased by Rhinanthus parasitism, low nutrient and water use efficiency results in relatively low biomass of Rhinanthus plants, which cannot compensate for total biomass loss [62]. A similar overall negative effect due to low resource efficiency on total productivity has also been noted in controlled conditions [37]. In practice, biomass loss can be up to 30%, and is thought to be synergistically affected by other biotic factors, such as arbuscular mycorrhizal fungi and pathogens [63].
9. How Does Contact with Host Plants Affect Rhinanthus Plants?
Water and solute uptake from host xylem through open xylem–xylem connections through open conductive tubes, osculae, is the main mechanism of resource transfer in Rhinanthus species. Therefore, possible selectivity for solute uptake initially seems doubtful. This mechanism also provides an opportunity for parasite species to exploit non-nutrient biologically active solutes of host plants—hormones, proteins and RNAs [64]. It would be logical to assume that the exchange of signals could be reciprocal and also take place in the direction from the parasite to the host, or, in the case of attachment of several hosts to the parasite, also between several hosts. Thus, both the adaptation of the parasite to the developmental stage of the host and the direct influence of the parasite on the host’s physiological processes and growth are possible.
Early studies with R. serotinus and Hordeum vulgare provided information on changes in inorganic and organic constituents in different tissues of a parasite before and immediately after attachment, coinciding with fast stimulation of growth [65]. The most striking increase was evident for N, P, K, Mg and Na concentration in parasite leaves, with pronounced increase of N and P also in roots. Among organic substances, a large increase in both leaves and roots of R. serotinus after attachment was evident for concentration of DNA, RNA and amino acids. However, concentration of carbohydrates as well as chlorophyll content decreased probably due to an initial decrease in photosynthesis rate, but significantly increased in later stages.
While in general xylem sap is thought to be low in organic substances, possible transfer of organic solutes from host to Rhinanthus plant cannot be excluded. It has been reported that around 20 to 80% of dry biomass of different hemiparasites is derived from heterotrophic carbon gain [66]. In particular, using natural abundance stable isotope profiles of 13C, it was estimated that about 50% of organic carbon in R. minor plants in controlled conditions was derived from their host plants [67]. Experiments with closely related species R. alectorolophus confirmed importance of heterotrophic carbon for growth of attached Rhinanthus plants, where decrease in light intensity increased the fraction of heterotrophically derived carbon from 10 to 50% [68].
Attachment to a host root results in drastic changes in basic physiological features of the parasite. The characteristic physiological response of Rhinanthus plants after successful attachment to host roots is an increased transpiration rate and constantly open stomata. As a result, stomatal conductivity of attached hemiparasite plants is higher than that of their hosts, optimizing xylem sap extraction [69]. Unattached R. minor plants usually had closed stomata, whereas attached plants kept their stomata open throughout day and night [70]. If host shoots were removed but Rhinanthus plants remained attached to the roots, that again resulted in changes in stomatal behavior: plant stomata were open at day and closed at night. The increased transpiration rate of parasitic plants is one of the main reasons for establishing high water potential gradient between host and parasite, leading to intensive solute transfer.
R. serotinus plants attaching to host roots earlier in their life cycle had advantages both in respect of higher growth rate as well as seed production in comparison to late-attaching plants [61]. Thus, early-attaching parasite plants were about four times taller at the end of their life cycle, and produced 23 times more seeds with higher viability.
Transfer of xylem-translocated cytokinin has been shown to be an important signal in parasitic interaction of R. minor and Hordeum vulgare plants [71]. Cytokinin synthesis was extremely low in both unattached and host-attached R. minor plants, and decreased by 35% after attachment, but cytokinin concentration in a form of zeatin and zeatin riboside was relatively high in attached plants due to import from the host. It is suggested that cytokinins are important factors for increased size of leaves and interveinal tissues after attachment to host [71]. Rhinanthus plants were able to restore cytokinin biosynthesis when the parasitized host plant died due to shoot cutting [70].
One of the biochemical peculiarities of metabolism in Rhinanthus species and some other hemiparasites from Orobanchaceae is use of mannitol instead of sucrose as the major assimilate, leading to a high rate of abscisic acid (ABA) biosynthesis [71]. ABA, in turn, is necessary for formation and differentiation of haustoria [72]. Mannitol biosynthesis in R. minor shoots is increased 16-fold by parasitism to Hordeum vulgare, resulting in 10 times higher mannitol concentration in shoots [71]. However, no sucrose was detected in xylem of H. vulgare, indicating that no sucrose was transferred to the parasite. The presence of osmotically active mannitol in tissues of Rhinanthus plants can be crucially important for the parasitic lifestyle, including better protection against water shortage as well as diversion of nutrient flow from a host plant.
The effect of reducing the biomass of the host plant as a result of defoliation on the parasitic interaction was also studied. Thus, partial defoliation (50% of entire leaves removed) of Agrostis capillaris plants had no significant effect on shoot or root biomass of attached R. serotinus plants, but the number of flowers decreased significantly [73]. However, the effect was significant only when defoliation of the host plant was performed in the developmental phase of the parasite when the demand for resources was at its highest.
Transfer of host-derived toxins to the parasite is another intriguing possibility with a potentially large impact on defense [64]. Thus, obligate mutualistic fungal endophyte (Neothyphodium uncinatum) of Lolium pratense plants produces mycotoxins—pyrrolizidine alkaloid loline and N-propionylnorloline—that are transferred to hemiparasitic R. serotinus plants [74]. Importantly, uptake of mycotoxins in the parasite increased its performance against a generalist aphid herbivore, Aulacorthum solani.
Recently, information is emerging that the transfer of small RNAs between partners could be an essential part of the life cycle of parasitic plants. Most importantly, the transfer can be bidirectional and selective [2,75]. Trafficking of phloem-mobile mRNAs in both directions during holoparasitic as well as obligate hemiparasitic interaction has been demonstrated, and the process is thought to be highly selective [75,76]. Most abundant trafficked mRNA types are from regulatory genes, involving transcription factors and calmodulin-related proteins. The question of mRNA transfer between host and parasite is rather intriguing, as any physiological response would require translation of respective mRNAs into proteins and interaction of the synthesized proteins with certain targets.
Another possibility for RNA transfer within parasite–host plant interaction is related to RNA silencing signals, aka RNA interference (RNAi) [75,77]. Non protein-coding RNAs, processed into small interfering RNA(siRNA) or micro RNA (iRNA), can participate in sequence-specific regulation of gene expression, but high gene homology is a prerequisite for this type of influence. In particular, miRNAs seem to be crucial components in regulation of interactions between parasitic plants and their hosts [78,79]. Comprehensive updates on existing knowledge of plant parasite–host signal transfer can be found in the recent scientific literature [2,79]. Updates on horizontal gene transfer in parasitic plant relationships with their hosts can be found elsewhere [80,81,82].
The above-mentioned examples of transfer of small RNAs during parasite–host relationship mostly refers to studies with species of obligate parasites from genera Cuscuta, Orobanche, Phelipanche, and Striga [2]. The question of transduction of such signals in xylem hemiparasites is still unresolved. One important aspect in this respect is the specificity of the relationship, which is high for phloem parasites and quite low for typical xylem parasites like Rhinanthus. Therefore, it is important to perform detailed transcriptome studies using classical model species from genus Rhinanthus together with functionally different host species in order to obtain detailed understanding of control of parasitic interactions leading to either “neutral” or “beneficial” effects for parasitic plants.
10. How Do Rhinanthus Plants Affect Plant Diversity in Grasslands?
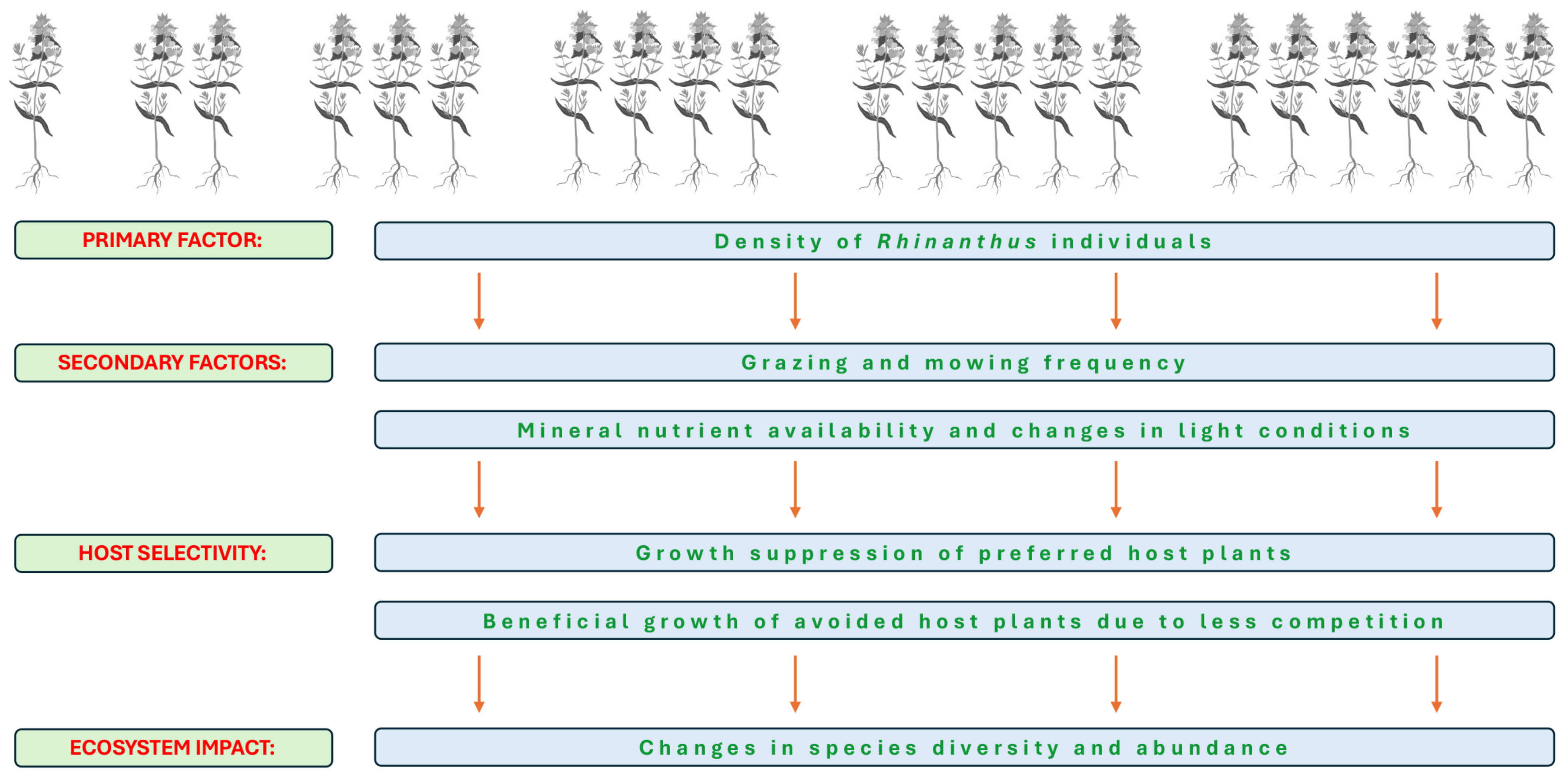
Host selectivity of Rhinanthus species, described in Section 4, seems to be the main mechanism leading to changes in species diversity in grasslands. The existence of selectivity has been proven also in experiments in controlled conditions even among taxonomically related host species, such as within grasses or legumes [20]. Most importantly, growth of more-preferred hosts (as indicated by a larger number of haustorial contacts in host roots) were more depressed in comparison to that of less-preferred hosts, and this effect was especially pronounced in conditions of low soil nutrient availability [20]. Moreover, parasitism by R. minor also changed the relative competitive ability of grass species cultivated in binary mixtures. Consequently, plant species interactions in grasslands are significantly affected by the presence of hemiparasitic plants in a similar manner to that of herbivores and pathogens. Spatial theoretical modelling has been used to predict an outcome of competitive interactions involving R. minor, and it was demonstrated that nutrient availability is one of the major factors determining the outcome [83]. The complex dynamics of interactions resulting in unpredictable shifts in vegetation structure might be associated with formation of locally unstable parasite populations, where even relatively small quantitative changes can result in significant qualitatively different outcomes. Thus, alternation between parasite-rich and parasite-poor phases can result from the fact that when the parasite suppresses growth of high quality host plants, the shortage of preferred host plants will become a limiting factor for parasite abundance [83]. Interaction between the main factors leading to changes in grassland biodiversity by the presence of Rhinanthus species is shown in Figure 4.
Figure 4.
Interactions between the main factors leading to changes in grassland biodiversity by the presence of Rhinanthus species.
Practical aspects for use of Rhinanthus species in grassland restoration have been analyzed several times in the recent decades [84]. It was initially suggested that five criteria need to be met for the species being used as a tool in biodiversity restoration practices: (i) the species needs to be a component of natural species-rich grasslands, (ii) seeds of the species are easily available at low cost, (iii) the species reduce growth and reproduction of several competitive species, especially grasses, (iv) the species has an ability for vigorous growth and rapid spread in different types of grasslands, and (v) population size of the species can be easily managed to prevent extensive damage to vegetation [84].
From a practical point of view, it is important that successful establishment of viable populations of R. minor is possible only in relatively unproductive grasslands. Several studies have shown that the species cannot form a long-term population when grassland productivity exceeds 4 or 5 t ha−1 of dry aboveground biomass [62]. One of the possible mechanisms discussed in this respect is the negative effect of shading, as R. minor plants are highly sensitive to decreased light [51]. However, it is also shown that reduced light is not a problem for already attached Rhinanthus plants [52].
Although it is accepted that the high availability of minerals in grassland soil does not allow the effect of the parasite to manifest itself due to their low ability to compete for light, in some situations a significant effect has also been observed in soils with a high supply of nutrients. When a relatively productive ex-arable field was sowed with a standard meadow mix containing six forb and six grass species against a background of three densities of R. minor (0, 600, 1000 seeds m−2), the immediate effect of parasite presence was evident as reduced grass biomass at both parasite densities, but forb biomass was reduced only at the higher sowing density [85]. However, no effect of parasite presence was evident in respect to species diversity. Obviously, in this particular case, as host plants also developed from seed, competition for light was not as active as it is in natural grassland conditions.
Experiments in field conditions involving removal of R. minor individuals gave further support to the evidence that plant species diversity in grassland communities is significantly affected by the presence of parasitic plants through both selective parasitism as well as modification of competitive ability between plants [20].
Population biology of R. angustifolius in grasslands in relation to vegetation structure due to management regime has been studied over the long term in several cases. As a rule, factors leading to increased abundance of Rhinanthus species resulted in decrease in grass cover and increase in that of forbs [63,86,87]. Thus, grazing in autumn by cattle and sheep produced gaps in vegetation resulting in increased abundance of R. minor during an eight-year trial [86]. Summer cutting alone had no such effect, and cutting in autumn only had a partial influence, but mineral fertilization had a mostly negative effect. However, environmental factors have a large impact on Rhinanthus populations, resulting in seemingly stochastic fluctuations in number of individuals over the seasons [87]. Thus, for R. angustifolius, it was suggested that spring drought could be the main negative factor [87].
In addition to effects of Rhinanthus presence on taxonomic diversity of grassland species, it is also valuable to look at its influence on changes in dominant functional characteristics. Thus, when presence of R. minor was manipulated (removal vs. no removal) in an oligotrophic meadow community, it appeared that functional diversity in general was higher in populations with the parasite [88]. The presence of competitively weak species (with low clonal growth capacity and small biomass) was promoted at a community level. In particular, Rhinanthus positively affected species with persistent taproot, such as Plantago lanceolata, or monocyclic species. Surprisingly, clonal species with extensive lateral spread were especially vulnerable to presence of R. minor.
Development of a dense moss layer as a result of nutrient depletion-associated soil acidification could have a negative effect on Rhinanthus populations due to mechanical restrictions on seedling emergence and establishment [89]. However, this possibility has not been clearly demonstrated in other studies [62,90,91].
Successful attempts to use parasitic Rhinanthus species to improve plant diversity under natural conditions have been described. Thus, in a meadow with patches highly infested with a clonal grass Calamagrostis epigejos, sowing of R. major led to reduced biomass of C. epigejos, and the effect was additive to that of mowing twice in the season [92]. Besides, community composition was significantly changed by parasite introduction, with some diagnostic or constant species of the association increasing in abundance. Such a positive response was not seen in the case of mowing twice; in contrast, several important or threatened species responded in a clearly negative way. In addition, Central European species R. alectorolophus was successfully used to suppress C. epigejos in both dry meadow and industrial areas, but the effect in wet meadow was variable due to unequal establishment of Rhinanthus population [93]. Similar to the above study, increased mowing frequency had additional negative effects on C. epigejos. More experimental evidence derived from field studies is available to support the use of introduction of R. minor in practical management for improvement of species-poor grasslands [90,94,95].
However, effects of Rhinanthus introduction in grasslands does not necessarily lead to a positive immediate effect on biodiversity. Thus, sowing of R. minor in a species-poor calcareous grassland had a negative influence on forb richness, and the effect was directly proportional to the density of parasites [96]. Due to these contrasting results, the overall general suggestion still holds that effects of Rhinanthus plants on community structure are extremely variable and highly unpredictable.
No less important is the possibility of reducing the presence of invasive plants with the help of Rhinanthus and other root hemiparasites, which has been shown in experiments under controlled conditions [97]. Thus, biomass accumulation and shoot density was significantly suppressed for two clonal invasive species, Solidago gigantea and Symphyotrichum lanceolatum, by Melampyrum arvense and R. alectorolophus. The degree of clonal integration of the host plants was associated with the pronounced species-specificity of the interaction.
11. Ecological Complexity of Parasitic Plant Interactions and Future Perspectives
To some extent, the non-specific nature of the parasitism of Rhinanthus species, on the one hand, and the selectivity of action, on the other, can explain the fact that their influence in the ecosystem is quite complex. There is increasing evidence that hemiparasitic species, including Rhinanthus, not only directly alter plant biodiversity through parasitism, but also affect broader ecosystem functions at multiple trophic levels [98]. Among them, plant–herbivore interactions seem to be significantly affected by the presence of parasitic Rhinanthus plants [25,99,100].
As discussed above, symbiotic nitrogen fixation in legume species makes them attractive as hosts to Rhinanthus species. However, in this case, the efficiency of N-fixing bacteria, which is largely influenced by the level of soil N availability, should also be taken into account. Since legumes are usually also characterized by the presence of mycorrhizal symbiosis, quite complex interactions can develop under natural conditions. Studies with other root hemiparasites, such as Pedicularis species, have shown that, in general, both rhizobial and arbuscular mycorrhizal symbiosis can alleviate negative effect of parasites on host plants [101]. On the other hand, the presence of R. minor significantly diminished colonization of Lolium perenne roots with arbuscular mycorrhizal fungi, but parasite growth drastically increased when the host plants were mycorrhizal [102].
Another interesting aspect for further exploration of biological complexity is relatedness of endophytic microbiomes of parasitic plants and their hosts. For example, it has been argued that parasitic plants are likely to be able to decrease the extent of colonization by fungal endophytes with further consequences for host plant physiology [103]. Considering the generally limited understanding of the role of endophytes in the functioning of different ecosystems, research on the relationship between hemiparasites, host plants and their endophytes could be of critical importance.
Although the emphasis in parasite resistance research has so far been on the so-called economically important parasite species [104,105], there is no doubt that root hemiparasitic species such as Rhinanthus, with their ability to influence the diversity of grasslands, stand out for the possibility of obtaining biologically and ecologically important information about the critical mechanisms of the interaction between parasitic plants and their hosts. The exchange of non-resource compounds with a signaling nature, especially at the level of small RNA molecules, seems to be one of the most important directions of research with Rhinanthus species. Expanding the models to include several host plant species with different resistance to specific environmental factors would broaden the understanding of the importance of parasitic plants in the functioning of the ecosystem and the maintenance of plant diversity.
Development of new model systems is an important aspect in any type of study of biological interactions between organisms, and this is true also for the Rhinanthus–host plant relationship. There is no doubt that ecologically significant results can be obtained in vegetation pot or microcosm experiments using soil or other soil-like substrates with model species native for a particular habitat. However, simpler model systems offer several advantages over vegetation pot experiments, including the need for a smaller space, as well as better repeatability and controllability. One of the underexplored directions in this respect is the use of tissue culture-based models. These could be extremely important as tools for deciphering host resistance mechanisms to root hemiparasites as well as in environmental resilience studies. So far, root cultures have been used mostly with obligate parasite species, as the ones of genus Striga [106,107]. However, there are also successful attempts using root and callus cultures of root hemiparasitic species, such as Triphysaria versicolor, to study early responses of parasitic interaction [108]. Most importantly, cultivated roots of hemiparasites retained competence for haustoria formation in the presence of host roots, exudates from host roots, or chemical inducers.
Due to the fact that some Rhinanthus species have been used in traditional medicine, there is an interest in revealing phytochemical composition and potential bioactive compounds in Rhinanthus plants [109,110,111]. In particular, R. angustifolius was characterized as a rich source of antioxidants and enzyme inhibitors [111]. In another study, methanolic extracts of R. angustifolius exhibited a wide-spectrum antibiotic activity [112]. Essential oil of R. angustifolius contains terpenes, aldehydes, alcohols, esters, acids, etc., and it has prominent antimicrobial activity [110]. Dihydroxylated derivate of methylindene, α-cubebene, 1-hexadecene, and hexadecanoic acid were identified as the major constituents of the essential oil. When compared to other grassland species, R. minor has an especially high level of benzoic acid [113]. In light of an effect of host species on chemical composition of parasitic plants, and especially, due to possible transfer of secondary metabolites from host to parasite, more attention should be paid to research on the effect of different host plants on the chemical composition of Rhinanthus plants, especially if the host plants are active secondary-compound-producing species. This effect has not been explored with Rhinanthus species, but it was shown with another hemiparasite, Castilleja miniata, that these plants receive alkaloids from a host, Lupinus argenteus, leading to decreased herbivory of the parasite [114].
12. Conclusions
The performed analysis shows that Rhinanthus species have gained scientific interest due to their ecological importance in increasing grassland biodiversity. Although significant progress has been made in the practical use of Rhinanthus species in grassland improvement, this activity is often empirical in nature due to a lack of fundamental knowledge of the factors affecting the parasite–host relationship in the ecosystem. Soil edaphic factors and the level of mineral availability could be among the influences that should receive more attention in this regard.
From the functional point of view of the interaction, the most significant lack of knowledge is about the signal exchange between the Rhinanthus parasite and the host plant. It would be necessary to understand how differences in signal exchange between Rhinanthus and host plants of different species affect the nature of subsequent relationships, distinguishing between “high-quality” and “low-quality” hosts. A particularly interesting model system could be one involving apparently resistant host plants such as Anthyllis vulneraria. This could make it possible to understand whether the differences in the resistance of different host plants to Rhinanthus are genetically determined by a gene-to-gene mechanism.
Funding
This research received no external funding.
Conflicts of Interest
The author declare no conflicts of interest.
References
- Heide-Jørgensen, H.S. Introduction: The parasitic syndrome in higher plants. In Parasitic Orobanchaceae: Parasitic Mechanisms and Control Strategies; Joel, D.M., Gressel, J., Musselman, L.J., Eds.; Springer: Berlin/Heidelberg, Germany, 2013; pp. 1–18. [Google Scholar]
- Clarke, C.R.; Timko, M.P.; Yoder, J.I.; Axtell, M.J.; Westwood, J.H. Molecular dialog between parasitic plants and their hosts. Annu. Rev. Plant Biol. 2019, 57, 279–299. [Google Scholar] [CrossRef] [PubMed]
- Nickrent, D.L. Parasitic angiosperms: How often and how many? Taxon 2020, 69, 5–27. [Google Scholar] [CrossRef]
- Bouwmeester, H.; Sinha, N.; Scholes, J. Parasitic plants: Physiology, development, signaling, and ecosystem interactions. Plant Physiol. 2021, 185, 1267–1269. [Google Scholar] [CrossRef] [PubMed]
- Krasylenko, Y.; Tšitel, J.; Ceccantini, G.; Oliveira-da-Silva, M.; Dvořák, V.; Steele, D.; Sosnovsky, Y.; Piwowarczyk, R.; Watson, D.M.; Teixeira-Costa, L. Parasites on parasites: Hyper-, epi-, and autoparasitism. Amer. J. Bot. 2021, 108, 8–21. [Google Scholar] [CrossRef] [PubMed]
- Mutuku, J.M.; Cui, S.; Yoshida, S.; Shirasu, K. Orobanchaceae parasite–host interactions. New Phytol. 2021, 230, 46–59. [Google Scholar] [CrossRef]
- Tešitel, J.; Li, A.-R.; Knotková, K.; McLellan, R.; Bandaranayake, P.C.G.; Watson, D.M. The bright side of parasitic plants: What are they good for? Plant Physiol. 2021, 185, 1309–1324. [Google Scholar] [CrossRef] [PubMed]
- Tešitel, J.; Říha, P.; Svobodova, Š.; Malinová, T.; Štech, M. Phylogeny, life history evolution and biogeography of the Rhinanthoid Orobanchaceae. Folia Geobot. 2010, 45, 347–367. [Google Scholar] [CrossRef]
- Vrancken, J.; Brochmann, C.; Wesselingh, R.A. A European phylogeography of Rhinanthus minor compared to Rhinanthus angustifolius: Unexpected splits and signs of hybridization. Ecol. Evol. 2012, 2, 1531–1548. [Google Scholar] [CrossRef] [PubMed]
- WFO. World Flora Online. Published on the Internet. 2024. Available online: http://www.worldfloraonline.org (accessed on 29 March 2024).
- Ducarme, V.; Wesselingh, R.A. Detecting hybridization in mixed populations of Rhinanthus minor and Rhinanthus angustifolius. Folia Geobot. 2005, 50, 151–161. [Google Scholar] [CrossRef]
- Talve, T.; Mürk, M.; Lindell, T.; Oja, T. Rhinanthus plants found in calcareous fens on Gotland (Sweden): Are they related to Rhinanthus osiliensis from Saaremaa (Estonia)? Bioch. Syst. Ecol. 2014, 54, 113–122. [Google Scholar] [CrossRef]
- Zopfi, H.-J. Ecotypic variation in Rhinanthus alectorolophus (Scopoli) Pollich (Scrophulariaceae) in relation to grassland management II. The genotypic basis of seasonal ecotypes. Flora 1993, 188, 153–173. [Google Scholar] [CrossRef]
- Jonstrup, A.; Hedrén, M.; Andersson, S. Host environment and local genetic adaptation determine phenotype in parasitic Rhinanthus angustifolius. Bot. J. Linn. Soc. 2016, 180, 89–103. [Google Scholar] [CrossRef]
- Vrancken, J.; Brochmann, C.; Wesselingh, R.A. How did annual plant react to Pleistocene glaciations? Postglacial history of Rhinanthus angustifolius in Europe. Biol. J. Linn. Soc. 2009, 98, 1–13. [Google Scholar] [CrossRef]
- Jonstrup, A.; Andersson, S.; Hedrén, M. Genetic structure in parasitic Rhinanthus angustifolius is determined by geographical distance rather than habitat—Implications for taxonomy and conservation. Nordic J. Bot. 2018, 2018, e01941. [Google Scholar] [CrossRef]
- Ahonen, R.; Puustinen, S.; Mutikainen, P. Host use of a hemiparasitic plant: No trade-offs in performance on different hosts. J. Evol. Biol. 2006, 19, 513–521. [Google Scholar] [CrossRef] [PubMed]
- Ter Borg, S.J. Population biology and habitat relations of some hemiparasitic Scrophulariaceae. In The Population Structure of Vegetation; Handbook of Vegetation Science; White, J., Ed.; Springer: Dordrecht, The Netherlands, 1985; Volume 3, pp. 463–487. [Google Scholar]
- Westbury, D.B. Rhinanthus minor L. J. Ecol. 2004, 92, 906–927. [Google Scholar] [CrossRef]
- Gibson, C.C.; Watkinson, A.R. Host selectivity and the mediation of competition by the root hemiparasite Rhinanthus minor. Oecologia 1991, 86, 81–87. [Google Scholar] [CrossRef] [PubMed]
- Ter Borg, S.J. Dormancy and germination of six Rhinanthus species in relation to climate. Folia Geobot. 2005, 40, 243–260. [Google Scholar] [CrossRef]
- Marin, M.; Laverack, G.; Powell, A.A. Germination characteristics of Rhinanthus minor influence field emergence, competitiveness and potential use in restoration projects. Plant Biol. 2019, 21, 470–479. [Google Scholar] [CrossRef]
- Matthies, D. Positive and negative interactions among individuals of a root hemiparasite. Plant Biol. 2003, 5, 79–84. [Google Scholar] [CrossRef]
- Gibson, C.C.; Watkinson, A.R. The host range and selectivity of a parasitic plant: Rhinanthus minor L. Oecologia 1989, 78, 401–406. [Google Scholar] [CrossRef] [PubMed]
- Rowntree, J.K.; Barham, D.F.; Stewart, A.J.A.; Hartley, S.E. The effect of multiple host species on a keystone parasitic plant and its aphid herbivores. Funct. Ecol. 2014, 28, 829–836. [Google Scholar] [CrossRef]
- Sandner, T.M.; Matthies, D. Multiple choice: Hemiparasite performance in multi-species mixtures. Oikos 2018, 127, 1291–1303. [Google Scholar] [CrossRef]
- Matthies, D. Closely related parasitic plants have similar host requirements and related effects on hosts. Ecol. Evol. 2021, 11, 12011–12024. [Google Scholar] [CrossRef] [PubMed]
- Cameron, D.D.; Coats, A.M.; Seel, W.E. Differential resistance among host and non-host species underlies the variable success of the hemi-parasitic plant Rhinanthus minor. Ann. Bot. 2006, 98, 1289–1299. [Google Scholar] [CrossRef] [PubMed]
- Marvier, M.A. A mixed diet improves performance and herbivore resistance of a parasitic plant. Ecology 1998, 79, 1272–1280. [Google Scholar] [CrossRef]
- Jiang, F.; Jeschke, W.D.; Hartung, W.; Cameron, D.D. Does legume nitrogen fixation underpin host quality for the hemiparasitic plant Rhinanthus minor? J. Exp. Bot. 2008, 59, 917–925. [Google Scholar] [CrossRef] [PubMed]
- Rümer, S.; Cameron, D.D.; Wacker, R.; Hartung, W.; Jiang, F. An anatomical study of the haustoria of Rhinanthus minor attached to roots of different hosts. Flora 2007, 202, 194–200. [Google Scholar] [CrossRef]
- Mutikainen, P.; Salonen, P.; Puustinen, S.; Koskela, T. Local adaptation, resistance, and virulence in a hemiparasitic plant–host plant interaction. Ecolution 2000, 54, 433–440. [Google Scholar]
- Goyet, V.; Wada, S.; Cui, S.; Wakatake, T.; Shirasu, K.; Montiel, G.; Simier, P.; Yoshida, S. Haustorium inducing factors for parasitic Orobanchaceae. Front. Plant Sci. 2019, 10, 1056. [Google Scholar] [CrossRef]
- Joel, D.M. Functional structure of the mature haustorium. In Parasitic Orobanchaceae: Parasitic Mechanisms and Control Strategies; Joel, D.M., Gressel, J., Musselman, L.J., Eds.; Springer: Berlin/Heidelberg, Germany, 2013; pp. 25–60. [Google Scholar]
- Yoshida, S.; Cui, S.; Ichihashi, Y.; Shirasu, K. The haustorium, a specialized invasive organ in parasitic plants. Annu. Rev. Plant Biol. 2016, 67, 643–667. [Google Scholar] [CrossRef] [PubMed]
- Sandner, T.M.; Schoppan, L.; Matthies, D. Seedlings of a hemiparasite recognize legumes, but do not distinguish good from poor host species. Folia Geobot. 2022, 57, 117–126. [Google Scholar] [CrossRef]
- Matthies, D. Parasitic and competitive interactions between the hemiparasites Rhinanthus serotinus and Odontites rubra and their host Medicago sativa. J. Ecol. 1995, 83, 245–251. [Google Scholar] [CrossRef]
- Svensson, B.M.; Seel, W.E.; Nilsson, C.H.; Carlsson, B.Å. Roles played by timing of seedling development and host identity in determining fitness of an annual, subarctic, hemiparasitic plant. Artic Anarctic Alipne Res. 2001, 33, 299–305. [Google Scholar] [CrossRef]
- Spallek, T.; Gan, P.; Kadota, Y.; Shirasu, K. Same tune, different song—Cytokinins as virulence factors in plant–pathogen interaction? Curr. Opin. Plant Biol. 2018, 44, 82–87. [Google Scholar] [CrossRef] [PubMed]
- Cameron, D.D.; Seel, W.E. Functional anatomy of haustoria formed by Rhinanthus minor: Linking evidence from histology and isotope tracing. New Phytol. 2007, 174, 412–419. [Google Scholar] [CrossRef] [PubMed]
- Seel, W.E.; Parsons, A.N.; Press, M.C. Do inorganic solutes limit growth of the facultative hemiparasite Rhinanthus minor L. in the absence of a host? New Phytol. 1993, 124, 283–289. [Google Scholar] [CrossRef] [PubMed]
- Prati, D.; Matthies, D.; Schmid, B. Reciprocal parasitization in Rhinanthus serotinus: A model system of physiological integration in clonal plants. OIKOS 1997, 78, 221–229. [Google Scholar] [CrossRef]
- Davies, D.M.; Graves, J.D. The impact of phosphorus on interactions of the hemiparasitic angiosperm Rhinanthus minor and its host Lolium perenne. Oecologia 2000, 124, 100–106. [Google Scholar] [CrossRef]
- De Hullu, E.; Brouwer, T.; ter Borg, S. Analysis of the demography of Rhinanthus angustifolius populations. Acta Bot. Neerl. 1985, 34, 5–22. [Google Scholar] [CrossRef]
- Korell, L.; Sandner, T.M.; Matthies, D.; Ludewig, K. Effects of drought and N level on the interactions of the root hemiparasite Rhinanthus alectorolophus with a combination of three host species. Plant Biol. 2020, 22, 84–92. [Google Scholar] [CrossRef] [PubMed]
- Těšitel, J.; Těšitelová, T.; Fisher, J.P.; Lepš, J.; Cameron, D.D. Integrating ecology and physiology of root-hemiparasitic interaction: Interactive effects of abiotic resources shape the interplay between parasitism and autotrophy. New Phytol. 2015, 205, 350–360. [Google Scholar] [CrossRef] [PubMed]
- Fresco, L.F.M. Ecological response curves of Rhinanthus serotinus: A synecological study. Acta Bot. Neerl. 1980, 29, 533–539. [Google Scholar] [CrossRef]
- Ducarme, V.; Wesselingh, R.A. Performance of two Rhinanthus species under different hydric conditions. Plant Ecol. 2010, 206, 263–277. [Google Scholar] [CrossRef]
- Jiang, F.; Jeschke, W.D.; Hartung, W. Contents and flows of assimilates (mannitol and sucrose) in the hemiparasaitic Rhinanthus minor/Hordeum vulgare association. Folia Geobot. 2005, 40, 195–203. [Google Scholar] [CrossRef]
- Svĕtliková, P.; Hájek, T.; Tĕšitel, J. Water-stress physiology of Rhinanthus alectorolophus, a root-hemiparasitic plant. PLoS ONE 2018, 13, e0200927. [Google Scholar] [CrossRef]
- Keith, A.M.; Cameron, D.D.; Seel, W.E. Spatial interactions between the hemiparasitic angiosperm Rhinanthus minor and its host are species-specific. Funct. Ecol. 2004, 18, 435–442. [Google Scholar] [CrossRef]
- Hwangbo, J.-K.; Seel, W.E. Effects of light availability on attached Rhinanthus minor (L.), an angiospermatic root hemiparasite. J. Plant Biol. 2002, 45, 102–106. [Google Scholar] [CrossRef]
- Ruberti, I.; Sessa, G.; Ciolfi, A.; Possenti, M.; Carabelli, M.; Morelli, G. Plant adaptation to dynamically changing environment: The shade avoidance response. Biotechnol. Adv. 2012, 30, 1047–1058. [Google Scholar] [CrossRef]
- Hwangbo, J.-K.; Seel, W.E.; Woodin, S.J. Short-term exposure to elevated atmospheric CO2 benefits the growth of a facultative annual root hemiparasite, Rhinanthus minor (L.), more than that of its host, Poa pratensis (L.). J. Exp. Bot. 2003, 54, 1951–1955. [Google Scholar] [CrossRef]
- Watling, J.R.; Press, M.C. How does the C4 grass Eragrostis pilosa respond to elevated carbon dioxide and infection with parasitic angiosperm Striga hermonthica? New Phytol. 1998, 140, 667–675. [Google Scholar] [CrossRef] [PubMed]
- Ievinsh, G. Disentangling the belowground web of biotic interactions in temperate coastal grasslands: From fundamental knowledge to novel applications. Land 2023, 12, 1209. [Google Scholar] [CrossRef]
- Cameron, D.D.; Hwangbo, J.-K.; Keith, A.M.; Geniez, J.-M.; Kraushaar, D.; Rowntree, J.; Seel, W.E. Interactions between the hemiparasitic angiosperm Rhinanthus minor and its hosts: From the cell to the ecosystem. Folia Geobot. 2005, 40, 217–229. [Google Scholar] [CrossRef]
- Cameron, D.D.; Geniez, J.-M.; Seel, W.E.; Irving, L.J. Suppression of photosynthesis by the parasitic plant Rhinanthus minor. Ann. Bot. 2008, 101, 573–578. [Google Scholar] [CrossRef] [PubMed]
- Seel, W.E.; Press, M.C. Effects of repeated parasitism by Rhinanthus minor on the growth and photosynthesis of a perennial grass, Poa alpina. New Phytol. 1996, 134, 495–502. [Google Scholar] [CrossRef]
- Puustinen, S.; Salonen, V. Effects of intensity and duration of infection by a hemiparasitic plant, Rhinanthus serotinus, on growth and reproduction of a perennial grass, Agrostis capillaris. Ecography 1999, 22, 160–168. [Google Scholar] [CrossRef]
- Svensson, B.M.; Carlsson, B.Å. Significance of time of attachment, host type, and neighbouring hemiparasites in determining fitness in two endangered grassland parasites. Ann. Bot. Fennici 2004, 41, 63–75. [Google Scholar]
- Hejcman, M.; Schellberg, J.; Pavlů, V. Competitive ability of Rhinanthus minor L. in relation to productivity in the Rengen Grassland Experiment. Plant Soil Environ. 2011, 57, 45–51. [Google Scholar] [CrossRef]
- Ameloot, E.; Verheyen, K.; Hermy, M. Meta-analysis of standing crop reduction by Rhinathus spp. and its effect on vegetation structure. Folia Geobot. 2005, 40, 289–310. [Google Scholar] [CrossRef]
- Smith, J.D.; Mescher, M.C.; De Moraes, C.M. Implications of bioactive solute transfer from hosts to parasitic plants. Curr. Opin. Plant Biol. 2013, 16, 464–472. [Google Scholar] [CrossRef]
- Klaren, C.H.; Janssen, G. Physiological changes in the hemiparasite Rhinanthus serotinus before and after attachment. Physiol. Plant. 1978, 42, 151–155. [Google Scholar] [CrossRef]
- Tĕšitel, J.; Plavcová, L.; Cameron, D.D. Interactions between hemniparasitic plants and their hosts: The importance of organic carbon transfer. Plant Signal. Behav. 2010, 5, 1072–1076. [Google Scholar] [CrossRef] [PubMed]
- Tešitel, J.; Plavcová, L.; Cameron, D.D. Heterotrophic carbon gain by the root hemiparasites, Rhinanthus minor and Euphrasia rostkoviana (Orobanchaceae). Planta 2010, 231, 1137–1144. [Google Scholar] [CrossRef] [PubMed]
- Tešitel, J.; Lepš, J.; Vráblová, M.; Cameron, D.D. The role of heterotrophic carbon acquisition by the hemiparasitic plant Rhinanthus alectorolophus in seedling establishment in natural communities: A physiological perspective. New Phytol. 2011, 192, 188–199. [Google Scholar] [CrossRef] [PubMed]
- Jiang, F.; Jeschke, W.D.; Hartung, W.; Cameron, D.D. Interactions between Rhinanthus minor and its hosts: A review of water, mineral nutrient and hormone flows and exchanges in the hemiparasitic association. Folia Geobot. 2010, 45, 369–385. [Google Scholar] [CrossRef]
- Jiang, F.; Timergalina, L.; Kudoyarova, G.; Jeschke, D.; Hartung, W. Growth and development of the facultative root hemiparasite Rhinanthus minor after removal of its host. Funct. Plant Biol. 2007, 34, 237–245. [Google Scholar] [CrossRef] [PubMed]
- Jiang, F.; Veselova, S.; Veselov, D.; Kudoyarova, G.; Jeschke, W.D. Cytokinin flows from Hordeum vulgare to the hemiparasite Rhinanthus minor and the influence of infection on host and parasite cytokinins relations. Funct. Plant Biol. 2005, 32, 619–629. [Google Scholar] [CrossRef] [PubMed]
- Jiang, F.; Jeschke, W.D.; Hartung, W. Abscisic acid (ABA) flows from Hordeum vulgare to the hemiparasite Rhinanthus minor and the influence of infection on host and parasite abscisic acid relations. J. Exp. Bot. 2004, 55, 2323–2329. [Google Scholar] [CrossRef]
- Salonen, V.; Puustinen, S. Success of a root hemiparasitic plant is influenced by soil quality and by defoliation of its host. Ecology 1996, 77, 1290–1293. [Google Scholar] [CrossRef]
- Lehtonen, P.; Helander, M.; Wink, M.; Sporer, F.; Saikkonen, K. Transfer of endophyte-origin defensive alkaloids from a grass to a hemiparasitic plant. Ecol. Lett. 2005, 8, 1256–1263. [Google Scholar] [CrossRef]
- Westwood, J.H.; Roney, J.K.; Khatibi, P.A.; Stromberg, V.K. RNA translocation between parasitic plants and their hosts. Pest Manag. Sci. 2009, 65, 533–539. [Google Scholar] [CrossRef] [PubMed]
- Aly, R. Trafficking of molecules between parasitic plants and their hosts. Weed Res. 2013, 53, 231–241. [Google Scholar] [CrossRef]
- Yoder, J.I.; Gunathilake, P.; Wu, B.; Tomilova, N.; Tomilov, A.A. Engineering host resistance against parasitic weeds with RNA interference. Pest Manage. Sci. 2009, 65, 460–466. [Google Scholar] [CrossRef] [PubMed]
- Wu, J. miRNAs as a secret weapon in the battlefield of haustoria, the interface between parasites and host plants. Molec. Plant 2018, 11, 354–356. [Google Scholar] [CrossRef] [PubMed]
- Shen, G.; Zhang, J.; Lei, Y.; Xu, Y.; Wu, J. Between-plant signaling. Annu. Rev. Plant Physiol. 2023, 74, 367–386. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Zhang, Y.; Wafula, E.K.; Honaas, L.A.; Ralph, P.E.; Jones, S.; Clarke, C.R.; Liu, S.; Su, C.; Zhang, H.; et al. Horizontal gene transfer is more frequent with increased heterotrophy and contributes to parasite adaptation. Proc. Natl. Acad. Sci. USA 2016, 113, E7010–E7019. [Google Scholar] [CrossRef] [PubMed]
- Góralski, G.; Denysenko-Bennett, M.; Burda, A.; Staszecka-Moskal, N.; Kwolek, D. Achievements in horizontal gene transfer studies in parasitic plants. Acta Biol. Cracov. Ser. Botanica 2021, 63, 17–28. [Google Scholar]
- Davis, C.C.; Xi, Z. Horizontal gene transfer in parasitic plants. Curr. Opin. Plant Biol. 2015, 26, 14–19. [Google Scholar] [CrossRef] [PubMed]
- Cameron, D.D.; White, A.; Antonovics, J. Parasite–Grass–Forb interactions and Rock–Paper–Scissor dynamics: Predicting the effects of the parasitic plant Rhinanthus minor on host plant communities. J. Ecol. 2009, 97, 1311–1319. [Google Scholar] [CrossRef]
- Bullock, J.M.; Pywell, R.F. Rhinanthus: A tool for restoring diverse grassland? Folia Geobot. 2005, 40, 273–288. [Google Scholar] [CrossRef]
- Westbury, D.B.; Dunnett, N.P. The impact of Rhinanthus minor in newly established meadows on a productive site. Appl. Veget. Sci. 2007, 10, 121–129. [Google Scholar] [CrossRef]
- Smith, R.S.; Shiel, R.S.; Millward, D.; Corkhill, P. The interactive effects of management on the productivity and plant community structure of an upland meadow: An 8-year field trial. J. Appl. Ecol. 2000, 37, 1029–1043. [Google Scholar] [CrossRef]
- Ameloot, E.; Verheyen, K.; Bakker, J.P.; De Vries, Y.; Hermy, M. Long-term dynamics of the hemiparasite Rhinanthus angustifolius and its relationship with vegetation structure. J. Veget. Sci. 2006, 17, 637–646. [Google Scholar] [CrossRef]
- Mudrák, O.; de Bello, F.; Doležal, J.; Lepš, J. Changes in the functional trait composition and diversity of meadow communities induced by Rhinanthus minor L. Folia Geobot. 2016, 51, 1–11. [Google Scholar] [CrossRef]
- Van Tooren, B.F. The fate of seeds after dispersal in chalk grassland: The role of the bryophyte layer. Oikos 1988, 53, 41–48. [Google Scholar] [CrossRef]
- Westbury, D.B.; Davies, A.; Woodecock, B.A.; Dunnett, N.P. Seeds of change: The value of using Rhinanthus minor in grassland restoration. J. Veget. Sci. 2006, 17, 435–446. [Google Scholar] [CrossRef]
- Mudrák, O.; Lepš, J. Interactions of the hemiparasitic species Rhinanthus minor L. with its host plant community at two nutrient levels. Folia Geobot. 2010, 45, 407–424. [Google Scholar] [CrossRef]
- Tešitel, J.; Mládek, J.; Fajmon, K.; Blažek, P.; Mudrák, O. Reversing expansion of Calamagrostis epigejos in a grassland biodiversity hotspot: Hemiparasitic Rhinanthus major does a better job than increased mowing intensity. Appl. Veget. Sci. 2018, 21, 104–112. [Google Scholar] [CrossRef]
- Těšitel, J.; Mládek, J.; Horník, J.; Těšitelová, T.; Adamec, V.; Tichý, L. Suppressing competitive dominants and community restoration with native parasitic plants using the hemiparasitic Rhinanthus alectorolophus and the dominant grass Calamagrostis epigejos. J. Appl. Ecol. 2017, 54, 1487–1495. [Google Scholar] [CrossRef]
- Pywell, R.F.; Bullock, J.M.; Walker, K.J.; Coulson, S.J.; Gregory, S.J.; Stevenson, M.J. Facilitating grassland diversificationn using the hemiparasitic plant Rhinanthus minor. J. Appl. Ecol. 2004, 41, 880–887. [Google Scholar] [CrossRef]
- Rowntree, J.K.; Craig, H. The contrasting roles of host species diversity and parasite population genetic diversity in the infection dynamics of a keystone parasitic plant. J. Ecol. 2019, 107, 23–33. [Google Scholar] [CrossRef]
- Wagner, M.; Peyton, J.; Heard, M.S.; Bullock, J.M.; Pywell, R.F. Effects of yellow-rattle (Rhinanthus minor) establishment on the vegetation of species-poor grassland. Aspects Appl. Biol. 2011, 108, 59–66. [Google Scholar]
- Těšitelová, T.; Knotková, K.; Knotek, A.; Cempírková, H.; Tešitel, J. Root hemiparasites suppress invasive clonal plants: Evidence from a cultivation experiment. NeoBiota 2024, 90, 97–121. [Google Scholar] [CrossRef]
- Chaudron, C.; Mazalová, M.; Kuras, T.; Malenovsk, I.; Mládek, J. Introducing ecosystem engineers for grassland biodiversity conservation: A review of the effects of hemiparasitic Rhinanthus species on plant and animal communities at multiple trophic levels. Perspect. Plant Ecol. Evol. Syst. 2021, 52, 125633. [Google Scholar] [CrossRef]
- Puustinen, S.; Mutikainen, P. Host–Parasite–Herbivore interactions: Implications of host cyanogenesis. Ecology 2001, 82, 2059–2071. [Google Scholar]
- Zytinska, S.E.; Frantz, L.; Hurst, B.; Johnson, A.; Preziosi, R.F.; Rowntree, J.K. Host-plant genotypic diversity and community genetic interactions mediate aphid spatial distribution. Ecol. Evol. 2014, 4, 121–131. [Google Scholar] [CrossRef] [PubMed]
- Sui, X.-L.; Zhang, T.; Tian, Y.-Q.; Xue, R.-J.; Li, A.-R. A neglected alliance in battles against parasitic plants: Arbuscular mycorrhizal and rhizobial symbiosis alleviate damage to a legume host by root hemiparasitic Pedicularis species. New Phytol. 2019, 221, 470–481. [Google Scholar] [CrossRef] [PubMed]
- Davies, D.M.; Graves, J.D. Interactions between arbuscular mycorrhizal fungi and the hemiparasitic angiosperm Rhinanthus minor during co-infection of a host. New Phytol. 1998, 139, 555–563. [Google Scholar] [CrossRef]
- De Paula, C.C.P.; Macek, P.; Bárta, J.; Borovec, J.; Svobodová, I.; Holá, E.; Lepš, J.; Sirová, D. Parasitic trophic mode of plant host affects the extent of colonization, but does not induce systemic shifts in the composition of foliar endophytic assemblages in temperate meadow ecosystems. Funct. Ecol. 2022, 36, 1177–1190. [Google Scholar] [CrossRef]
- Jhu, M.-Y.; Sinha, N.R. Parasitic plants: An overview of mechanisms by which plants perceive and respond to parasites. Annu. Rev. Plant Biol. 2022, 73, 433–455. [Google Scholar] [CrossRef]
- Albanova, I.A.; Zagorchev, L.I.; Teofanova, D.R.; Odjakova, M.K.; Kutueva, L.I.; Ashapkin, V.V. Host resistance to parasitic plants—Current knowledge and future perspectives. Plants 2023, 12, 1447. [Google Scholar] [CrossRef] [PubMed]
- Cai, T.; Babiker, A.G.; Ejeta, G.; Butler, L.G. Morphological response of witchweed (Striga asiatica) to in vitro culture. J. Exp. Bot. 1993, 44, 1377–1384. [Google Scholar] [CrossRef]
- Waweru, D.N.; Kuria, E.K.; Bradley, J.M.; Scholes, J.D.; Runo, S. Tissue culture protocols for the obligate parasitic plant Striga hermonthica and implications for host-parasite co-cultivation. Plant Cell Tissue Organ Cult. 2019, 138, 247–256. [Google Scholar] [CrossRef]
- Tomilov, A.; Tomilova, N.; Yoder, J.I. In vitro haustorium development in roots and root cultures of the hemiparasitic plant Triphysaria versicolor. Plant Cell Tissue Organ Cult. 2004, 77, 257–265. [Google Scholar] [CrossRef]
- Denes, T.; Papp, N.; Marton, K.; Kaszas, A.; Felinger, A.; Varga, E.; Boros, B. Polyphenol content of Ononis arvensis L. and Rhinanthus serotinus (Schönh. ex Halácsy & Heinr.Braun) Oborny used in the Transylvanian ethnomedicine. Int. J. Pharmacogn. Phytochem. 2018, 30, 1301–1307. [Google Scholar]
- Kaya, A.A.; Üçüncü, O.; İlter, Ș.M.; Baltacı, C.; Öztürk, S. Chemical composition and bioactive properties of the essential oil of Rhinanthus angustifolius subsp. grandiflorus. Bulg. Chem. Commun. 2017, 49, 923–927. [Google Scholar]
- Zhang, L.; Zengin, G.; Rocchetti, G.; Senkardes, I.; Sharmeen, J.B.; Mahomoodally, M.F.; Behl, T.; Rouphael, Y.; Lucini, L. Phytochemical constituents and biological activities of the unexplored plant Rhinanthus angustifiolius subsp. grandiflorus. Appl. Sci. 2021, 11, 9162. [Google Scholar]
- Usta, C.; Yildirim, A.B.; Turker, A.U. Antibacterial and antitumor activities of some plants grown in Turkey. Biotechnol. Biotechnol. Equipm. 2014, 28, 306–315. [Google Scholar] [CrossRef]
- French, K.E.; Harvey, J.; McCullagh, J.S.O. Targeted and untargeted metabolic profiling of wild grassland plants identifies antibiotic and anthelmintic compounds targeting pathogen physiology, metabolism and reproduction. Sci. Rep. 2018, 8, 1695. [Google Scholar] [CrossRef]
- Adler, L.S. Host effects on herbivory and pollination in a hemiparasitic plant. Ecology 2002, 83, 2700–2710. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).