Better Safe Than Sorry: A Model to Assess Anthropic Impacts on a River System in Order to Take Care of the Landscape
Abstract
1. Introduction
2. Materials and Methods
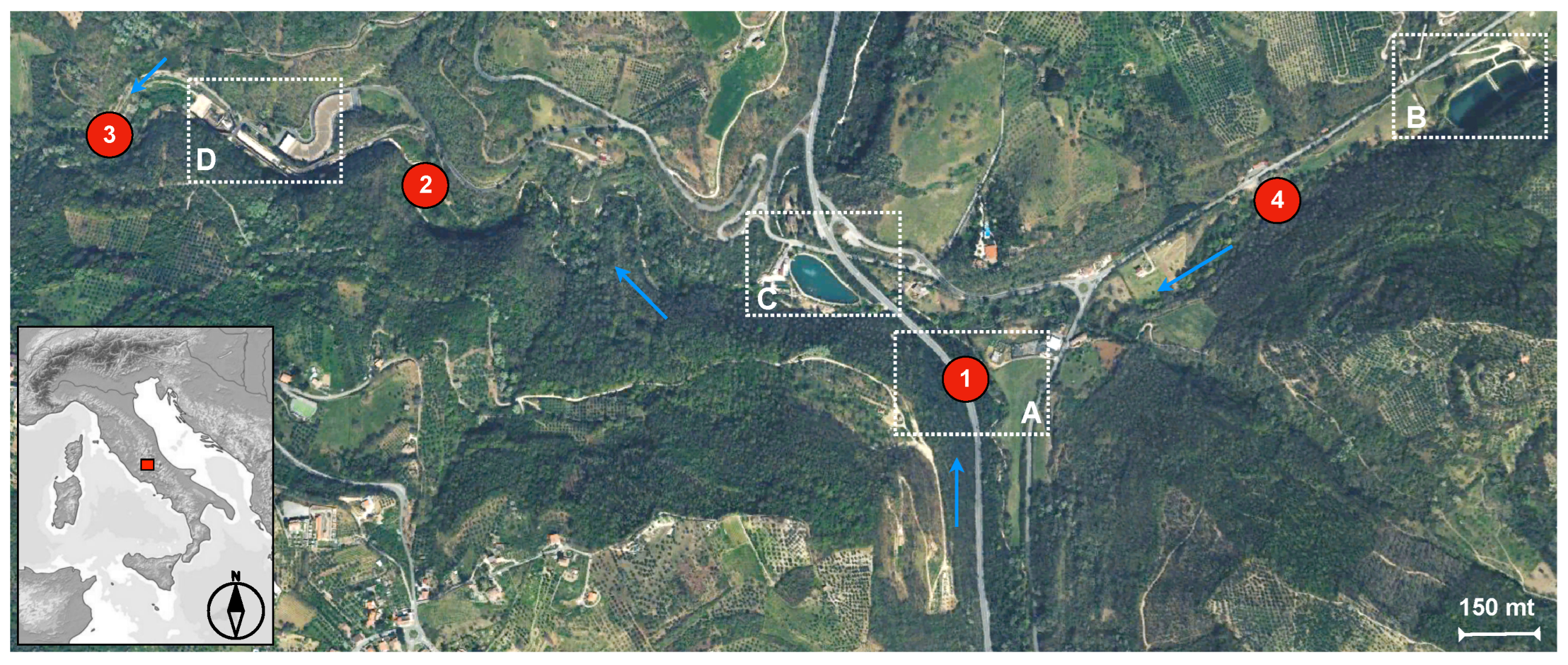
2.1. Study Area
- -
- Station 1: the Fosso della Mola (UTM WGS84 33T: E 320673.760–N 4677220.717) that is at the point where the river collects urban wastewater from the municipality of Monteleone; a center of 1.159 inhabitants (ISTAT, EU Statistical Institutes). Here, the river is 1.5 m long, a few tens of millimeters in depth, and has dense bank vegetation surrounded by an agricultural and arable environment.
- -
- Station 2: the Fosso della Mola that is upstream of the “Le Capore” springs (Frasso Sabino, central Italy) (UTM WGS84 33T: E 319624.434–N 4677876.461); this station is representative of the ecological conditions of the watercourse after the water supplied by Fosso delle Mole.
- -
- Station 3: Fiume Farfa that is downstream of the “Le Capore” springs (UTM WGS84 33T: E 319192.977–N 4678085.328); this station is representative of the ecological conditions of the watercourse after the release of the springs. It also corresponds to the ARPA Lazio surveillance station named “Farfa 1”. The Le Capore springs are characterized by an artesian aquifer system with an annual mean discharge of about 5 m3/s. These springs are managed by ACEA ATO2 SpA, which is a water utility that operates mainly in central Italy, supplying drinking water to more than 4 million users in the city of Rome and its hinterland. The springs of “Le Capore” and Peschiera fed the homonymous aqueduct of Peschiera-Capore, which is a strategic aqueduct system that supplied over 80% of the water needs of the Roman water distribution network.
- -
- Station 4: the Fosso delle Mole ditch that is downstream of a fish farming factory (UTM WGS84 33T: E 321398.253–N 4677945.623). This station is representative of one of the main waterways that supplies the Farfa river. It originates between Tommasella and Capannaccia and is also fed by the Fosso Venella in a north-east direction with respect to the “Le Capore” springs.
2.2. Sampling
2.3. Taxonomic Identification of the Macrobenthos
2.4. Ecological Quality Status Assessment
2.5. Flora and Vegetation Characterization
2.6. Correlation between Vegetation and Benthonic Macroinvertebrates
3. Results
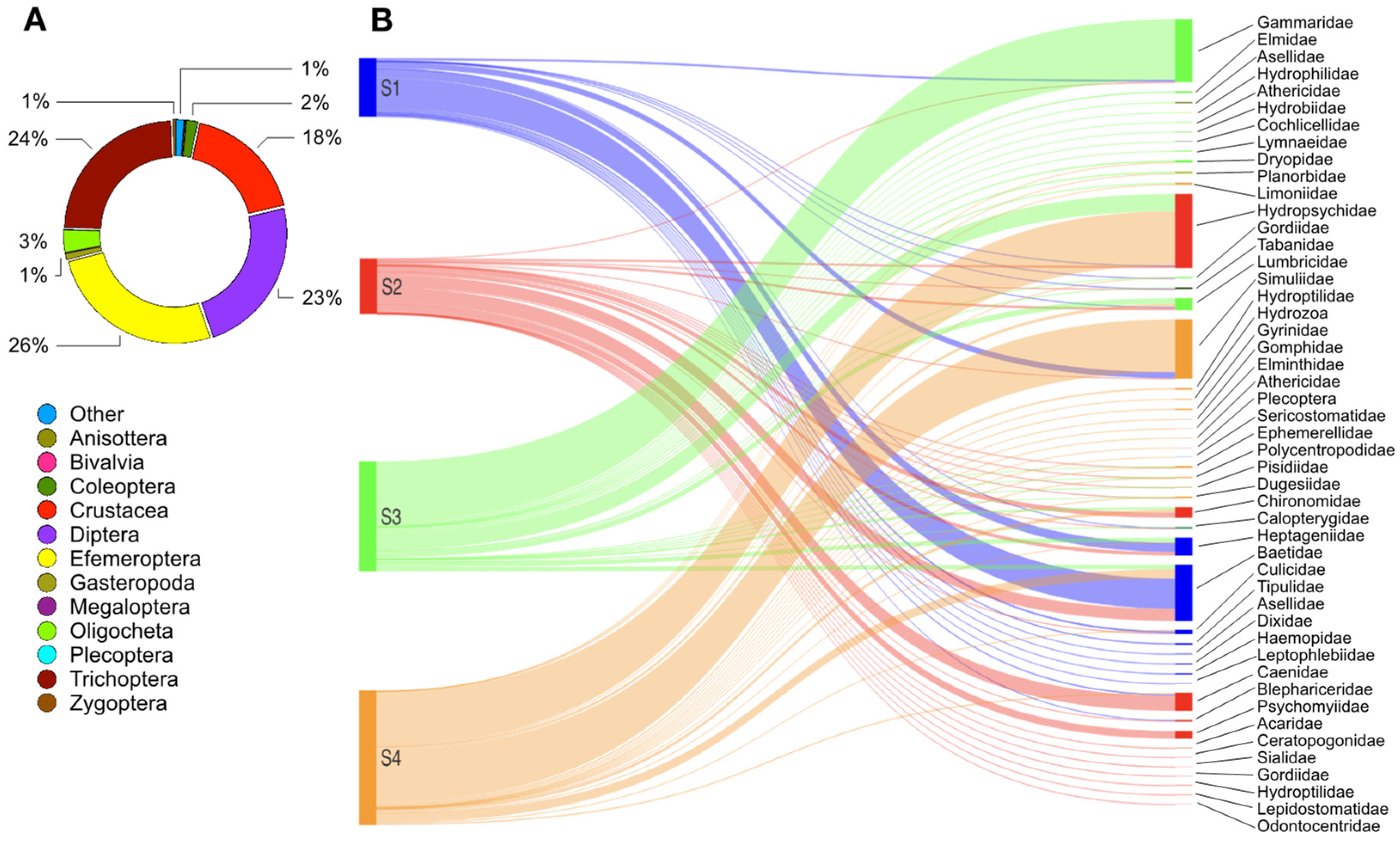
3.1. Ecological Quality by Macrobenthos
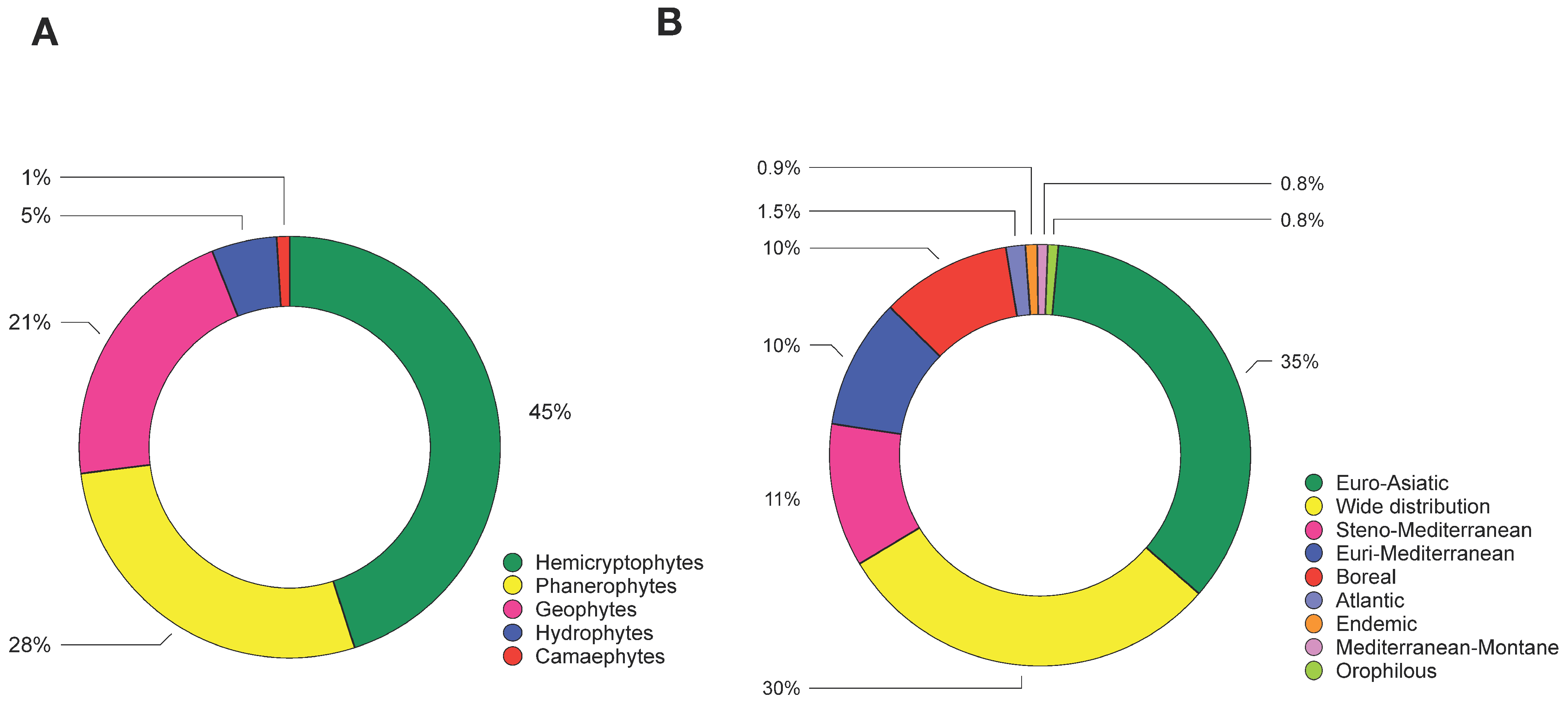
3.2. Characterization of Vegetation Pattern
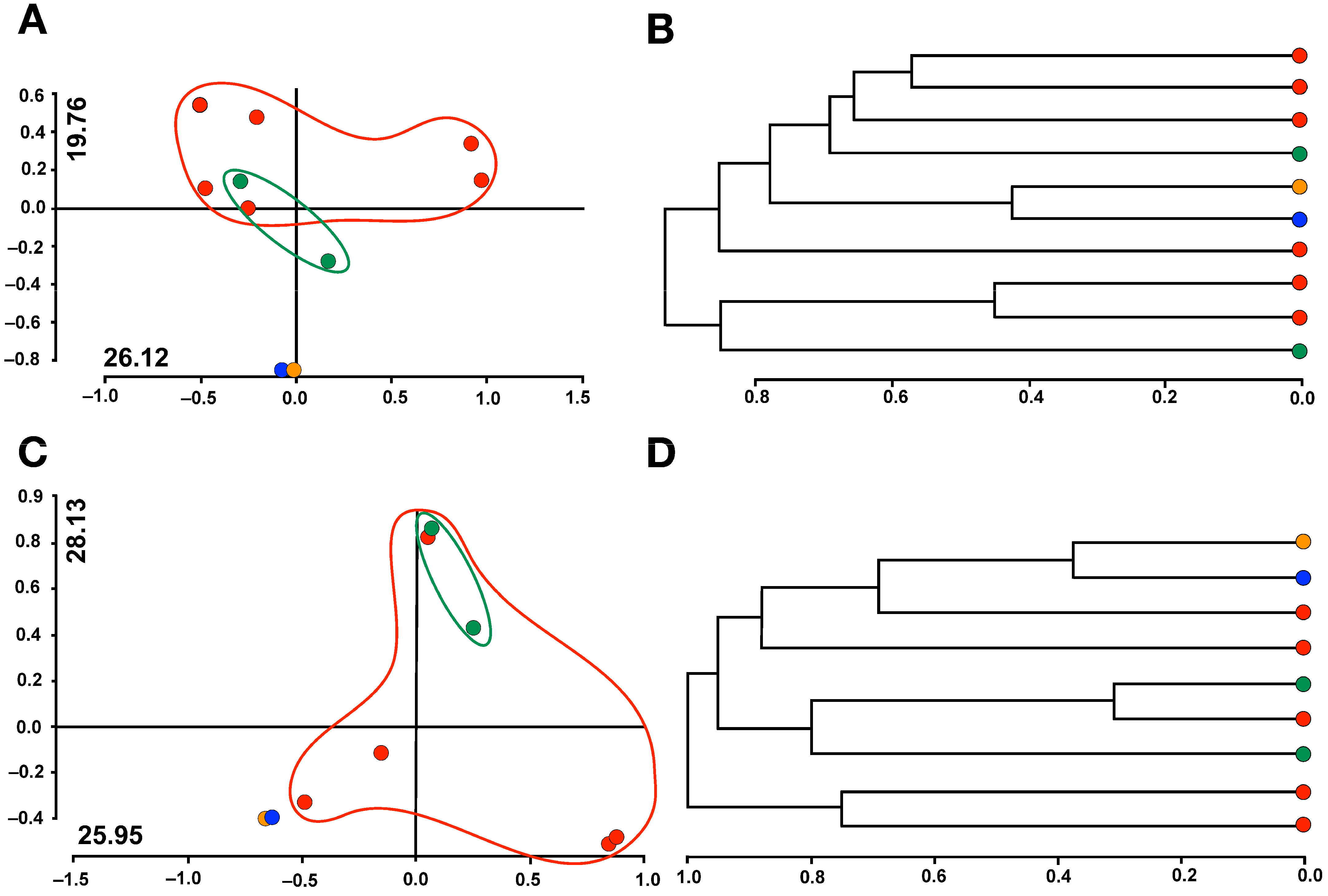
3.3. Correlation between Riparian Vegetation and Macrobenthic Community
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Naiman, R.J.; Magnuson, J.J.; McKnight, D.M.; Stanford, J.A.; Karr, J.R. Freshwater Ecosystems and Their Management: A National Initiative. Science 1995, 270, 584–585. [Google Scholar] [CrossRef]
- Naiman, R.J.; Dudgeon, D. Global Alteration of Freshwaters: Influences on Human and Environmental Well-Being. Ecol. Res. 2011, 26, 865–873. [Google Scholar] [CrossRef]
- Department for Environment Food & Rural Affair. Managing Abstraction and the Water Environment; Welsh Government: London, UK, 2013; pp. 1–16. [Google Scholar]
- Lynch, A.J.; Cooke, S.J.; Arthington, A.H.; Baigun, C.; Bossenbroek, L.; Dickens, C.; Harrison, I.; Kimirei, I.; Langhans, S.D.; Murchie, K.J.; et al. People Need Freshwater Biodiversity. WIREs Water 2023, 10, e1633. [Google Scholar] [CrossRef]
- Loh, J.; Green, R.E.; Ricketts, T.; Lamoreux, J.; Jenkins, M.; Kapos, V.; Randers, J. The Living Planet Index: Using Species Population Time Series to Track Trends in Biodiversity. Phil. Trans. R. Soc. B 2005, 360, 289–295. [Google Scholar] [CrossRef] [PubMed]
- Butchart, S.; Dieme-Amting, E.; Gitay, H.; Raaijmakers, S.; Douglas, T. Ecosystems and Human Well-Being: Wetlands and Water Synthesis: A Report of the Millennium Ecosystem Assessment; Millennium Ecosystem Assessment (Program), Ed.; World Resources Institute: Washington, DC, USA, 2005; ISBN 978-1-56973-597-8. [Google Scholar]
- Arroita, M.; Flores, L.; Larrañaga, A.; Martínez, A.; Martínez-Santos, M.; Pereda, O.; Ruiz-Romera, E.; Solagaistua, L.; Elosegi, A. Water Abstraction Impacts Stream Ecosystem Functioning via Wetted-Channel Contraction. Freshw. Biol. 2017, 62, 243–257. [Google Scholar] [CrossRef]
- Mancini, L.; Marcheggiani, S.; Puccinelli, C.; Lacchetti, I.; Carere, M.; Bouley, T. Global Environmental Changes and the Impact on Ecosystems and Human Health. Energ. Ambiente Innov. 2017, 3, 98–105. [Google Scholar] [CrossRef]
- Buglione, M.; Petrelli, S.; Troiano, C.; Notomista, T.; Rivieccio, E.; Fulgione, D. The Diet of Otters (Lutra lutra) on the Agri River System, One of the Most Important Presence Sites in Italy: A Molecular Approach. PeerJ 2020, 8, e9606. [Google Scholar] [CrossRef] [PubMed]
- Filipe, A.F.; Feio, M.J.; Garcia-Raventós, A.; Ramião, J.P.; Pace, G.; Martins, F.M.; Magalhães, M.F. The European Water Framework Directive Facing Current Challenges: Recommendations for a More Efficient Biological Assessment of Inland Surface Waters. Inland Waters 2019, 9, 95–103. [Google Scholar] [CrossRef]
- Bruno, D.; Gutiérrez-Cánovas, C.; Sánchez-Fernández, D.; Velasco, J.; Nilsson, C. Impacts of Environmental Filters on Functional Redundancy in Riparian Vegetation. J. Appl. Ecol. 2016, 53, 846–855. [Google Scholar] [CrossRef]
- Capon, S.J.; Stewart-Koster, B.; Bunn, S.E. Future of Freshwater Ecosystems in a 1.5 °C Warmer World. Front. Environ. Sci. 2021, 9, 784642. [Google Scholar] [CrossRef]
- Rosenberg, R. Benthic Faunal Dynamics during Succession Following Pollution Abatement in a Swedish Estuary. Oikos 1976, 27, 414. [Google Scholar] [CrossRef]
- Snelgrove, P.V. The Importance of Marine Sediment Biodiversity in Ecosystem Processes. Ambio 1997, 28, 578–583. [Google Scholar]
- Ysebaert, T.; Herman, P. Spatial and Temporal Variation in Benthic Macrofauna and Relationships with Environmental Variables in an Estuarine, Intertidal Soft-Sediment Environment. Mar. Ecol. Prog. Ser. 2002, 244, 105–124. [Google Scholar] [CrossRef]
- Hawkins, C.P.; Murphy, M.L.; Anderson, N.H. Effects of Canopy, Substrate Composition, and Gradient on the Structure of Macroinvertebrate Communities in Cascade Range Streams of Oregon. Ecology 1982, 63, 1840. [Google Scholar] [CrossRef]
- Ormerod, S.J.; Donald, A.P.; Brown, S.J. The Influence of Plantation Forestry on the pH and Aluminium Concentration of Upland Welsh Streams: A Re-Examination. Environ. Pollut. 1989, 62, 47–62. [Google Scholar] [CrossRef] [PubMed]
- Rutt, G.P.; Weatherley, N.S.; Ormerod, S.J. Microhabitat Availability in Welsh Moorland and Forest Streams as a Determinant of Macroinvertebrate Distribution. Freshw. Biol. 1989, 22, 247–261. [Google Scholar] [CrossRef]
- Rundle, S.D.; Clare Lloyd, E.; Ormerod, S.J. The Effects of Riparian Management and Physicochemistry on Macroinvertebrate Feeding Guilds and Community Structure in Upland British Streams. Aquat. Conserv. 1992, 2, 309–324. [Google Scholar] [CrossRef]
- Ormerod, S.J.; Rundle, S.D.; Lloyd, E.C.; Douglas, A.A. The Influence of Riparian Management on the Habitat Structure and Macroinvertebrate Communities of Upland Streams Draining Plantation Forests. J. Appl. Ecol. 1993, 30, 13. [Google Scholar] [CrossRef]
- Borja, Á.; Dauer, D.M.; Grémare, A. The Importance of Setting Targets and Reference Conditions in Assessing Marine Ecosystem Quality. Ecol. Indic. 2012, 12, 1–7. [Google Scholar] [CrossRef]
- Cabral-Oliveira, J.; Mendes, S.; Maranhão, P.; Pardal, M.A. Effects of Sewage Pollution on the Structure of Rocky Shore Macroinvertebrate Assemblages. Hydrobiologia 2014, 726, 271–283. [Google Scholar] [CrossRef]
- Hynes, H.B.N. Symposium on water pollution: The use of invertebrates as indicators of river pollution. Proc. Linn. Soc. Lond. 1959, 170, 165–169. [Google Scholar] [CrossRef]
- Khatri, N.; Tyagi, S. Influences of Natural and Anthropogenic Factors on Surface and Groundwater Quality in Rural and Urban Areas. Front. Life Sci. 2015, 8, 23–39. [Google Scholar] [CrossRef]
- Tampo, L.; Kaboré, I.; Alhassan, E.H.; Ouéda, A.; Bawa, L.M.; Djaneye-Boundjou, G. Benthic Macroinvertebrates as Ecological Indicators: Their Sensitivity to the Water Quality and Human Disturbances in a Tropical River. Front. Water 2021, 3, 662765. [Google Scholar] [CrossRef]
- Dubois, G.; Girard, C.; Lapointe, F.-J.; Shapiro, B.J. The Inuit Gut Microbiome Is Dynamic over Time and Shaped by Traditional Foods. Microbiome 2017, 5, 151. [Google Scholar] [CrossRef] [PubMed]
- Howarth, W. Going with the Flow: Integrated Water Resources Management, the EU Water Framework Directive and Ecological Flows. Legal Stud. 2018, 38, 298–319. [Google Scholar] [CrossRef]
- González del Tánago, M.; García de Jalón, D. Attributes for Assessing the Environmental Quality of Riparian Zones. Limnética 2006, 25, 389–402. [Google Scholar] [CrossRef]
- Fogliata, P.; Cislaghi, A.; Sala, P.; Giupponi, L. An Ecological Analysis of the Riparian Vegetation for Improving the Riverine Ecosystem Management: The Case of Lombardy Region (North Italy). Landsc. Ecol. Eng. 2021, 17, 375–386. [Google Scholar] [CrossRef]
- Riis, T.; Kelly-Quinn, M.; Aguiar, F.C.; Manolaki, P.; Bruno, D.; Bejarano, M.D.; Clerici, N.; Fernandes, M.R.; Franco, J.C.; Pettit, N.; et al. Global Overview of Ecosystem Services Provided by Riparian Vegetation. BioScience 2020, 70, 501–514. [Google Scholar] [CrossRef]
- Macfarlane, W.W.; Gilbert, J.T.; Jensen, M.L.; Gilbert, J.D.; Hough-Snee, N.; McHugh, P.A.; Wheaton, J.M.; Bennett, S.N. Riparian Vegetation as an Indicator of Riparian Condition: Detecting Departures from Historic Condition across the North American West. J. Environ. Manag. 2017, 202, 447–460. [Google Scholar] [CrossRef]
- Królak, E.; Strzałek, M.; Korycińska, M. The Usefulness of Various Indices in the Assessment of Water Quality of a Lowland River. Ecohydrol. Hydrobiol. 2009, 9, 271–280. [Google Scholar] [CrossRef]
- Erba, S.; Cazzola, M.; Belfiore, C.; Buffagni, A. Macroinvertebrate Metrics Responses to Morphological Alteration in Italian Rivers. Hydrobiologia 2020, 847, 2169–2191. [Google Scholar] [CrossRef]
- Apollonio, C.; Petroselli, A.; Cornelini, P.; Manzari, V.; Preti, F.; Grimaldi, S. Riparian Vegetation as a Marker for Bankfull and Management Discharge Evaluation: The Case Study of Rio Torbido River Basin (Central Italy). J. Agric. Eng. 2021, 52, 1140. [Google Scholar] [CrossRef]
- García-Barreras, E.; Martínez-Fernández, V.; García De Jalón, D. Long-Term Macrobentos Responses to Environmental Flows and Water Quality Improvement along River Jarama. Ecohydrol. Hydrobiol. 2023, 23, 304–315. [Google Scholar] [CrossRef]
- Calabrese, S.; Mezzanotte, V.; Marazzi, F.; Canobbio, S.; Fornaroli, R. The Influence of Multiple Stressors on Macroinvertebrate Communities and Ecosystem Attributes in Northern Italy Pre-Alpine Rivers and Streams. Ecol. Indic. 2020, 115, 106408. [Google Scholar] [CrossRef]
- Aschonitis, V.G.; Feld, C.K.; Castaldelli, G.; Turin, P.; Visonà, E.; Fano, E.A. Environmental Stressor Gradients Hierarchically Regulate Macrozoobenthic Community Turnover in Lotic Systems of Northern Italy. Hydrobiologia 2016, 765, 131–147. [Google Scholar] [CrossRef]
- Ferranti, C.; Paolella, A. La Pianificazione del Bacino del Fiume Tevere 1992–2000; Autorità di bacino del Tevere, A., Ed.; Gangemi: Roma, Italy, 2001; ISBN 978-88-492-0166-6. [Google Scholar]
- Balzamo, S.; Martone, C. Metodi Biologici Per le Acque Superficiali Interne 2014; Manuali e Linee Guida 111/2014; Parte I.; ISPRA: Roma, Italy, 2014; ISBN 978-88-448-0651. [Google Scholar]
- Naman, S.M.; Rosenfeld, J.S.; Richardson, J.S. Causes and Consequences of Invertebrate Drift in Running Waters: From Individuals to Populations and Trophic Fluxes. Can. J. Fish. Aquat. Sci. 2016, 73, 1292–1305. [Google Scholar] [CrossRef]
- Ruffo, S. Guide per il Riconoscimento delle Specie Animali delle Acque Interne Italiane; CNR: Rome, Italy, 1977; ISBN 88-8080-061-2. [Google Scholar]
- Belfiore, C. Efemerotteri (Ephemeroptera). In Guide: Specie Animali delle Acque Interne Italiane; CNR: Verona, Italy, 1983; Volume 24. [Google Scholar]
- Campaioli, S.; Ghetti, P.F.; Minelli, A.; Ruffo, S. Manuale per il Riconoscimento dei Macroinvertebrati delle Acque Dolci Italiane; Museo di Storia Naturale di Trento: Trento, Italy, 1994; Volume 1, ISBN 0299600000002. [Google Scholar]
- Campaioli, S.; Ghetti, P.F.; Minelli, A.; Ruffo, S. Manuale per il Riconoscimento dei Macroinvertebrati delle Acque Dolci Italiane; Museo di Storia Naturale di Trento: Trento, Italy, 1999; Volume 2, ISBN 978-88-7702-089-5. [Google Scholar]
- Sansoni, G.; Ghetti, P.F. Atlante per il Riconoscimento dei Macroinvertebrati dei Corsi D’acqua Italiani, 5th ed.; APR&B, Provincia Autonoma di trento: Trento, Italy, 2005; ISBN 88-86602-29-4. [Google Scholar]
- Buffagni, A.; Erba, E.; Birk, S.; Cazzola, M.; Feld, C.; Ofenboeck, T.; Murray-Bligh, J.; Furse, M.; Clarke, R.; Hering, D.; et al. Towards European Inter-Calibration for the Water Framework Directive Procedures and Examples for Different River Types from the BC Project STAR; Istituto di Ricerca sulle Acque-CNR: Rome, Italy, 2005. [Google Scholar]
- Buffagni, A.; Belfiore, C. ICMeasy 1.2: A Software for the Intercalibration Common Metrics and Index Easy Calculation. User Guide. IRSA-CNR Not. Metod. Anal. 2007, 1, 101–114. [Google Scholar]
- Pignatti, S. Flora d’Italia. Volume 1, 2nd ed.; Edagricole: Milano, Italy, 2017; Volume 4, ISBN 978-88-506-5242-6. [Google Scholar]
- Raunkiær, C. Life Forms of Plants and Statistical Plant Geography; Facsimile Publisher: New Delhi, India, 2019; ISBN 978-93-333-9336-2. [Google Scholar]
- R CORE Team. A Language and Environment for Statistical Computing R Foundation for Statistical Computing. In Foundation for Statistical Computing; R CORE Team: Vienna, Austria, 2021. [Google Scholar]
- Wickham, H.; Averick, M.; Bryan, J.; Chang, W.; McGowan, L.; François, R.; Grolemund, G.; Hayes, A.; Henry, L.; Hester, J.; et al. Welcome to the Tidyverse. J. Open Source Softw. 2019, 4, 1686. [Google Scholar] [CrossRef]
- Oksanen, J.; Simpson, G.L.; Blanchet, F.G.; Kindt, R.; Legendre, P.; Minchin, P.R.; O’Hara, R.B.; Solymos, P.; Stevens, M.H.H.; Szoecs, E.; et al. Vegan: Community Ecology Package 2021, 2.6-6.1. Available online: https://cran.r-project.org/web/packages/vegan/vegan.pdf (accessed on 4 May 2023).
- Gumiero, B.; Maiolini, B.; Comiti, F.; Moroni, F. The Italian Rivers. In Rivers of Europe; Elsevier: Amsterdam, The Netherlands, 2022; pp. 657–685. ISBN 978-0-08-102612-0. [Google Scholar]
- Stephenson, P.J.; Londoño-Murcia, M.C.; Borges, P.A.V.; Claassens, L.; Frisch-Nwakanma, H.; Ling, N.; McMullan-Fisher, S.; Meeuwig, J.J.; Unter, K.M.M.; Walls, J.L.; et al. Measuring the Impact of Conservation: The Growing Importance of Monitoring Fauna, Flora and Funga. Diversity 2022, 14, 824. [Google Scholar] [CrossRef]
- Ifabiyi, I.P. Self Purification of a Freshwater Stream in Ile-Ife: Lessons for Water Management. J. Hum. Ecol. 2008, 24, 131–137. [Google Scholar] [CrossRef]
- Nugraha, W.D.; Sarminingsih, A.; Alfisya, B. The Study of Self Purification Capacity Based on Biological Oxygen Demand (BOD) and Dissolved Oxygen (DO) Parameters. IOP Conf. Ser. Earth Environ. Sci. 2020, 448, 012105. [Google Scholar] [CrossRef]
- Buffagni, A.; Tenchini, R.; Cazzola, M.; Erba, S.; Balestrini, R.; Belfiore, C.; Pagnotta, R. Detecting the Impact of Bank and Channel Modification on Invertebrate Communities in Mediterranean Temporary Streams (Sardinia, SW Italy). Sci. Total Environ. 2016, 565, 1138–1150. [Google Scholar] [CrossRef] [PubMed]
- Hasselquist, E.; Polvi, L.; Kahlert, M.; Nilsson, C.; Sandberg, L.; McKie, B. Contrasting Responses among Aquatic Organism Groups to Changes in Geomorphic Complexity Along a Gradient of Stream Habitat Restoration: Implications for Restoration Planning and Assessment. Water 2018, 10, 1465. [Google Scholar] [CrossRef]
- Rios, S.L.; Bailey, R.C. Relationship between Riparian Vegetation and Stream Benthic Communities at Three Spatial Scales. Hydrobiologia 2006, 553, 153–160. [Google Scholar] [CrossRef]
- Fierro, P.; Bertrán, C.; Tapia, J.; Hauenstein, E.; Peña-Cortés, F.; Vergara, C.; Cerna, C.; Vargas-Chacoff, L. Effects of Local Land-Use on Riparian Vegetation, Water Quality, and the Functional Organization of Macroinvertebrate Assemblages. Science of The Total Environment 2017, 609, 724–734. [Google Scholar] [CrossRef]
- Ono, E.R.; Manoel, P.S.; Melo, A.L.U.; Uieda, V.S. Effects of Riparian Vegetation Removal on the Functional Feeding Group Structure of Benthic Macroinvertebrate Assemblages. Community Ecol. 2020, 21, 145–157. [Google Scholar] [CrossRef]
- Palt, M.; Hering, D.; Kail, J. Context-specific Positive Effects of Woody Riparian Vegetation on Aquatic Invertebrates in Rural and Urban Landscapes. J. Appl. Ecol. 2023, 60, 1010–1021. [Google Scholar] [CrossRef]
- Alcaraz-Hernández, J.D.; Sánchez-Hernández, J.; Muñoz-Mas, R.; Martínez-Capel, F. Drivers of Macroinvertebrate Communities in Mediterranean Rivers: A Mesohabitat Approach. Sustainability 2024, 16, 3075. [Google Scholar] [CrossRef]
- Dos Santos, C.; Simeone, D.; Beasley, C.R. Lower Diversity in Benthic Macrofaunal Assemblages Associated with Increasing Riparian Deforestation in First-Order Lowland Tropical Streams. Stud. Neotrop. Fauna Environ. 2024, 1–15. [Google Scholar] [CrossRef]
- Aguiar, F.C.; Segurado, P.; Martins, M.J.; Bejarano, M.D.; Nilsson, C.; Portela, M.M.; Merritt, D.M. The Abundance and Distribution of Guilds of Riparian Woody Plants Change in Response to Land Use and Flow Regulation. J. Appl. Ecol. 2018, 55, 2227–2240. [Google Scholar] [CrossRef]
- Nilsson, C.; Berggren, K. Alterations of Riparian Ecosystems Caused by River Regulation. BioScience 2000, 50, 783. [Google Scholar] [CrossRef]
- Greet, J.; Cousens, R.D.; Webb, J.A. Flow Regulation Affects Temporal Patterns of Riverine Plant Seed Dispersal: Potential Implications for Plant Recruitment. Freshw. Biol. 2012, 57, 2568–2579. [Google Scholar] [CrossRef]
- Johnson, S.E.; Waller, D.M. Influence of Dam Regulation on 55-Year Canopy Shifts in Riparian Forests. Can. J. For. Res. 2013, 43, 159–170. [Google Scholar] [CrossRef]
- Rood, S.B.; Braatne, J.H.; Goater, L.A. Favorable Fragmentation: River Reservoirs Can Impede Downstream Expansion of Riparian Weeds. Ecol. Appl. 2010, 20, 1664–1677. [Google Scholar] [CrossRef] [PubMed]
- Bejarano, M.D.; Sordo-Ward, Á. Riparian Woodland Encroachment Following Flow Regulation: A Comparative Study of Mediterranean and Boreal Streams. Knowl. Manag. Aquat. Ecosyst. 2011, 402, 20. [Google Scholar] [CrossRef]
- Webb, J.A.; Padgham, M. How Does Network Structure and Complexity in River Systems Affect Population Abundance and Persistence? Limnologica 2013, 43, 399–403. [Google Scholar] [CrossRef]
- Sand-Jensen, K.; Jeppesen, E.; Nielsen, K.; Van Der Bijl, L.; Hjermind, L.; Nielsen, L.W.; Ivlrsln, T.M. Growth of Macrophytes and Ecosystem Consequences in a Lowland Danish Stream. Freshw. Biol. 1989, 22, 15–32. [Google Scholar] [CrossRef]
- Westhoff, V.; Van Der Maarel, E. The Braun-Blanquet Approach. In Classification of Plant Communities; Whittaker, R.H., Ed.; Springer: Dordrecht, The Netherlands, 1978; pp. 287–399. ISBN 978-90-6193-566-7. [Google Scholar]


| Station | ||||
|---|---|---|---|---|
| 1 | 2 | 3 | 4 | |
| ASTP (EQR) | 0.78 | 0.88 | 0.83 | 0.83 |
| N_EPT (EQR) | 0.41 | 0.83 | 0.50 | 0.75 |
| Log (SelEPTD + 1) (EQR) | 0.81 | 0.65 | 0.63 | 0.50 |
| 1-GOLD (EQR) | 0.97 | 1.00 | 0.92 | 0.66 |
| Shannon–Weiner (EQR) | 0.76 | 0.96 | 1.02 | 0.66 |
| STAR_ICM normalised | 0.74 | 0.81 | 0.77 | 0.69 |
| Ecological quality | good | good | good | good |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rivieccio, E.; Fulgione, D.; de Filippo, G.; De Natale, A.; Paturzo, V.; Mineo, C.; Passaretti, S.; Varriale, A.; Buglione, M. Better Safe Than Sorry: A Model to Assess Anthropic Impacts on a River System in Order to Take Care of the Landscape. Land 2024, 13, 1076. https://doi.org/10.3390/land13071076
Rivieccio E, Fulgione D, de Filippo G, De Natale A, Paturzo V, Mineo C, Passaretti S, Varriale A, Buglione M. Better Safe Than Sorry: A Model to Assess Anthropic Impacts on a River System in Order to Take Care of the Landscape. Land. 2024; 13(7):1076. https://doi.org/10.3390/land13071076
Chicago/Turabian StyleRivieccio, Eleonora, Domenico Fulgione, Gabriele de Filippo, Antonino De Natale, Vincenzo Paturzo, Claudio Mineo, Stefania Passaretti, Anna Varriale, and Maria Buglione. 2024. "Better Safe Than Sorry: A Model to Assess Anthropic Impacts on a River System in Order to Take Care of the Landscape" Land 13, no. 7: 1076. https://doi.org/10.3390/land13071076
APA StyleRivieccio, E., Fulgione, D., de Filippo, G., De Natale, A., Paturzo, V., Mineo, C., Passaretti, S., Varriale, A., & Buglione, M. (2024). Better Safe Than Sorry: A Model to Assess Anthropic Impacts on a River System in Order to Take Care of the Landscape. Land, 13(7), 1076. https://doi.org/10.3390/land13071076






