Soil Quality Assessment and Its Spatial Variability in an Intensively Cultivated Area in India
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Soil Sampling and Analysis
2.3. Assessment of Soil Quality Index (SQI)
2.3.1. Minimum Dataset
2.3.2. Scoring of Indicator Soil Properties
- (i)
- Linear scoring—The indicator scores were assigned based on the ‘more is better’ and ‘less is better’ criteria using linear scoring equations (Equations (1) and (2)) as described by Biswas et al. [51]:
- (ii)
2.3.3. Development of Soil Quality Indices
2.3.4. Sensitivity Index
2.4. Spatial Assessment of Soil Quality Index
2.4.1. Prediction of Soil Properties
2.4.2. Spatial Mapping of Soil Quality Index
3. Results
3.1. Descriptive Statistics of Soil Properties
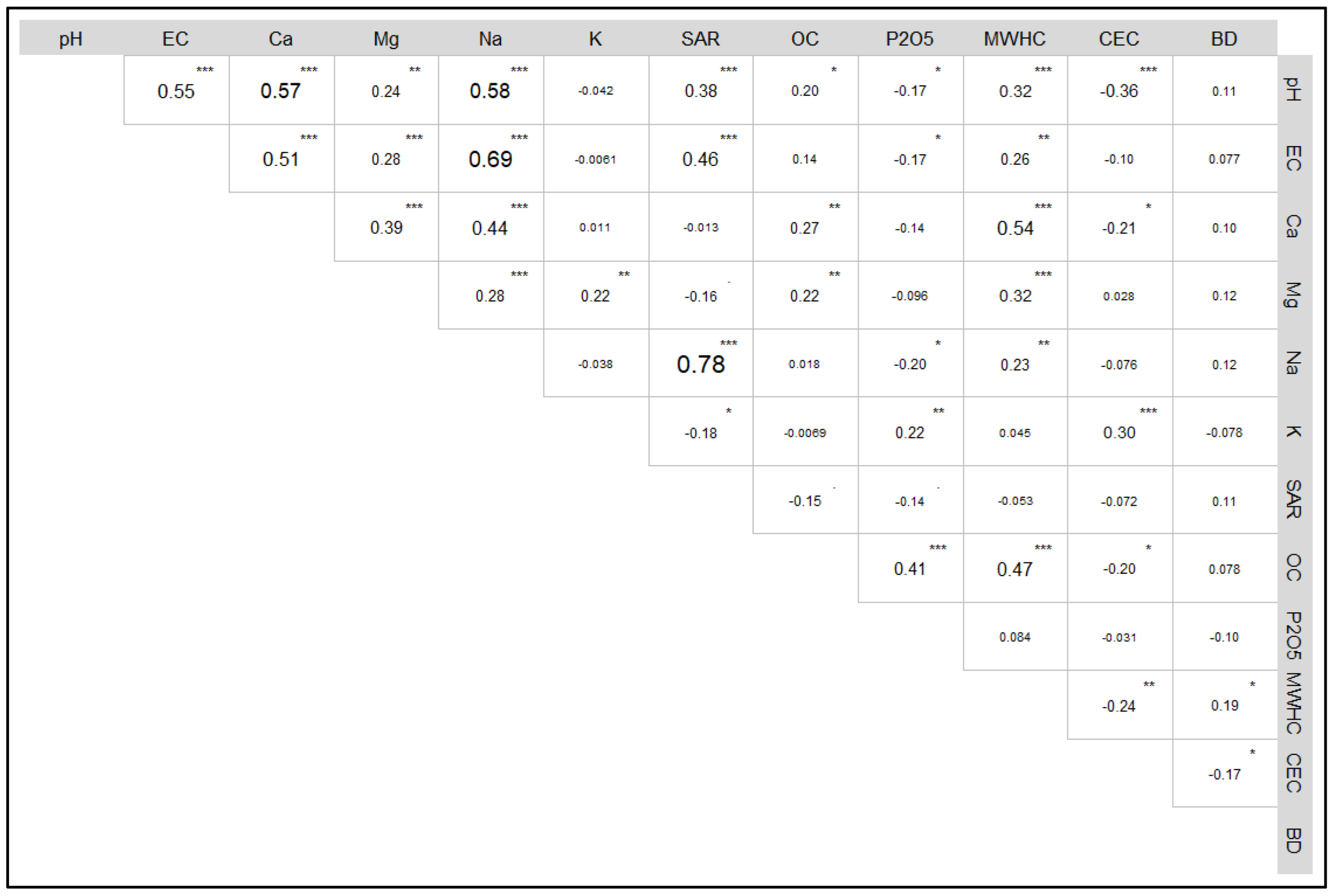
3.2. Principal Component Analysis and MDS
3.3. Assessment of Soil Quality Index (SQI)
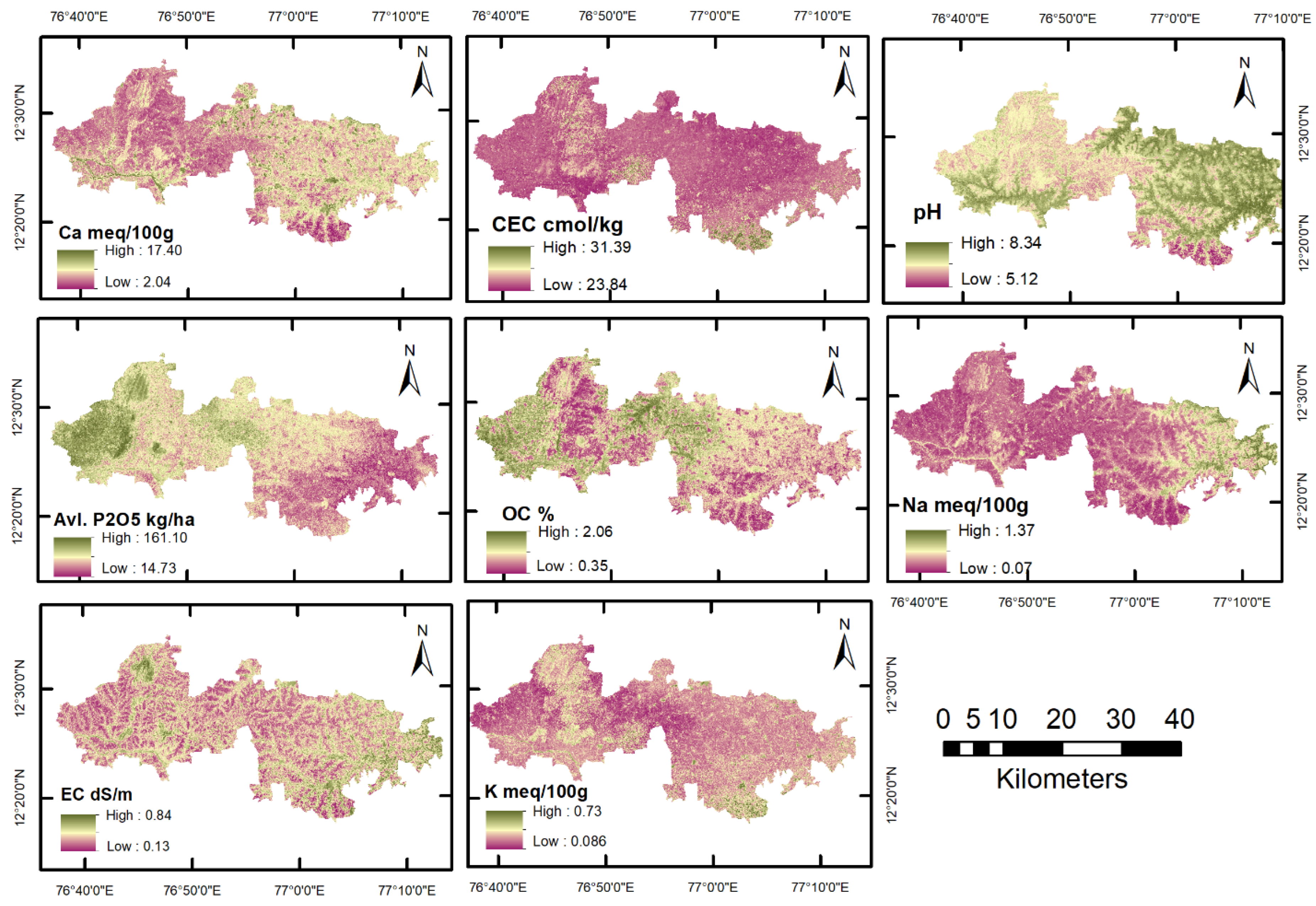
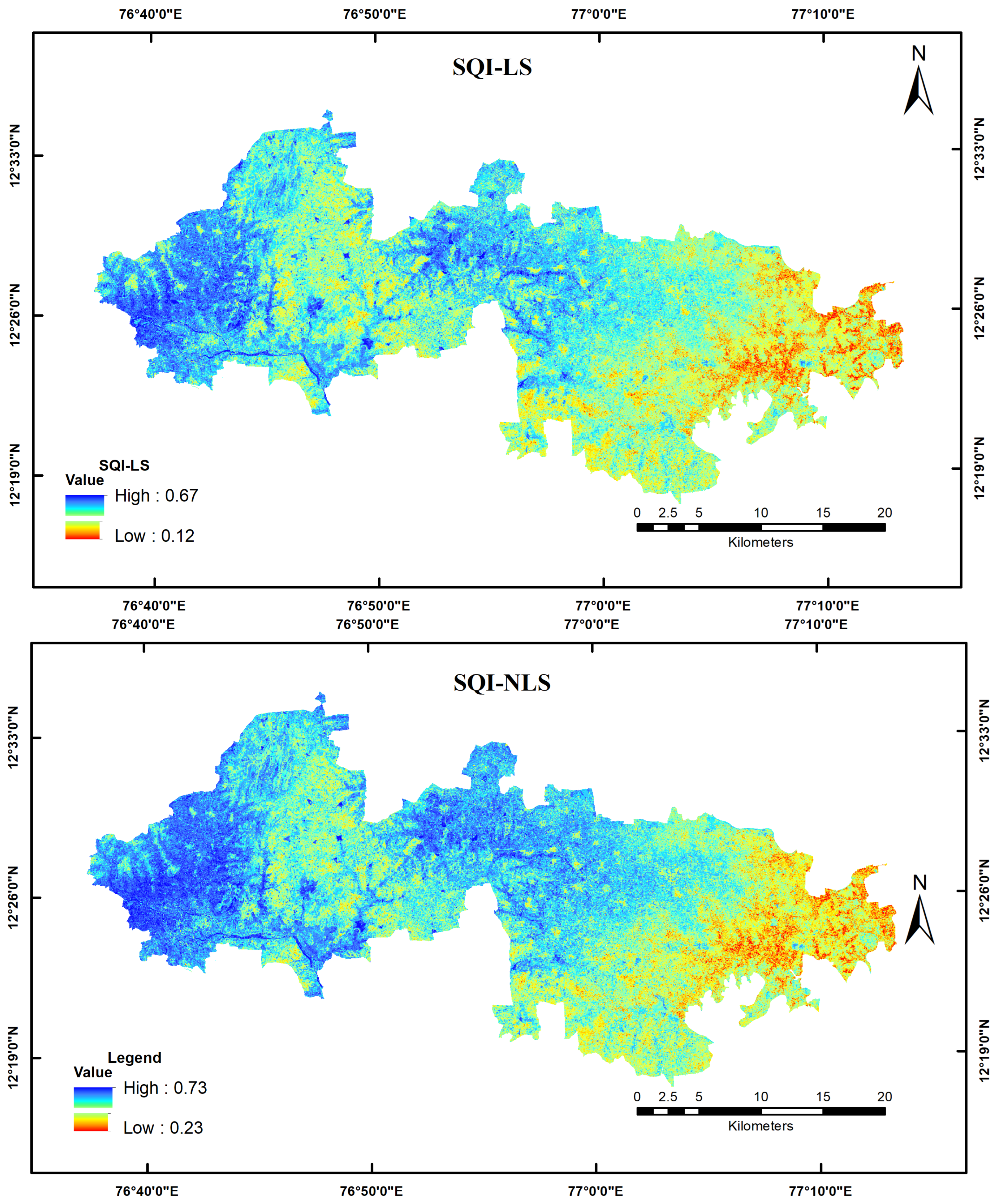
3.4. Spatial Assessment of SQI
4. Discussion
4.1. PCA, Scoring, and SQI Assessment
4.2. Spatial Assessment of Soil Properties and Soil Quality
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sánchez-Navarro, A.; Gil-Vázquez, J.M.; Delgado-Iniesta, M.J.; Marín-Sanleandro, P.; Blanco-Bernardeau, A.; Ortiz-Silla, R. Establishing an Index and Identification of Limiting Parameters for Characterizing Soil Quality in Mediterranean Ecosystems. Catena 2015, 131, 35–45. [Google Scholar] [CrossRef]
- Zhu, W.; Pan, Y.; He, H.; Wang, L.; Mou, M.; Liu, J. A Changing-Weight Filter Method for Reconstructing a High-Quality NDVI Time Series to Preserve the Integrity of Vegetation Phenology. IEEE Trans. Geosci. Remote Sens. 2012, 50, 1085–1094. [Google Scholar] [CrossRef]
- Weis, T. The Accelerating Biophysical Contradictions of Industrial Capitalist Agriculture. J. Agrar. Change 2010, 10, 315–341. [Google Scholar] [CrossRef]
- Power, A.G. Ecosystem Services and Agriculture: Tradeoffs and Synergies. Philos. Trans. R. Soc. B Biol. Sci. 2010, 365, 2959–2971. [Google Scholar] [CrossRef] [PubMed]
- Lorenz, K.; Lal, R. Biochar Application to Soil for Climate Change Mitigation by Soil Organic Carbon Sequestration. Wiley Online Libr. 2014, 177, 651–670. [Google Scholar] [CrossRef]
- Singh, D.K.; Jaiswal, C.S.; Reddy, K.S.; Singh, R.M.; Bhandarkar, D.M. Optimal Cropping Pattern in a Canal Command Area. Agric. Water Manag. 2001, 50, 1–8. [Google Scholar] [CrossRef]
- Yi, J.; Li, H.; Zhao, Y.; Shao, M.; Zhang, H.; Liu, M. Assessing Soil Water Balance to Optimize Irrigation Schedules of Flood-Irrigated Maize Fields with Different Cultivation Histories in the Arid Region. Agric. Water Manag. 2022, 265, 107543. [Google Scholar] [CrossRef]
- Kalambukattu, J.G.; Johns, B.; Kumar, S.; Raj, A.D.; Ellur, R. Temporal Remote Sensing Based Soil Salinity Mapping in Indo-Gangetic Plain Employing Machine-Learning Techniques. Proc. Indian Natl. Sci. Acad. 2023, 89, 290–305. [Google Scholar] [CrossRef]
- Das, A.; Lal, R.; Patel, D.P.; Idapuganti, R.G.; Layek, J.; Ngachan, S.V.; Ghosh, P.K.; Bordoloi, J.; Kumar, M. Effects of Tillage and Biomass on Soil Quality and Productivity of Lowland Rice Cultivation by Small Scale Farmers in North Eastern India. Soil Tillage Res. 2014, 143, 50–58. [Google Scholar] [CrossRef]
- Zhang, T.; Li, H.; Yan, T.; Shaheen, S.M.; Niu, Y.; Xie, S.; Zhang, Y.; Abdelrahman, H.; Ali, E.F.; Bolan, N.S.; et al. Organic Matter Stabilization and Phosphorus Activation during Vegetable Waste Composting: Multivariate and Multiscale Investigation. Sci. Total Environ. 2023, 891, 164608. [Google Scholar] [CrossRef]
- He, M.Y.; Dong, J.B.; Jin, Z.; Liu, C.Y.; Xiao, J.; Zhang, F.; Sun, H.; Zhao, Z.Q.; Gou, L.F.; Liu, W.G.; et al. Pedogenic Processes in Loess-Paleosol Sediments: Clues from Li Isotopes of Leachate in Luochuan Loess. Geochim. Cosmochim. Acta 2021, 299, 151–162. [Google Scholar] [CrossRef]
- Andrews, S.S.; Mitchell, J.P.; Mancinelli, R.; Karlen, D.L.; Hartz, T.K.; Horwath, W.R.; Pettygrove, G.S.; Scow, K.M.; Munk, D.S. On-Farm Assessment of Soil Quality in California’s Central Valley. Agron. J. 2002, 94, 12–23. [Google Scholar] [CrossRef]
- Adelana, A.; Aduramigba-Modupe, V.; Oke, A.; Are, K.; Ojo, O.; Adeyolanu, O. Soil Quality Assessment under Different Long-Term Rice-Based Cropping Systems in a Tropical Dry Savanna Ecology of Northern Nigeria. Acta Ecol. Sin. 2022, 42, 312–321. [Google Scholar] [CrossRef]
- Maleki, S.; Zeraatpisheh, M.; Karimi, A.; Sareban, G.; Wang, L. Assessing Variation of Soil Quality in Agroecosystem in an Arid Environment Using Digital Soil Mapping. Agronomy 2022, 12, 578. [Google Scholar] [CrossRef]
- Mahajan, G.; Das, B.; Morajkar, S.; Desai, A.; Murgaokar, D.; Kulkarni, R.; Sale, R.; Patel, K. Soil Quality Assessment of Coastal Salt-Affected Acid Soils of India. Environ. Sci. Pollut. Res. 2020, 27, 26221–26238. [Google Scholar] [CrossRef]
- Rezaee, L.; Moosavi, A.A.; Davatgar, N.; Sepaskhah, A.R. Soil Quality Indices of Paddy Soils in Guilan Province of Northern Iran: Spatial Variability and Their Influential Parameters. Ecol. Indic. 2020, 117, 106566. [Google Scholar] [CrossRef]
- Cooper, P.J.M.; Dimes, J.; Rao, K.P.C.; Shapiro, B.; Shiferaw, B.; Twomlow, S. Coping Better with Current Climatic Variability in the Rain-Fed Farming Systems of Sub-Saharan Africa: An Essential First Step in Adapting to Future Climate Change? Agric. Ecosyst. Environ. 2008, 126, 24–35. [Google Scholar] [CrossRef]
- Shiferaw, B.A.; Okello, J.; Reddy, R.V. Adoption and Adaptation of Natural Resource Management Innovations in Smallholder Agriculture: Reflections on Key Lessons and Best Practices. Environ. Dev. Sustain. 2009, 11, 601–619. [Google Scholar] [CrossRef]
- Nabiollahi, K.; Taghizadeh-Mehrjardi, R.; Eskandari, S. Assessing and Monitoring the Soil Quality of Forested and Agricultural Areas Using Soil-Quality Indices and Digital Soil-Mapping in a Semi-Arid Environment. Arch. Agron. Soil Sci. 2017, 64, 696–707. [Google Scholar] [CrossRef]
- Zhang, T.; Song, B.; Han, G.; Zhao, H.; Hu, Q.; Zhao, Y.; Liu, H. Effects of Coastal Wetland Reclamation on Soil Organic Carbon, Total Nitrogen, and Total Phosphorus in China: A Meta-Analysis. Land Degrad. Dev. 2023, 34, 3340–3349. [Google Scholar] [CrossRef]
- McBratney, A.B.; Mendonça Santos, M.L.; Minasny, B. On Digital Soil Mapping. Geoderma 2003, 117, 3–52. [Google Scholar] [CrossRef]
- Parent, E.J.; Parent, S.É.; Parent, L.E. Determining Soil Particle-Size Distribution from Infrared Spectra Using Machine Learning Predictions: Methodology and Modeling. PLoS ONE 2021, 16, e0233242. [Google Scholar] [CrossRef] [PubMed]
- Choudhury, B.U.; Mandal, S. Indexing Soil Properties through Constructing Minimum Datasets for Soil Quality Assessment of Surface and Profile Soils of Intermontane Valley (Barak, North East India). Ecol. Indic. 2021, 123, 107369. [Google Scholar] [CrossRef]
- Rosemary, F.; Vitharana, U.W.A.; Indraratne, S.P.; Weerasooriya, R.; Mishra, U. Exploring the Spatial Variability of Soil Properties in an Alfisol Soil Catena. Catena 2017, 150, 53–61. [Google Scholar] [CrossRef]
- Dharumarajan, S.; Kalaiselvi, B.; Suputhra, A.; Lalitha, M.; Vasundhara, R.; Kumar, K.A.; Nair, K.M.; Hegde, R.; Singh, S.K.; Lagacherie, P. Digital Soil Mapping of Soil Organic Carbon Stocks in Western Ghats, South India. Geoderma Reg. 2021, 25, e00387. [Google Scholar] [CrossRef]
- Mitran, T.; Mishra, U.; Lal, R.; Ravisankar, T.; Sreenivas, K. Spatial Distribution of Soil Carbon Stocks in a Semi-Arid Region of India. Geoderma Reg. 2018, 15, e00192. [Google Scholar] [CrossRef]
- Peng, Y.; Zhao, L.; Hu, Y.; Wang, G.; Wang, L.; Liu, Z. Prediction of Soil Nutrient Contents Using Visible and Near-Infrared Reflectance Spectroscopy. ISPRS Int. J. Geo-Inf. 2019, 8, 437. [Google Scholar] [CrossRef]
- Mamehpour, N.; Rezapour, S.; Ghaemian, N. Quantitative Assessment of Soil Quality Indices for Urban Croplands in a Calcareous. Geoderma 2021, 382, 114781. [Google Scholar] [CrossRef]
- Astle, W.L.; Webster, R.; Lawrance, C.J. Land Classification for Management Planning in the Luangwa Valley of Zambia. J. Appl. Ecol. 1969, 6, 143. [Google Scholar] [CrossRef]
- Jiang, H.; Rusuli, Y.; Amuti, T.; He, Q. Quantitative Assessment of Soil Salinity Using Multi-Source Remote Sensing Data Based on the Support Vector Machine and Artificial Neural Network. Int. J. Remote Sens. 2019, 40, 284–306. [Google Scholar] [CrossRef]
- Tiwari, S.K.; Saha, S.K.; Kumar, S. Prediction Modeling and Mapping of Soil Carbon Content Using Artificial Neural Network, Hyperspectral Satellite Data and Field Spectroscopy. Adv. Remote Sens. 2015, 04, 63–72. [Google Scholar] [CrossRef]
- Were, K.; Bui, D.T.; Dick, Ø.B.; Singh, B.R. A Comparative Assessment of Support Vector Regression, Artificial Neural Networks, and Random Forests for Predicting and Mapping Soil Organic Carbon Stocks across an Afromontane Landscape. Ecol. Indic. 2015, 52, 394–403. [Google Scholar] [CrossRef]
- Hogland, J.; Billor, N.; Anderson, N. Comparison of Standard Maximum Likelihood Classification and Polytomous Logistic Regression Used in Remote Sensing. Eur. J. Remote Sens. 2013, 46, 623–640. [Google Scholar] [CrossRef]
- Lenka, N.K.; Meena, B.P.; Lal, R.; Khandagle, A.; Lenka, S.; Shirale, A.O. Comparing Four Indexing Approaches to Define Soil Quality in an Intensively Cropped Region of Northern India. Front. Environ. Sci. 2022, 10, 865473. [Google Scholar] [CrossRef]
- Lal, R. World reference base for soil resources. In Encyclopedia of Soil Science—Two-Volume Set; CRC Press: Boca Raton, FL, USA, 2005; pp. 1918–1923. [Google Scholar] [CrossRef]
- Meena, R.S.; Natarajan, A.; Hegde, R.; Dhanorkar, B.A.; Koyal, A.; Naidu, L.G.K. Characterization and Classification of Upland Soils of Chikkarsinkere Hobli, Maddur Taluk, Mandya District of Karnataka. Agropedology 2014, 25, 154–160. [Google Scholar]
- Jackson, M.L. Aluminum Bonding in Soils: A Unifying Principle in Soil Science. Soil Sci. Soc. Am. J. 1963, 27, 1–10. [Google Scholar] [CrossRef]
- Pansu, M.; Gautheyrou, J. Exchangeable cations. In Handbook of Soil Analysis; Springer: Berlin/Heidelberg, Germany, 2006; pp. 667–676. [Google Scholar] [CrossRef]
- Olsen, S.R.; Watanabe, F.S. A Method to Determine a Phosphorus Adsorption Maximum of Soils as Measured by the Langmuir Isotherm. Soil Sci. Soc. Am. J. 1957, 21, 144–149. [Google Scholar] [CrossRef]
- Walkley, A. A Critical Examination of A Rapid Method For Determining Organic Carbon In Soils—Effect of Variations In Digestion Conditions and of Inorganic Soil Constituents. Soil Sci. 1947, 64, 251–264. [Google Scholar] [CrossRef]
- Karlen, D.L.; Mausbach, M.J.; Doran, J.W.; Cline, R.G.; Harris, R.F.; Schuman, G.E. Soil Quality: A Concept, Definition, and Framework for Evaluation (A Guest Editorial). Soil Sci. Soc. Am. J. 1997, 61, 4–10. [Google Scholar] [CrossRef]
- Carter, M.R. Soil Quality for Sustainable Land Management. Agron. J. 2002, 94, 38–47. [Google Scholar] [CrossRef]
- Kibblewhite, M.G.; Ritz, K.; Swift, M.J. Soil Health in Agricultural Systems. Philos. Trans. R. Soc. B Biol. Sci. 2008, 363, 685–701. [Google Scholar] [CrossRef]
- Hegde, R.; Natarajan, A.; Meena, R.S.; Niranjana, K.V.; Thayalan, S.; Singh, S.K. Status of Soil Degradation in an Irrigated Command Area in Chikkarasinakere Hobli, Mandya District, Karnataka. Curr. Sci. 2015, 108, 1501–1511. [Google Scholar]
- Wander, M.M.; Bollero, G.A. Soil Quality Assessment of Tillage Impacts in Illinois. Soil Sci. Soc. Am. J. 1999, 63, 961–971. [Google Scholar] [CrossRef]
- Govaerts, B.; Sayre, K.D.; Deckers, J. A Minimum Data Set for Soil Quality Assessment of Wheat and Maize Cropping in the Highlands of Mexico. Soil Tillage Res. 2006, 87, 163–174. [Google Scholar] [CrossRef]
- Andrews, S.S.; Karlen, D.L.; Cambardella, C.A. The Soil Management Assessment Framework. Soil Sci. Soc. Am. J. 2004, 68, 1945–1962. [Google Scholar] [CrossRef]
- Lamichhane, S.; Kumar, L.; Wilson, B. Digital Soil Mapping Algorithms and Covariates for Soil Organic Carbon Mapping and Their Implications: A Review. Geoderma 2019, 352, 395–413. [Google Scholar] [CrossRef]
- Mukherjee, A.; Lal, R. Comparison of Soil Quality Index Using Three Methods. PLoS ONE 2014, 9, e105981. [Google Scholar] [CrossRef] [PubMed]
- Biswas, S.; Hazra, G.C.; Purakayastha, T.J.; Saha, N.; Mitran, T.; Singha Roy, S.; Basak, N.; Mandal, B. Establishment of Critical Limits of Indicators and Indices of Soil Quality in Rice-Rice Cropping Systems under Different Soil Orders. Geoderma 2017, 292, 34–48. [Google Scholar] [CrossRef]
- Nabiollahi, K.; Taghizadeh-Mehrjardi, R.; Kerry, R.; Moradian, S. Assessment of Soil Quality Indices for Salt-Affected Agricultural Land in Kurdistan Province, Iran. Ecol. Indic. 2017, 83, 482–494. [Google Scholar] [CrossRef]
- Zornoza, R.; Mataix-Solera, J.; Guerrero, C.; Arcenegui, V.; Mataix-Beneyto, J.; Gómez, I. Validating the Effectiveness and Sensitivity of Two Soil Quality Indices Based on Natural Forest Soils under Mediterranean Conditions. Soil Biol. Biochem. 2008, 40, 2079–2087. [Google Scholar] [CrossRef]
- Dharumarajan, S.; Hegde, R.; Singh, S.K. Spatial Prediction of Major Soil Properties Using Random Forest Techniques—A Case Study in Semi-Arid Tropics of South India. Geoderma Reg. 2017, 10, 154–162. [Google Scholar] [CrossRef]
- Khanal, S.; Fulton, J.; Klopfenstein, A.; Douridas, N. Integration of High Resolution Remotely Sensed Data and Machine Learning Techniques for Spatial Prediction of Soil Properties and Corn Yield. Comput. Electron. Agric. 2018, 153, 213–225. [Google Scholar] [CrossRef]
- Guo, L.; Linderman, M.; Shi, T.; Chen, Y.; Duan, L.; Zhang, H. Exploring the Sensitivity of Sampling Density in Digital Mapping of Soil Organic Carbon and Its Application in Soil Sampling. Remote Sens. 2018, 10, 888. [Google Scholar] [CrossRef]
- Zeraatpisheh, M.; Ayoubi, S.; Jafari, A.; Tajik, S.; Finke, P. Digital Mapping of Soil Properties Using Multiple Machine Learning in a Semi-Arid Region, Central Iran. Geoderma 2019, 338, 445–452. [Google Scholar] [CrossRef]
- Sreenivas, K.; Dadhwal, V.K.; Kumar, S.; Harsha, G.S.; Mitran, T.; Sujatha, G.; Rama, G.J.; Fyzee, M.A.; Ravisankar, T. Digital Mapping of Soil Organic and Inorganic Carbon Status in India. Geoderma 2016, 269, 160–173. [Google Scholar] [CrossRef]
- Fick, S.E.; Hijmans, R.J. WorldClim 2: New 1-Km Spatial Resolution Climate Surfaces for Global Land Areas. Int. J. Climatol. 2017, 37, 4302–4315. [Google Scholar] [CrossRef]
- Li, J.; Heap, A.D. Spatial Interpolation Methods Applied in the Environmental Sciences: A Review. Environ. Model. Softw. 2014, 53, 173–189. [Google Scholar] [CrossRef]
- Carter, M.R.; Gregorich, E.G.; Anderson, D.W.; Doran, J.W.; Janzen, H.H.; Pierce, F.J. Concepts of Soil Quality and Their Significance. Dev. Soil Sci. 1997, 25, 1–19. [Google Scholar] [CrossRef]
- Sheidai Karkaj, E.; Sepehry, A.; Barani, H.; Motamedi, J.; Shahbazi, F. Establishing a Suitable Soil Quality Index for Semi-Arid Rangeland Ecosystems in Northwest of Iran. J. Soil Sci. Plant Nutr. 2019, 19, 648–658. [Google Scholar] [CrossRef]
- Sims, J.T. Soil PH Effects on the Distribution and Plant Availability of Manganese, Copper, and Zinc. Soil Sci. Soc. Am. J. 1986, 50, 367–373. [Google Scholar] [CrossRef]
- Weaver, S.E.; Hamill, A.S. Effects of Soil PH on Competitive Ability and Leaf Nutrient Content of Corn (Zea Mays L.) and Three Weed Species. Weed Sci. 1985, 33, 447–451. [Google Scholar] [CrossRef]
- Rhoades, J.D.; Manteghi, N.A.; Shouse, P.J.; Alves, W.J. Soil Electrical Conductivity and Soil Salinity: New Formulations and Calibrations. Soil Sci. Soc. Am. J. 1989, 53, 433–439. [Google Scholar] [CrossRef]
- Fan, Y.; Zhang, Y.; Kalkhajeh, Y.K.; Hu, W.; Tian, K.; Yu, D.; Huang, B. Effective Indicators and Drivers of Soil Organic Matter in Intensive Orchard Production Systems. Soil Tillage Res. 2024, 238, 105999. [Google Scholar] [CrossRef]
- Dexter, A.R.; Richard, G.; Arrouays, D.; Czyz, E.A.; Jolivet, C.; Duval, O. Complexed Organic Matter Controls Soil Physical Properties. Geoderma 2008, 144, 620–627. [Google Scholar] [CrossRef]
- Hemmati, S.; Yaghmaeian, N.; Farhangi, M.B.; Sabouri, A. Soil Quality Assessment of Paddy Fields (in Northern Iran) with Different Productivities: Establishing the Critical Limits of Minimum Data Set Indicators. Environ. Sci. Pollut. Res. 2023, 30, 10286–10296. [Google Scholar] [CrossRef] [PubMed]
- Smith, J.L.; Doran, J.W. Measurement and Use of PH and Electrical Conductivity for Soil Quality Analysis. Methods Assess. Soil Qual. 2015, 169–185. [Google Scholar] [CrossRef]
- Ismayilov, A.I.; Mamedov, A.I.; Fujimaki, H.; Tsunekawa, A.; Levy, G.J. Soil Salinity Type Effects on the Relationship between the Electrical Conductivity and Salt Content for 1:5 Soil-to-Water Extract. Sustainability 2021, 13, 3395. [Google Scholar] [CrossRef]
- Subbarao, G.V.; Ito, O.; Berry, W.L.; Wheeler, R.M. Sodium—A Functional Plant Nutrient. Crit. Rev. Plant Sci. 2003, 22, 391–416. [Google Scholar] [CrossRef]
- Rengasamy, P.; Olsson, K.A. Sodicity and Soil Structure. Soil Res. 1991, 29, 935–952. [Google Scholar] [CrossRef]
- Naimi, S.; Ayoubi, S.; Demattê, J.A.M.; Zeraatpisheh, M.; Amorim, M.T.A.; Mello, F.A.d.O. Spatial Prediction of Soil Surface Properties in an Arid Region Using Synthetic Soil Image and Machine Learning. Geocarto Int. 2022, 37, 8230–8253. [Google Scholar] [CrossRef]
- Meroni, M.; Rossini, M.; Guanter, L.; Alonso, L.; Rascher, U.; Colombo, R.; Moreno, J. Remote Sensing of Environment Remote Sensing of Solar-Induced Chlorophyll Fl Uorescence: Review of Methods and Applications. Remote Sens. Environ. 2009, 113, 2037–2051. [Google Scholar] [CrossRef]
- Nawar, S.; Corstanje, R.; Halcro, G.; Mulla, D.; Mouazen, A.M. Delineation of Soil Management Zones for Variable-Rate Fertilization: A Review, 1st ed.; Elsevier: Amsterdam, The Netherlands, 2017; Volume 143. [Google Scholar]
- Tekin, A.B. Variable Rate Fertilizer Application in Turkish Wheat Agriculture: Economic Assessment. Afr. J. Agric.Res. 2010, 5, 647–652. [Google Scholar] [CrossRef]
- Rajath, E.; Anush Kumar, K.; Setia, R.; Taneja, S.; Galohda, A.; Ansari, J.; Gupta, S.K.; Nigam, R.; Pateriya, B. Remote and Proximal Sensing for Optimising Input Use Efficiency for Sustainable Agriculture. Input Use Effic. Food Environ. Secur. 2022, 513–540. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, Y.F.; Wu, W.H. Potassium and Phosphorus Transport and Signaling in Plants. J. Integr. Plant Biol. 2021, 63, 34–52. [Google Scholar] [CrossRef] [PubMed]
- Surendran, U.; Ramesh, V.; Jayakumar, M.; Marimuthu, S.; Sridevi, G. Improved Sugarcane Productivity with Tillage and Trash Management Practices in Semi Arid Tropical Agro Ecosystem in India. Soil Tillage Res. 2016, 158, 10–21. [Google Scholar] [CrossRef]
- Yadav, R.L.; Prasad, S.R.; Singh, R.; Srivastava, V.K. Recycling Sugarcane Trash to Conserve Soil Organic Carbon for Sustaining Yields of Successive Ratoon Crops in Sugarcane. Bioresour. Technol. 1994, 49, 231–235. [Google Scholar] [CrossRef]
- Ketterings, Q.M.; Gami, S.K.; Mathur, R.R.; Woods, M. A Simple Method for Estimating Effective Cation Exchange Capacity, Cation Saturation Ratios, and Sulfur across a Wide Range of Soils. Soil Sci. 2014, 179, 230–236. [Google Scholar] [CrossRef]
- Nabiollahi, K.; Golmohamadi, F.; Taghizadeh-Mehrjardi, R.; Kerry, R.; Davari, M. Assessing the Effects of Slope Gradient and Land Use Change on Soil Quality Degradation through Digital Mapping of Soil Quality Indices and Soil Loss Rate. Geoderma 2018, 318, 16–28. [Google Scholar] [CrossRef]
- Lagacherie, P.; Arrouays, D.; Bourennane, H.; Gomez, C.; Nkuba-Kasanda, L. Analysing the Impact of Soil Spatial Sampling on the Performances of Digital Soil Mapping Models and Their Evaluation: A Numerical Experiment on Quantile Random Forest Using Clay Contents Obtained from Vis-NIR-SWIR Hyperspectral Imagery. Geoderma 2020, 375, 114503. [Google Scholar] [CrossRef]





| Auxiliary Data | Land Surface Parameters | Definition | Reference/ Source and Resolution | Soil Forming Factors |
|---|---|---|---|---|
| Terrain data | Aspect | The compass direction of the maximum rate of change | SRTM DEM (30 m) | R |
| Analytical hill shading | Distribution and characteristics of soils across the landscape. | R | ||
| Channel network base level | Represents the ultimate destination for water within the watershed | R | ||
| Channel network distance | Provides information about the connectivity and flow dynamics of river systems within a watershed | R | ||
| Closed depression | Geological processes such as erosion, dissolution of soluble rocks like limestone or gypsum, or tectonic activity. | R | ||
| Convergence index | Quantifies the tendency of surface water to accumulate at a specific location | R | ||
| LS factor | Slope length factor | R | ||
| Plan curvature | Plan curvature is perpendicular to the slope | R | ||
| Profile curvature | Profile curvature is parallel to the direction of the maximum slope | R | ||
| Relative slope position | Spatial distribution of landforms and their influence on various environmental processes | R | ||
| Slope | Average gradient above flow path | |||
| Topographic wetness index | TWI is made up of three components: flow width, total catchment area, and slope for each pixel | R, Cl | ||
| Valley depth | Relative position of the valley (meters) | R | ||
| Remote sensing data | SI_1 | Landsat-9 (30 m) | PM, S | |
| SI_2 | PM, S | |||
| SI_3 | PM, S | |||
| SI_4 | PM, S | |||
| SI_5 | PM, S | |||
| SI_7 | PM, S | |||
| BI | PM, S | |||
| CRSI | O | |||
| NDSI | O | |||
| RI | O | |||
| SAVI | O, S | |||
| VSSI | 2 ∗ Green − 5 ∗ (Red + NIR) | O, S | ||
| Long-term vegetation indices | EVI | MODIS (250 m) | O | |
| NDVI | O | |||
| Climate | WC_Mean_Precp | Mean annual precipitation | WorldClim (1 Km) | C |
| WC_Mean_Temp | Mean annual temperature | C |
| Index | Soil Quality Grades | ||||
|---|---|---|---|---|---|
| I (Very High) | II (High) | III (Moderate) | IV (Low) | V (Very Low) | |
| SQI-LS | >0.547 | 0.49–0.547 | 0.433–0.49 | 0.381–0.433 | <0.381 |
| SQI-NLS | >0.705 | 0.581–0.643 | 0.519–0.581 | 0.457–0.519 | <0.457 |
| pH | EC (dSm−1) | Ca | Mg | Na | K | SAR | OC (%) | Avl. P2O5 (kg ha−1) | MWHC (%) | CEC cmol/kg | BD (g cm−3) | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (meq/100 g) | ||||||||||||
| Max | 9.05 | 1.39 | 20.70 | 17.70 | 1.99 | 1.18 | 19.18 | 2.54 | 233.15 | 65.40 | 33.3 | 1.4 |
| Min | 4.46 | 0.06 | 0.80 | 0.40 | 0.03 | 0.04 | 0.36 | 0.15 | 2.09 | 17.60 | 22.9 | 1.33 |
| Mean | 6.90 | 0.36 | 8.18 | 4.82 | 0.36 | 0.21 | 2.83 | 0.97 | 62.08 | 41.55 | 25.83 | 1.36 |
| Std Dev | 0.96 | 0.24 | 4.35 | 3.06 | 0.33 | 0.15 | 2.39 | 0.52 | 58.06 | 7.37 | 16.07 | 1.38 |
| CV (%) | 13.90 | 65.77 | 53.27 | 63.45 | 89.48 | 72.89 | 84.42 | 53.48 | 93.52 | 17.73 | 6.22 | 1.01 |
| Skewness | −0.46 | 1.65 | 0.58 | 1.17 | 2.42 | 2.79 | 3.66 | 0.69 | 1.19 | −0.13 | 1.71 | −0.19 |
| Kurtosis | −0.47 | 3.38 | −0.35 | 1.58 | 7.28 | 12.54 | 19.30 | 0.02 | 0.40 | 0.73 | 5.26 | −0.07 |
| PC1 | PC2 | PC3 | PC4 | Communalities | Weightage | |
|---|---|---|---|---|---|---|
| pH | 0.417 | 0.033 | 0.168 | 0.141 | 0.223 | 0.084 |
| EC | 0.402 | 0.093 | 0.065 | 0.288 | 0.258 | 0.098 |
| Ca | 0.412 | 0.133 | 0.082 | 0.298 | 0.283 | 0.107 |
| Mg | 0.264 | 0.043 | 0.407 | 0.288 | ||
| Na | 0.406 | 0.339 | 0.015 | 0.211 | 0.324 | 0.123 |
| K | 0.022 | 0.124 | 0.533 | 0.331 | 0.409 | 0.155 |
| SAR | 0.293 | 0.418 | 0.211 | 0.381 | ||
| OC | 0.213 | 0.478 | 0.002 | 0.108 | 0.285 | 0.108 |
| Avl_P2O5 | 0.025 | 0.397 | 0.128 | 0.572 | 0.501 | 0.19 |
| MWHC | 0.318 | 0.352 | 0.086 | 0.178 | ||
| BD_cg/cm3 | 0.012 | 0.301 | 0.434 | 0.224 | ||
| CEC_cmol/kg | 0.165 | 0.255 | 0.505 | 0.078 | 0.353 | 0.134 |
| Eigenvalues | 3.2577 | 2.0704 | 1.4919 | 1.376 | ||
| Proportion of variance | 0.2715 | 0.1725 | 0.1243 | 0.1147 | ||
| Cumulative variance | 0.2715 | 0.444 | 0.5683 | 0.683 |
| Soil Property | R2 | Concordance | RMSE | Bias | ntree | mtry |
|---|---|---|---|---|---|---|
| pH | 0.31 | 0.36 | 0.80 | −0.03 | 280 | 8 |
| EC | 0.18 | 0.20 | 223 | 7.02 | 100 | 3 |
| SOC | 0.34 | 0.40 | 0.43 | 0.01 | 350 | 4 |
| Ca | 0.37 | 0.42 | 3.52 | 0.03 | 50 | 9 |
| Na | 0.24 | 0.32 | 0.27 | 0.01 | 48 | 9 |
| CEC | 0.33 | 0.36 | 12 | 0.18 | 500 | 9 |
| K | 0.09 | 0.05 | 0.14 | 0.01 | 1000 | 4 |
| Avl. P2O5 | 0.07 | 0.09 | 59 | 3.88 | 500 | 9 |
| Grade | SQI-LS | SQI-NLS |
|---|---|---|
| I (very high) | 17,121 (18.82) | 21,168 (23.27) |
| II (high) | 26,058 (28.64) | 26,130 (28.72) |
| III (moderate) | 26,105 (28.69) | 24,330 (26.74) |
| IV (low) | 17,131 (18.83) | 15,012 (16.50) |
| V (very low) | 4545 (4.99) | 4320 (4.74) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ellur, R.; Ankappa, A.M.; Dharumarajan, S.; Puttavenkategowda, T.; Nanjundegowda, T.M.; Sannegowda, P.S.; Pratap Mishra, A.; Đurin, B.; Dogančić, D. Soil Quality Assessment and Its Spatial Variability in an Intensively Cultivated Area in India. Land 2024, 13, 970. https://doi.org/10.3390/land13070970
Ellur R, Ankappa AM, Dharumarajan S, Puttavenkategowda T, Nanjundegowda TM, Sannegowda PS, Pratap Mishra A, Đurin B, Dogančić D. Soil Quality Assessment and Its Spatial Variability in an Intensively Cultivated Area in India. Land. 2024; 13(7):970. https://doi.org/10.3390/land13070970
Chicago/Turabian StyleEllur, Rajath, Ananthakumar Maddur Ankappa, Subramanian Dharumarajan, Thimmegowda Puttavenkategowda, Thimmegowda Matadadoddi Nanjundegowda, Prakash Salekoppal Sannegowda, Arun Pratap Mishra, Bojan Đurin, and Dragana Dogančić. 2024. "Soil Quality Assessment and Its Spatial Variability in an Intensively Cultivated Area in India" Land 13, no. 7: 970. https://doi.org/10.3390/land13070970







