Climate and Land Use Changes Impact the Future of European Amphibian Functional Diversity
Abstract
:1. Introduction
2. Materials and Methods
2.1. Species List and Occurrence Data
2.2. Functional Traits
2.3. Climate and Land Use Data
2.4. Ensemble Species Distribution Modelling
2.5. Analysis of Temporal Changes of Taxonomic and Functional Diversity
3. Results
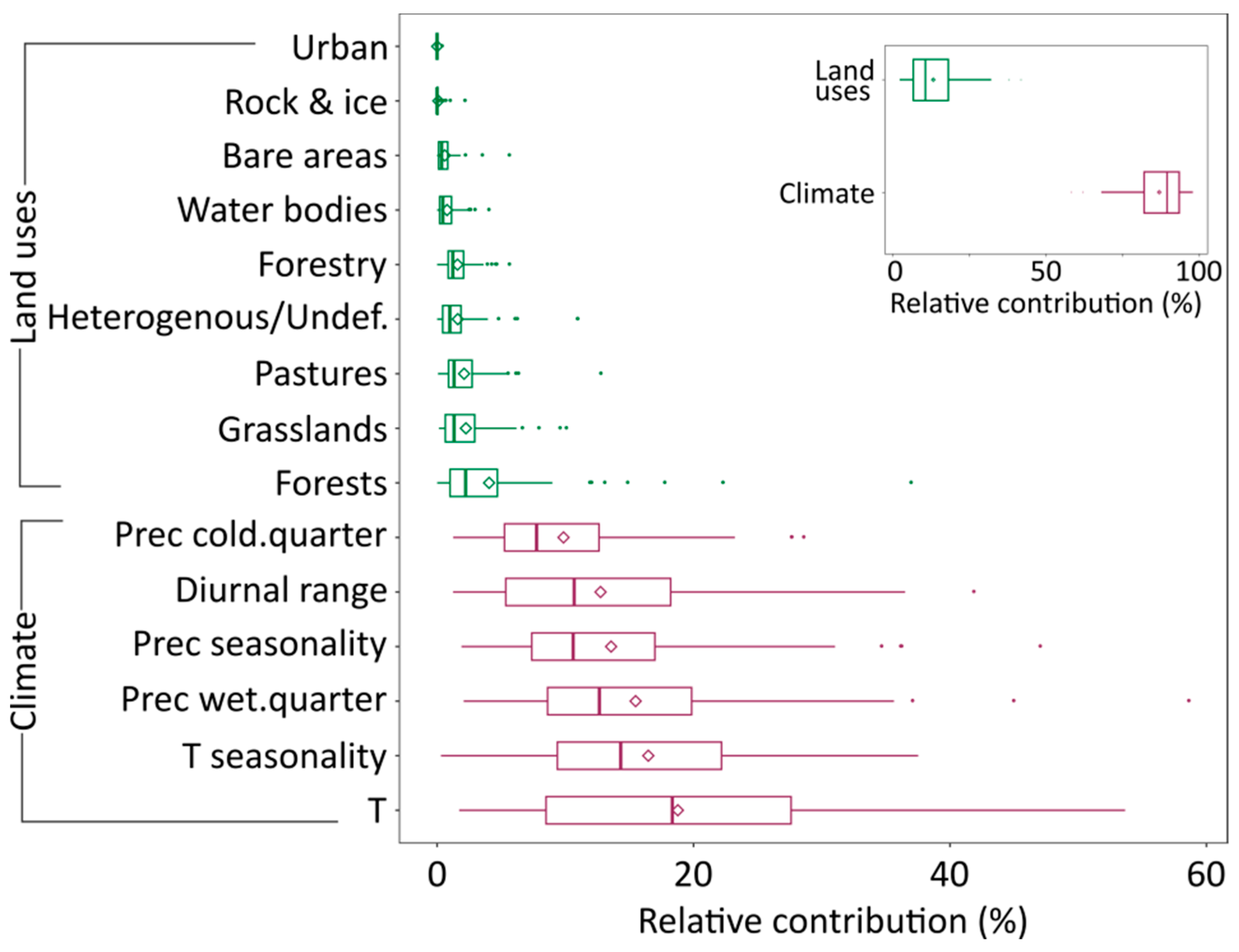
3.1. Contribution of Climate and Land Use to Projected Species Distributions
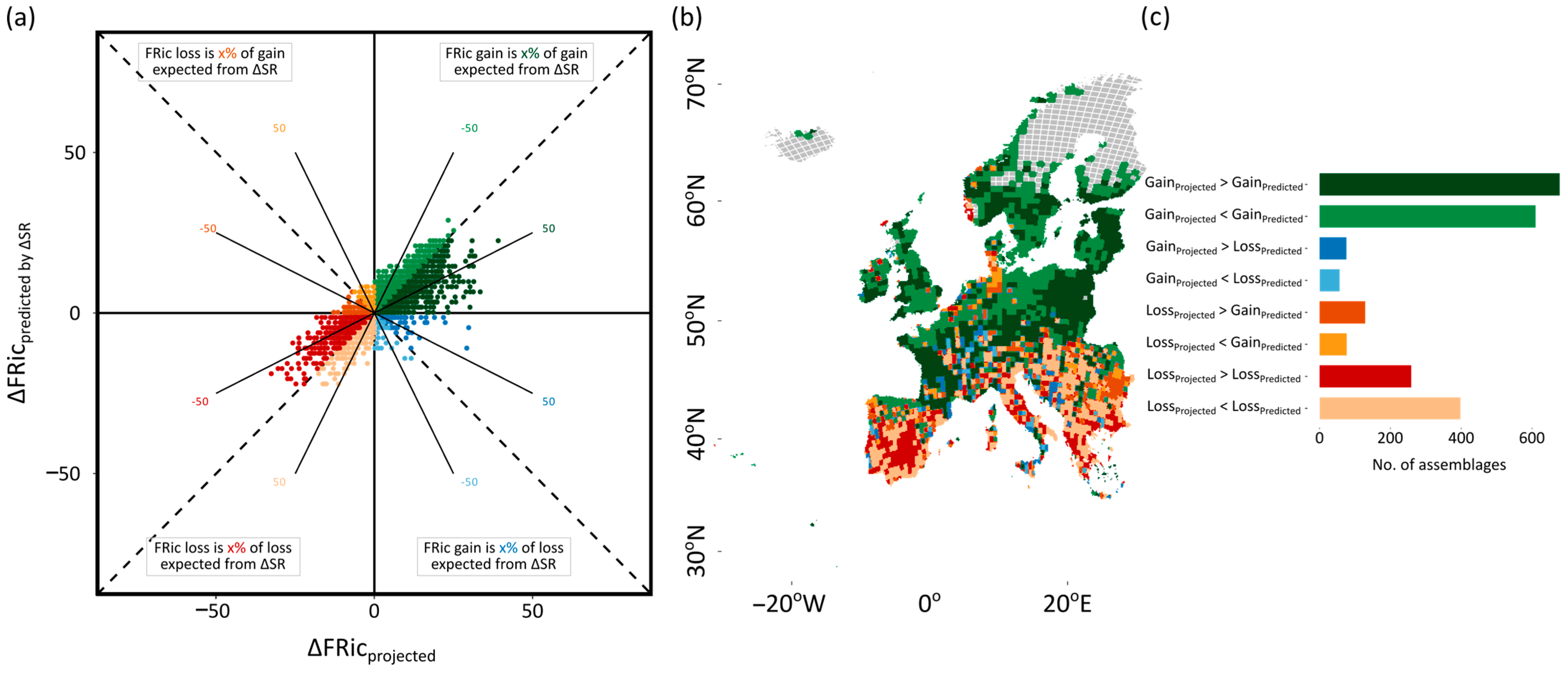
3.2. Temporal Differences in Taxonomic and Functional Diversity
3.3. The Influence of Species Richness on Functional Diversity
4. Discussion
4.1. Shifts in Species Richness and Functional Diversity: The Importance of Traits
4.2. Contribution of Climate and Land Use to Changes in Functional Diversity
4.3. Relationship between Species with Functional Richness
4.4. Limitations
5. Future Perspectives
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Morse, N.B.; Pellissier, P.A.; Cianciola, E.N.; Brereton, R.L.; Sullivan, M.M.; Shonka, N.K.; Wheeler, T.B.; McDowell, W.H. Novel ecosystems in the Anthropocene: A revision of the novel ecosystem concept for pragmatic applications. Ecol. Soc. 2014, 19. [Google Scholar] [CrossRef]
- Intergovernmental Panel on Climate Change. Climate Change 2021: The Physical Science Basis. In Contribution of Working Group I to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2021; p. 2391. [Google Scholar]
- Oliver, T.H.; Roy, D.B. The pitfalls of ecological forecasting. Biol. J. Linn. Soc. 2015, 115, 767–778. [Google Scholar] [CrossRef]
- Lenoir, J.; Bertrand, R.; Comte, L.; Bourgeaud, L.; Hattab, T.; Murienne, J.; Grenouillet, G. Species better track climate warming in the oceans than on land. Nat. Ecol. Evol. 2020, 4, 1044–1059. [Google Scholar] [CrossRef] [PubMed]
- Lenoir, J.; Svenning, J.C. Climate-related range shifts–a global multidimensional synthesis and new research directions. Ecography 2015, 38, 15–28. [Google Scholar] [CrossRef]
- Dale, V.H. The relationship between land-use change and climate change. Ecol. Appl. 1997, 7, 753–769. [Google Scholar] [CrossRef]
- Thuiller, W.; Lavorel, S.; Sykes, M.T.; Araújo, M.B. Using niche-based modelling to assess the impact of climate change on tree functional diversity in Europe. Divers. Distrib. 2006, 12, 49–60. [Google Scholar] [CrossRef]
- Buisson, L.; Grenouillet, G.; Villéger, S.; Canal, J.; Laffaille, P. Toward a loss of functional diversity in stream fish assemblages under climate change. Glob. Chang. Biol. 2013, 19, 387–400. [Google Scholar] [CrossRef]
- Gallagher, R.V.; Hughes, L.; Leishman, M.R. Species loss and gain in communities under future climate change: Consequences for functional diversity. Ecography 2013, 36, 531–540. [Google Scholar] [CrossRef]
- Del Toro, I.; Silva, R.R.; Ellison, A.M. Predicted impacts of climatic change on ant functional diversity and distributions in eastern N orth A merican forests. Divers. Distrib. 2015, 21, 781–791. [Google Scholar] [CrossRef]
- Benedetti, F.; Ayata, S.D.; Irisson, J.O.; Adloff, F.; Guilhaumon, F. Climate change may have minor impact on zooplankton functional diversity in the Mediterranean Sea. Divers. Distrib. 2019, 25, 568–581. [Google Scholar] [CrossRef]
- Bender, I.M.; Kissling, W.D.; Böhning-Gaese, K.; Hensen, I.; Kühn, I.; Nowak, L.; Töpfer, T.; Wiegand, T.; Dehling, D.M.; Schleuning, M. Projected impacts of climate change on functional diversity of frugivorous birds along a tropical elevational gradient. Sci. Rep. 2019, 9, 17708. [Google Scholar] [CrossRef] [PubMed]
- Jetz, W.; Wilcove, D.S.; Dobson, A.P. Projected impacts of climate and land-use change on the global diversity of birds. PLoS Biol. 2007, 5, e157. [Google Scholar] [CrossRef] [PubMed]
- Stewart, P.S.; Voskamp, A.; Santini, L.; Biber, M.F.; Devenish, A.J.; Hof, C.; Willis, S.G.; Tobias, J.A. Global impacts of climate change on avian functional diversity. Ecol. Lett. 2022, 25, 673–685. [Google Scholar] [CrossRef]
- Scherer, L.; Boom, H.A.; Barbarossa, V.; van Bodegom, P.M. Climate change threats to the global functional diversity of freshwater fish. Glob. Chang. Biol. 2023, 29, 3781–3793. [Google Scholar] [CrossRef]
- Walther, G.-R.; Post, E.; Convey, P.; Menzel, A.; Parmesan, C.; Beebee, T.J.; Fromentin, J.-M.; Hoegh-Guldberg, O.; Bairlein, F. Ecological responses to recent climate change. Nature 2002, 416, 389–395. [Google Scholar] [CrossRef]
- Buckley, L.B.; Hurlbert, A.H.; Jetz, W. Broad-scale ecological implications of ectothermy and endothermy in changing environments. Glob. Ecol. Biogeogr. 2012, 21, 873–885. [Google Scholar] [CrossRef]
- Ramalho, Q.; Vale, M.M.; Manes, S.; Diniz, P.; Malecha, A.; Prevedello, J.A. Evidence of stronger range shift response to ongoing climate change by ectotherms and high-latitude species. Biol. Conserv. 2023, 279, 109911. [Google Scholar] [CrossRef]
- Rodríguez, M.Á.; Belmontes, J.A.; Hawkins, B.A. Energy, water and large-scale patterns of reptile and amphibian species richness in Europe. Acta Oecologica 2005, 28, 65–70. [Google Scholar] [CrossRef]
- Currie, D.J. Energy and Large-Scale Patterns of Animal- and Plant-Species Richness. Am. Nat. 1991, 137, 27–49. [Google Scholar] [CrossRef]
- Rozen-Rechels, D.; Dupoué, A.; Lourdais, O.; Chamaillé-Jammes, S.; Meylan, S.; Clobert, J.; Le Galliard, J.F. When water interacts with temperature: Ecological and evolutionary implications of thermo-hydroregulation in terrestrial ectotherms. Ecol. Evol. 2019, 9, 10029–10043. [Google Scholar] [CrossRef]
- Bellard, C.; Bertelsmeier, C.; Leadley, P.; Thuiller, W.; Courchamp, F. Impacts of climate change on the future of biodiversity. Ecol. Lett. 2012, 15, 365–377. [Google Scholar] [CrossRef]
- Mouillot, D.; Villéger, S.; Scherer-Lorenzen, M.; Mason, N.W. Functional structure of biological communities predicts ecosystem multifunctionality. PLoS ONE 2011, 6, e17476. [Google Scholar] [CrossRef] [PubMed]
- Bregman, T.P.; Lees, A.C.; MacGregor, H.E.; Darski, B.; de Moura, N.G.; Aleixo, A.; Barlow, J.; Tobias, J.A. Using avian functional traits to assess the impact of land-cover change on ecosystem processes linked to resilience in tropical forests. Proc. R. Soc. B Biol. Sci. 2016, 283, 20161289. [Google Scholar] [CrossRef] [PubMed]
- Re:wild; Synchronicity Earth; IUCN SSC Amphibian Specialist Group. State of the World’s Amphibians: The Second Global Amphibian Assessment; Re:wild: Austin, TX, USA, 2023. [Google Scholar]
- Luedtke, J.A.; Chanson, J.; Neam, K.; Hobin, L.; Maciel, A.O.; Catenazzi, A.; Borzée, A.; Hamidy, A.; Aowphol, A.; Jean, A. Ongoing declines for the world’s amphibians in the face of emerging threats. Nature 2023, 622, 308–314. [Google Scholar] [CrossRef] [PubMed]
- Kingsford, R.T.; Basset, A.; Jackson, L. Wetlands: Conservation’s poor cousins. Aquat. Conserv. Mar. Freshw. Ecosyst. 2016, 26, 892–916. [Google Scholar] [CrossRef]
- Verhoeven, J.T. Wetlands in Europe: Perspectives for restoration of a lost paradise. Ecol. Eng. 2014, 66, 6–9. [Google Scholar] [CrossRef]
- Hocking, D.J.; Babbitt, K.J. Amphibian contributions to ecosystem services. Herpetol. Conserv. Biol. 2014, 9, 1–17. [Google Scholar]
- Frost, D. Amphibian Species of the World 6.2. Available online: https://amphibiansoftheworld.amnh.org/ (accessed on 1 November 2023).
- GBIF. GBIF Home Page. Available online: https://www.gbif.org (accessed on 15 March 2024).
- Chamberlain, S.; Barve, V.; Mcglinn, D.; Oldoni, D.; Desmet, P.; Geffert, L.; Ram, K. rgbif: Interface to the Global Biodiversity Information Facility API; Version 3.7.9. 2019. Available online: https://cran.r-project.org/web/packages/rgbif/index.html (accessed on 30 April 2024).
- Zizka, A.; Silvestro, D.; Andermann, T.; Azevedo, J.; Duarte Ritter, C.; Edler, D.; Farooq, H.; Herdean, A.; Ariza, M.; Scharn, R.; et al. CoordinateCleaner: Standardized cleaning of occurrence records from biological collection databases. Methods Ecol. Evol. 2019, 10, 744–751. [Google Scholar] [CrossRef]
- Speybroeck, J.; Beukema, W.; Dufresnes, C.; Fritz, U.; Jablonski, D.; Lymberakis, P.; Martínez-Solano, I.; Razzetti, E.; Vamberger, M.; Vences, M.; et al. Species list of the European herpetofauna—2020 update by the Taxonomic Committee of the Societas Europaea Herpetologica. Amphib. Reptil. 2020, 41, 139–189. [Google Scholar] [CrossRef]
- Bozkurt, E.; Tural, M.; Ulutaş, G.; Üzüm, N.; Olgun, K. Two new paedomorphic population records of the smooth newt, Lissotriton vulgaris schmidtleri (Raxworthy, 1988)(Urodela, Salamandridae), from Western Turkey. Russ. J. Herpetol. 2016, 23, 158–162. [Google Scholar]
- Sotiropoulos, K.; Moustakas, K.; Toli, E.-A. First record of facultative paedomorphosis in the Turkish smooth newt (Lissotriton schmidtleri) from Greece. Herpetol. Notes 2020, 13, 1041–1044. [Google Scholar]
- Güler, H.; Üzüm, N.; Olgun, K.; Avcı, A. Age and Body Size Comparison of the Turkish Smooth Newt, Lissotriton schmidtleri We (Raxworthy, 1988)(Amphibia: Urodela) Populations from Two Different Altitudes. Commagene J. Biol. 2023, 7, 24–30. [Google Scholar] [CrossRef]
- Furtula, M.; Ivanović, A.; Džukić, G.; Kalezić, M.L. Egg size variation in crested newts from the western Balkans (Caudata, Salamandridae, Triturus cristatus superspecies). Zool. Stud. 2008, 47, 585–590. [Google Scholar]
- Furtula, M.; Todorović, B.; Simić, V.; Ivanović, A. Interspecific differences in early life-history traits in crested newts (Triturus cristatus superspecies, Caudata, Salamandridae) from the Balkan Peninsula. J. Nat. Hist. 2009, 43, 469–477. [Google Scholar] [CrossRef]
- Lukanov, S.; Doncheva, T.; Kostova, N.; Naumov, B. Effects of selected environmental parameters on the activity and body condition of the Buresch’s crested newt (Triturus ivanbureschi) with notes on skin secretions. North-West. J. Zool. 2021, 17, 34–38. [Google Scholar]
- Lukanov, S.; Tzankov, N. Life history, age and normal development of the Balkan-Anatolian crested newt (Triturus ivanbureschi Arntzen and Wielstra, 2013) from Sofia district. North-West. J. Zool. 2016, 12, 22–32. [Google Scholar]
- Recuero, E.; Canestrelli, D.; Vörös, J.; Szabó, K.; Poyarkov, N.; Arntzen, J.W.; Crnobrnja-Isailovic, J.; Kidov, A.; Cogălniceanu, D.; Caputo, F. Multilocus species tree analyses resolve the radiation of the widespread Bufo bufo species group (Anura, Bufonidae). Mol. Phylogenet. Evol. 2012, 62, 71–86. [Google Scholar] [CrossRef] [PubMed]
- International Union for Conservation of Nature. The IUCN Red List of Threatened Species. Available online: https://www.iucnredlist.org (accessed on 10 January 2024).
- Villéger, S.; Mason, N.W.; Mouillot, D. New multidimensional functional diversity indices for a multifaceted framework in functional ecology. Ecology 2008, 89, 2290–2301. [Google Scholar] [CrossRef] [PubMed]
- Zellmer, A.J.; Slezak, P.; Katz, T.S. Clearing up the crystal ball: Understanding uncertainty in future climate suitability projections for amphibians. Herpetologica 2020, 76, 108–120. [Google Scholar] [CrossRef]
- Anunciação, P.R.; Ernst, R.; Martello, F.; Vancine, M.H.; de Carvalho, L.M.T.; Ribeiro, M.C. Climate-driven loss of taxonomic and functional richness in Brazilian Atlantic Forest anurans. Perspect. Ecol. Conserv. 2023, 21, 274–285. [Google Scholar] [CrossRef]
- Tsianou, M.A.; Kallimanis, A.S. Different species traits produce diverse spatial functional diversity patterns of amphibians. Biodivers. Conserv. 2016, 25, 117–132. [Google Scholar] [CrossRef]
- Tsianou, M.A.; Kallimanis, A.S. Geographical patterns and environmental drivers of functional diversity and trait space of amphibians of Europe. Ecol. Res. 2020, 35, 123–138. [Google Scholar] [CrossRef]
- Tsianou, M.A.; Kallimanis, A.S. Trait selection matters! A study on European amphibian functional diversity patterns. Ecol. Res. 2019, 34, 225–234. [Google Scholar] [CrossRef]
- Albert, C.H.; de Bello, F.; Boulangeat, I.; Pellet, G.; Lavorel, S.; Thuiller, W. On the importance of intraspecific variability for the quantification of functional diversity. Oikos 2011, 121, 116–126. [Google Scholar] [CrossRef]
- Wong, M.K.L.; Carmona, C.P.; Ergon, T. Including intraspecific trait variability to avoid distortion of functional diversity and ecological inference: Lessons from natural assemblages. Methods Ecol. Evol. 2021, 12, 946–957. [Google Scholar] [CrossRef]
- Carmona, C.P.; de Bello, F.; Mason, N.W.H.; Leps, J. Traits without Borders: Integrating Functional Diversity Across Scales. Trends Ecol. Evol. 2016, 31, 382–394. [Google Scholar] [CrossRef]
- Palacio, F.; Ottaviani, G.; Mammola, S.; Graco-Roza, C.; de Bello, F.; Carmona, C. Integrating intraspecific trait variability in functional diversity: An overview of methods and a guide for ecologists. EcoEvoRxiv 2024. [Google Scholar] [CrossRef]
- Goncalves-Souza, T.; Chaves, L.S.; Boldorini, G.X.; Ferreira, N.; Gusmao, R.A.F.; Peronico, P.B.; Sanders, N.J.; Teresa, F.B. Bringing light onto the Raunkiaeran shortfall: A comprehensive review of traits used in functional animal ecology. Ecol. Evol. 2023, 13, e10016. [Google Scholar] [CrossRef]
- Hevia, V.; Martin-Lopez, B.; Palomo, S.; Garcia-Llorente, M.; de Bello, F.; Gonzalez, J.A. Trait-based approaches to analyze links between the drivers of change and ecosystem services: Synthesizing existing evidence and future challenges. Ecol. Evol. 2017, 7, 831–844. [Google Scholar] [CrossRef]
- Etard, A.; Morrill, S.; Newbold, T.; Sheard, C. Global gaps in trait data for terrestrial vertebrates. Glob. Ecol. Biogeogr. 2020, 29, 2143–2158. [Google Scholar] [CrossRef]
- Schleuning, M.; García, D.; Tobias, J.A. Animal functional traits: Towards a trait-based ecology for whole ecosystems. Funct. Ecol. 2023, 37, 4–12. [Google Scholar] [CrossRef]
- Michaels, C.J.; Gini, B.F.; Preziosi, R.F. The importance of natural history and species-specific approaches in amphibian ex-situ conservation. Herpetol. J. 2014, 24, 135–145. [Google Scholar]
- Chalmandrier, L.; Münkemüller, T.; Colace, M.P.; Renaud, J.; Aubert, S.; Carlson, B.Z.; Clément, J.C.; Legay, N.; Pellet, G.; Saillard, A.; et al. Spatial scale and intraspecific trait variability mediate assembly rules in alpine grasslands. J. Ecol. 2016, 105, 277–287. [Google Scholar] [CrossRef]
- Siefert, A.; Violle, C.; Chalmandrier, L.; Albert, C.H.; Taudiere, A.; Fajardo, A.; Aarssen, L.W.; Baraloto, C.; Carlucci, M.B.; Cianciaruso, M.V.; et al. A global meta-analysis of the relative extent of intraspecific trait variation in plant communities. Ecol. Lett. 2015, 18, 1406–1419. [Google Scholar] [CrossRef]
- Albert, C.H.; Grassein, F.; Schurr, F.M.; Vieilledent, G.; Violle, C. When and how should intraspecific variability be considered in trait-based plant ecology? Perspect. Plant Ecol. Evol. Syst. 2011, 13, 217–225. [Google Scholar] [CrossRef]
- Ibarra-Isassi, J.; Handa, I.T.; Lessard, J.P. Community-wide trait adaptation, but not plasticity, explains ant community structure in extreme environments. Funct. Ecol. 2022, 37, 139–149. [Google Scholar] [CrossRef]
- Callaghan, C.T.; Palacio, F.X.; Benedetti, Y.; Morelli, F.; Bowler, D.E. Large-scale spatial variability in urban tolerance of birds. J. Anim. Ecol. 2023, 92, 403–416. [Google Scholar] [CrossRef]
- Santini, L.; BENÍTEZ-LÓPEZ, A.; Ficetola, G.F.; Huijbregts, M.A. Length–mass allometries in amphibians. Integr. Zool. 2018, 13, 36–45. [Google Scholar] [CrossRef] [PubMed]
- Alzate, A.; Onstein, R.E. Understanding the relationship between dispersal and range size. Ecol. Lett. 2022, 25, 2303–2323. [Google Scholar] [CrossRef]
- Trakimas, G.; Whittaker, R.J.; Borregaard, M.K. Do biological traits drive geographical patterns in European amphibians? Glob. Ecol. Biogeogr. 2016, 25, 1228–1238. [Google Scholar] [CrossRef]
- Trochet, A.; Moulherat, S.; Calvez, O.; Stevens, V.M.; Clobert, J.; Schmeller, D.S. A database of life-history traits of European amphibians. Biodivers. Data J. 2014, 2, e4123. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, B.F.; São-Pedro, V.A.; Santos-Barrera, G.; Penone, C.; Costa, G.C. AmphiBIO, a global database for amphibian ecological traits. Sci. Data 2017, 4, 170123. [Google Scholar] [CrossRef]
- de Bello, F.; Botta-Dukát, Z.; Lepš, J.; Fibich, P. Towards a more balanced combination of multiple traits when computing functional differences between species. Methods Ecol. Evol. 2021, 12, 443–448. [Google Scholar] [CrossRef]
- Karger, D.N.; Conrad, O.; Böhner, J.; Kawohl, T.; Kreft, H.; Soria-Auza, R.W.; Zimmermann, N.E.; Linder, H.P.; Kessler, M. Climatologies at high resolution for the earth’s land surface areas. Sci. Data 2017, 4, 170122. [Google Scholar] [CrossRef]
- Karger, D.N.; Conrad, O.; Böhner, J.; Kawohl, T.; Kreft, H.; Soria-Auza, R.W.; Zimmermann, N.E.; Linder, H.P.; Kessler, M. Climatologies at high resolution for the earth’s land surface areas. EnviDat 2021. [Google Scholar] [CrossRef] [PubMed]
- Boucher, O.; Servonnat, J.; Albright, A.L.; Aumont, O.; Balkanski, Y.; Bastrikov, V.; Bekki, S.; Bonnet, R.; Bony, S.; Bopp, L. Presentation and evaluation of the IPSL-CM6A-LR climate model. J. Adv. Model. Earth Syst. 2020, 12, e2019MS002010. [Google Scholar] [CrossRef]
- Eyring, V.; Bony, S.; Meehl, G.A.; Senior, C.A.; Stevens, B.; Stouffer, R.J.; Taylor, K.E. Overview of the Coupled Model Intercomparison Project Phase 6 (CMIP6) experimental design and organization. Geosci. Model Dev. 2016, 9, 1937–1958. [Google Scholar] [CrossRef]
- Fujimori, S.; Hasegawa, T.; Masui, T.; Takahashi, K.; Herran, D.S.; Dai, H.; Hijioka, Y.; Kainuma, M. SSP3: AIM implementation of shared socioeconomic pathways. Glob. Environ. Chang. 2017, 42, 268–283. [Google Scholar] [CrossRef]
- Riahi, K.; Van Vuuren, D.P.; Kriegler, E.; Edmonds, J.; O’neill, B.C.; Fujimori, S.; Bauer, N.; Calvin, K.; Dellink, R.; Fricko, O. The Shared Socioeconomic Pathways and their energy, land use, and greenhouse gas emissions implications: An overview. Glob. Environ. Chang. 2017, 42, 153–168. [Google Scholar] [CrossRef]
- Schipper, A.M.; Hilbers, J.P.; Meijer, J.R.; Antão, L.H.; Benítez-López, A.; de Jonge, M.M.; Leemans, L.H.; Scheper, E.; Alkemade, R.; Doelman, J.C. Projecting terrestrial biodiversity intactness with GLOBIO 4. Glob. Chang. Biol. 2020, 26, 760–771. [Google Scholar] [CrossRef]
- Thuiller, W.; Georges, D.; Engler, R.; Breiner, F. Biomod2: Ensemble Platform for Species Distribution Modelling; R Package Version 3.4.6. 2020. Available online: https://biomodhub.github.io/biomod2/ (accessed on 30 April 2024).
- Polaina, E.; Pärt, T.; Recio, M.R. Identifying hotspots of invasive alien terrestrial vertebrates in Europe to assist transboundary prevention and control. Sci. Rep. 2020, 10, 11655. [Google Scholar] [CrossRef] [PubMed]
- Michailidou, D.-E.; Lazarina, M.; Sgardelis, S.P. Temperature and prey species richness drive the broad-scale distribution of a generalist predator. Diversity 2021, 13, 169. [Google Scholar] [CrossRef]
- Aguirre-Gutiérrez, J.; Carvalheiro, L.G.; Polce, C.; van Loon, E.E.; Raes, N.; Reemer, M.; Biesmeijer, J.C. Fit-for-purpose: Species distribution model performance depends on evaluation criteria–Dutch hoverflies as a case study. PLoS ONE 2013, 8, e63708. [Google Scholar] [CrossRef] [PubMed]
- Comte, L.; Grenouillet, G. Species distribution modelling and imperfect detection: Comparing occupancy versus consensus methods. Divers. Distrib. 2013, 19, 996–1007. [Google Scholar] [CrossRef]
- Marmion, M.; Parviainen, M.; Luoto, M.; Heikkinen, R.K.; Thuiller, W. Evaluation of consensus methods in predictive species distribution modelling. Divers. Distrib. 2009, 15, 59–69. [Google Scholar] [CrossRef]
- Crossman, N.D.; Bass, D.A. Application of common predictive habitat techniques for post-border weed risk management. Divers. Distrib. 2008, 14, 213–224. [Google Scholar] [CrossRef]
- Araújo, M.B.; New, M. Ensemble forecasting of species distributions. Trends Ecol. Evol. 2007, 22, 42–47. [Google Scholar] [CrossRef] [PubMed]
- Allouche, O.; Tsoar, A.; Kadmon, R. Assessing the accuracy of species distribution models: Prevalence, kappa and the true skill statistic (TSS). J. Appl. Ecol. 2006, 43, 1223–1232. [Google Scholar] [CrossRef]
- Wisz, M.S.; Guisan, A. Do pseudo-absence selection strategies influence species distribution models and their predictions? An information-theoretic approach based on simulated data. BMC Ecol. 2009, 9, 8. [Google Scholar] [CrossRef]
- Barbet-Massin, M.; Jiguet, F.; Albert, C.H.; Thuiller, W. Selecting pseudo-absences for species distribution models: How, where and how many? Methods Ecol. Evol. 2012, 3, 327–338. [Google Scholar] [CrossRef]
- Liu, C.; White, M.; Newell, G. Selecting thresholds for the prediction of species occurrence with presence-only data. J. Biogeogr. 2013, 40, 778–789. [Google Scholar] [CrossRef]
- Sillero, N.; Campos, J.; Bonardi, A.; Corti, C.; Creemers, R.; Crochet, P.-A.; Isailović, J.C.; Denoël, M.; Ficetola, G.F.; Gonçalves, J. Updated distribution and biogeography of amphibians and reptiles of Europe. Amphib. Reptil. 2014, 35, 1–31. [Google Scholar] [CrossRef]
- Mouillot, D.; Graham, N.A.; Villéger, S.; Mason, N.W.; Bellwood, D.R. A functional approach reveals community responses to disturbances. Trends Ecol. Evol. 2013, 28, 167–177. [Google Scholar] [CrossRef] [PubMed]
- Rao, C.R. Diversity and dissimilarity coefficients: A unified approach. Theor. Popul. Biol. 1982, 21, 24–43. [Google Scholar] [CrossRef]
- Mouillot, D.; Villéger, S.; Parravicini, V.; Kulbicki, M.; Arias-González, J.E.; Bender, M.; Chabanet, P.; Floeter, S.R.; Friedlander, A.; Vigliola, L. Functional over-redundancy and high functional vulnerability in global fish faunas on tropical reefs. Proc. Natl. Acad. Sci. USA 2014, 111, 13757–13762. [Google Scholar] [CrossRef] [PubMed]
- Magneville, C.; Loiseau, N.; Albouy, C.; Casajus, N.; Claverie, T.; Escalas, A.; Leprieur, F.; Maire, E.; Mouillot, D.; Villéger, S. mFD: An R package to compute and illustrate the multiple facets of functional diversity. Ecography 2021, 2022. [Google Scholar] [CrossRef]
- Ripley, B.; Venables, B.; Bates, D.M.; Hornik, K.; Gebhardt, A.; Firth, D.; Ripley, M.B. Package ‘MASS’; CRAN R. 2013. Available online: https://cran.r-project.org/web/packages/MASS/index.html (accessed on 30 April 2024).
- Hollister, J.W.; Robitaille, A.L.; Beck, M.W.; MikeJohnson-NOAA; Shah, T. jhollist/elevatr: CRAN Release v0.99.0. 2023. Available online: https://zenodo.org/records/8335450 (accessed on 30 April 2024).
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2023. [Google Scholar]
- Arntzen, J. From descriptive to predictive distribution models: A working example with Iberian amphibians and reptiles. Front. Zool. 2006, 3, 8. [Google Scholar] [CrossRef] [PubMed]
- Girardello, M.; Griggio, M.; Whittingham, M.J.; Rushton, S.P. Models of climate associations and distributions of amphibians in Italy. Ecol. Res. 2010, 25, 103–111. [Google Scholar] [CrossRef]
- Boyer, I.; Cayuela, H.; Bertrand, R.; Isselin-Nondedeu, F. Improving biological relevance of model projections in response to climate change by considering dispersal amongst lineages in an amphibian. J. Biogeogr. 2021, 48, 561–576. [Google Scholar] [CrossRef]
- Campos, J.C.; Rodrigues, S.; Freitas, T.; Santos, J.A.; Honrado, J.P.; Regos, A. Climatic variables and ecological modelling data for birds, amphibians and reptiles in the Transboundary Biosphere Reserve of Meseta Ibérica (Portugal-Spain). Biodivers. Data J. 2021, 9, e66509. [Google Scholar] [CrossRef]
- Arntzen, J.W. Coexistence of two newt species in a transition zone of range overlap. Contrib. Zool. 2022, 91, 133–151. [Google Scholar] [CrossRef]
- Araújo, M.B.; Thuiller, W.; Pearson, R.G. Climate warming and the decline of amphibians and reptiles in Europe. J. Biogeogr. 2006, 33, 1712–1728. [Google Scholar] [CrossRef]
- Markovic, D.; Carrizo, S.; Freyhof, J.; Cid, N.; Lengyel, S.; Scholz, M.; Kasperdius, H.; Darwall, W. Europe’s freshwater biodiversity under climate change: Distribution shifts and conservation needs. Divers. Distrib. 2014, 20, 1097–1107. [Google Scholar] [CrossRef]
- Lourenço-de-Moraes, R.; Campos, F.S.; Ferreira, R.B.; Solé, M.; Beard, K.H.; Bastos, R.P. Back to the future: Conserving functional and phylogenetic diversity in amphibian-climate refuges. Biodivers. Conserv. 2019, 28, 1049–1073. [Google Scholar] [CrossRef]
- Villacampa, J.; Whitworth, A.; Allen, L.; Malo, J.E. Altitudinal differences in alpha, beta and functional diversity of an amphibian community in a biodiversity hotspot. Neotrop. Biodivers. 2019, 5, 60–68. [Google Scholar] [CrossRef]
- González-del-Pliego, P.; Scheffers, B.R.; Freckleton, R.P.; Basham, E.W.; Araújo, M.B.; Acosta-Galvis, A.R.; Medina Uribe, C.A.; Haugaasen, T.; Edwards, D.P. Thermal tolerance and the importance of microhabitats for Andean frogs in the context of land use and climate change. J. Anim. Ecol. 2020, 89, 2451–2460. [Google Scholar] [CrossRef]
- Sillero, N. Climate change in action: Local elevational shifts on Iberian amphibians and reptiles. Reg. Environ. Chang. 2021, 21, 101. [Google Scholar] [CrossRef]
- Tiberti, R.; Mangiacotti, M.; Bennati, R. The upward elevational shifts of pond breeding amphibians following climate warming. Biol. Conserv. 2021, 253, 108911. [Google Scholar] [CrossRef]
- Alves-Ferreira, G.; Talora, D.C.; Solé, M.; Cervantes-López, M.J.; Heming, N.M. Unraveling global impacts of climate change on amphibians distributions: A life-history and biogeographic-based approach. Front. Ecol. Evol. 2022, 10, 987237. [Google Scholar] [CrossRef]
- Guirguis, J.; Goodyear, L.E.; Finn, C.; Johnson, J.V.; Pincheira-Donoso, D. Risk of extinction increases towards higher elevations across the world’s amphibians. Glob. Ecol. Biogeogr. 2023, 32, 1952–1963. [Google Scholar] [CrossRef]
- Lawler, J.J.; Shafer, S.L.; White, D.; Kareiva, P.; Maurer, E.P.; Blaustein, A.R.; Bartlein, P.J. Projected climate-induced faunal change in the Western Hemisphere. Ecology 2009, 90, 588–597. [Google Scholar] [CrossRef] [PubMed]
- Duan, R.-Y.; Kong, X.-Q.; Huang, M.-Y.; Varela, S.; Ji, X. The potential effects of climate change on amphibian distribution, range fragmentation and turnover in China. PeerJ 2016, 4, e2185. [Google Scholar] [CrossRef] [PubMed]
- Mi, C.; Huettmann, F.; Li, X.; Jiang, Z.; Du, W.; Sun, B. Effects of climate and human activity on the current distribution of amphibians in China. Conserv. Biol. 2022, 36, e13964. [Google Scholar] [CrossRef] [PubMed]
- Cancellario, T.; Miranda, R.; Baquero, E.; Fontaneto, D.; Martínez, A.; Mammola, S. Climate change will redefine taxonomic, functional, and phylogenetic diversity of Odonata in space and time. npj Biodivers. 2022, 1, 1. [Google Scholar] [CrossRef]
- Zhang, L.; Lu, X. Amphibians live longer at higher altitudes but not at higher latitudes. Biol. J. Linn. Soc. 2012, 106, 623–632. [Google Scholar] [CrossRef]
- Morrison, C.; Hero, J.M. Geographic variation in life-history characteristics of amphibians: A review. J. Anim. Ecol. 2003, 72, 270–279. [Google Scholar] [CrossRef]
- Amat, F.; Meiri, S. Geographical, climatic and biological constraints on age at sexual maturity in amphibians. Biol. J. Linn. Soc. 2018, 123, 34–42. [Google Scholar] [CrossRef]
- Forero-Medina, G.; Joppa, L.; Pimm, S.L. Constraints to species’ elevational range shifts as climate changes. Conserv. Biol. 2011, 25, 163–171. [Google Scholar] [CrossRef] [PubMed]
- Slavenko, A.; Meiri, S. Mean body sizes of amphibian species are poorly predicted by climate. J. Biogeogr. 2015, 42, 1246–1254. [Google Scholar] [CrossRef]
- Jacob, D.; Petersen, J.; Eggert, B.; Alias, A.; Christensen, O.B.; Bouwer, L.M.; Braun, A.; Colette, A.; Déqué, M.; Georgievski, G. EURO-CORDEX: New high-resolution climate change projections for European impact research. Reg. Environ. Chang. 2014, 14, 563–578. [Google Scholar] [CrossRef]
- Blaustein, A.R.; Wake, D.B. The puzzle of declining amphibian populations. Sci. Am. 1995, 272, 52–57. [Google Scholar] [CrossRef]
- Buckley, L.B.; Jetz, W. Environmental and historical constraints on global patterns of amphibian richness. Proc. R. Soc. B Biol. Sci. 2007, 274, 1167–1173. [Google Scholar] [CrossRef]
- Whittaker, R.J.; Nogués-Bravo, D.; Araújo, M.B. Geographical gradients of species richness: A test of the water-energy conjecture of Hawkins et al.(2003) using European data for five taxa. Glob. Ecol. Biogeogr. 2007, 16, 76–89. [Google Scholar] [CrossRef]
- Araújo, M.B.; Nogués-Bravo, D.; Diniz-Filho, J.A.F.; Haywood, A.M.; Valdes, P.J.; Rahbek, C. Quaternary climate changes explain diversity among reptiles and amphibians. Ecography 2008, 31, 8–15. [Google Scholar] [CrossRef]
- Brown, S.C.; Wigley, T.M.; Otto-Bliesner, B.L.; Rahbek, C.; Fordham, D.A. Persistent Quaternary climate refugia are hospices for biodiversity in the Anthropocene. Nat. Clim. Chang. 2020, 10, 244–248. [Google Scholar] [CrossRef]
- López-Alcaide, S.; Macip-Ríos, R. Effects of climate change in amphibians and reptiles. In Biodiversity Loss in A Changing Planet; Grillo, O., Venora, G., Eds.; InTech: Rijeka, Croatia, 2011; pp. 163–184. [Google Scholar]
- Blaustein, A.R.; Walls, S.C.; Bancroft, B.A.; Lawler, J.J.; Searle, C.L.; Gervasi, S.S. Direct and indirect effects of climate change on amphibian populations. Diversity 2010, 2, 281–313. [Google Scholar] [CrossRef]
- Munguía, M.; Rahbek, C.; Rangel, T.F.; Diniz-Filho, J.A.F.; Araújo, M.B. Equilibrium of global amphibian species distributions with climate. PLoS ONE 2012, 7, e34420. [Google Scholar] [CrossRef]
- Li, Y.; Cohen, J.M.; Rohr, J.R. Review and synthesis of the effects of climate change on amphibians. Integr. Zool. 2013, 8, 145–161. [Google Scholar] [CrossRef]
- Winter, M.; Fiedler, W.; Hochachka, W.M.; Koehncke, A.; Meiri, S.; De la Riva, I. Patterns and biases in climate change research on amphibians and reptiles: A systematic review. R. Soc. Open Sci. 2016, 3, 160158. [Google Scholar] [CrossRef]
- Carilo Filho, L.M.; Gomes, L.; Katzenberger, M.; Sole, M.; Orrico, V.G. There and back again: A meta-analytical approach on the influence of acclimation and altitude in the upper thermal tolerance of amphibians and reptiles. Front. Ecol. Evol. 2022, 10, 1017255. [Google Scholar] [CrossRef]
- Pottier, P.; Lin, H.-Y.; Oh, R.R.; Pollo, P.; Rivera-Villanueva, A.N.; Valdebenito, J.O.; Yang, Y.; Amano, T.; Burke, S.; Drobniak, S.M. A comprehensive database of amphibian heat tolerance. Sci. Data 2022, 9, 600. [Google Scholar] [CrossRef]
- Vacek, Z.; Vacek, S.; Cukor, J. European forests under global climate change: Review of tree growth processes, crises and management strategies. J. Environ. Manag. 2023, 332, 117353. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Liao, Z.; Chen, X.; Chen, Y. Climatic debts for global amphibians: Who, where and why? Biol. Conserv. 2023, 279, 109929. [Google Scholar] [CrossRef]
- Lazarina, M.; Sgardelis, S.P.; Michailidou, D.E.; Tsianou, M.; Andrikou-Charitidou, A.; Touloumis, K.; Kallimanis, A.S. Replacement drives native β-diversity of British avifauna, while richness differences shape alien β-diversity. Divers. Distrib. 2023, 29, 61–74. [Google Scholar] [CrossRef]
- Maiorano, L.; Amori, G.; Capula, M.; Falcucci, A.; Masi, M.; Montemaggiori, A.; Pottier, J.; Psomas, A.; Rondinini, C.; Russo, D. Threats from climate change to terrestrial vertebrate hotspots in Europe. PLoS ONE 2013, 8, e74989. [Google Scholar] [CrossRef] [PubMed]
- MacLean, S.A.; Beissinger, S.R. Species’ traits as predictors of range shifts under contemporary climate change: A review and meta-analysis. Glob. Chang. Biol. 2017, 23, 4094–4105. [Google Scholar] [CrossRef] [PubMed]
- Wu, J. The hazard and unsureness of reducing habitat ranges in response to climate warming for 91 amphibian species in China. Acta Oecologica 2020, 108, 103640. [Google Scholar] [CrossRef]
- Eggert, C.; Cogălniceanu, D.; Veith, M.; Dzukic, G.; Taberlet, P. The declining Spadefoot toad, Pelobates fuscus (Pelobatidae): Paleo and recent environmental changes as a major influence on current population structure and status. Conserv. Genet. 2006, 7, 185–195. [Google Scholar] [CrossRef]
- Hof, C.; Araújo, M.B.; Jetz, W.; Rahbek, C. Additive threats from pathogens, climate and land-use change for global amphibian diversity. Nature 2011, 480, 516–519. [Google Scholar] [CrossRef] [PubMed]
- Falaschi, M.; Manenti, R.; Thuiller, W.; Ficetola, G.F. Continental-scale determinants of population trends in European amphibians and reptiles. Glob. Chang. Biol. 2019, 25, 3504–3515. [Google Scholar] [CrossRef]
- Allentoft, M.; O’Brien, J. Global Amphibian Declines, Loss of Genetic Diversity and Fitness: A Review. Diversity 2010, 2, 47–71. [Google Scholar] [CrossRef]
- Hu, J.; Huang, Y.; Jiang, J.; Guisan, A. Genetic diversity in frogs linked to past and future climate changes on the roof of the world. J. Anim. Ecol. 2019, 88, 953–963. [Google Scholar] [CrossRef] [PubMed]
- Pearman, P.B.; Broennimann, O.; Aavik, T.; Albayrak, T.; Alves, P.C.; Aravanopoulos, F.A.; Bertola, L.D.; Biedrzycka, A.; Buzan, E.; Cubric-Curik, V.; et al. Monitoring of species’ genetic diversity in Europe varies greatly and overlooks potential climate change impacts. Nat. Ecol. Evol. 2024, 8, 267–281. [Google Scholar] [CrossRef] [PubMed]
- Pupins, M.; Nekrasova, O.; Marushchak, O.; Tytar, V.; Theissinger, K.; Čeirāns, A.; Skute, A.; Georges, J.-Y. Potential Threat of an Invasive Fish Species for Two Native Newts Inhabiting Wetlands of Europe Vulnerable to Climate Change. Diversity 2023, 15, 201. [Google Scholar] [CrossRef]
- Tytar, V.; Nekrasova, O.; Pupins, M.; Skute, A.; Kirjusina, M.; Gravele, E.; Mezaraupe, L.; Marushchak, O.; Ceirans, A.; Kozynenko, I.; et al. Modeling the Distribution of the Chytrid Fungus Batrachochytrium dendrobatidis with Special Reference to Ukraine. J. Fungi 2023, 9, 607. [Google Scholar] [CrossRef]
- IUCN SSC Amphibian Specialist Group. Alytes dickhilleni. The IUCN Red List of Threatened Species 2022: e.T979A89697540. 2022. Available online: https://www.iucnredlist.org/species/979/89697540 (accessed on 30 April 2024).
- Brum, F.T.; Gonçalves, L.O.; Cappelatti, L.; Carlucci, M.B.; Debastiani, V.J.; Salengue, E.V.; dos Santos Seger, G.D.; Both, C.; Bernardo-Silva, J.S.; Loyola, R.D. Land use explains the distribution of threatened new world amphibians better than climate. PLoS ONE 2013, 8, e60742. [Google Scholar] [CrossRef] [PubMed]
- Mouchet, M.; Levers, C.; Zupan, L.; Kuemmerle, T.; Plutzar, C.; Erb, K.; Lavorel, S.; Thuiller, W.; Haberl, H. Testing the effectiveness of environmental variables to explain European terrestrial vertebrate species richness across biogeographical scales. PLoS ONE 2015, 10, e0131924. [Google Scholar] [CrossRef] [PubMed]
- Chaudhary, A.; Mooers, A.O. Terrestrial vertebrate biodiversity loss under future global land use change scenarios. Sustainability 2018, 10, 2764. [Google Scholar] [CrossRef]
- Tsianou, M.A.; Koutsias, N.; Mazaris, A.D.; Kallimanis, A.S. Climate and landscape explain richness patterns depending on the type of species’ distribution data. Acta Oecol. 2016, 74, 19–27. [Google Scholar] [CrossRef]
- Newbold, T. Future effects of climate and land-use change on terrestrial vertebrate community diversity under different scenarios. Proc. R. Soc. B 2018, 285, 20180792. [Google Scholar] [CrossRef]
- Tsianou, M.A.; Lazarina, M.; Michailidou, D.-E.; Andrikou-Charitidou, A.; Sgardelis, S.P.; Kallimanis, A.S. The effect of climate and human pressures on functional diversity and species richness patterns of amphibians, reptiles and mammals in Europe. Diversity 2021, 13, 275. [Google Scholar] [CrossRef]
- Cordier, J.M.; Aguilar, R.; Lescano, J.N.; Leynaud, G.C.; Bonino, A.; Miloch, D.; Loyola, R.; Nori, J. A global assessment of amphibian and reptile responses to land-use changes. Biol. Conserv. 2021, 253, 108863. [Google Scholar] [CrossRef]
- Finn, C.; Grattarola, F.; Pincheira-Donoso, D. More losers than winners: Investigating Anthropocene defaunation through the diversity of population trends. Biol. Rev. 2023, 98, 1732–1748. [Google Scholar] [CrossRef]
- Toussaint, A.; Brosse, S.; Bueno, C.G.; Pärtel, M.; Tamme, R.; Carmona, C.P. Extinction of threatened vertebrates will lead to idiosyncratic changes in functional diversity across the world. Nat. Commun. 2021, 12, 5162. [Google Scholar] [CrossRef] [PubMed]
- Nowakowski, A.J.; Frishkoff, L.O.; Thompson, M.E.; Smith, T.M.; Todd, B.D. Phylogenetic homogenization of amphibian assemblages in human-altered habitats across the globe. Proc. Natl. Acad. Sci. USA 2018, 115, E3454–E3462. [Google Scholar] [CrossRef]
- Newbold, T.; Adams, G.L.; Albaladejo Robles, G.; Boakes, E.H.; Braga Ferreira, G.; Chapman, A.S.; Etard, A.; Gibb, R.; Millard, J.; Outhwaite, C.L. Climate and land-use change homogenise terrestrial biodiversity, with consequences for ecosystem functioning and human well-being. Emerg. Top. Life Sci. 2019, 3, 207–219. [Google Scholar]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Proios, K.; Michailidou, D.-E.; Lazarina, M.; Tsianou, M.A.; Kallimanis, A.S. Climate and Land Use Changes Impact the Future of European Amphibian Functional Diversity. Land 2024, 13, 1206. https://doi.org/10.3390/land13081206
Proios K, Michailidou D-E, Lazarina M, Tsianou MA, Kallimanis AS. Climate and Land Use Changes Impact the Future of European Amphibian Functional Diversity. Land. 2024; 13(8):1206. https://doi.org/10.3390/land13081206
Chicago/Turabian StyleProios, Konstantinos, Danai-Eleni Michailidou, Maria Lazarina, Mariana A. Tsianou, and Athanasios S. Kallimanis. 2024. "Climate and Land Use Changes Impact the Future of European Amphibian Functional Diversity" Land 13, no. 8: 1206. https://doi.org/10.3390/land13081206





