Revetment Affects Nitrogen Removal and N2O Emission at the Urban River–Riparian Interface
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area and Field Sampling
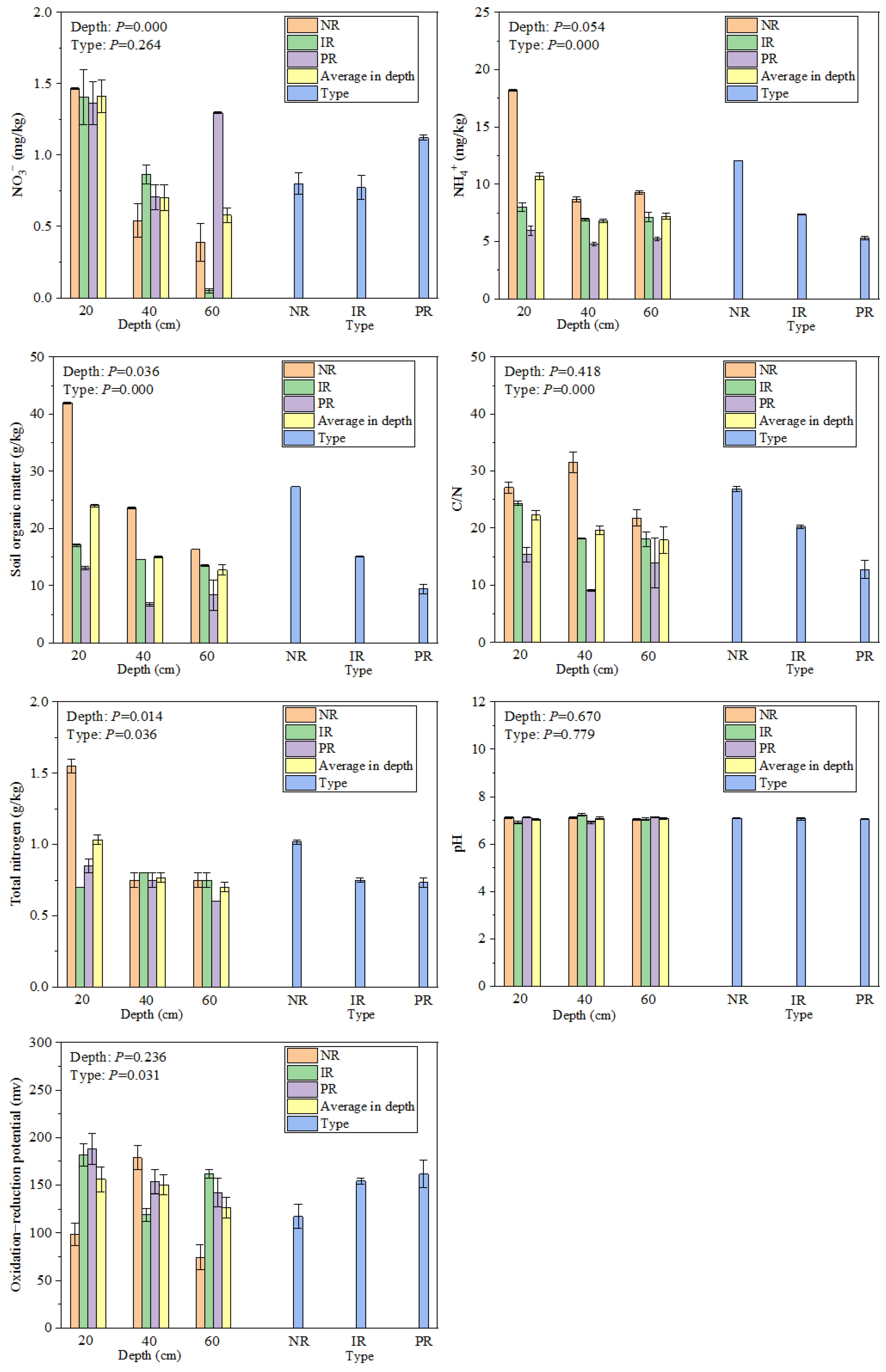
2.2. Soil Physical and Chemical Properties
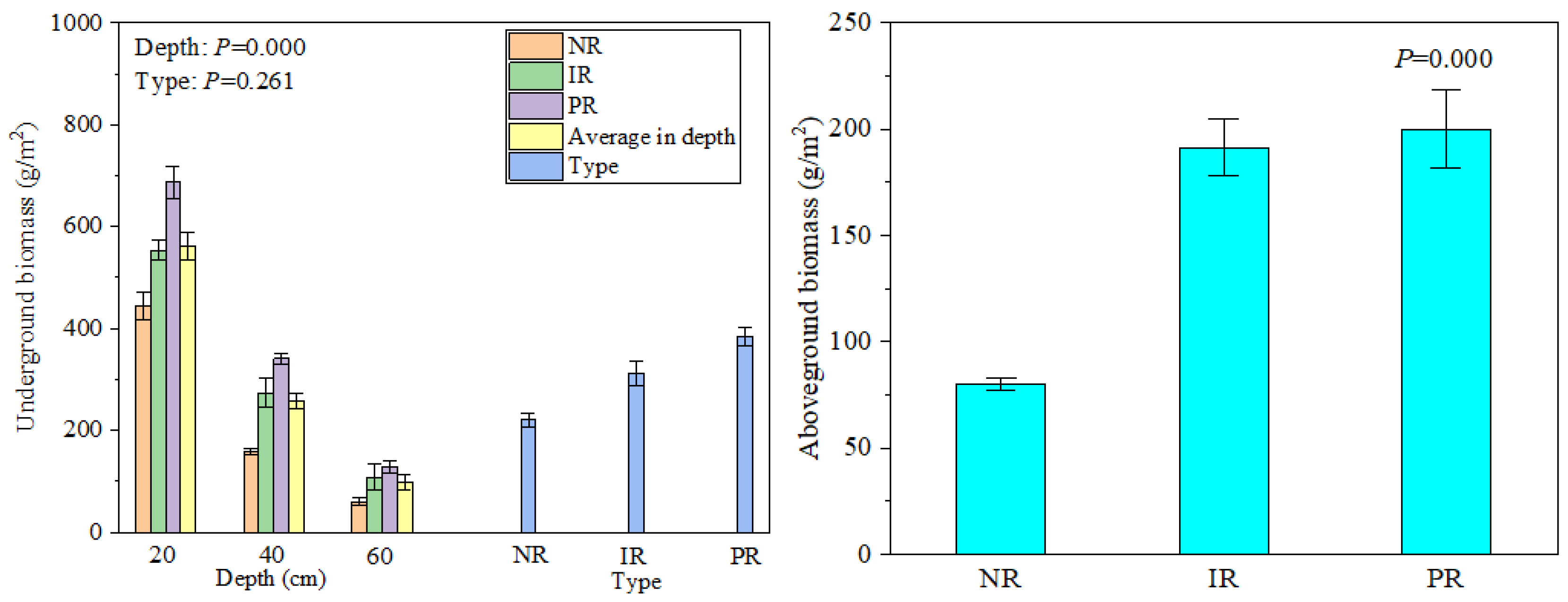
2.3. Plant Biomass Measurements
2.4. Nitrifying and Denitrifying Microorganisms
2.5. Nitrification and Denitrification Potentials
2.6. N2O Emission Rate
2.7. Statistical Analysis
3. Results
3.1. Soil Physical and Chemical Properties and Plant Biomass Characteristics
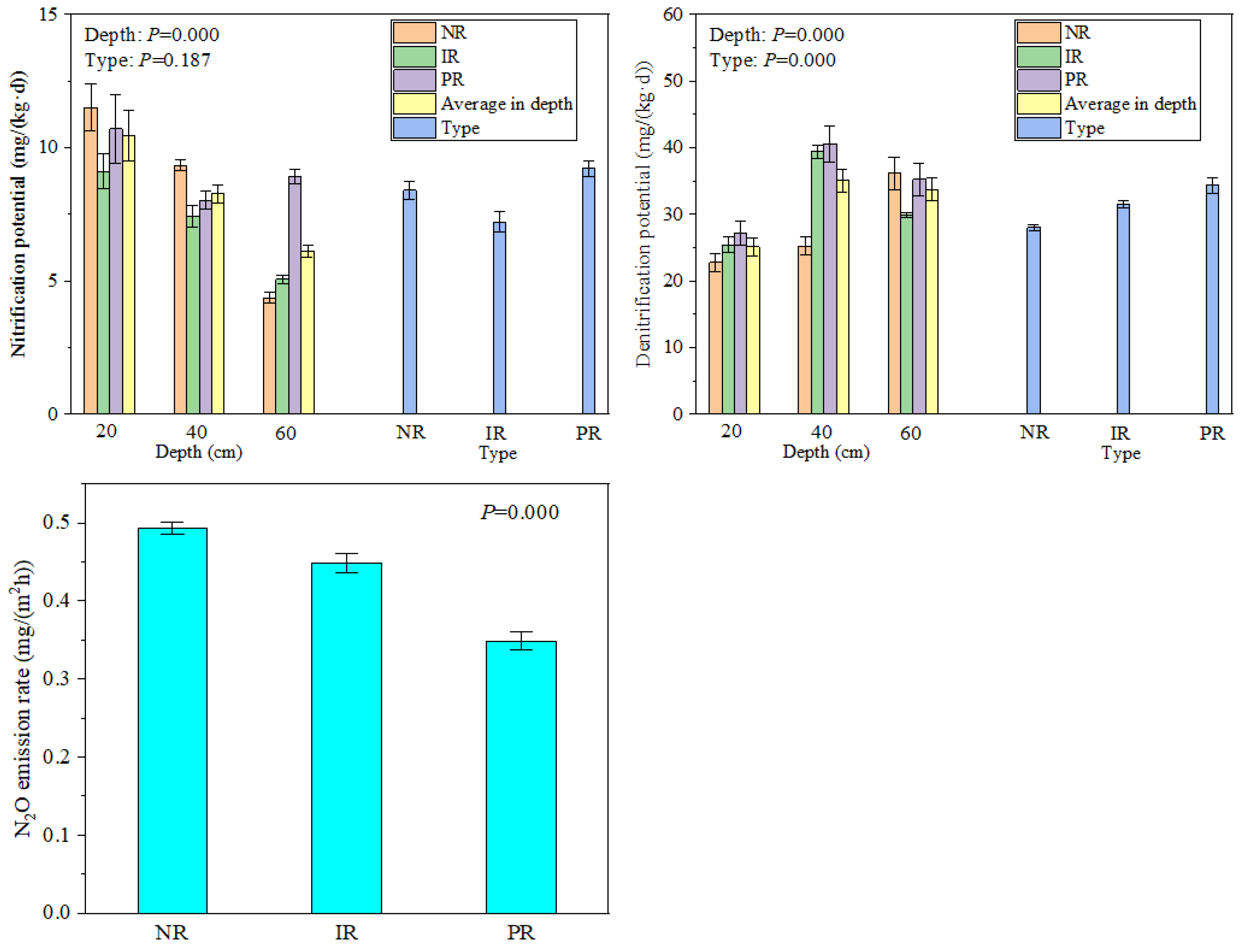
3.2. Nitrification, Denitrification Potential, and N2O Emission Characteristics
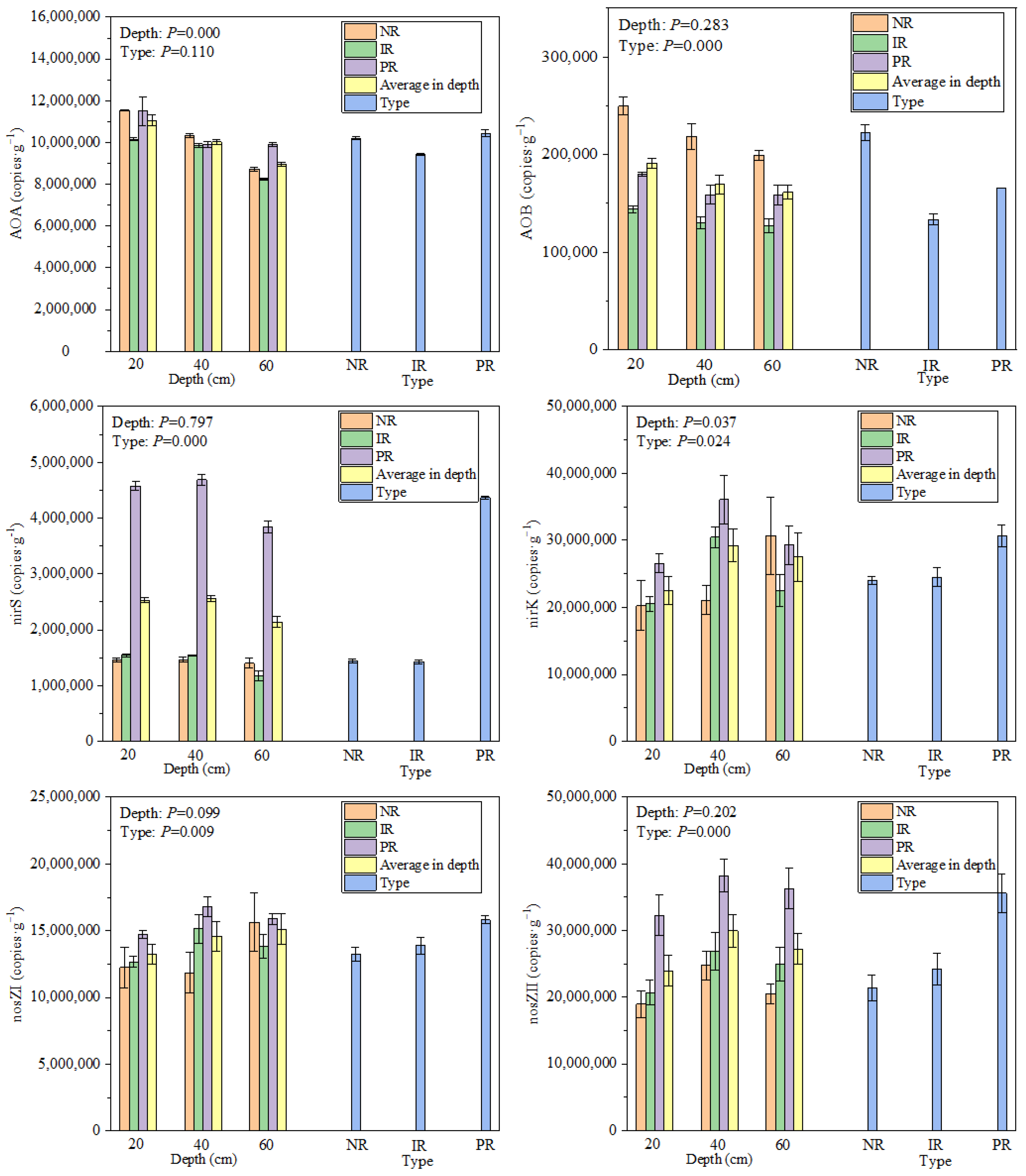
3.3. Nitrification and Denitrification Gene Abundance Characteristics
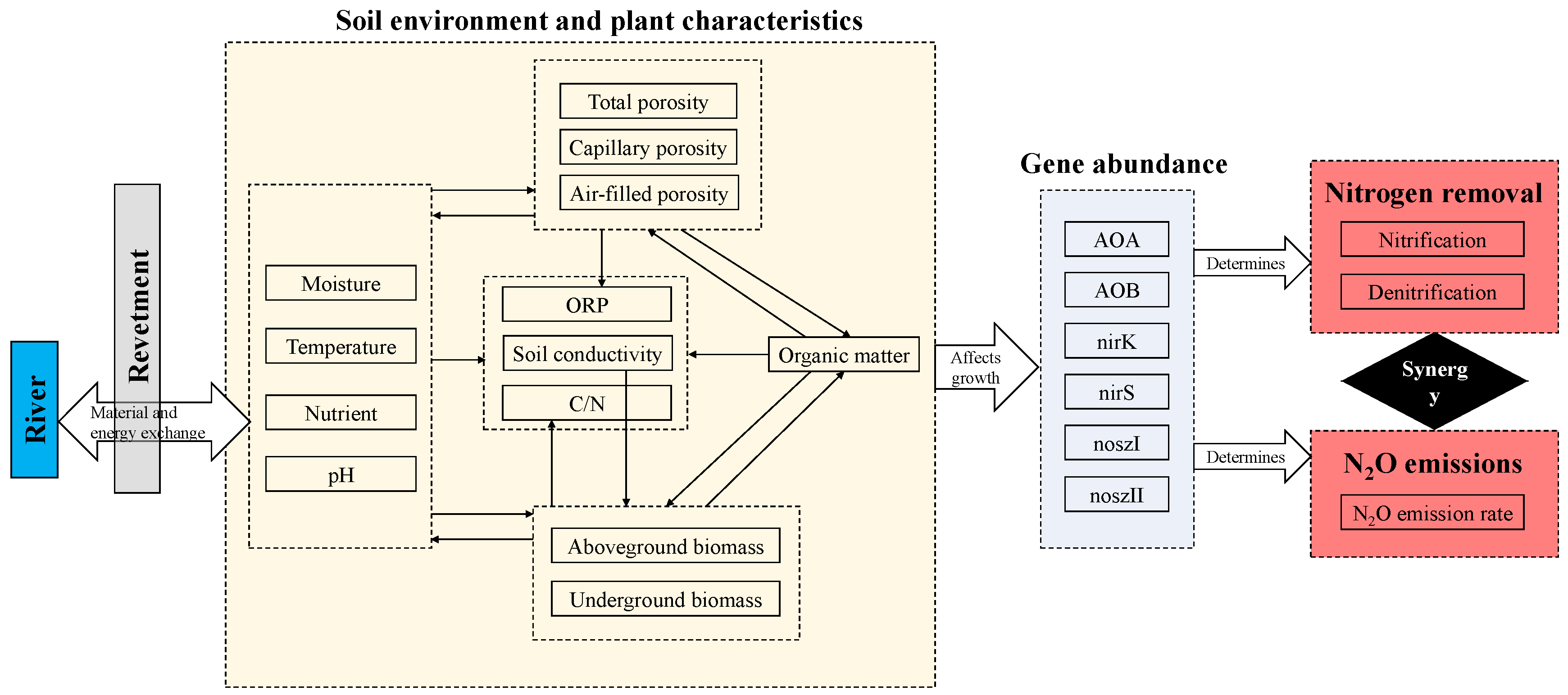
3.4. Interactions within Soil Ecosystems
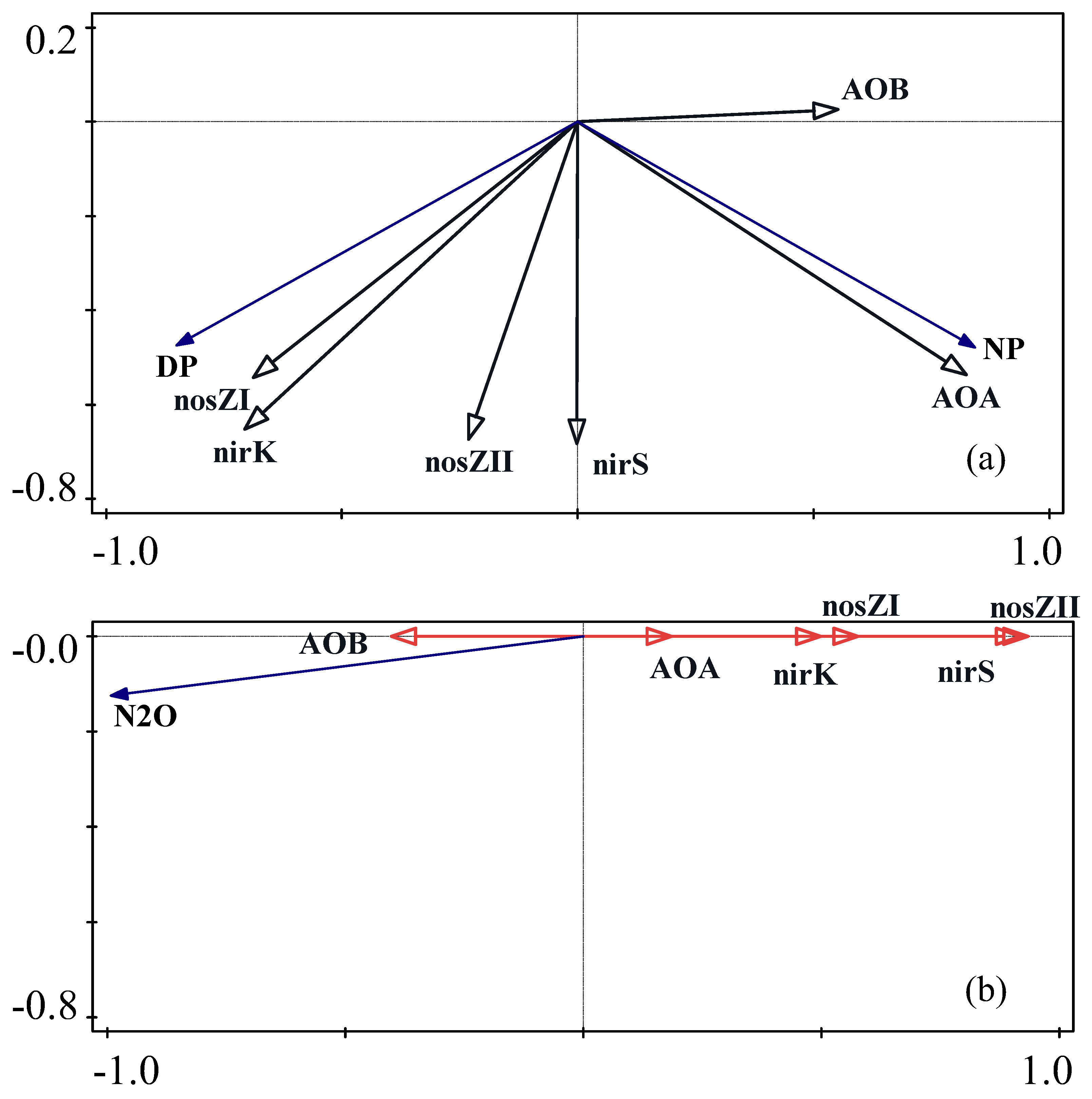
3.5. Correlation and Redundancy Analysis of Soil Ecosystems and the Abundance of Nitrification and Denitrification Microbial Genes
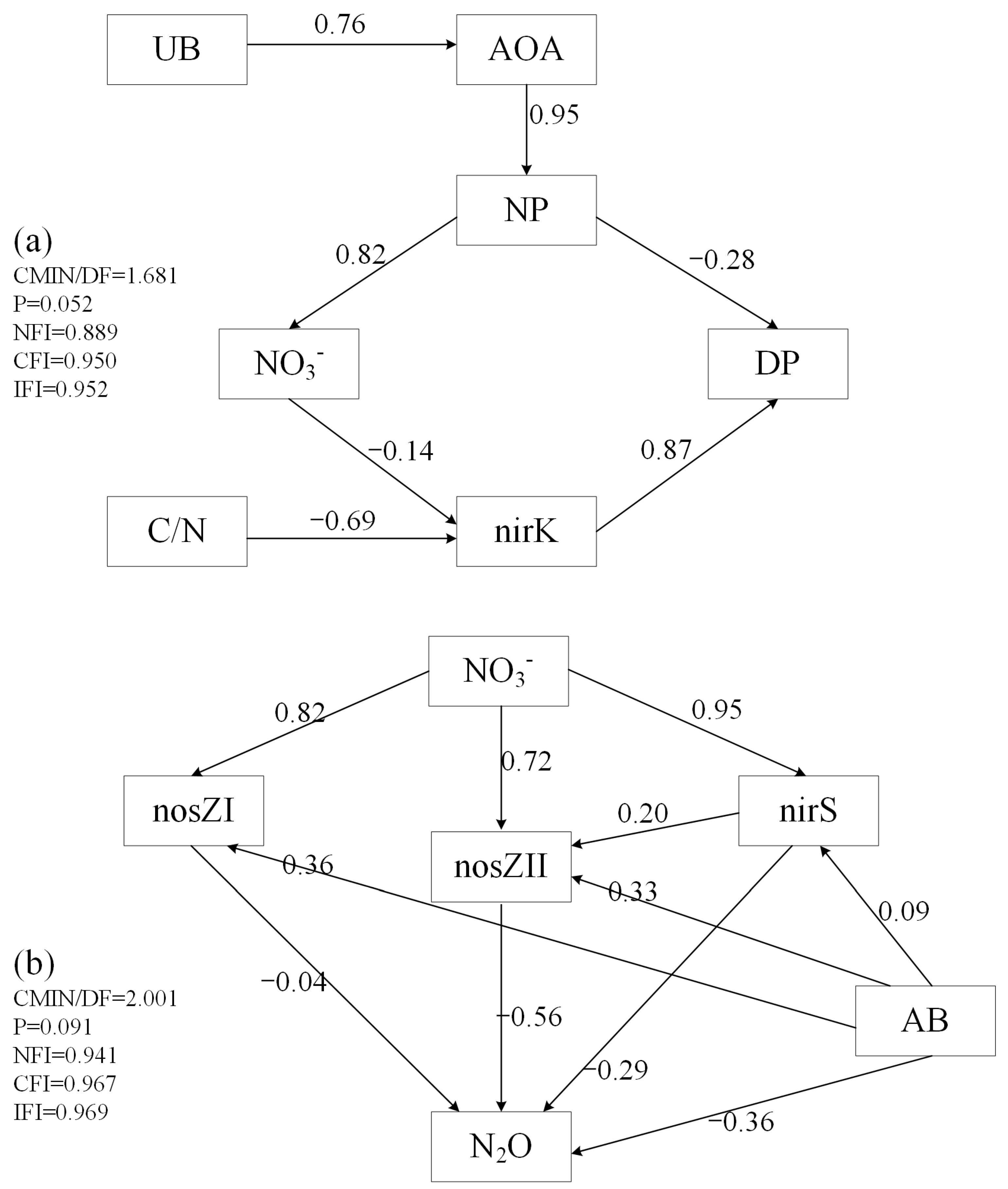
3.6. Structural Equation Model
4. Discussion
4.1. Revetment Affects AOA, AOB, nirS, nirK, nosZI, nosZII at RRI
4.2. Revetment Affects Denitrification Potential at RRI
4.3. Revetment Affects N2O Emissions at RRI
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| Abbreviations | Full Name |
| RRI | River–riparian interface |
| PR | Permeable revetment |
| NR | Natural revetment |
| IR | Impermeable revetment |
| NP | Nitrification potential |
| DP | Denitrification potential |
| TP | Total porosity |
| CP | Capillary porosity |
| AFP | Air-filled porosity |
| ORP | Oxidation–reduction potential |
| TN | Total nitrogen |
| SOM | Soil organic matter |
| AB | Plant aboveground biomass |
| UB | Plant underground biomass |
References
- Man, Z.; Xie, C.; Jiang, R.; Liang, A.; Wu, H.; Che, S. Effects of revetments on soil ecosystems in the urban river-riparian interface. iScience 2022, 25, 105277. [Google Scholar] [CrossRef] [PubMed]
- Adekalu, K.O.; Olorunfemi, I.A.; Osunbitan, J.A. Grass mulching effect on infiltration, surface runoff and soil loss of three agricultural soils in Nigeria. Bioresour. Technol. 2007, 98, 912–917. [Google Scholar] [CrossRef]
- Liu, X.; Vidon, P.; Jacinthe, P.-A.; Fisher, K.; Baker, M. Seasonal and geomorphic controls on N and P removal in riparian zones of the US Midwest. Biogeochemistry 2014, 119, 245–257. [Google Scholar] [CrossRef]
- Hester, E.T.; Brooks, K.E.; Scott, D.T. Comparing reach scale hyporheic exchange and denitrification induced by in-stream restoration structures and natural streambed morphology. Ecol. Eng. 2018, 115, 105–121. [Google Scholar] [CrossRef]
- Mayer, P.M.; Reynolds, S.K., Jr.; McCutchen, M.D.; Canfield, T.J. Meta-Analysis of Nitrogen Removal in Riparian Buffers. J. Environ. Qual. 2007, 36, 1172–1180. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.-P.; Zhang, L.-M.; Di, H.J.; He, J.-Z. A review of ammonia-oxidizing bacteria and archaea in Chinese soils. Front. Microbiol. 2012, 3, 296. [Google Scholar] [CrossRef] [PubMed]
- Hou, S.; Ai, C.; Zhou, W.; Liang, G.; He, P. Structure and assembly cues for rhizospheric nirK- and nirS-type denitrifier communities in long-term fertilized soils. Soil Biol. Biochem. 2018, 119, 32–40. [Google Scholar] [CrossRef]
- Yan, L.; Xie, C.; Xu, X.; Che, S. The influence of revetment types on soil denitrification in the adjacent tidal urban riparian zones. J. Hydrol. 2019, 574, 398–407. [Google Scholar] [CrossRef]
- Yan, L.; Xie, C.; Liang, A.; Jiang, R.; Che, S. Effects of revetments on soil denitrifying communities in the urban river-riparian interface. Chemosphere 2021, 263, 128077. [Google Scholar] [CrossRef]
- Ravishankara, A.R.; Daniel, J.S.; Portmann, R.W. Nitrous Oxide (N2O): The Dominant Ozone-Depleting Substance Emitted in the 21st Century. Science 2009, 326, 123–125. [Google Scholar] [CrossRef]
- Stocker, T. Climate Change 2013: The Physical Science Basis: Working Group I Contribution to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Cambridge University Press: Cambridge, UK, 2014. [Google Scholar]
- Man, Z.; Xie, C.; Qin, Y.; Che, S. Effects of Revetments on Nitrification and Denitrification Potentials in the Urban River–Riparian Interface. Land 2024, 13, 333. [Google Scholar] [CrossRef]
- Xu, X.; Ran, Y.; Li, Y.; Zhang, Q.; Liu, Y.; Pan, H.; Guan, X.; Li, J.; Shi, J.; Dong, L.; et al. Warmer and drier conditions alter the nitrifier and denitrifier communities and reduce N2O emissions in fertilized vegetable soils. Agric. Ecosyst. Environ. 2016, 231, 133–142. [Google Scholar] [CrossRef]
- E Taylor, A.; Giguere, A.T.; Zoebelein, C.M.; Myrold, D.D.; Bottomley, P.J. Modeling of soil nitrification responses to temperature reveals thermodynamic differences between ammonia-oxidizing activity of archaea and bacteria. ISME J. 2016, 11, 896–908. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Liu, X.; Li, Y.; Ran, Y.; Liu, Y.; Zhang, Q.; Li, Z.; He, Y.; Xu, J.; Di, H. High temperatures inhibited the growth of soil bacteria and archaea but not that of fungi and altered nitrous oxide production mechanisms from different nitrogen sources in an acidic soil. Soil Biol. Biochem. 2017, 107, 168–179. [Google Scholar] [CrossRef]
- Portilho, I.I.R.; Savin, M.C.; Borges, C.D.; Tsai, S.M.; Mercante, F.M.; Roscoe, R.; de Carvalho, L.A. Maintenance of N cycling gene communities with crop-livestock integration management in tropical agriculture systems. Agric. Ecosyst. Environ. 2018, 267, 52–62. [Google Scholar] [CrossRef]
- Mukhtar, H.; Lin, Y.-P.; Lin, C.-M.; Petway, J.R. Assessing thermodynamic parameter sensitivity for simulating temperature responses of soil nitrification. Environ. Sci. Process. Impacts 2019, 21, 1596–1608. [Google Scholar] [CrossRef]
- Tzanakakis, V.A.; Taylor, A.E.; Bakken, L.R.; Bottomley, P.J.; Myrold, D.D.; D¨orsch, P. Relative activity of ammonia oxidizing archaea and bacteria determine nitrification-dependent N2O emissions in Oregon forest soils. Soil Biol. Biochem. 2019, 139, 107612. [Google Scholar] [CrossRef]
- Sun, H.; Jiang, S. A review on nirS-type and nirK-type denitrifiers via a scientometric approach coupled with case studies. Environ. Sci. Process. Impacts 2022, 24, 221–232. [Google Scholar] [CrossRef]
- Andrade, R.; Mancini, M.; Teixeira, A.F.d.S.; Silva, S.H.G.; Weindorf, D.C.; Chakraborty, S.; Guilherme, L.R.G.; Curi, N. Proximal sensor data fusion and auxiliary information for tropical soil property prediction: Soil texture. Geoderma 2022, 422, 115936. [Google Scholar] [CrossRef]
- Man, Z.; Weng, B.; Yang, Y.; Gong, X.; Li, M.; Yu, Z. Effects of the Freezing–Thawing Cycle Mode on Alpine Vegetation in the Nagqu River Basin of the Qinghai–Tibet Plateau. Water 2019, 11, 2122. [Google Scholar] [CrossRef]
- Yan, L.; Xie, C.; Xu, X.; Che, S. Effects of revetment type on the spatial distribution of soil nitrification and denitrification in adjacent tidal urban riparian zones. Ecol. Eng. 2019, 132, 65–74. [Google Scholar] [CrossRef]
- Liu, W.; Xiong, Z.; Liu, H.; Zhang, Q.; Liu, G. Catchment agriculture and local environment affecting the soil denitrification potential and nitrous oxide production of riparian zones in the Han River Basin, China. Agric. Ecosyst. Environ. 2016, 216, 147–154. [Google Scholar] [CrossRef]
- Zhao, S.; Wang, Q.; Zhou, J.; Yuan, D.; Zhu, G. Linking abundance and community of microbial N2O-producers and N2O-reducers with enzymatic N2O production potential in a riparian zone. Sci. Total. Environ. 2018, 642, 1090–1099. [Google Scholar] [CrossRef] [PubMed]
- Stewart, F.J.; Ulloa, O.; DeLong, E.F. Microbial meta transcriptomics in a permanent marine oxygen minimum zone. Environ. Microbiol. 2012, 14, 23–40. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Hu, B.; He, Z.; Zhang, B.; Tian, G.; Zheng, P.; Fang, F. Ammonia-oxidizing archaea have better adaptability in oxygenated/hypoxic alternant conditions compared to ammonia-oxidizing bacteria. Appl. Microbiol. Biotechnol. 2015, 99, 8587–8596. [Google Scholar] [CrossRef]
- Du, J.; Meng, L.; Qiu, M.; Chen, S.; Zhang, B.; Song, W.; Cong, P.; Zheng, X. Ammonia-oxidizing archaea and ammonia-oxidizing bacteria communities respond differently in oxy-gen-limited habitats. Front. Environ. Sci. 2022, 10, 976618. [Google Scholar] [CrossRef]
- Rajaram, G.; Erbach, D. Effect of wetting and drying on soil physical properties. J. Terramechanics 1999, 36, 39–49. [Google Scholar] [CrossRef]
- Cosentino, D.; Chenu, C.; Le Bissonnais, Y. Aggregate stability and microbial community dynamics under drying–wetting cycles in a silt loam soil. Soil Biol. Biochem. 2006, 38, 2053–2062. [Google Scholar] [CrossRef]
- Jozefaciuk, G.; Czachor, H. Impact of organic matter, iron oxides, alumina, silica and drying on mechanical and water stability of artificial soil aggregates. Assessment of new method to study water stability. Geoderma 2014, 221–222, 1–10. [Google Scholar] [CrossRef]
- Xu, X.-T.; Shao, L.-J.; Huang, J.-B.; Xu, X.; Liu, D.-Q.; Xian, Z.-X.; Jian, W.-B. Effect of wet-dry cycles on shear strength of residual soil. Soils Found. 2021, 61, 782–797. [Google Scholar] [CrossRef]
- Wang, T.; Franz, T.E.; Zlotnik, V.A.; You, J.; Shulski, M.D. Investigating soil controls on soil moisture spatial variability: Numerical simulations and field observations. J. Hydrol. 2015, 524, 576–586. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, Z.; Guo, Z.; Xiong, P.; Peng, X. The dynamic changes of soil air-filled porosity associated with soil shrinkage in a Vertisol. Eur. J. Soil Sci. 2022, 73, e13313. [Google Scholar] [CrossRef]
- Morugán-Coronado, A.; García-Orenes, F.; Mataix-Solera, J.; Arcenegui, V.; Mataix-Beneyto, J. Short-term effects of treated wastewater irrigation on Mediterranean calcareous soil. Soil Tillage Res. 2011, 112, 18–26. [Google Scholar] [CrossRef]
- Rabbi, S.M.; Warren, C.R.; Swarbrick, B.; Minasny, B.; McBratney, A.B.; Young, I.M. Microbial decomposition of organic matter and wetting–drying promotes aggregation in artificial soil but porosity increases only in wet-dry condition. Geoderma 2024, 447, 116924. [Google Scholar] [CrossRef]
- Martens-Habbena, W.; Berube, P.M.; Urakawa, H.; de la Torre, J.R.; Stahl, D.A. Ammonia oxidation kinetics determine niche separation of nitrifying Archaea and Bacteria. Nature 2009, 461, 976–979. [Google Scholar] [CrossRef]
- Verhamme, D.T.; I Prosser, J.; Nicol, G.W. Ammonia concentration determines differential growth of ammonia-oxidising archaea and bacteria in soil microcosms. ISME J. 2011, 5, 1067–1071. [Google Scholar] [CrossRef]
- Wang, C.; Tang, S.; He, X.; Ji, G. The abundance and community structure of active ammonia-oxidizing archaea and ammonia-oxidizing bacteria shape their activities and contributions in coastal wetlands. Water Res. 2020, 171, 115464. [Google Scholar] [CrossRef] [PubMed]
- Desnues, C.; Michotey, V.D.; Wieland, A.; Zhizang, C.; Fourçans, A.; Duran, R.; Bonin, P.C. Seasonal and diel distributions of denitrifying and bacterial communities in a hypersaline microbial mat (Camargue, France). Water Res. 2007, 41, 3407–3419. [Google Scholar] [CrossRef] [PubMed]
- Knapp, C.W.; Dodds, W.K.; Wilson, K.C.; O’brien, J.M.; Graham, D.W. Spatial Heterogeneity of Denitrification Genes in a Highly Homogenous Urban Stream. Environ. Sci. Technol. 2009, 43, 4273–4279. [Google Scholar] [CrossRef]
- Graham, D.W.; Trippett, C.; Dodds, W.K.; O’brien, J.M.; Banner, E.B.; Head, I.M.; Smith, M.S.; Yang, R.K.; Knapp, C.W. Correlations between in situ denitrification activity and nir-gene abundances in pristine and impacted prairie streams. Environ. Pollut. 2010, 158, 3225–3229. [Google Scholar] [CrossRef]
- Zhang, L.M.; Hu, H.W.; Shen, J.P.; He, J.Z. Ammonia-oxidizing archaea have more important role than ammo-nia-oxidizing bacteria in ammonia oxidation of strongly acidic soils. ISME J. 2012, 6, 1032–1045. [Google Scholar] [CrossRef] [PubMed]
- Duan, P.; Wu, Z.; Zhang, Q.; Fan, C.; Xiong, Z. Thermodynamic responses of ammonia-oxidizing archaea and bacteria explain N2O production from greenhouse vegetable soils. Soil Biol. Biochem. 2018, 120, 37–47. [Google Scholar] [CrossRef]
- Lin, Y.-P.; Ansari, A.; Wunderlich, R.F.; Lur, H.-S.; Cao, T.N.-D.; Mukhtar, H. Assessing the influence of environmental niche segregation in ammonia oxidizers on N2O fluxes from soil and sediments. Chemosphere 2022, 289, 133049. [Google Scholar] [CrossRef]
- Kim, J.; Jung, M.; Park, S.; Rijpstra, W.; Sinninghe Damsté, J.; Madsen, E.; Rhee, S. Cultivation of a highly enriched ammonia-oxidizing archaeon of thaumarchaeotal group I.1b from an agricultural soil. Environ. Microbiol. 2012, 14, 1528–1543. [Google Scholar] [CrossRef]
- Jin, K.; Shen, J.; Ashton, R.W.; White, R.P.; Dodd, I.C.; Parry, M.A.J.; Whalley, W.R. Wheat root growth responses to horizontal stratification of fertiliser in a water-limited environment. Plant Soil 2014, 386, 77–88. [Google Scholar] [CrossRef]
- Lange, M.; Eisenhauer, N.; Sierra, C.A.; Bessler, H.; Engels, C.; Griffiths, R.I.; Mellado-Vázquez, P.G.; Malik, A.A.; Roy, J.; Scheu, S.; et al. Plant diversity increases soil microbial activity and soil carbon storage. Nat. Commun. 2015, 6, 6707. [Google Scholar] [CrossRef] [PubMed]
- Bengough, A.G.; Loades, K.; McKenzie, B.M. Root hairs aid soil penetration by anchoring the root surface to pore walls. J. Exp. Bot. 2016, 64, 1071–1078. [Google Scholar] [CrossRef]
- Huang, T.; Gao, B.; Hu, X.-K.; Lu, X.; Well, R.; Christie, P.; Bakken, L.R.; Ju, X.-T. Ammonia-oxidation as an engine to generate nitrous oxide in an intensively managed calcareous Fluvo-aquic soil. Sci. Rep. 2014, 4, 3950. [Google Scholar] [CrossRef]
- Jones, C.; Stres, B.; Rosenquist, M.; Hallin, S. Phylogenetic Analysis of Nitrite, Nitric Oxide, and Nitrous Oxide Res-piratory Enzymes Reveal a Complex Evolutionary History for Denitrification. Mol. Biol. Evol. 2008, 25, 1955–1966. [Google Scholar] [CrossRef] [PubMed]
- Graf, D.R.H.; Jones, C.M.; Hallin, S. Intergenomic Comparisons Highlight Modularity of the Denitrification Pathway and Underpin the Importance of Community Structure for N2O Emissions. PLoS ONE 2014, 9, e114118. [Google Scholar] [CrossRef]
- Yoon, S.; Nissen, S.; Park, D.; Sanford, R.A.; Löffler, F.E. Nitrous Oxide Reduction Kinetics Distinguish Bacteria Harboring Clade I NosZ from Those Harboring Clade II NosZ. Appl. Environ. Microbiol. 2016, 82, 3793–3800. [Google Scholar] [CrossRef] [PubMed]
- Suenaga, T.; Hori, T.; Riya, S.; Hosomi, M.; Terada, A. Enrichment, isolation, and characterization of high-affinity N2O-reducing bacteria in a gas-permeable membrane reactor. Environ. Sci. Technol. 2019, 53, 12101–12112. [Google Scholar] [CrossRef] [PubMed]
- Suenaga, T.; Riya, S.; Hosomi, M.; Terada, A. Biokinetic characterization and activities of N2O-reducing bacteria in response to various oxygen levels. Front. Microbiol. 2018, 9, 697–707. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Zhou, J.; Yuan, D.; Wang, W.; Zhou, L.; Pi, Y.; Zhu, G. NirS-type N2O-producers and nosZ II-type N2O-reducers determine the N2O emission potential in farmland rhizosphere soils. J. Soils Sediments 2019, 20, 461–471. [Google Scholar] [CrossRef]
- Thomson, A.J.; Giannopoulos, G.; Pretty, J.; Baggs, E.M.; Richardson, D.J. Biological sources and sinks of nitrous oxide and strategies to mitigate emissions. Philos. Trans. R. Soc. B Biol. Sci. 2012, 367, 1157–1168. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.; Ding, W.; Luo, J. Nitrous oxide emissions from Chinese maizeewheat rotation systems: A 3-year field measurement. Atmos. Environ. 2013, 65, 112–122. [Google Scholar] [CrossRef]
- Wu, L.; Tang, S.; He, D.; Wu, X.; Shaaban, M.; Wang, M.; Zhao, J.; Khan, I.; Zheng, X.; Hu, R.; et al. Conversion from rice to vegetable production increases N2O emission via increased soil organic matter mineralization. Sci. Total Environ. 2017, 583, 190–201. [Google Scholar] [CrossRef] [PubMed]
- Felgate, F.; Giannopoulos, G.; Sullivan, M.J.; Gates, A.J.; Clarke, T.A.; Baggs, E.; Rowley, G.; Richardson, D.J. The impact of copper, nitrate and carbon status on the emission of nitrous oxide by two species of bacteria with biochemically distinct denitri-fication pathways. Environ. Microbiol. 2012, 14, 1788–1800. [Google Scholar] [CrossRef] [PubMed]
- Juhanson, J.; Hallin, S.; Söderström, M.; Stenberg, M.; Jones, C.M. Spatial and phyloecological analyses of nosZ genes underscore niche differentiation amongst terrestrial N2O reducing communities. Soil Biol. Biochem. 2017, 115, 82–91. [Google Scholar] [CrossRef]
- Domeignoz-Horta, L.; Putz, M.; Spor, A.; Bru, D.; Breuil, M.; Hallin, S.; Philippot, L. Non-denitrifying nitrous oxide-reducing bacteria—An effective N2O sink in soil. Soil Biol. Biochem. 2016, 103, 376–379. [Google Scholar] [CrossRef]






| Denitrification | Direct Effects | Indirect Effects | Total Effects |
|---|---|---|---|
| nirK | 0.87 | 0 | 0.87 |
| C/N | 0 | −0.6003 | −0.6003 |
| NO3− | 0 | −0.1218 | −0.1218 |
| NP | −0.28 | −0.099876 | −0.379876 |
| AOA | 0 | −0.266 | −0.266 |
| UB | 0 | −0.20216 | −0.20216 |
| N2O Emissions | Direct Effects | Indirect Effects | Total Effects |
| nosZII | −0.56 | 0 | −0.56 |
| nosZI | −0.04 | 0 | −0.04 |
| nirS | −0.29 | −0.112 | −0.402 |
| NO3− | 0 | −0.7115 | −0.7115 |
| AB | −0.36 | −0.2253 | −0.5853 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Man, Z.; Xie, C.; Jiang, R.; Wang, J.; Qin, Y.; Che, S. Revetment Affects Nitrogen Removal and N2O Emission at the Urban River–Riparian Interface. Land 2024, 13, 1310. https://doi.org/10.3390/land13081310
Man Z, Xie C, Jiang R, Wang J, Qin Y, Che S. Revetment Affects Nitrogen Removal and N2O Emission at the Urban River–Riparian Interface. Land. 2024; 13(8):1310. https://doi.org/10.3390/land13081310
Chicago/Turabian StyleMan, Zihao, Changkun Xie, Ruiyuan Jiang, Jin Wang, Yifeng Qin, and Shengquan Che. 2024. "Revetment Affects Nitrogen Removal and N2O Emission at the Urban River–Riparian Interface" Land 13, no. 8: 1310. https://doi.org/10.3390/land13081310
APA StyleMan, Z., Xie, C., Jiang, R., Wang, J., Qin, Y., & Che, S. (2024). Revetment Affects Nitrogen Removal and N2O Emission at the Urban River–Riparian Interface. Land, 13(8), 1310. https://doi.org/10.3390/land13081310









