Analysis of Spatial Divergence in Bird Diversity Driven by Built Environment Characteristics of Ecological Corridors in High-Density Urban Areas
Abstract
:1. Introduction
2. Materials and Methods
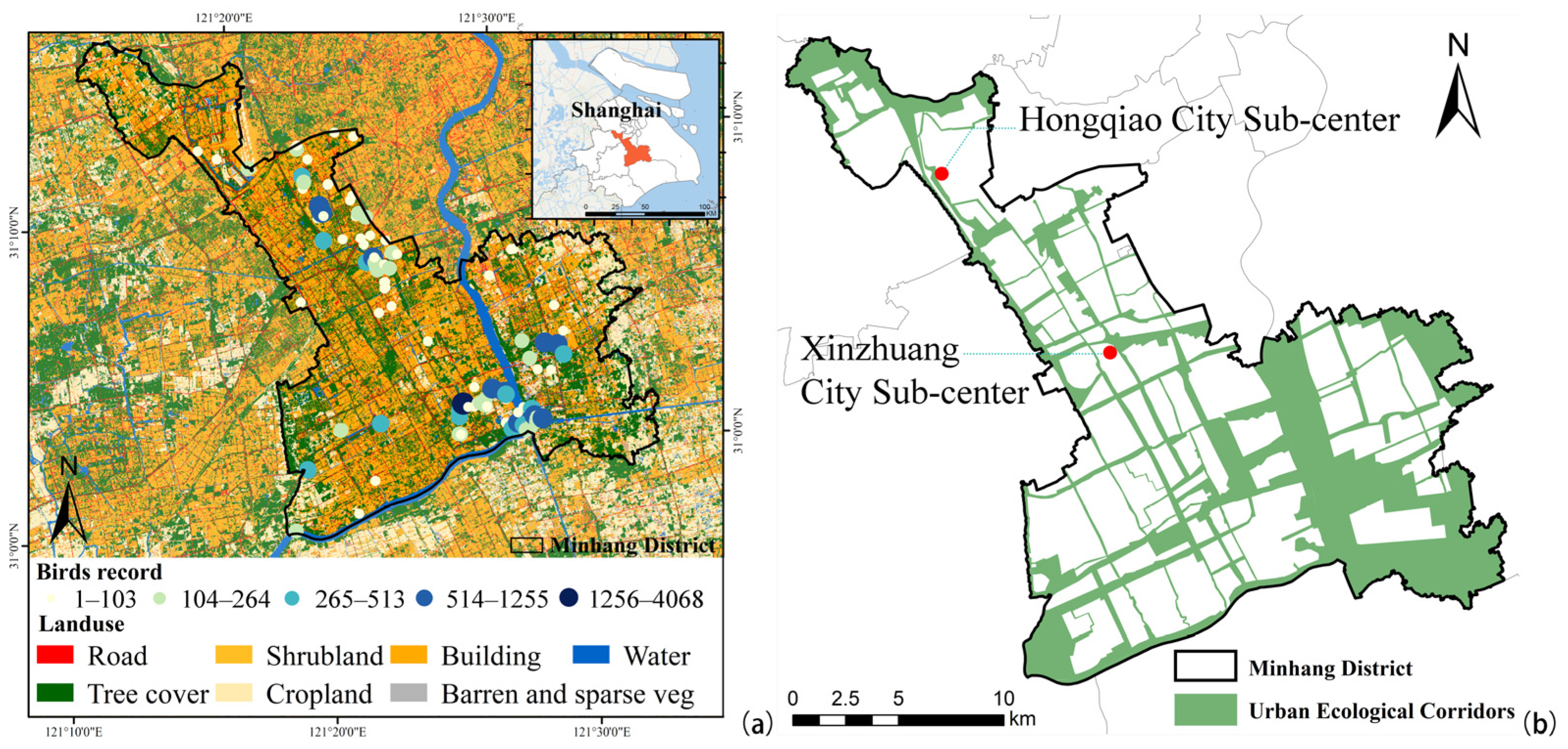
2.1. Study Area
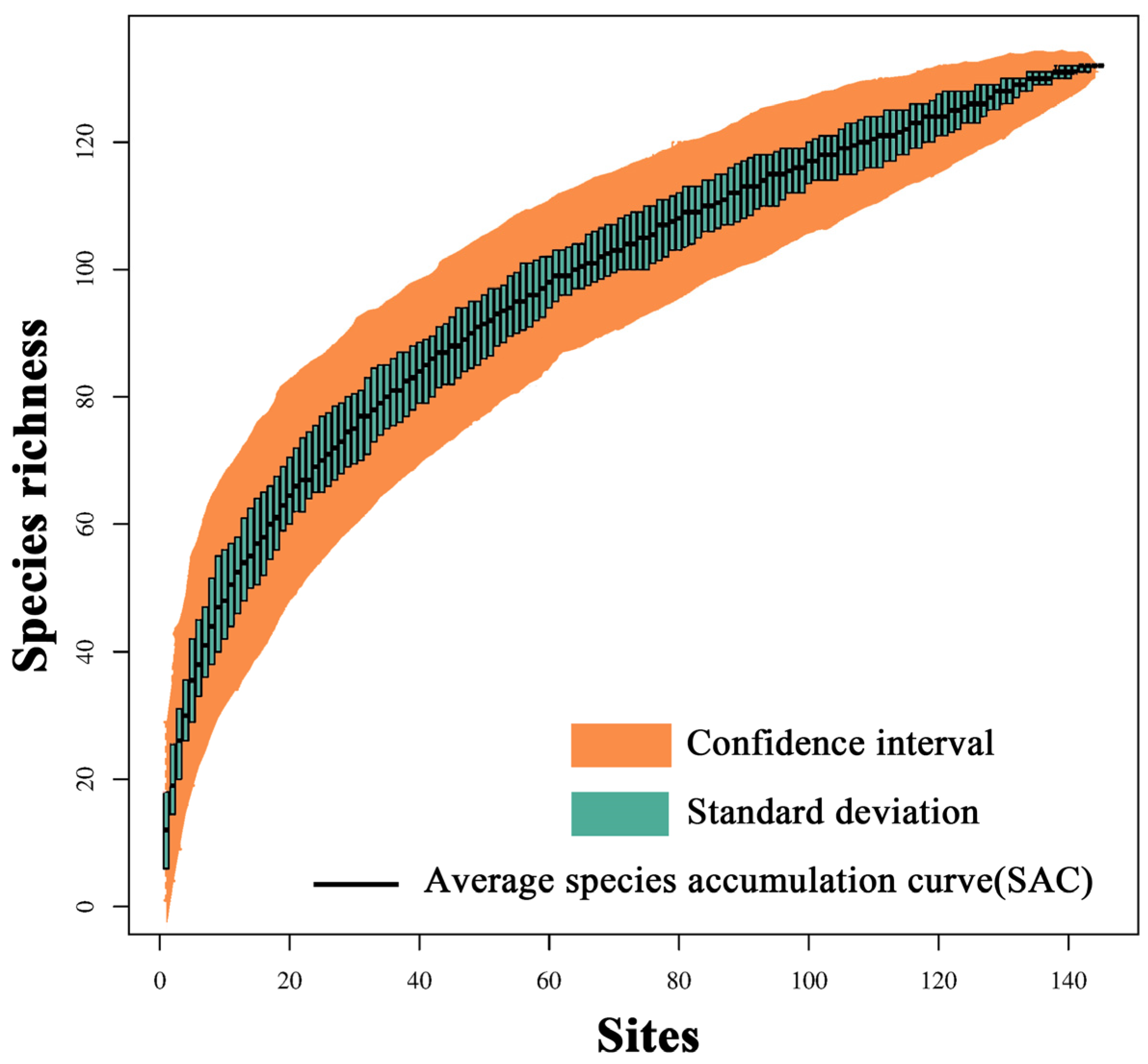
2.2. Bird Surveys and Diversity
2.3. Built Environment Factors for Urban Ecological Corridors
2.4. Generalized Linear Model
2.5. Data Processing and Analysis
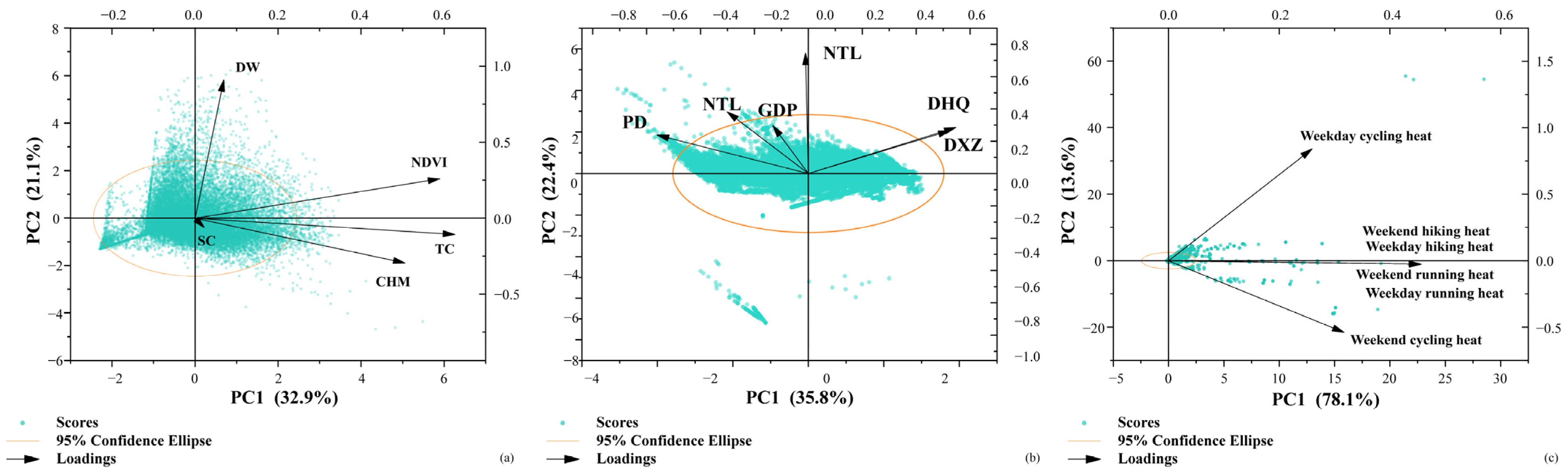
2.5.1. Characteristics of the Built Environment of Urban Ecological Corridors
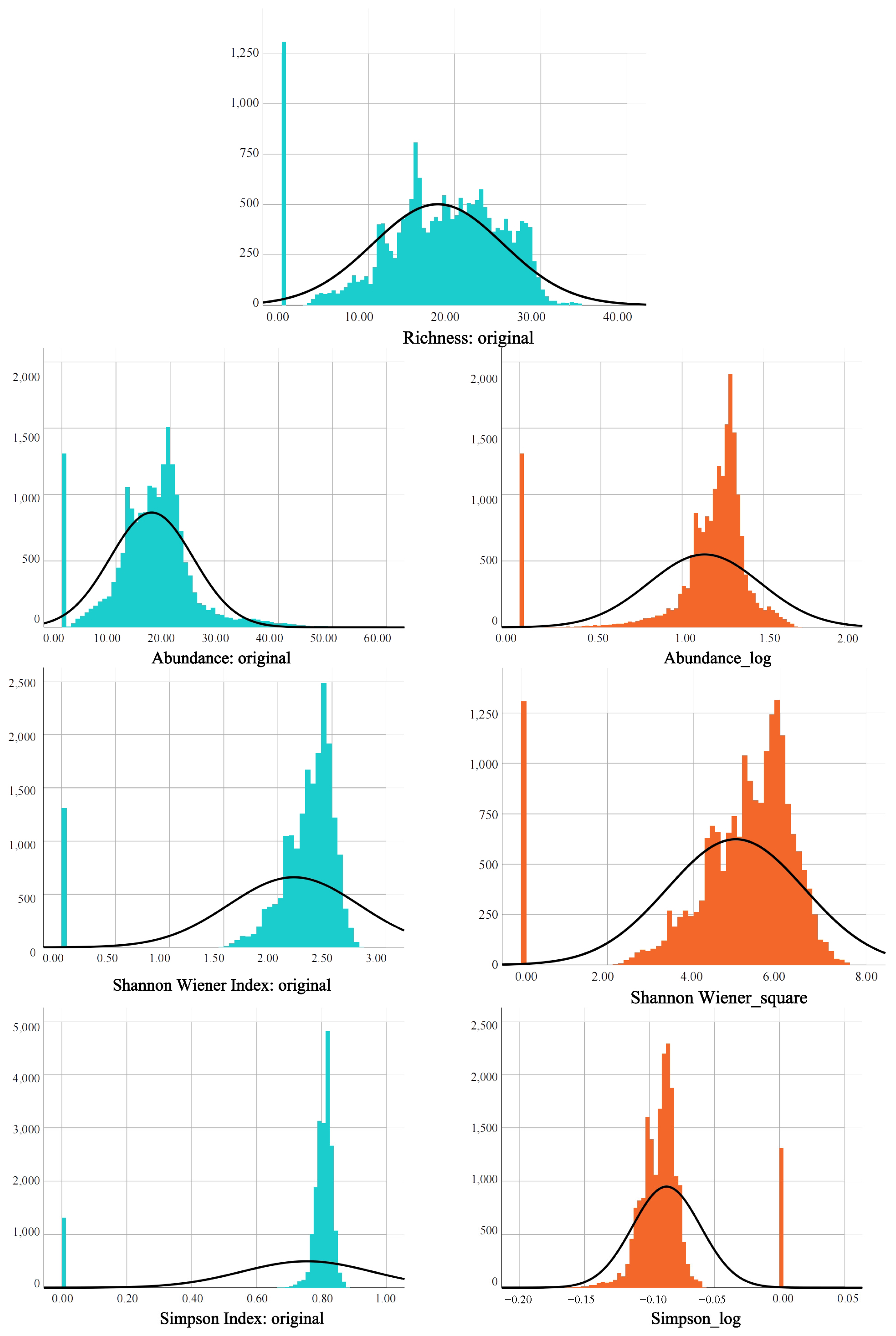
2.5.2. Bird Diversity Data
3. Results
3.1. Impacts of Built Environment Characteristics of Urban Ecological Corridors on Bird Diversity
3.1.1. Habitat Environment
3.1.2. Degree of Urbanization
3.1.3. Slow-Traffic Connectivity
3.2. Characteristics of Built Environments in Urban Ecological Corridors and Spatial Differentiation Patterns of Bird Diversity
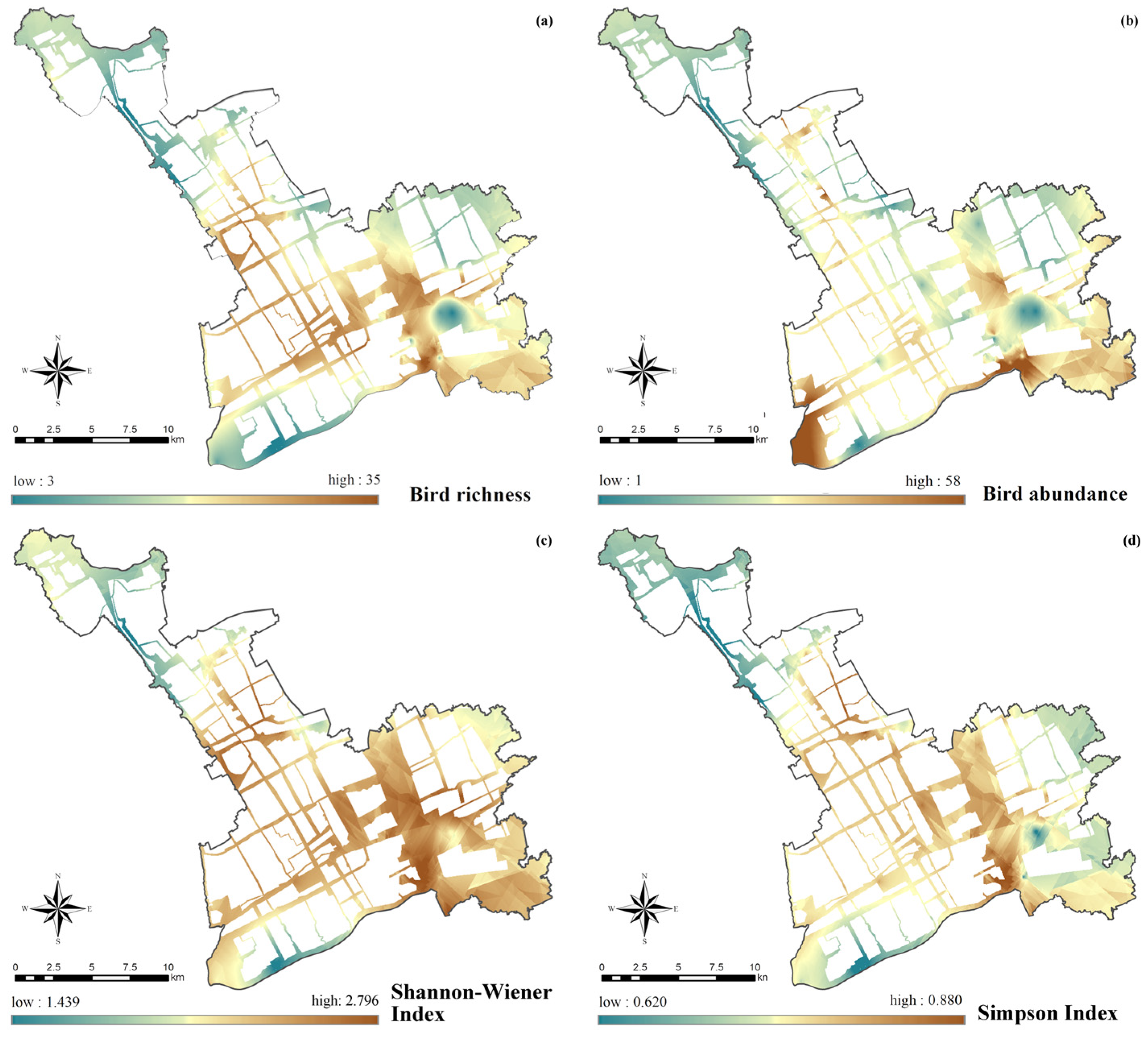
3.2.1. Habitat Suitability Index
3.2.2. Degree of Urbanization
3.2.3. Slow-Traffic Connectivity
4. Discussion
4.1. Characterization of the Built Environment of Urban Ecological Corridors Drives Attribution of Spatial Divergence in Bird Diversity
4.2. Planning and Management of Urban Ecological Corridors Based on Biodiversity Conservation
4.3. Shortcomings and Prospects
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ganaie, T.A.; Jamal, S.; Ahmad, W.S. Changing land use/land cover patterns and growing human population in Wular catchment of Kashmir Valley, India. GeoJournal 2021, 86, 1589–1606. [Google Scholar] [CrossRef]
- Afrifa, J.K.; Monney, K.A.; Deikumah, J.P. Effects of urban land-use types on avifauna assemblage in a rapidly developing urban settlement in Ghana. Urban Ecosyst. 2023, 26, 67–79. [Google Scholar] [CrossRef]
- Van den Bosch, M.; Sang, O. Urban natural environments as nature-based solutions for improved public health—A systematic review of reviews. Environ. Res. 2017, 158, 373–384. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Yu, H.; Zhong, Q.; Zhang, G.; Zhang, Q. Research on the Spatial Demarcation of Urban Ecological Corridors Based on Functional Connectivity. Chin. Landsc. Archit. 2024, 40, 6–14. (In Chinese) [Google Scholar]
- Jordán, F. A reliability-theory approach to corridor design. Ecol. Model. 2000, 128, 211–220. [Google Scholar] [CrossRef]
- Hastedt, A.; Tietze, D.T. The importance of unsealed areas in the urban core and periphery for bird diversity in a large central european city. Urban Ecosyst. 2023, 26, 1015–1028. [Google Scholar] [CrossRef]
- LaPoint, S.; Balkenhol, N.; Hale, J.; Sadler, J.; van der Ree, R.; Evans, K. Ecological connectivity research in urban areas. Funct. Ecol. 2015, 29, 868–878. [Google Scholar] [CrossRef]
- Zhang, Z.; Meerow, S.; Newell, J.P.; Lindquist, M. Enhancing landscape connectivity through multifunctional green infrastructure corridor modeling and design. Urban For. Urban Green. 2019, 38, 305–317. [Google Scholar] [CrossRef]
- Muderere, T.; Murwira, A.; Kativu, S.; Tagwireyi, P.; Chiweshe, N. The influence of landscape structure on the diversity of avifauna species in tropical urban areas of Northeastern Zimbabwe. Biodiversity 2020, 21, 182–197. [Google Scholar] [CrossRef]
- Hedblom, M.; Knez, I.; Gunnarsson, B. Bird Diversity Improves the Well-Being of City Residents. Ecol. Conserv. Birds Urban Environ. 2017, 287–306. [Google Scholar] [CrossRef]
- Brodie, J.F.; Mohd-Azlan, J.; Chen, C.; Wearn, O.R.; Deith, M.C.; Ball, J.G.; Luskin, M.S. Landscape-scale benefits of protected areas for tropical biodiversity. Nature 2023, 620, 807–812. [Google Scholar] [CrossRef] [PubMed]
- Herrando, S.; Weiserbs, A.; Quesada, J.; Ferrer, X.; Paquet, J.-Y. Development of urban bird indicators using data from monitoring schemes in two large European cities. Anim. Biodivers. Conserv. 2012, 35, 141–150. [Google Scholar] [CrossRef]
- McDonald, R.I.; Forman, R.T.T.; Kareiva, P.; Neugarten, R.; Salzer, D.; Fisher, J. Urban effects, distance, and protected areas in an urbanizing world. Landsc. Urban Plan. 2009, 93, 63–75. [Google Scholar] [CrossRef]
- Gan, J.; Breuste, J. Relationship Between Built Environment and Biodiversity in High-Density Metropolitan Areas: The Case of Shanghai, China. In Making Green Cities; Springer: Cham, Switzerland, 2023; pp. 377–400. [Google Scholar] [CrossRef]
- Hernández-Dávila, O.A.; Sosa, V.J.; Laborde, J. Effects of landscape context and vegetation attributes on understorey bird communities of cloud forest riparian belts. Ecol. Eng. 2021, 167, 106269. [Google Scholar] [CrossRef]
- Souza, F.L.; Valente-Neto, F.; Severo-Neto, F.; Bueno, B.; Ochoa-Quintero, J.M.; Laps, R.R.; de Oliveira Roque, F. Impervious surface and heterogeneity are opposite drivers to maintain bird richness in a Cerrado cit. Landsc. Urban Plan. 2019, 192, 103643. [Google Scholar] [CrossRef]
- Latta, S.C.; Musher, L.J.; Latta, K.N.; Katzner, T.E. Influence of human population size and the built environment on avian assemblages in urban green spaces. Urban Ecosyst. 2013, 16, 463–479. [Google Scholar] [CrossRef]
- Matsuba, M.; Nishijima, S.; Katoh, K. Effectiveness of corridor vegetation depends on urbanization tolerance of forest birds in central Tokyo, Japan. Urban For. Urban Green. 2016, 18, 173–181. [Google Scholar] [CrossRef]
- Threlfall, C.G.; Williams, N.S.G.; Hahs, A.K.; Livesley, S.J. Approaches to urban vegetation management and the impacts on urban bird and bat assemblages. Landsc. Urban Plan. 2016, 153, 28–39. [Google Scholar] [CrossRef]
- Cristaldi, M.A.; Giraudo, A.R.; Arzamendia, V.; Bellini, G.P.; Claus, J. Urbanization impacts on the trophic guild composition of bird communities. J. Nat. Hist. 2017, 51, 2385–2404. [Google Scholar] [CrossRef]
- Opoku, A. Biodiversity and the built environment: Implications for the Sustainable Development Goals (SDGs). Resour. Conserv. Recycl. 2019, 141, 1–7. [Google Scholar] [CrossRef]
- Petit, M.; Celis, C.; Weideman, C.; Gouin, N.; Bertin, A. Effects of land cover and habitat condition on the bird community along a gradient of agricultural development within an arid watershed of Chile. Agric. Ecosyst. Environ. 2023, 356, 108635. [Google Scholar] [CrossRef]
- Zhang, R.; Zhang, L.; Zhong, Q.; Zhang, Q.; Ji, Y.; Song, P.; Wang, Q. An optimized evaluation method of an urban ecological network: The case of the Minhang District of Shanghai. Urban For. Urban Green. 2021, 62, 127158. [Google Scholar] [CrossRef]
- Zheng, G. (Ed.) A List of the Classification and Distribution of Birds in China, 3rd ed.; China Science Publishing & Media Ltd.: Beijing, China, 2017. (In Chinese) [Google Scholar]
- Bevilacqua, S.; Ugland, K.I.; Plicanti, A.; Scuderi, D.; Terlizzi, A. An approach based on the total-species accumulation curve and higher taxon richness to estimate realistic upper limits in regional species richness. Ecol. Evol. 2018, 8, 405–415. [Google Scholar] [CrossRef]
- Dixon, P. VEGAN, a package of R functions for community ecology. J. Veg. Sci. 2003, 14, 927–930. [Google Scholar] [CrossRef]
- Sun, B.; Lu, Y.; Yang, Y.; Yu, M.; Yuan, J.; Yu, R.; Bullock, J.M.; Stenseth, N.C.; Li, X.; Cao, Z.; et al. Urbanization affects spatial variation and species similarity of bird diversity distribution. Sci. Adv. 2022, 8, eade3061. [Google Scholar] [CrossRef]
- Mahmoudi, S.; Ilanloo, S.S.; Shahrestanaki, A.K.; Valizadegan, N.; Yousefi, M. Effect of human-induced forest edges on the understory bird community in Hyrcanian forests in Iran: Implication for conservation and management. For. Ecol. Manag. 2016, 382, 120–128. [Google Scholar] [CrossRef]
- Zhang, W.; Zhou, Y.; Fang, X.; Zhao, S.; Wu, Y.; Zhang, H.; Cui, L.; Cui, P. Effects of Environmental Factors on Bird Communities in Different Urbanization Grades: An Empirical Study in Lishui, a Mountainous Area of Eastern China. Animals 2023, 13, 882. [Google Scholar] [CrossRef]
- Hsieh, T.; Ma, K.; Chao, A. iNEXT: An R package for rarefaction and extrapolation of species diversity (Hill numbers). Methods Ecol. Evol. 2016, 7, 1451–1456. [Google Scholar] [CrossRef]
- Liu, Z.; Zhou, Y.; Yang, H.; Liu, Z. Urban green infrastructure affects bird biodiversity in the coastal megalopolis region of Shenzhen city. Appl. Geogr. 2023, 151, 102860. [Google Scholar] [CrossRef]
- Wu, W.-B. A first Chinese building height estimate at 10 m resolution (CNBH-10 m) using multi-source earth observations and machine learning. Remote Sens. Environ. 2023, 291, 113578. [Google Scholar] [CrossRef]
- Dai, S.; Zhao, W.; Wang, Y.; Huang, X.; Chen, Z.; Lei, J.; Stein, A.; Jia, P. Assessing spatiotemporal bikeability using multi-source geospatial big data: A case study of Xiamen, China. Int. J. Appl. Earth Obs. Geoinf. 2023, 125, 103539. [Google Scholar] [CrossRef]
- Powell, B.F.; Steidl, R.J. Influence of vegetation on montane riparian bird communities in the Sky Islands of Arizona, USA. Southwest. Nat. 2015, 60, 65–71. [Google Scholar] [CrossRef]
- Cantonati, M.; Poikane, S.; Pringle, C.M.; Stevens, L.E.; Turak, E.; Heino, J.; Richardson, J.S.; Bolpagni, R.; Borrini, A.; Cid, N.; et al. Characteristics, Main Impacts, and Stewardship of Natural and Artificial Freshwater Environments: Consequences for Biodiversity Conservation. Water 2020, 12, 260. [Google Scholar] [CrossRef]
- Fahrig, L. Rethinking patch size and isolation effects: The habitat amount hypothesis. J. Biogeogr. 2013, 40, 1649–1663. [Google Scholar] [CrossRef]
- Ferenc, M.; Sedláček, O.; Fuchs, R. How to improve urban greenspace for woodland birds: Site and local-scale determinants of bird species richness. Urban Ecosyst. 2014, 17, 625–640. [Google Scholar] [CrossRef]
- Jasmani, Z.; Ravn, H.; Bosch, C. The influence of small urban parks characteristics on bird diversity: A case study of Petaling Jaya, Malaysia. Urban Ecosyst. 2017, 20, 227–243. [Google Scholar] [CrossRef]
- Zhenhuan, L.; Qiandu, H.; Tang, G. Identification of urban flight corridors for migratory birds in the coastal regions of Shenzhen city based on three-dimensional landscapes. Landsc. Ecol. 2021, 36, 2043–2057. [Google Scholar] [CrossRef]
- de Groot, M.; Flajšman, K.; Mihelič, T.; Vilhar, U.; Simončič, P.; Verlič, A. Green space area and type affect bird communities in a South-eastern European city. Urban For. Urban Green. 2021, 63, 127212. [Google Scholar] [CrossRef]
- James Barth, B.; Ian FitzGibbon, S.; Stuart Wilson, R. New urban developments that retain more remnant trees have greater bird diversity. Landsc. Urban Plan. 2015, 136, 122–129. [Google Scholar] [CrossRef]
- Leveau, L.M.; Leveau, C.M. Does urbanization affect the seasonal dynamics of bird communities in urban parks? Urban Ecosyst. 2016, 19, 631–647. [Google Scholar] [CrossRef]
- Van Doren, B.M.; Willard, D.E.; Hennen, M.; Horton, K.G.; Stuber, E.F.; Sheldon, D.; Sivakumar, A.H.; Wang, J.; Farnsworth, A.; Winger, B.M. Drivers of fatal bird collisions in an urban center. Proc. Natl. Acad. Sci. USA 2021, 118, e2101666118. [Google Scholar] [CrossRef] [PubMed]
- Lao, S.; Robertson, B.A.; Anderson, A.W.; Blair, R.B.; Eckles, J.W.; Turner, R.J.; Loss, S.R. The influence of artificial night at night and polarized light on bird-building collisions. Biol. Conserv. 2020, 241, 108358. [Google Scholar] [CrossRef]
- Loss, S.R.s.l.o.e.; Will, T.; Loss, S.S.; Marra, P.P. Bird-building collisions in the United States: Estimates of annual mortality and species vulnerability. Condor 2014, 116, 8–23. [Google Scholar] [CrossRef]
- Adams, C.A.; Fernández-Juricic, E.; Bayne, E.M.; Clair, C.C.S. Effects of artificial light on bird movement and distribution: A systematic map. Environ. Evid. 2021, 10, 37. [Google Scholar] [CrossRef]
- Evans, B.S.; Reitsma, R.; Hurlbert, A.H.; Marra, P.P. Environmental filtering of avian communities along a rural_to_urban gradient in Greater Washington, D.C., USA. Ecosphere 2018, 9, 1. [Google Scholar] [CrossRef]
- Shaffer, J.A.; Loesch, C.R.; Buhl, D.A. Estimating offsets for avian displacement effects of anthropogenic impacts. Ecol. Appl. 2019, 29, e01983. [Google Scholar] [CrossRef]
- Jameson, F.; Chace, J.J.W. Urban effects on native avifauna: A review. Landsc. Urban Plan. 2004, 74, 46–69. [Google Scholar] [CrossRef]
- Zhang, Z.; Xia, F.; Yang, D.; Huo, J.; Wang, G.; Chen, H. Spatiotemporal characteristics in ecosystem service value and its interaction with human activities in Xinjiang, China. Ecol. Indic. 2020, 110, 105826. [Google Scholar] [CrossRef]
- Rico-Silva, J.F.; Cruz-Trujillo, E.J.; Colorado, Z.G.J. Influence of environmental factors on bird diversity in greenspaces in an Amazonian city. Urban Ecosyst. 2020, 24, 365–374. [Google Scholar] [CrossRef]
- Tellez-Hernandez, E.; Dominguez-Vega, H.; Zuria, I.; Marin-Togo, M.C.; Gomez-Ortiz, Y. Functional and ecological diversity of urban birds: Conservation and redesign of biocultural landscapes. J. Nat. Conserv. 2023, 73, 126395. [Google Scholar] [CrossRef]
- Puan, C.L.; Yeong, K.L.; Ong, K.W.; Fauzi, M.I.A.; Yahya, M.S.; Khoo, S.S. Influence of landscape matrix on urban bird abundance: Evidence from Malaysian citizen science data. J. Asia-Pac. Biodivers. 2019, 12, 369–375. [Google Scholar] [CrossRef]
- Xie, S.; Marzluff, J.M.; Su, Y.; Wang, Y.; Meng, N.; Wu, T.; Ouyang, Z. The role of urban waterbodies in maintaining bird species diversity within built area of Beijing. Sci. Total Environ. 2022, 806, 150430. [Google Scholar] [CrossRef] [PubMed]
- Kang, W.; Minor, E.; Park, C.-R.; Lee, D. Effects of habitat structure, human disturbance, and habitat connectivity on urban forest bird communities. Urban Ecosyst. 2015, 18, 857–870. [Google Scholar] [CrossRef]
- Wang, Y.; Qu, Z.; Zhong, Q.; Zhang, Q.; Zhang, L.; Zhang, R.; Yi, Y.; Zhang, G.; Li, X.; Liu, J. Delimitation of ecological corridors in a highly urbanizing region based on circuit theory and MSPA. Ecol. Indic. 2022, 142, 109258. [Google Scholar] [CrossRef]
- Radford, J.Q.; Bennett, A.F.; Cheers, G.J. Landscape-level thresholds of habitat cover for woodland-dependent birds. Biol. Conserv. 2005, 124, 317–337. [Google Scholar] [CrossRef]
- Shih, W.-Y. Bird diversity of greenspaces in the densely developed city centre of Taipei. Urban Ecosyst. 2017, 21, 379–393. [Google Scholar] [CrossRef]
- Zhang, R. Impact of spatial structure on the functional connectivity of urban ecological corridors based on quantitative analysis. Urban For. Urban Green. 2023, 89, 128121. [Google Scholar] [CrossRef]
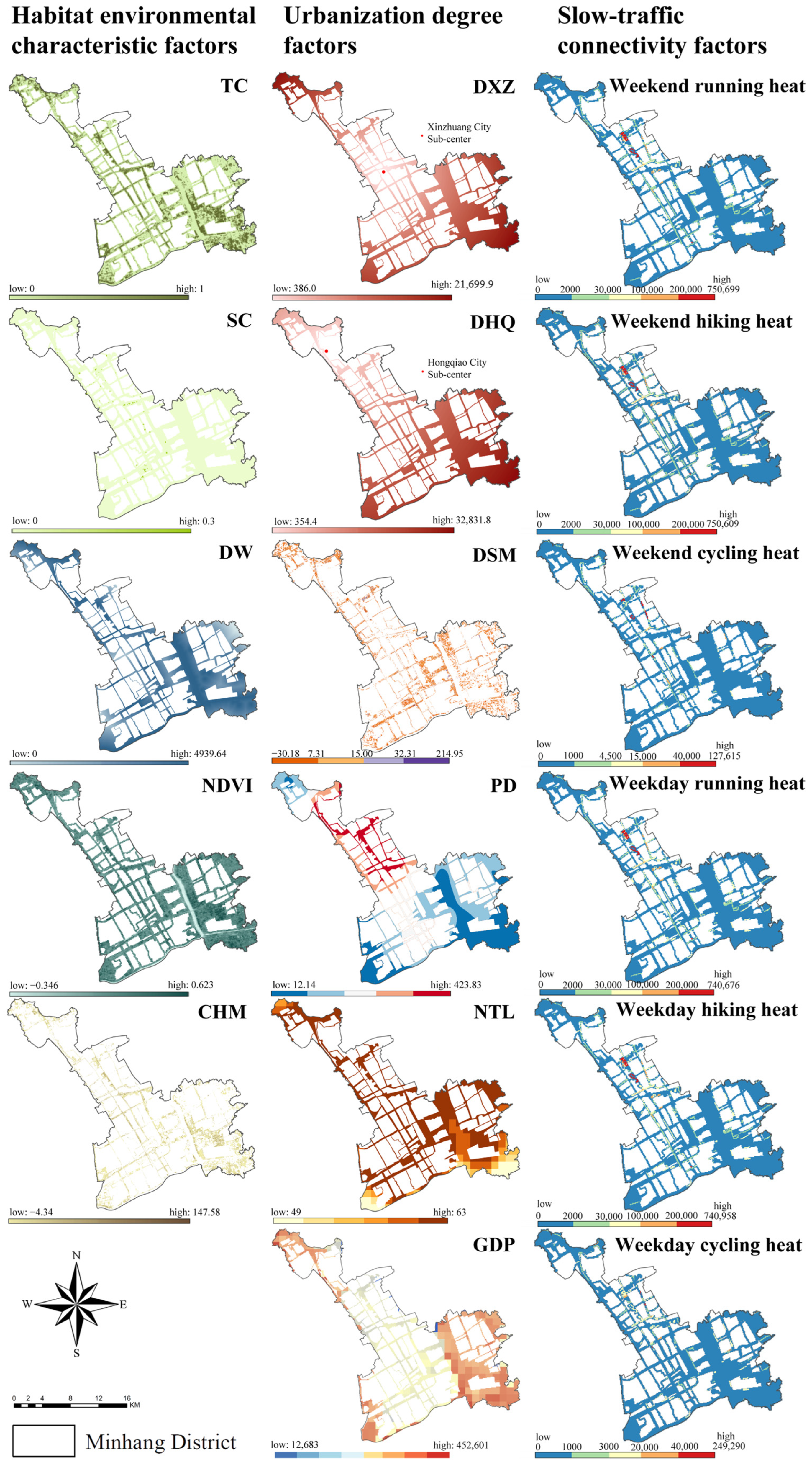

| Factors | Indices (Abbreviation) | Data Source | Citation |
|---|---|---|---|
| Habitat environmental characteristics | |||
| Land use type | Proportion of area covered by tree (TC) | SinoLC-1 (https://doi.org/10.5281/zenodo.7707461, accessed on 2 February 2024) | [31] |
| Proportion of area covered by shrubland (SC) | |||
| Mean Normalized Difference Vegetation Index | NDVI | Copernicus Sentinel-2 at a spatial resolution of 10 m | |
| Vegetation canopy height | CHM | https://glad.umd.edu/dataset/gedi/, accessed on 2 February 2024 | |
| Distance to water | Distance to waters >1 ha in size (DW) | SinoLC-1 (https://doi.org/10.5281/zenodo.7707461, accessed on 2 February 2024) | |
| Degree of urbanization | |||
| Distance to regional centers | Distance to Hongqiao City Sub-center (DHQ) | NGCC, https://www.ngcc.cn, accessed on 2 February 2024 | [27] |
| Distance to Xinzhuang City Sub-center (DXZ) | |||
| Building height | DSM | [32] | |
| Nighttime Lighting Index | NTL | Luojia-1 image (http://59.175.109.173:8888/app/login.html, accessed on 2 February 2024) | |
| Population density | PD | http://www.pbl.nl/hyde, accessed on 2 February 2024 | |
| Economic development | gross domestic production (GDP) | Resourse and environment science data platform (http://www.resdc.cn, accessed on 2 February 2024) | |
| Slow-traffic connectivity | |||
| Heat of slow-traffic movement trajectories of urban residents | Weekend cycling heat | Exercise trajectories of urban residents in Minhang District, Shanghai, China, in 2023 in the Keep app. | [33] |
| Weekend hiking heat | |||
| Weekend running heat | |||
| Weekday cycling heat | |||
| Weekday running heat | |||
| Weekday hiking heat | |||
| PC1Hab | PC2Hab | PC1Urb | PC2Urb | PCStc | |||
|---|---|---|---|---|---|---|---|
| TC | 0.49 | −0.096 | DXZ | 0.526 | 0.266 | Weekend cycling heat | 0.146 |
| SC | 0.017 | −0.301 | DHQ | 0.548 | 0.286 | Weekend hiking heat | 0.211 |
| DW | 0.019 | 0.864 | DSM | −0.301 | 0.383 | Weekend running heat | 0.211 |
| CHM | 0.398 | −0.199 | NTL | −0.010 | 0.746 | Weekday cycling heat | 0.12 |
| NDVI | 0.456 | 0.252 | PD | −0.561 | 0.238 | Weekday running heat | 0.21 |
| Propertion of variance | 32.89 | 21.08 | GDP | −0.131 | 0.297 | Weekday hiking heat | 0.21 |
| Cumualative proportion | 32.89 | 43.342 | Propertion of variance | 35.8 | 22.4 | Standard deviation | 4.688 |
| Cumualative proportion | 35.8 | 58.2 | Cumualative proportion | 78.14 |
| TC | SC | DW | CHM | NDVI | PD | DXZ | DHQ | NTL | DSM | |
|---|---|---|---|---|---|---|---|---|---|---|
| Richness | 0.214 *** | 0.012 | 0.113 *** | 0.117 *** | 0.099 *** | 0.065 *** | 0.004 | 0.375 *** | 0.326 *** | 0.096 *** |
| Abundance_log | 0.222 *** | 0.004 | 0.048 *** | 0.078 *** | 0.081 | 0.083 *** | 0.253 *** | 0.407 *** | 0.416 *** | 0.078 *** |
| Simpson_log | 0.223 *** | 0.016 * | 0.045 *** | 0.125 *** | 0.084 *** | 0.281 *** | 0.136 *** | 0.272 *** | 0.427 *** | 0.150 *** |
| Shannon Wiener _square | 0.237 *** | 0.011 | 0.088 *** | 0.125 *** | 0.113 *** | 0.138 *** | 0.068 *** | 0.398 *** | 0.514 *** | 0.112 *** |
| GHab | −0.5 *** | −0.017 *** | 0.964 *** | 0.036 *** | 0.115 *** | 0.029 *** | 0.037 *** | 0.014 *** | 0.010 | −0.003 |
| GUrb | 0.091 *** | −0.054 *** | 0.016 *** | −0.128 *** | 0.113 *** | −0.553 *** | 0.817 *** | 0.857 *** | 0.011 *** | −0.140 *** |
| GStc | 0.143 *** | 0.031 *** | −0.003 | 0.083 *** | 0.045 *** | 0.207 *** | −0.144 *** | −0.120 *** | 0.037 *** | 0.002 |
| Weekend cycling | Weekend hiking | Weekend running | Weekday cycling | Weekday hiking | Weekday running | GDP | GHab | GUrb | GStc | |
| Richness | 0.031 *** | 0.031 *** | 0.031 *** | 0.023 *** | 0.032 *** | 0.032 *** | −0.109 *** | −0.113 *** | 0.272 *** | 0.032 *** |
| Abundance_log | 0.012 | 0.034 *** | 0.018 *** | 0.034 *** | 0.034 *** | 0.033 *** | −0.117 *** | −0.048 *** | 0.419 *** | 0.034 *** |
| Simpson_log | 0.030 *** | 0.040 *** | 0.040 *** | 0.031 *** | 0.040 *** | 0.040 *** | −0.147 *** | −0.087 *** | 0.328 *** | 0.041 *** |
| Shannon Wiener _square | 0.031 *** | 0.038 *** | 0.038 *** | 0.031 *** | 0.039 *** | 0.039 *** | −0.130 *** | −0.045 *** | 0.300 *** | 0.039 *** |
| GHab | −0.009 | −0.003 | −0.003 | −0.012 | −0.002 | −0.002 | −0.049 *** | 0.107 *** | −0.003 | |
| GUrb | −0.106 *** | −0.143 *** | −0.143 *** | −0.032 *** | −0.143 *** | −0.143 *** | −0.192 *** | 0.107 *** | −0.146 *** | |
| GStc | 0.632 *** | 0.998 *** | 0.998 *** | 0.503 *** | 0.997 *** | 0.997 *** | 0.048 *** | −0.003 | −0.146 *** |
| Richness | Abundance_log | |||||
| Estimates | CI | p | Estimates | CI | p | |
| Intercept | 2.170 | [2.153; 2.187] | <0.001 | 0.418 | [0.401; 0.435] | <0.001 |
| GHab | −0.036 | [−0.000; −0.000] | <0.001 | −0.001 | [−0.001; −0.000] | <0.001 |
| PD | 0.002 | [0.002; 0.002] | <0.001 | 0.002 | [0.002; 0.002] | <0.001 |
| DXZ | −0.000 | [−0.000; −0.000] | <0.001 | 0.000 | [0.000; 0.000] | <0.001 |
| DHQ | 0.000 | [0.000; 0.000] | <0.001 | 0.000 | [0.000; 0.000] | <0.001 |
| Num. obs. | 19,959 | 19,959 | ||||
| R2 | 0.654 | 0.319 | ||||
| Shannon Wiener_square | Simpson_log | |||||
| Estimates | CI | p | Estimates | CI | p | |
| Intercept | 2.066 | [1.991; 2.141] | <0.001 | 0.250 | [0.242; 0.258] | <0.001 |
| GHab | −0.001 | [−0.001; −0.001] | <0.001 | −0.001 | [−0.001; −0.000] | <0.001 |
| PD | 0.009 | [0.008; 0.009] | <0.001 | 0.001 | [0.001; 0.001] | <0.001 |
| DXZ | −0.000 | [−0.000; −0.000] | <0.001 | 0.000 | [0.000; 0.000] | <0.001 |
| DHQ | 0.000 | [0.000; 0.000] | <0.001 | 0.000 | [0.000; 0.000] | <0.001 |
| Num. obs. | 19,959 | 19,959 | ||||
| R2 | 0.354 | 0.352 | ||||
| Richness | Abundance_log | |||||
| Estimates | CI | p | Estimates | CI | p | |
| Intercept | 0.391 | [3.528; 3.625] | <0.001 | 0.948 | [0.909; 0.986] | <0.001 |
| GUrb | 1.412 | [1.364; 1.460] | <0.001 | 0.436 | [0.408; 0.463] | <0.001 |
| PD | 1.517 | [1.481; 1.553] | <0.001 | 0.855 | [0.832; 0.878] | <0.001 |
| DXZ | −1.901 | [−1.952; −1.850] | <0.001 | −0.295 | [−0.334; −0.257] | <0.001 |
| DHQ | −0.221 | [−0.275; −0.166] | <0.001 | −0.226 | [−0.266; −0.186] | <0.001 |
| Num. obs. | 19,959 | 19,959 | ||||
| R2 | 0.715 | 0.345 | ||||
| Shannon Wiener_square | Simpson_log | |||||
| Estimates | CI | p | Estimates | CI | p | |
| Intercept | 4.872 | [4.699; 5.044] | 0.030 | 0.610 | [0.592; 0.627] | 0.011 |
| GUrb | 2.330 | [2.206; 2.453] | <0.001 | 0.292 | [0.280; 0.305] | <0.001 |
| PD | 4.138 | [4.035; 4.241] | <0.001 | 0.562 | [0.552; 0.573] | <0.001 |
| DSZ | −3.385 | [−3.556; −3.214] | <0.001 | −0.257 | [−0.275; −0.240] | <0.001 |
| DHQ | −1.458 | [−1.637; −1.279] | <0.001 | −0.016 | [−0.034; −0.002] | <0.001 |
| Num. obs. | 19,959 | 19,959 | ||||
| R2 | 0.386 | 0.409 | ||||
| Richness | Abundance_log | |||||
| Estimates | CI | p | Estimates | CI | p | |
| Intercept | 2.131 | [2.115; 2.148] | <0.001 | 0.396 | [0.380; 0.413] | <0.001 |
| GStc | 0.001 | [0.000; 0.000] | <0.001 | 0.000 | [0.000; 0.000] | <0.001 |
| PD | 0.002 | [0.002; 0.002] | <0.001 | 0.002 | [0.002; 0.002] | <0.001 |
| DXZ | −0.000 | [−0.000; −0.000] | <0.001 | 0.000 | [0.000; 0.000] | <0.001 |
| DHQ | 0.000 | [−0.000; −0.000] | <0.001 | 0.000 | [0.000; 0.000] | <0.001 |
| Num. obs. | 19,959 | 19,959 | ||||
| R2 | 0.638 | 0.314 | ||||
| Shannon Wiener_square | Simpson_log | |||||
| Estimates | CI | p | Estimates | CI | p | |
| Intercept | 1.923 | [1.848; 1.997] | <0.001 | 0.240 | [0.232; 0.247] | <0.001 |
| GStc | 0.000 | [−0.000; 0.000] | 0.060 | −0.000 | [−0.000; 0.000] | 0.005 |
| PD | 0.008 | [0.008; 0.009] | <0.001 | 0.001 | [0.001; 0.001] | <0.001 |
| DXZ | −0.000 | [−0.000; −0.000] | <0.001 | 0.000 | [0.000; 0.000] | <0.001 |
| DHQ | 0.000 | [0.000; 0.000] | <0.001 | 0.000 | [0.000; 0.000] | <0.001 |
| Num. obs. | 19,959 | 19,959 | ||||
| R2 | 0.343 | 0.346 | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, D.; Zhang, L.; Zhong, Q.; Zhang, G.; Chen, X.; Zhang, Q. Analysis of Spatial Divergence in Bird Diversity Driven by Built Environment Characteristics of Ecological Corridors in High-Density Urban Areas. Land 2024, 13, 1359. https://doi.org/10.3390/land13091359
Wang D, Zhang L, Zhong Q, Zhang G, Chen X, Zhang Q. Analysis of Spatial Divergence in Bird Diversity Driven by Built Environment Characteristics of Ecological Corridors in High-Density Urban Areas. Land. 2024; 13(9):1359. https://doi.org/10.3390/land13091359
Chicago/Turabian StyleWang, Di, Lang Zhang, Qicheng Zhong, Guilian Zhang, Xuanying Chen, and Qingping Zhang. 2024. "Analysis of Spatial Divergence in Bird Diversity Driven by Built Environment Characteristics of Ecological Corridors in High-Density Urban Areas" Land 13, no. 9: 1359. https://doi.org/10.3390/land13091359






