Immunochemistry-Based Diagnosis of Extrapulmonary Tuberculosis: A Strategy for Large-Scale Production of MPT64-Antibodies for Use in the MPT64 Antigen Detection Test
Abstract
:1. Introduction
2. Materials and Methods
2.1. Production of MPT64 Antigen
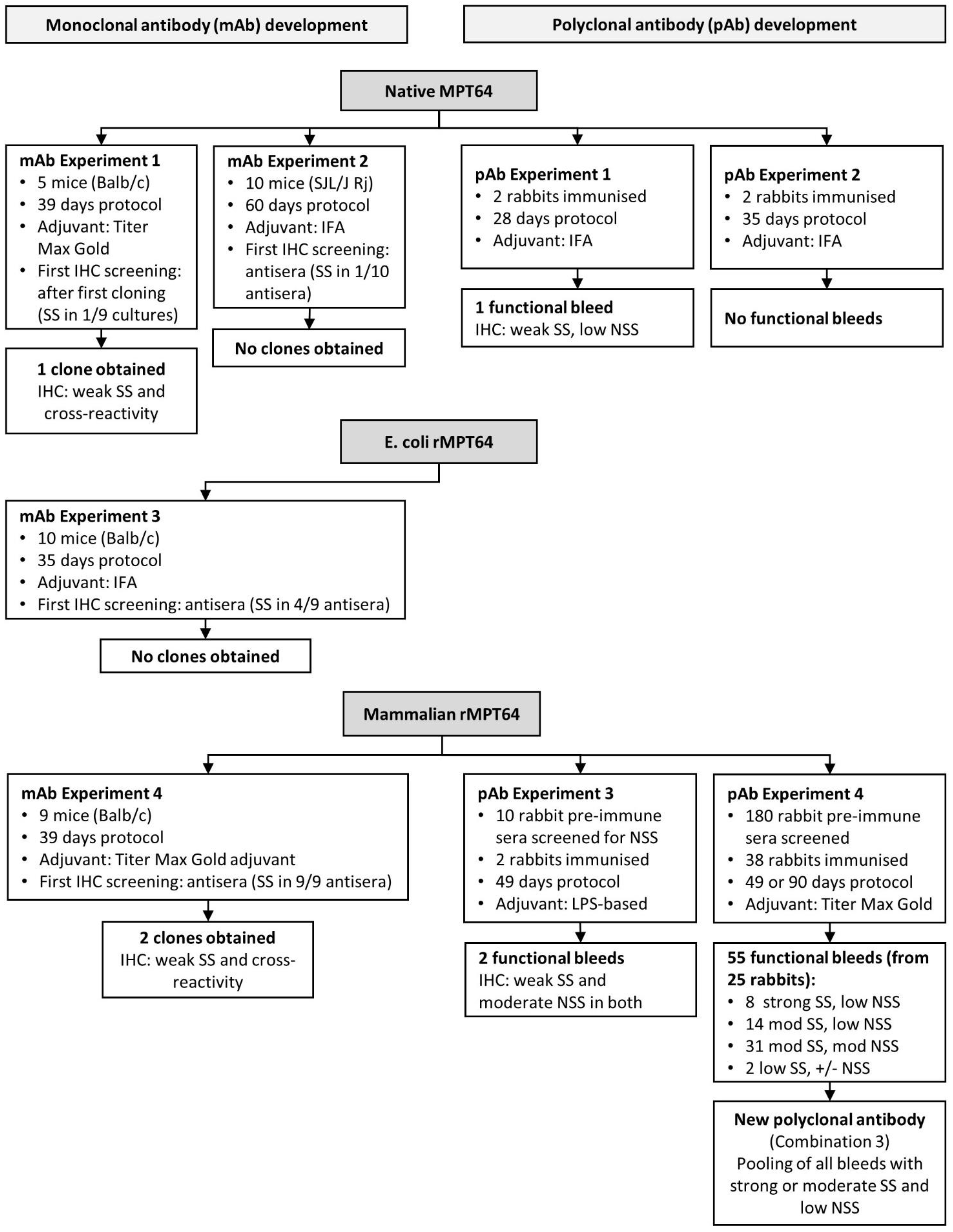
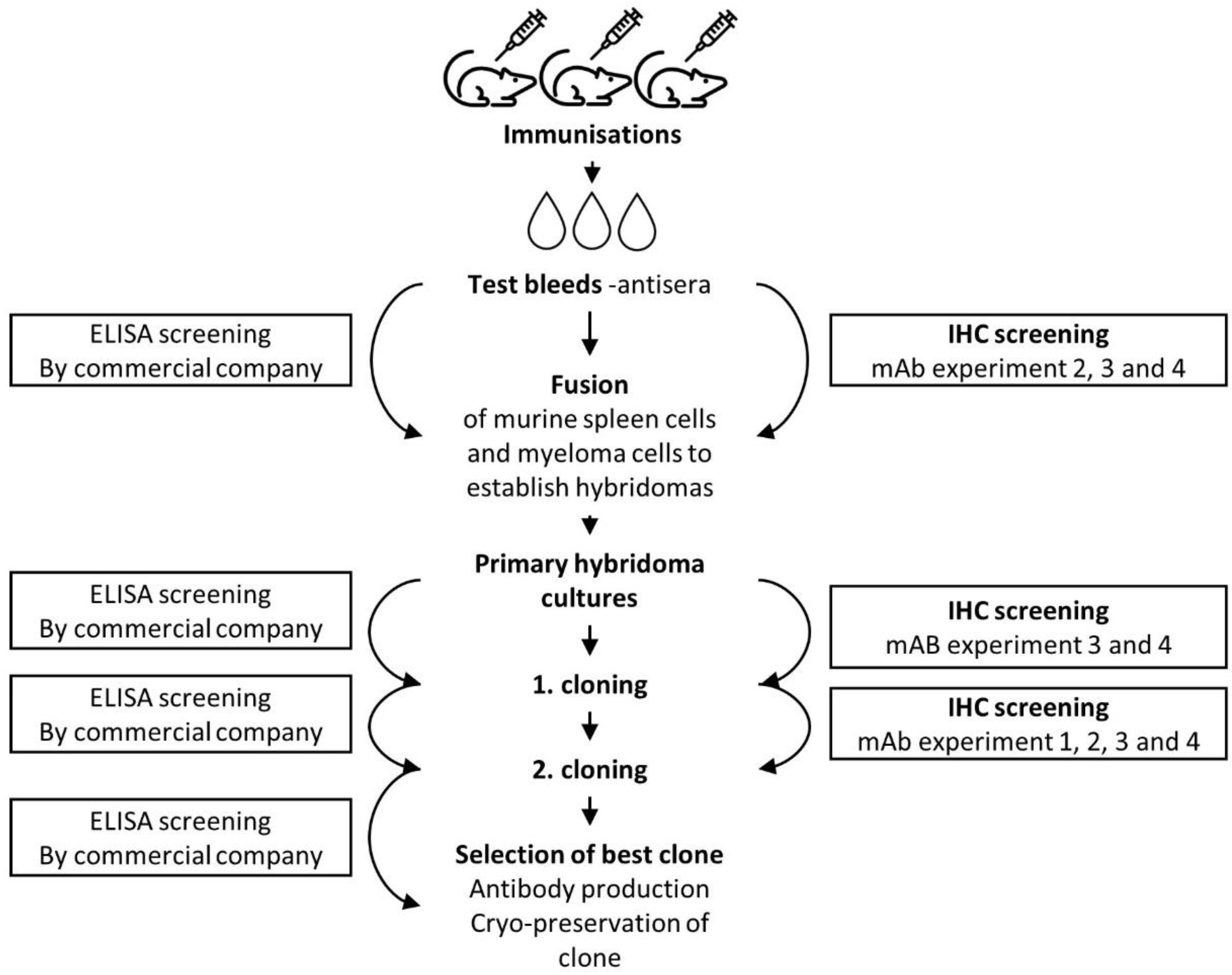
2.2. Development of a Monoclonal Anti-MPT64 Antibody
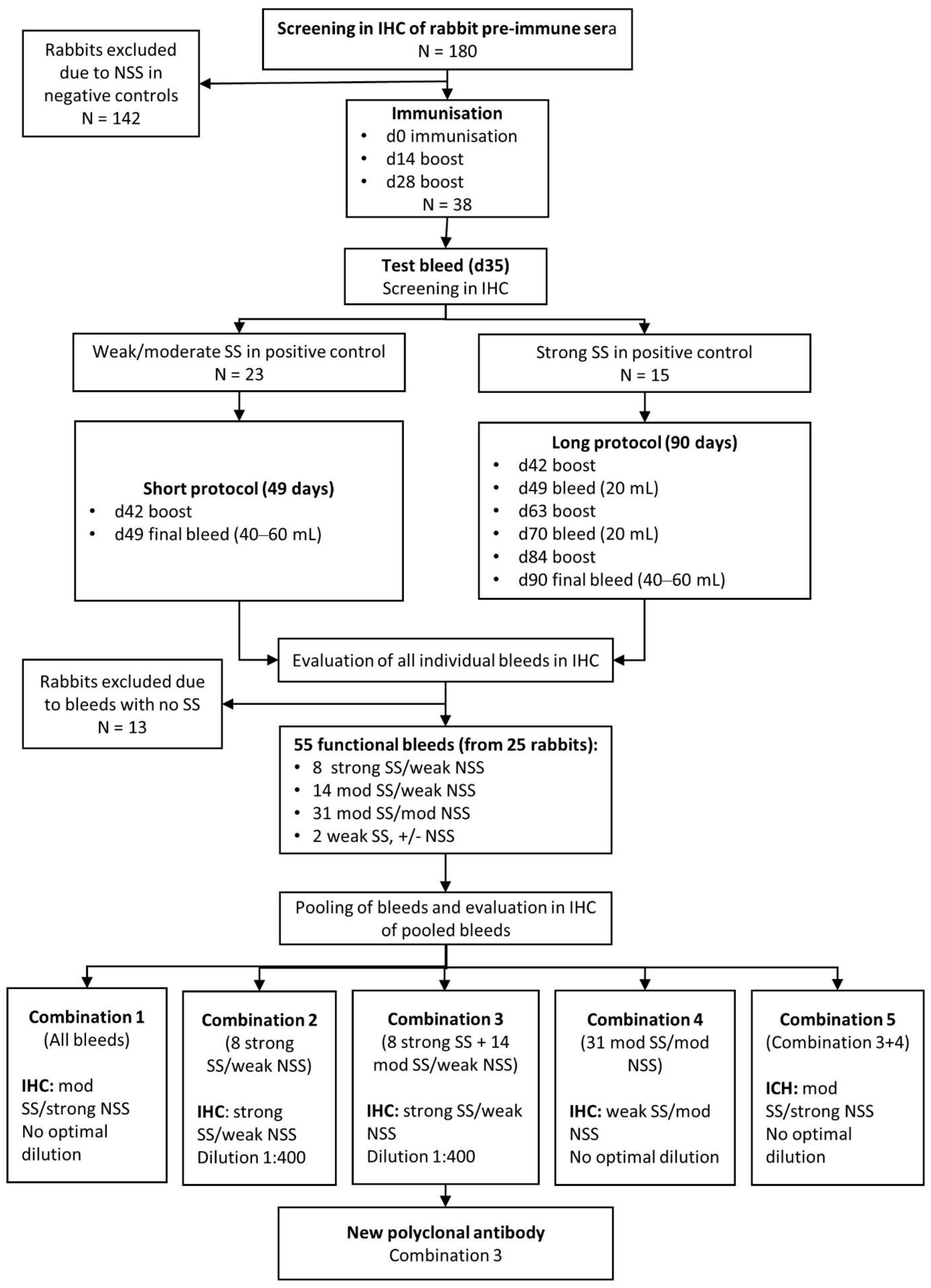
2.3. Development of Polyclonal Rabbit Anti-MPT64 Antibodies
2.3.1. Immunisations
2.3.2. Selection and Pooling of MPT64-Specific Antibodies
2.4. Background Blocking and Antibody Absorption Experiments
2.5. Immunohistochemistry (the MPT64 Test)
2.6. Validation of the New Polyclonal Antibodies
2.7. Statistical Methods
2.8. Ethical Considerations
3. Results
3.1. Production of MPT64 Antigen
3.2. Development of Monoclonal MPT64 Antibody
3.3. Development of a Polyclonal Antibody
3.4. Validation of the New Polyclonal Antibody
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Global Tuberculosis Report. 2020. Available online: https://www.who.int/publications/i/item/9789240013131 (accessed on 13 November 2020).
- Nelson, L.; Wells, C. Global epidemiology of childhood tuberculosis. Int. J. Tuberc. Lung Dis. 2004, 8, 636–647. [Google Scholar]
- Smith, S.; Jacobs, R.F.; Wilson, C.B. Immunobiology of childhood tuberculosis: A window on the ontogeny of cellular immunity. J. Pediatrics 1997, 131, 16–26. [Google Scholar] [CrossRef]
- Jones, B.E.; Young, S.M.; Antoniskis, D.; Davidson, P.T.; Kramer, F.; Barnes, P.F. Relationship of the manifestations of tuberculosis to CD4 cell counts in patients with human immunodeficiency virus infection. Am. J. Respir. Crit. Care Med. 1993, 148, 1292–1297. [Google Scholar] [CrossRef]
- Harries, A. Tuberculosis and human immunodeficiency virus infection in developing countries. Lancet Infect. Dis. 1990, 335, 387–390. [Google Scholar] [CrossRef]
- Small, P.M.; Schecter, G.F.; Goodman, P.C.; Sande, M.A.; Chaisson, R.E.; Hopewell, P.C. Treatment of tuberculosis in patients with advanced human immunodeficiency virus infection. N. Engl. J. Med. 1991, 324, 289–294. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Improving the Diagnosis and Treatment of Smear-Negative Pulmonary and Extrapulmonary Tuberculosis among Adults and Adolescents: Recommendations for HIV-Prevalent and Resource-Constrained Settings. 2007. Available online: http://www.who.int/iris/handle/10665/69463 (accessed on 26 August 2021).
- World Health Organization. High-Priority Target Product Profiles for New Tuberculosis Diagnostics. Report of a Consensus Meeting. 2014. Available online: https://www.who.int/tb/publications/tpp_report/en/ (accessed on 5 February 2020).
- Broger, T.; Sossen, B.; du Toit, E.; Kerkhoff, A.D.; Schutz, C.; Reipold, E.I.; Ward, A.; Barr, D.A.; Macé, A.; Trollip, A.; et al. Novel lipoarabinomannan point-of-care tuberculosis test for people with HIV: A diagnostic accuracy study. Lancet Infect. Dis. 2019, 19, 852–861. [Google Scholar] [CrossRef] [Green Version]
- World Health Organization. Lateral Flow Urine Lipoarabinomannan Assay (LF-LAM) for the Diagnosis of Active Tuberculosis in People Living with HIV-Policy Update (2019). 2019. Available online: https://www.who.int/tb/publications/2019/LAMPolicyUpdate2019/en/ (accessed on 23 June 2020).
- Mustafa, T.; Wiker, H.G.; Mfinanga, S.G.; Mørkve, O.; Sviland, L. Immunohistochemistry using a Mycobacterium tuberculosis complex specific antibody for improved diagnosis of tuberculous lymphadenitis. Mod. Pathol. 2006, 19, 1606–1614. [Google Scholar] [CrossRef] [PubMed]
- Purohit, M.R.; Mustafa, T.; Wiker, H.G.; Mørkve, O.; Sviland, L. Immunohistochemical diagnosis of abdominal and lymph node tuberculosis by detecting Mycobacterium tuberculosis complex specific antigen MPT64. Diagn. Pathol. 2007, 2, 36. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Purohit, M.R.; Mustafa, T.; Wiker, H.G.; Sviland, L. Rapid diagnosis of tuberculosis in aspirate, effusions, and cerebrospinal fluid by immunocytochemical detection of Mycobacterium tuberculosis complex specific antigen MPT64. Diagn. Cytopathol. 2012, 40, 782–791. [Google Scholar] [CrossRef] [PubMed]
- Tadele, A.; Beyene, D.; Hussein, J.; Gemechu, T.; Birhanu, A.; Mustafa, T.; Tsegaye, A.; Aseffa, A.; Sviland, L. Immunocytochemical detection of Mycobacterium Tuberculosis complex specific antigen, MPT64, improves diagnosis of tuberculous lymphadenitis and tuberculous pleuritis. BMC Infect. Dis. 2014, 14, 585. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Purohit, M.R.; Sviland, L.; Wiker, H.; Mustafa, T. Rapid and specific diagnosis of extrapulmonary tuberculosis by immunostaining of tissues and aspirates with anti-MPT64. Appl. Immunohistochem. Mol. Morphol. 2017, 25, 282–288. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jørstad, M.D.; Marijani, M.; Dyrhol-Riise, A.M.; Sviland, L.; Mustafa, T. MPT64 antigen detection test improves routine diagnosis of extrapulmonary tuberculosis in a low-resource setting: A study from the tertiary care hospital in Zanzibar. PLoS ONE 2018, 13, e0196723. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baba, K.; Dyrhol-Riise, A.M.; Sviland, L.; Langeland, N.; Hoosen, A.A.; Wiker, H.G.; Mustafa, T. Rapid and specific diagnosis of tuberculous pleuritis with immunohistochemistry by detecting Mycobacterium tuberculosis complex specific antigen MPT64 in patients from a HIV endemic area. Appl. Immunohistochem. Mol. Morphol. 2008, 16, 554–561. [Google Scholar] [CrossRef]
- Hoel, I.M.; Sviland, L.; Syre, H.; Dyrhol-Riise, A.M.; Skarstein, I.; Jebsen, P.; Jørstad, M.D.; Wiker, H.; Mustafa, T. Diagnosis of extrapulmonary tuberculosis using the MPT64 antigen detection test in a high-income low tuberculosis prevalence setting. BMC Infect. Dis. 2020, 20, 130. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lipman, N.S.; Jackson, L.R.; Trudel, L.J.; Weis-Garcia, F. Monoclonal versus polyclonal antibodies: Distinguishing characteristics, applications, and information resources. ILAR J. 2005, 46, 258–268. [Google Scholar] [CrossRef] [Green Version]
- Nagai, S.; Wiker, H.G.; Harboe, M.; Kinomoto, M. Isolation and partial characterization of major protein antigens in the culture fluid of Mycobacterium tuberculosis. Infect. Immun. 1991, 59, 372–382. [Google Scholar] [CrossRef] [Green Version]
- Harboe, M.; Nagai, S.; Patarroyo, M.E.; Torres, M.; Ramirez, C.; Cruz, N. Properties of proteins MPB64, MPB70, and MPB80 of Mycobacterium bovis BCG. Infect. Immun. 1986, 52, 293–302. [Google Scholar] [CrossRef] [Green Version]
- Nagai, S.; Matsumoto, J.; Nagasuga, T. Specific skin-reactive protein from culture filtrate of Mycobacterium bovis BCG. Infect. Immun. 1981, 31, 1152–1160. [Google Scholar] [CrossRef] [Green Version]
- Oettinger, T.; Andersen, A.B. Cloning and B-cell-epitope mapping of MPT64 from Mycobacterium tuberculosis H37Rv. Infect. Immun. 1994, 62, 2058–2064. [Google Scholar] [CrossRef] [Green Version]
- Snapper, S.; Melton, R.; Mustafa, S.; Kieser, T.; Jr, W.J. Isolation and characterization of efficient plasmid transformation mutants of Mycobacterium smegmatis. Mol. Microbiol. 1990, 4, 1911–1919. [Google Scholar] [CrossRef]
- Ehrt, S.; Guo, X.V.; Hickey, C.M.; Ryou, M.; Monteleone, M.; Riley, L.W.; Schnappinger, D. Controlling gene expression in mycobacteria with anhydrotetracycline and Tet repressor. Nucleic Acids Res. 2005, 33, e21. [Google Scholar] [CrossRef] [PubMed]
- Parish, T.; Roberts, D.M. Mycobacteria Protocols, 3rd ed.; Springer Science+Business Media: New York, NY, USA, 2015. [Google Scholar]
- Guo, X.V.; Monteleone, M.; Klotzsche, M.; Kamionka, A.; Hillen, W.; Braunstein, M.; Ehrt, S.; Schnappinger, D. Silencing essential protein secretion in Mycobacterium smegmatis by using tetracycline repressors. J. Bacteriol. 2007, 189, 4614–4623. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Köhler, G.; Milstein, C. Continuous cultures of fused cells secreting antibody of predefined specificity. Nature 1975, 256, 495–497. [Google Scholar] [CrossRef] [PubMed]
- Harboe, M.; Closs, O.; Bjorvatn, B.; Kronvall, G.; Axelsen, N. Antibody response in rabbits to immunization with Mycobacterium leprae. Infect. Immun. 1977, 18, 792–805. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tahseen, S.; Ambreen, A.; Masood, F.; Qadir, M.; Hussain, A.; Jamil, M.; Safdar, N.; Sviland, L.; Mustafa, T. Primary drug resistance in extra-pulmonary tuberculosis: A hospital-based prospective study from Pakistan. Int. J. Tuberc. Lung Dis. 2019, 23, 900–906. [Google Scholar] [CrossRef]
- Bergmann-Leitner, E.S.; Leitner, W.W. Adjuvants in the driver’s seat: How magnitude, type, fine specificity and longevity of immune responses are driven by distinct classes of immune potentiators. Vaccines 2014, 2, 252–296. [Google Scholar] [CrossRef] [Green Version]
- Schunk, M.K.; Macallum, G.E. Applications and optimization of immunization procedures. ILAR J. 2005, 46, 241–257. [Google Scholar] [CrossRef]
- Leenaars, M.; Hendriksen, C.F. Critical steps in the production of polyclonal and monoclonal antibodies: Evaluation and recommendations. ILAR J. 2005, 46, 269–279. [Google Scholar] [CrossRef] [Green Version]
- Stils, H., Jr. Adjuvants and antibody production: Dispelling the myths associated with Freund’s complete and other adjuvants. ILAR J. 2005, 46, 280–293. [Google Scholar] [CrossRef] [Green Version]
- Mason, J.T.; O’leary, T.J. Effects of formaldehyde fixation on protein secondary structure: A calorimetric and infrared spectroscopic investigation. J. Histochem. Cytochem. 1991, 39, 225–229. [Google Scholar] [CrossRef]
- Roche, P.; Winter, N.; Triccas, J.; Feng, C.; Britton, W. Expression of Mycobacterium tuberculosis MPT64 in recombinant Myco. smegmatis: Purification, immunogenicity and application to skin tests for tuberculosis. Clin. Exp. Immunol. 1996, 103, 226–232. [Google Scholar] [CrossRef]
- Sartain, M.J.; Belisle, J.T. N-Terminal clustering of the O-glycosylation sites in the Mycobacterium tuberculosis lipoprotein SodC. Glycobiology 2009, 19, 38–51. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dobos, K.M.; Khoo, K.-H.; Swiderek, K.M.; Brennan, P.J.; Belisle, J.T. Definition of the full extent of glycosylation of the 45-kilodalton glycoprotein of Mycobacterium tuberculosis. J. Bacteriol. 1996, 178, 2498–2506. [Google Scholar] [CrossRef] [Green Version]
- Daugelat, S.; Kowall, J.; Mattow, J.; Bumann, D.; Winter, R.; Hurwitz, R.; Kaufmann, S.H. The RD1 proteins of Mycobacterium tuberculosis: Expression in Mycobacterium smegmatis and biochemical characterization. Microbes Infect. 2003, 5, 1082–1095. [Google Scholar] [CrossRef]
- Garbe, T.; Harris, D.; Vordermeier, M.; Lathigra, R.; Ivanyi, J.; Young, D. Expression of the Mycobacterium tuberculosis 19-kilodalton antigen in Mycobacterium smegmatis: Immunological analysis and evidence of glycosylation. Infect. Immun. 1993, 61, 260–267. [Google Scholar] [CrossRef] [Green Version]
- Herrmann, J.; O’Gaora, P.; Gallagher, A.; Thole, J.; Young, D.B. Bacterial glycoproteins: A link between glycosylation and proteolytic cleavage of a 19 kDa antigen from Mycobacterium tuberculosis. EMBO J. 1996, 15, 3547–3554. [Google Scholar] [CrossRef] [PubMed]
- Herrmann, J.L.; Delahay, R.; Gallagher, A.; Robertson, B.; Young, D. Analysis of post-translational modification of mycobacterial proteins using a cassette expression system. FEBS Lett. 2000, 473, 358–362. [Google Scholar] [CrossRef] [Green Version]
- Parra, J.; Marcoux, J.; Poncin, I.; Canaan, S.; Herrmann, J.L.; Nigou, J.; Burlet-Schiltz, O.; Rivière, M. Scrutiny of Mycobacterium tuberculosis 19 kDa antigen proteoforms provides new insights in the lipoglycoprotein biogenesis paradigm. Sci. Rep. 2017, 7, 43682. [Google Scholar] [CrossRef]
- World Health Organization. Global Tuberculosis Report. 2019. Available online: https://www.who.int/tb/publications/global_report/en/ (accessed on 22 November 2019).
- Torres-González, P.; Niembro-Ortega, M.D.; Martínez-Gamboa, A.; Ahumada-Topete, V.H.; Andrade-Villanueva, J.; Araujo-Meléndez, J.; Chaparro-Sánchez, A.; Crabtree-Ramírez, B.; Cruz-Martínez, S.; Gamboa-Domínguez, A.; et al. Diagnostic accuracy cohort study and clinical value of the Histoplasma urine antigen (ALPHA Histoplasma EIA) for disseminated histoplasmosis among HIV infected patients: A multicenter study. PLoS Negl. Trop. Dis. 2018, 12, e0006872. [Google Scholar] [CrossRef] [Green Version]
- Petrini, I.; Zucali, P.A.; Lee, H.S.; Pineda, M.A.; Meltzer, P.S.; Walter-Rodriguez, B.; Roncalli, M.; Santoro, A.; Wang, Y.; Giaccone, G. Expression and mutational status of c-kit in thymic epithelial tumors. J. Thorac. Oncol. 2010, 5, 1447–1453. [Google Scholar] [CrossRef]
- Eyzaguirre, E.; Haque, A.K. Application of immunohistochemistry to infections. Arch. Pathol. Lab. Med. 2008, 132, 424–431. [Google Scholar] [CrossRef] [PubMed]
- Otali, D. The Combined Effect of Formalin Fixation and Individual Steps in Tissue Processing on Immunorecognition; University of Alabama at Birmingham: Birmingham, AL, USA, 2007. [Google Scholar]
- de Almeida, R.; Nakamura, C.N.; de Lima Fontes, M.; Deffune, E.; Felisbino, S.L.; Kaneno, R.; Fávaro, W.J.; Billis, A.; Cerri, M.O.; Fusco-Almeida, A.M.; et al. Enhanced Immunization Techniques to Obtain Highly Specific Monoclonal Antibodies; Taylor & Francis: Boca Raton, FL, USA, 2018. [Google Scholar]

| Protein | Amino Acid Sequence |
|---|---|
| Native MPT64 | MRIKIFMLVTAVVLLCCSGVATAAPKTYCEELKGTDTGQACQIQMSDPAYNINISLPSYYPDQKSLENYIAQTRDKFLSAATSSTPREAPYELNITSATYQSAIPPRGTQAVVLKVYQNAGGTHPTTTYKAFDWDQAYRKPITYDTLWQADTDPLPVVFPIVQGELSKQTGQQVSIAPNAGLDPVNYQNFAVTNDGVIFFFNPGELLPEAAGPTQVLVPRSAIDSMLA |
| E. coli rMPT64 | MAPKTYCEELKGTDTGQACQIQMSDPAYNINISLPSYYPDQKSLENYIAQTRDKFLSAATSSTPREAPYELNITSATYQSAIPPRGTQAVVLKVYQNAGGTHPTTTYKAFDWDQAYRKPITYDTLWQADTDPLPVVFPIVQGELSKQTGQQVSIAPNAGLDPVNYQNFAVTNDGVIFFFNPGELLPEAAGPTQVLVPRSAIDSMLAVLVPRGSAAALEHHHHHHHH |
| Mammalian rMPT64 | MKWVTFISLLFLFSSAYSAPKTYCEELKGTDTGQACQIQMSDPAYNINISLPSYYPDQKSLENYIAQTRDKFLSAATSSTPREAPYELNITSATYQSAIPPRGTQAVVLKVYQNAGGTHPTTTYKAFDWDQAYRKPITYDTLWQADTDPLPVVFPIVQGELSKQTGQQVSIAPNAGLDPVNYQNFAVTNDGVIFFFNPGELLPEAAGPTQVLVPRSAIDSMLAHHHHHH |
| Level of Non-Specific Staining | ||
|---|---|---|
| Strategy | Positive TB Control | Negative Non-TB Control |
| Blocking experiments | ||
| Serum free block (12 min, 30 min, 60 min, or overnight) | - | - |
| BSA 3% or 10% and NGS 10% (60 min), followed by serum free block (60 min) | -/↓ | -/↓ |
| BSA 3% or 10% and NGS 10% (overnight), followed by serum free block (12 min) | ↓↓ | ↓↓ |
| Fc block | - | - |
| Absorption experiments | ||
| M. bovis BCG Copenhagen, culture filtrates | -/↓ | -/↓ |
| M. bovis BCG Copenhagen, cell sonicate | - | - |
| Homogenised non-TB lung and lymph node tissue sections (deparaffinised and hydrated) | - | ↑ |
| Routine Diagnostic Tests Positive/Total (%) | The MPT64 Test Positive/Total (%) | ||||
|---|---|---|---|---|---|
| N | Ziehl-Neelsen | Xpert MTB/RIF | Culture LJ | New Polyclonal MPT64 Antibody | |
| TB cases total | 20 | 1/9 (11) | 6/9 (67) | 6/15 (40) | 19/20 (95) |
| Lymph node biopsies | 14 | 1/6 (17) | 3/4 (75) | 6/12 (50) | 14/14 (100) |
| Other biopsies | 6 | 0/3 (0) | 3/5 (60) | 0/3 (0) | 5/6 (83) |
| Confirmed TB cases | 12 | 0/5 (0) | 6/6 (100) | 6/11 (55) | 12/12 (100) |
| Clinically diagnosed TB cases | 8 | 1/4 (25) | 0/4 (0) | 0/3 (0) | 7/8 (88) |
| Non-TB cases total | 24 | N/A | N/A | N/A | 4/24 (17) |
| Lymph node biopsies | 7 | N/A | N/A | N/A | 2/7 (29) |
| Other biopsies | 17 | N/A | N/A | N/A | 2/17 (12) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hoel, I.M.; Ali, I.A.M.; Ishtiaq, S.; Sviland, L.; Wiker, H.; Mustafa, T. Immunochemistry-Based Diagnosis of Extrapulmonary Tuberculosis: A Strategy for Large-Scale Production of MPT64-Antibodies for Use in the MPT64 Antigen Detection Test. Antibodies 2021, 10, 34. https://doi.org/10.3390/antib10030034
Hoel IM, Ali IAM, Ishtiaq S, Sviland L, Wiker H, Mustafa T. Immunochemistry-Based Diagnosis of Extrapulmonary Tuberculosis: A Strategy for Large-Scale Production of MPT64-Antibodies for Use in the MPT64 Antigen Detection Test. Antibodies. 2021; 10(3):34. https://doi.org/10.3390/antib10030034
Chicago/Turabian StyleHoel, Ida Marie, Iman A Mohammed Ali, Sheeba Ishtiaq, Lisbet Sviland, Harald Wiker, and Tehmina Mustafa. 2021. "Immunochemistry-Based Diagnosis of Extrapulmonary Tuberculosis: A Strategy for Large-Scale Production of MPT64-Antibodies for Use in the MPT64 Antigen Detection Test" Antibodies 10, no. 3: 34. https://doi.org/10.3390/antib10030034
APA StyleHoel, I. M., Ali, I. A. M., Ishtiaq, S., Sviland, L., Wiker, H., & Mustafa, T. (2021). Immunochemistry-Based Diagnosis of Extrapulmonary Tuberculosis: A Strategy for Large-Scale Production of MPT64-Antibodies for Use in the MPT64 Antigen Detection Test. Antibodies, 10(3), 34. https://doi.org/10.3390/antib10030034





