Conditionally Active, pH-Sensitive Immunoregulatory Antibodies Targeting VISTA and CTLA-4 Lead an Emerging Class of Cancer Therapeutics
Abstract
:1. Introduction
2. VISTA and CTLA-4: Two Immune Checkpoints That Illustrate Distinct Hurdles to Overcome in Immuno-Oncology Drug Development
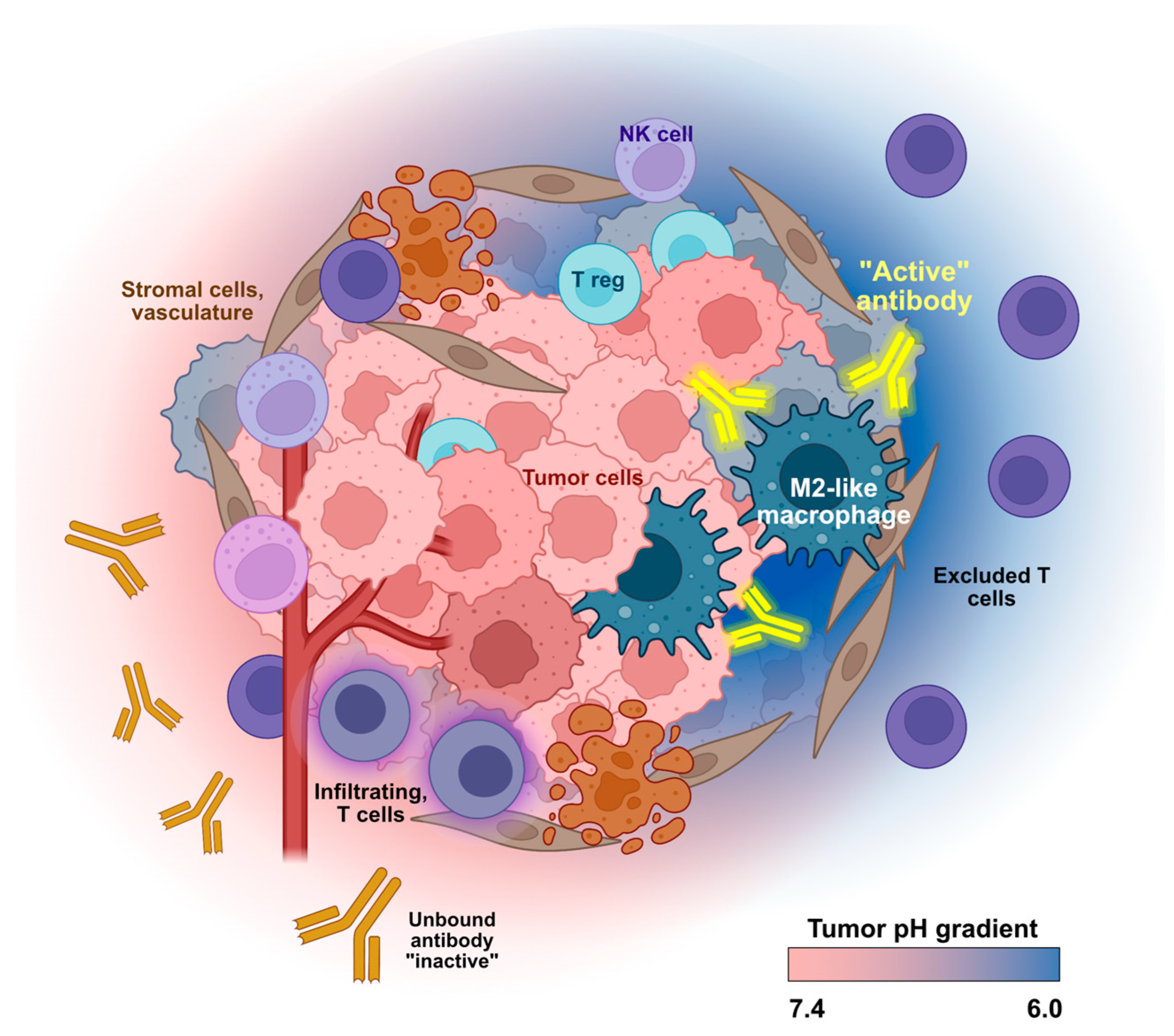
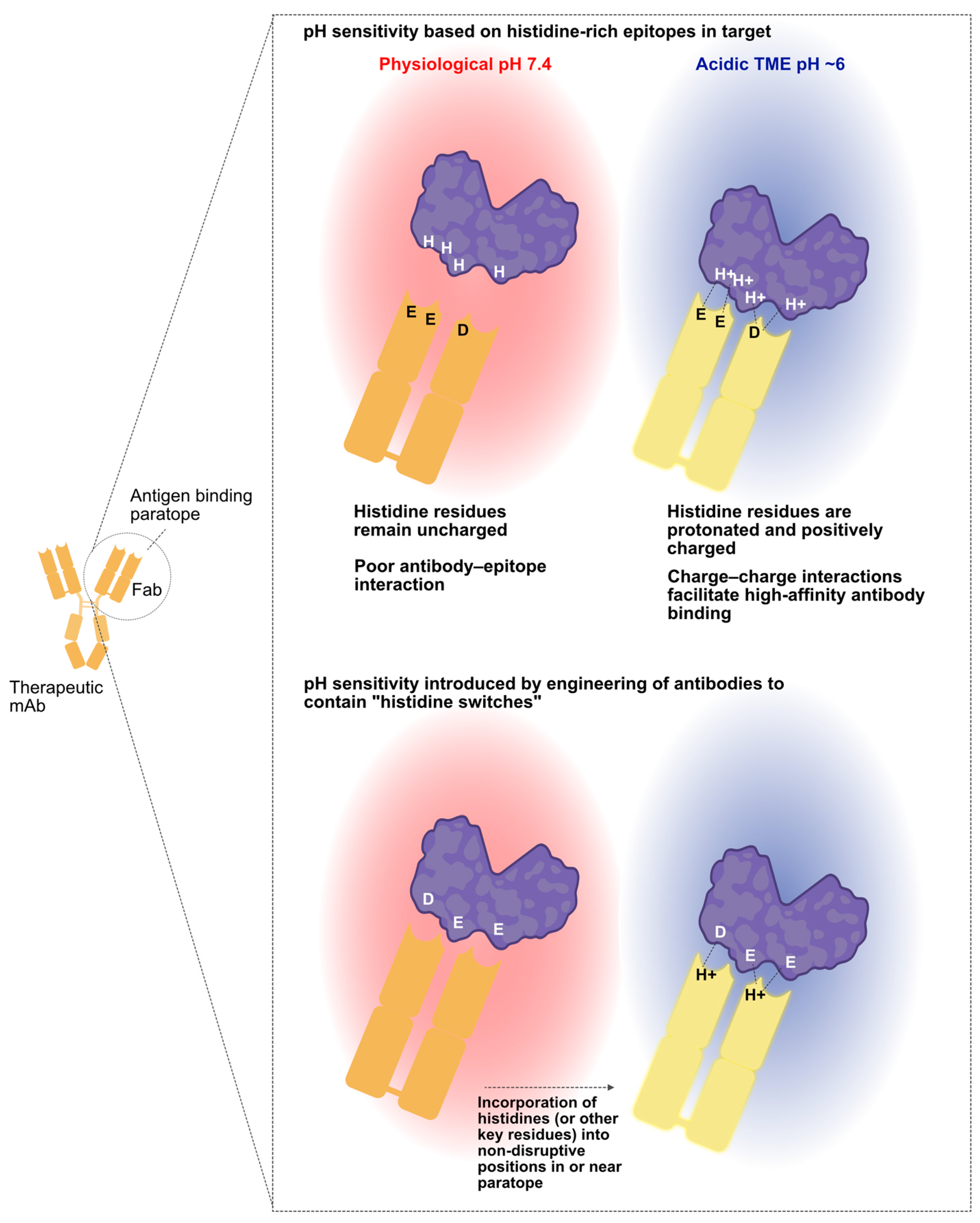
3. Harnessing Unique Features of the TME for Conditional mAb Activity
4. Conclusions and Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Marks, L. The birth pangs of monoclonal antibody therapeutics: The failure and legacy of Centoxin. mAbs 2012, 4, 403–412. [Google Scholar] [CrossRef] [PubMed]
- Mullard, A. FDA approves 100th monoclonal antibody product. Nat. Rev. Drug Discov. 2021, 20, 491–495. [Google Scholar] [CrossRef] [PubMed]
- Brennan, F.R.; Morton, L.D.; Spindeldreher, S.; Kiessling, A.; Allenspach, R.; Hey, A.; Müller, P.; Frings, W.; Sims, J. Safety and immunotoxicity assessment of immunomodulatory monoclonal antibodies. mAbs 2010, 2, 233–255. [Google Scholar] [CrossRef] [PubMed]
- Traxlmayr, M.W.; Lobner, E.; Hasenhindl, C.; Stadlmayr, G.; Oostenbrink, C.; Rüker, F.; Obinger, C. Construction of pH-sensitive Her2-binding IgG1-Fc by directed evolution. Biotechnol. J. 2014, 9, 1013–1022. [Google Scholar] [CrossRef]
- Engelen, W.; Zhu, K.; Subedi, N.; Idili, A.; Ricci, F.; Tel, J.; Merkx, M. Programmable Bivalent Peptide–DNA Locks for pH-Based Control of Antibody Activity. ACS Cent. Sci. 2020, 6, 22–31. [Google Scholar] [CrossRef] [PubMed]
- Lucchi, R.; Bentanachs, J.; Oller-Salvia, B. The Masking Game: Design of Activatable Antibodies and Mimetics for Selective Therapeutics and Cell Control. ACS Cent. Sci. 2021, 7, 724–738. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.W.; Frey, G.; Liu, H.; Xing, C.; Steinman, L.; Boyle, W.J.; Short, J.M. Generating tumor-selective conditionally active biologic anti-CTLA4 antibodies via protein-associated chemical switches. Proc. Natl. Acad. Sci. USA 2021, 118, e2020606118. [Google Scholar] [CrossRef]
- Lee, P.S.; MacDonald, K.G.; Massi, E.; Chew, P.V.; Bee, C.; Perkins, P.; Chau, B.; Thudium, K.; Lohre, J.; Nandi, P.; et al. Improved therapeutic index of an acidic pH-selective antibody. mAbs 2022, 14, 2024642. [Google Scholar] [CrossRef]
- Liu, Y.; Nguyen, A.W.; Maynard, J.A. Engineering antibodies for conditional activity in the solid tumor microenvironment. Curr. Opin. Biotechnol. 2022, 78, 102809. [Google Scholar] [CrossRef]
- Yuan, L.; Tatineni, J.; Mahoney, K.M.; Freeman, G.J. VISTA: A Mediator of Quiescence and a Promising Target in Cancer Immunotherapy. Trends Immunol. 2021, 42, 209–227. [Google Scholar] [CrossRef]
- Wang, L.; Rubinstein, R.; Lines, J.L.; Wasiuk, A.; Ahonen, C.; Guo, Y.; Lu, L.-F.; Gondek, D.; Wang, Y.; Fava, R.A.; et al. VISTA, a novel mouse Ig superfamily ligand that negatively regulates T cell responses. J. Exp. Med. 2011, 208, 577–592. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Ward, J.F.; Pettaway, C.A.; Shi, L.Z.; Subudhi, S.K.; Vence, L.M.; Zhao, H.; Chen, J.; Chen, H.; Efstathiou, E.; et al. VISTA is an inhibitory immune checkpoint that is increased after ipilimumab therapy in patients with prostate cancer. Nat. Med. 2017, 23, 551–555. [Google Scholar] [CrossRef] [PubMed]
- Nowak, E.C.; Lines, J.L.; Varn, F.S.; Deng, J.; Sarde, A.; Mabaera, R.; Kuta, A.; Le Mercier, I.; Cheng, C.; Noelle, R.J. Immunoregulatory functions of VISTA. Immunol. Rev. 2017, 276, 66–79. [Google Scholar] [CrossRef] [PubMed]
- Haber, T.; Symons, J.; Dharmadhikari, B.; Müller, T.; Thakkar, D.; Rodon, J.A.; Gruber, J.; Telli, M.; Mita, M.; Mita, A.; et al. 623 Trial in Progress: A Phase 1 First-in-Human Study of HMBD-002, an IgG4 Monoclonal Antibody Targeting VISTA, As a Monotherapy and Combined with Pembrolizumab in Patients with Advanced Solid Malignancies. In Proceedings of the SITC Annual Meeting, Boston, MA, USA, 8–12 November 2022. [Google Scholar] [CrossRef]
- Lustig, K.; Frazier, E.; Kabi, N.; Katz, C.; Eyde, N.; Cross, J.; Lance, R.; Ovechkina, Y.; Peckham, D.; Sridhar, S.; et al. KVA12123: An anti-VISTA monoclonal antibody with strong single agent anti-tumor activity and no evidence of cytokine mediated toxicity. In Proceedings of the SITC Annual Meeting, Boston, MA, USA, 8–12 November 2022. [Google Scholar]
- Noelle, R.; Johnson, M.; Rodon, J.; Zauderer, M.; Lewis, L.; Severgnini, M.; Parker, J.; Lane, M.; von Roemeling, R.; Martin, A.; et al. 761 Pharmacokinetic and pharmacodynamic data from a phase 1 study of CI-8993 Anti-VISTA antibody in patients with advanced solid tumors. In Proceedings of the SITC Annual Meeting, Boston, MA, USA, 8–12 November 2022. [Google Scholar] [CrossRef]
- Park, C.H.; Byun, S.S.; An, J.Y.; Han, H.; Lee, W.S. PMC-309, a highly selective anti-VISTA antibody reverses immunosuppressive TME to immune-supportive TME. Cancer Res. 2022, 82 (Suppl. S12), 5557. [Google Scholar] [CrossRef]
- NCT02671955. A Study of Safety, Pharmacokinetics, Pharmacodynamics of JNJ-61610588 in Participants With Advanced Cancer. Available online: https://clinicaltrials.gov/ct2/show/NCT02671955 (accessed on 27 August 2023).
- Snyder, L.A. JNJ-61610588: A human anti-VISTA antibody induces antitumor responses via a unique mechanism of action. In Proceedings of the AACR Annual Meeting 2016, New Orleans, LA, USA, 6–10 December 2016. [Google Scholar]
- Mostböck, S.; Wu, H.H.; Fenn, T.; Riegler, B.; Strahlhofer, S.; Huang, Y.; Hansen, G.; Kroe-Barrett, R.; Tirapu, I.; Vogt, A.B. Distinct immune stimulatory effects of anti-human VISTA antibodies are determined by Fc-receptor interaction. Front. Immunol. 2022, 13, 862757. [Google Scholar] [CrossRef]
- Shimabukuro-Vornhagen, A.; Gödel, P.; Subklewe, M.; Stemmler, H.J.; Schlößer, H.A.; Schlaak, M.; Kochanek, M.; Böll, B.; von Bergwelt-Baildon, M.S. Cytokine release syndrome. J. Immunother. Cancer 2018, 6, 56. [Google Scholar] [CrossRef]
- Hodi, F.S.; O’Day, S.J.; McDermott, D.F.; Weber, R.W.; Sosman, J.A.; Haanen, J.B.; Gonzalez, R.; Robert, C.; Schadendorf, D.; Hassel, J.C.; et al. Improved Survival with Ipilimumab in Patients with Metastatic Melanoma. N. Engl. J. Med. 2010, 363, 711–723. [Google Scholar] [CrossRef]
- Korman, A.J.; Garrett-Thomson, S.C.; Lonberg, N. The foundations of immune checkpoint blockade and the ipilimumab approval decennial. Nat. Rev. Drug Discov. 2021, 21, 509–528. [Google Scholar] [CrossRef]
- Squibb, B.-M. YERVOY U.S. Prescribing Information. Available online: https://packageinserts.bms.com/pi/pi_yervoy.pdf (accessed on 27 August 2023).
- Tarhini, A. Immune-Mediated Adverse Events Associated with Ipilimumab CTLA-4 Blockade Therapy: The Underlying Mechanisms and Clinical Management. Scientifica 2013, 2013, 8575. [Google Scholar] [CrossRef]
- de Miguel, M.; Calvo, E. Clinical Challenges of Immune Checkpoint Inhibitors. Cancer Cell 2020, 38, 326–333. [Google Scholar] [CrossRef]
- Feng, Y.; Roy, A.; Masson, E.; Chen, T.-T.; Humphrey, R.; Weber, J.S. Exposure–Response Relationships of the Efficacy and Safety of Ipilimumab in Patients with Advanced Melanoma. Clin. Cancer Res. 2013, 19, 3977–3986. [Google Scholar] [CrossRef] [PubMed]
- Egen, J.G.; Kuhns, M.S.; Allison, J.P. CTLA-4: New insights into its biological function and use in tumor immunotherapy. Nat. Immunol. 2002, 3, 611–618. [Google Scholar] [CrossRef] [PubMed]
- Sakaguchi, S.; Yamaguchi, T.; Nomura, T.; Ono, M. Regulatory T Cells and Immune Tolerance. Cell 2008, 133, 775–787. [Google Scholar] [CrossRef] [PubMed]
- Wing, K.; Onishi, Y.; Prieto-Martin, P.; Yamaguchi, T.; Miyara, M.; Fehervari, Z.; Nomura, T.; Sakaguchi, S. CTLA-4 control over Foxp3+ regulatory T cell function. Science 2008, 322, 271–275. [Google Scholar] [CrossRef]
- Simpson, T.R.; Li, F.; Montalvo-Ortiz, W.; Sepulveda, M.A.; Bergerhoff, K.; Arce, F.; Roddie, C.; Henry, J.Y.; Yagita, H.; Wolchok, J.D.; et al. Fc-dependent depletion of tumor-infiltrating regulatory T cells co-defines the efficacy of anti–CTLA-4 therapy against melanoma. J. Exp. Med. 2013, 210, 1695–1710. [Google Scholar] [CrossRef] [PubMed]
- Vargas, F.A.; Furness, A.J.S.; Litchfield, K.; Joshi, K.; Rosenthal, R.; Ghorani, E.; Solomon, I.; Lesko, M.H.; Ruef, N.; Roddie, C.; et al. Fc Effector Function Contributes to the Activity of Human Anti-CTLA-4 Antibodies. Cancer Cell 2018, 33, 649–663.e644. [Google Scholar] [CrossRef]
- Ribas, A.; Hanson, D.C.; Noe, D.A.; Millham, R.; Guyot, D.J.; Bernstein, S.H.; Canniff, P.C.; Sharma, A.; Gomez-Navarro, J. Tremelimumab (CP-675,206), a Cytotoxic T Lymphocyte–Associated Antigen 4 Blocking Monoclonal Antibody in Clinical Development for Patients with Cancer. Oncologist 2007, 12, 873–883. [Google Scholar] [CrossRef]
- Chen, X.; Song, X.; Li, K.; Zhang, T. FcγR-Binding Is an Important Functional Attribute for Immune Checkpoint Antibodies in Cancer Immunotherapy. Front. Immunol. 2019, 10, 292. [Google Scholar] [CrossRef]
- Pereira, N.A.; Chan, K.F.; Lin, P.C.; Song, Z. The “less-is-more” in therapeutic antibodies: Afucosylated anti-cancer antibodies with enhanced antibody-dependent cellular cytotoxicity. mAbs 2018, 10, 693–711. [Google Scholar] [CrossRef]
- Plitas, G.; Rudensky, A.Y. Regulatory T cells in cancer. Annu. Rev. Cancer Biol. 2020, 4, 459–477. [Google Scholar] [CrossRef]
- Lau, A.N.; Vander Heiden, M.G. Metabolism in the tumor microenvironment. Annu. Rev. Cancer Biol. 2020, 4, 17–40. [Google Scholar] [CrossRef]
- DeBerardinis, R.J.; Chandel, N.S. We need to talk about the Warburg effect. Nat. Metab. 2020, 2, 127–129. [Google Scholar] [CrossRef]
- Desnoyers, L.R.; Vasiljeva, O.; Richardson, J.H.; Yang, A.; Menendez, E.E.M.; Liang, T.W.; Wong, C.; Bessette, P.H.; Kamath, K.; Moore, S.J.; et al. Tumor-Specific Activation of an EGFR-Targeting Probody Enhances Therapeutic Index. Sci. Transl. Med. 2013, 5, 207ra144. [Google Scholar] [CrossRef]
- Etxeberria, I.; Bolaños, E.; Teijeira, A.; Garasa, S.; Yanguas, A.; Azpilikueta, A.; Kavanaugh, W.M.; Vasiljeva, O.; Belvin, M.; Howng, B.; et al. Antitumor efficacy and reduced toxicity using an anti-CD137 Probody therapeutic. Proc. Natl. Acad. Sci. USA 2021, 118, e2025930118. [Google Scholar] [CrossRef] [PubMed]
- Autio, K.A.; Boni, V.; Humphrey, R.W.; Naing, A. Probody Therapeutics: An Emerging Class of Therapies Designed to Enhance On-Target Effects with Reduced Off-Tumor Toxicity for Use in Immuno-Oncology. Clin. Cancer Res. 2020, 26, 984–989. [Google Scholar] [CrossRef]
- Assi, H.H.; Wong, C.; Tipton, K.A.; Mei, L.; Wong, K.; Razo, J.; Chan, C.; Howng, B.; Sagert, J.; Krimm, M.; et al. Conditional PD-1/PD-L1 Probody Therapeutics Induce Comparable Antitumor Immunity but Reduced Systemic Toxicity Compared with Traditional Anti–PD-1/PD-L1 Agents. Cancer Immunol. Res. 2021, 9, 1451–1464. [Google Scholar] [CrossRef]
- Mimoto, F.; Tatsumi, K.; Shimizu, S.; Kadono, S.; Haraya, K.; Nagayasu, M.; Suzuki, Y.; Fujii, E.; Kamimura, M.; Hayasaka, A.; et al. Exploitation of Elevated Extracellular ATP to Specifically Direct Antibody to Tumor Microenvironment. Cell Rep. 2020, 33, 108542. [Google Scholar] [CrossRef] [PubMed]
- Kamata-Sakurai, M.; Narita, Y.; Hori, Y.; Nemoto, T.; Uchikawa, R.; Honda, M.; Hironiwa, N.; Taniguchi, K.; Shida-Kawazoe, M.; Metsugi, S.; et al. Antibody to CD137 Activated by Extracellular Adenosine Triphosphate Is Tumor Selective and Broadly Effective In Vivo without Systemic Immune Activation. Cancer Discov. 2021, 11, 158–175. [Google Scholar] [CrossRef]
- Warburg, O. On the Origin of Cancer Cells. Science 1956, 123, 309–314. [Google Scholar] [CrossRef] [PubMed]
- Pavlova, N.N.; Thompson, C.B. The Emerging Hallmarks of Cancer Metabolism. Cell Metab. 2016, 23, 27–47. [Google Scholar] [CrossRef]
- Pavlova, N.N.; Zhu, J.; Thompson, C.B. The hallmarks of cancer metabolism: Still emerging. Cell Metab. 2022, 34, 355–377. [Google Scholar] [CrossRef] [PubMed]
- DeBerardinis, R.J.; Chandel, N.S. Fundamentals of cancer metabolism. Sci. Adv. 2016, 2, e1600200. [Google Scholar] [CrossRef] [PubMed]
- Koshiji, M.; Kageyama, Y.; Pete, E.A.; Horikawa, I.; Barrett, J.C.; Huang, L.E. HIF-1α induces cell cycle arrest by functionally counteracting Myc. EMBO J. 2004, 23, 1949–1956. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Sun, X.-X.; Qian, D.Z.; Dai, M.-S. Molecular Crosstalk Between MYC and HIF in Cancer. Front. Cell Dev. Biol. 2020, 8, 590576. [Google Scholar] [CrossRef]
- Girdhar, K.; Powis, A.; Raisingani, A.; Chrudinová, M.; Huang, R.; Tran, T.; Sevgi, K.; Dogru, Y.D.; Altindis, E. Viruses and Metabolism: The Effects of Viral Infections and Viral Insulins on Host Metabolism. Annu. Rev. Virol. 2021, 8, 373–391. [Google Scholar] [CrossRef]
- Parks, S.K.; Mueller-Klieser, W.; Pouysségur, J. Lactate and Acidity in the Cancer Microenvironment. Annu. Rev. Cancer Biol. 2020, 4, 141–158. [Google Scholar] [CrossRef]
- Wu, H.; Estrella, V.; Beatty, M.; Abrahams, D.; El-Kenawi, A.; Russell, S.; Ibrahim-Hashim, A.; Longo, D.L.; Reshetnyak, Y.K.; Moshnikova, A.; et al. T-cells produce acidic niches in lymph nodes to suppress their own effector functions. Nat. Commun. 2020, 11, 4113. [Google Scholar] [CrossRef]
- Tannock, I.F.; Rotin, D. Acid pH in tumors and its potential for therapeutic exploitation. Cancer Res. 1989, 49, 4373–4384. [Google Scholar]
- Zhang, X.; Lin, Y.; Gillies, R.J. Tumor pH and Its Measurement. J. Nucl. Med. 2010, 51, 1167–1170. [Google Scholar] [CrossRef]
- Damaghi, M.; Wojtkowiak, J.W.; Gillies, R.J. pH sensing and regulation in cancer. Front. Physiol. 2013, 4, 370. [Google Scholar] [CrossRef]
- Hashim, A.I.; Zhang, X.; Wojtkowiak, J.W.; Martinez, G.V.; Gillies, R.J. Imaging pH and metastasis. NMR Biomed. 2011, 24, 582–591. [Google Scholar] [CrossRef] [PubMed]
- Gillies, R.J.; Raghunand, N.; Karczmar, G.S.; Bhujwalla, Z.M. MRI of the tumor microenvironment. J. Magn. Reson. Imaging 2002, 16, 430–450. [Google Scholar] [CrossRef] [PubMed]
- Stubbs, M.; McSheehy, P.M.; Griffiths, J.R.; Bashford, C. Causes and consequences of tumour acidity and implications for treatment. Mol. Med. Today 2000, 6, 15–19. [Google Scholar] [CrossRef] [PubMed]
- Estrella, V.; Chen, T.; Lloyd, M.; Wojtkowiak, J.; Cornnell, H.H.; Ibrahim-Hashim, A.; Bailey, K.; Balagurunathan, Y.; Rothberg, J.M.; Sloane, B.F.; et al. Acidity Generated by the Tumor Microenvironment Drives Local Invasion. Cancer Res. 2013, 73, 1524–1535. [Google Scholar] [CrossRef]
- Gerweck, L.E.; Seetharaman, K. Cellular pH gradient in tumor versus normal tissue: Potential exploitation for the treatment of cancer. Cancer Res. 1996, 56, 1194–1198. [Google Scholar]
- Corbet, C.; Feron, O. Tumour acidosis: From the passenger to the driver’s seat. Nat. Rev. Cancer 2017, 17, 577–593. [Google Scholar] [CrossRef]
- Damaghi, M.; Tafreshi, N.K.; Lloyd, M.C.; Sprung, R.; Estrella, V.; Wojtkowiak, J.W.; Morse, D.L.; Koomen, J.M.; Bui, M.M.; Gatenby, R.A.; et al. Chronic acidosis in the tumour microenvironment selects for overexpression of LAMP2 in the plasma membrane. Nat. Commun. 2015, 6, 8752. [Google Scholar] [CrossRef]
- Rohani, N.; Hao, L.; Alexis, M.S.; Joughin, B.A.; Krismer, K.; Moufarrej, M.N.; Soltis, A.R.; Lauffenburger, D.A.; Yaffe, M.B.; Burge, C.B.; et al. Acidification of Tumor at Stromal Boundaries Drives Transcriptome Alterations Associated with Aggressive Phenotypes. Cancer Res. 2019, 79, 1952–1966. [Google Scholar] [CrossRef]
- Klaus, T.; Deshmukh, S. pH-responsive antibodies for therapeutic applications. J. Biomed. Sci. 2021, 28, 11. [Google Scholar] [CrossRef]
- Liu, R.; Oldham, R.J.; Teal, E.; Beers, S.A.; Cragg, M.S. Fc-Engineering for Modulated Effector Functions—Improving Antibodies for Cancer Treatment. Antibodies 2020, 9, 64. [Google Scholar] [CrossRef]
- Igawa, T.; Ishii, S.; Tachibana, T.; Maeda, A.; Higuchi, Y.; Shimaoka, S.; Moriyama, C.; Watanabe, T.; Takubo, R.; Doi, Y.; et al. Antibody recycling by engineered pH-dependent antigen binding improves the duration of antigen neutralization. Nat. Biotechnol. 2010, 28, 1203–1207. [Google Scholar] [CrossRef] [PubMed]
- Johnston, R.J.; Su, L.J.; Pinckney, J.; Critton, D.; Boyer, E.; Krishnakumar, A.; Corbett, M.; Rankin, A.L.; DiBella, R.; Campbell, L.; et al. VISTA is an acidic pH-selective ligand for PSGL-1. Nature 2019, 574, 565–570. [Google Scholar] [CrossRef]
- Matsumoto, M.; Miyasaka, M.; Hirata, T. P-Selectin Glycoprotein Ligand-1 Negatively Regulates T-Cell Immune Responses. J. Immunol. 2009, 183, 7204–7211. [Google Scholar] [CrossRef]
- Tinoco, R.; Carrette, F.; Barraza, M.L.; Otero, D.C.; Magaña, J.; Bosenberg, M.W.; Swain, S.L.; Bradley, L.M. PSGL-1 Is an Immune Checkpoint Regulator that Promotes T Cell Exhaustion. Immunity 2016, 44, 1470. [Google Scholar] [CrossRef] [PubMed]
- Thisted, T.; Mukherjee, A.; Malhotra, K.; Biesova, Z.; Kleschenko, Y.; Jiang, Z.-G.; Cifuentes, A.; Boland, N.; Nielson, N.; Horst, E.H.v. Antagonistic pH-selective VISTA antibody SNS-101 potentiates anti-PD-1/PD-L1-induced anti-tumor immunity. In Proceedings of the SITC Annual Meeting 2021, Washington, DC, USA, 1–5 November 2021. [Google Scholar]
- Thisted, T.; Eitas, T.; Malhotra, K.; Kleschenko, Y.; Finley, F.; Jiang, Z.-G.; Mukherjee, A.; Biesova, Z.; Cifuentes, A.; Pierce, R.; et al. 856 SNS-101, a highly pH-selective VISTA:PSGL-1 inhibitory antibody, potentiates anti-PD-1 sensitivity, expands memory T-cells and enhances tumor infiltration of CD8 T-cells. In Proceedings of the SITC Annual Meeting 2022, Boston, MA, USA, 8–22 November 2022. [Google Scholar] [CrossRef]
- Tanokura, M. 1H-NMR study on the tautomerism of the imidazole ring of histidine residues. I. Microscopic pK values and molar ratios of tautomers in histidine-containing peptides. Biochim. Biophys. Acta 1983, 742, 576–585. [Google Scholar] [CrossRef] [PubMed]
- Guy, C.; Mitrea, D.M.; Chou, P.-C.; Temirov, J.; Vignali, K.M.; Liu, X.; Zhang, H.; Kriwacki, R.; Bruchez, M.P.; Watkins, S.C.; et al. LAG3 associates with TCR–CD3 complexes and suppresses signaling by driving co-receptor–Lck dissociation. Nat. Immunol. 2022, 23, 757–767. [Google Scholar] [CrossRef]
- NCT03504488. CAB-ROR2-ADC Safety and Efficacy Study in Patients with TNBC or Head & Neck Cancer (Ph1) and NSCLC or Melanoma (Ph2). Available online: https://clinicaltrials.gov/ct2/show/NCT03504488 (accessed on 27 August 2023).
- NCT05271604. A Phase 2 Open Label Study of BA3021 in Patients with Recurrent or Metastatic Squamous Cell Carcinoma of the Head and Neck. Available online: https://www.clinicaltrials.gov/ct2/show/NCT05271604 (accessed on 27 August 2023).
- Wilky, B.; Druta, M.; Ahnert, J.R.; Conley, A.P.; Falchook, G.; Burris III, H.A.; Ingham, M.A.; Mehmi, I.; Sievers, E.L. Interim Safety and Efficacy Results from Phase 1/2 Study of Mecbotamab Vedotin (BA3011), a CAB-AXL-ADC, in Patients with Advanced Sarcoma. In Proceedings of the Connective Tissue Oncology Society (CTOS) Annual Meeting, Virtual, 10–13 November 2021. [Google Scholar]
- Sulea, T.; Rohani, N.; Baardsnes, J.; Corbeil, C.R.; Deprez, C.; Cepero-Donates, Y.; Robert, A.; Schrag, J.D.; Parat, M.; Duchesne, M.; et al. Structure-based engineering of pH-dependent antibody binding for selective targeting of solid-tumor microenvironment. mAbs 2019, 12, 1682866. [Google Scholar] [CrossRef]
- Chen, T.T. Conditionally active T cell engagers for the treatment of solid tumors: Rationale and clinical development. Expert Opin. Biol. Ther. 2022, 22, 955–963. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Smith, F.D.; Pierce, R.H.; Thisted, T.; van der Horst, E.H. Conditionally Active, pH-Sensitive Immunoregulatory Antibodies Targeting VISTA and CTLA-4 Lead an Emerging Class of Cancer Therapeutics. Antibodies 2023, 12, 55. https://doi.org/10.3390/antib12030055
Smith FD, Pierce RH, Thisted T, van der Horst EH. Conditionally Active, pH-Sensitive Immunoregulatory Antibodies Targeting VISTA and CTLA-4 Lead an Emerging Class of Cancer Therapeutics. Antibodies. 2023; 12(3):55. https://doi.org/10.3390/antib12030055
Chicago/Turabian StyleSmith, F. Donelson, Robert H. Pierce, Thomas Thisted, and Edward H. van der Horst. 2023. "Conditionally Active, pH-Sensitive Immunoregulatory Antibodies Targeting VISTA and CTLA-4 Lead an Emerging Class of Cancer Therapeutics" Antibodies 12, no. 3: 55. https://doi.org/10.3390/antib12030055
APA StyleSmith, F. D., Pierce, R. H., Thisted, T., & van der Horst, E. H. (2023). Conditionally Active, pH-Sensitive Immunoregulatory Antibodies Targeting VISTA and CTLA-4 Lead an Emerging Class of Cancer Therapeutics. Antibodies, 12(3), 55. https://doi.org/10.3390/antib12030055






