Interaction between Floral Merism and Symmetry: Evidence from Fasciated Mutant of Lupinus angustifolius L. (Leguminosae)
Abstract
1. Introduction
2. Materials and Methods
3. Results
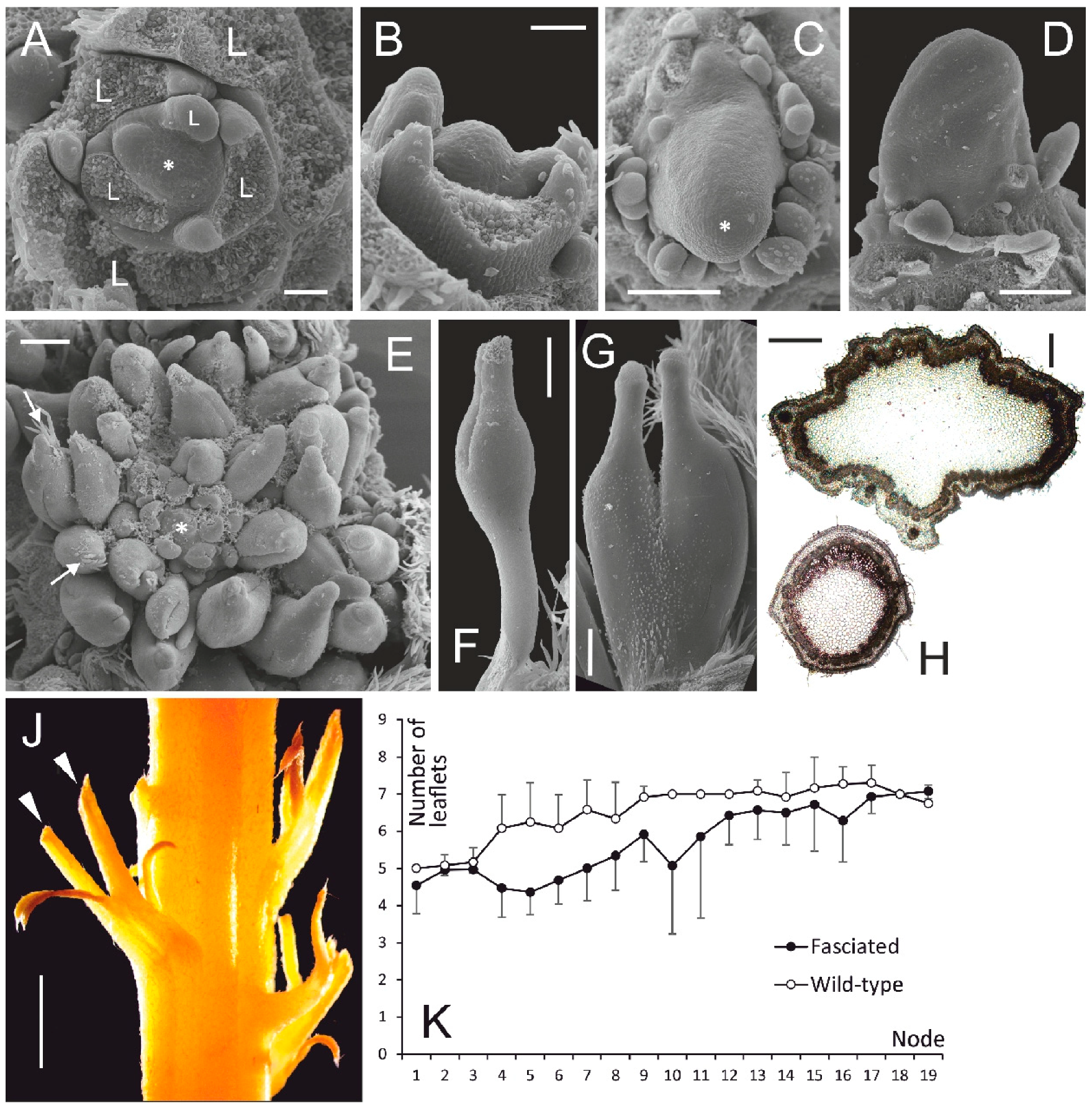
3.1. Vegetative Morphology of Fasciated vs. Normal Plants
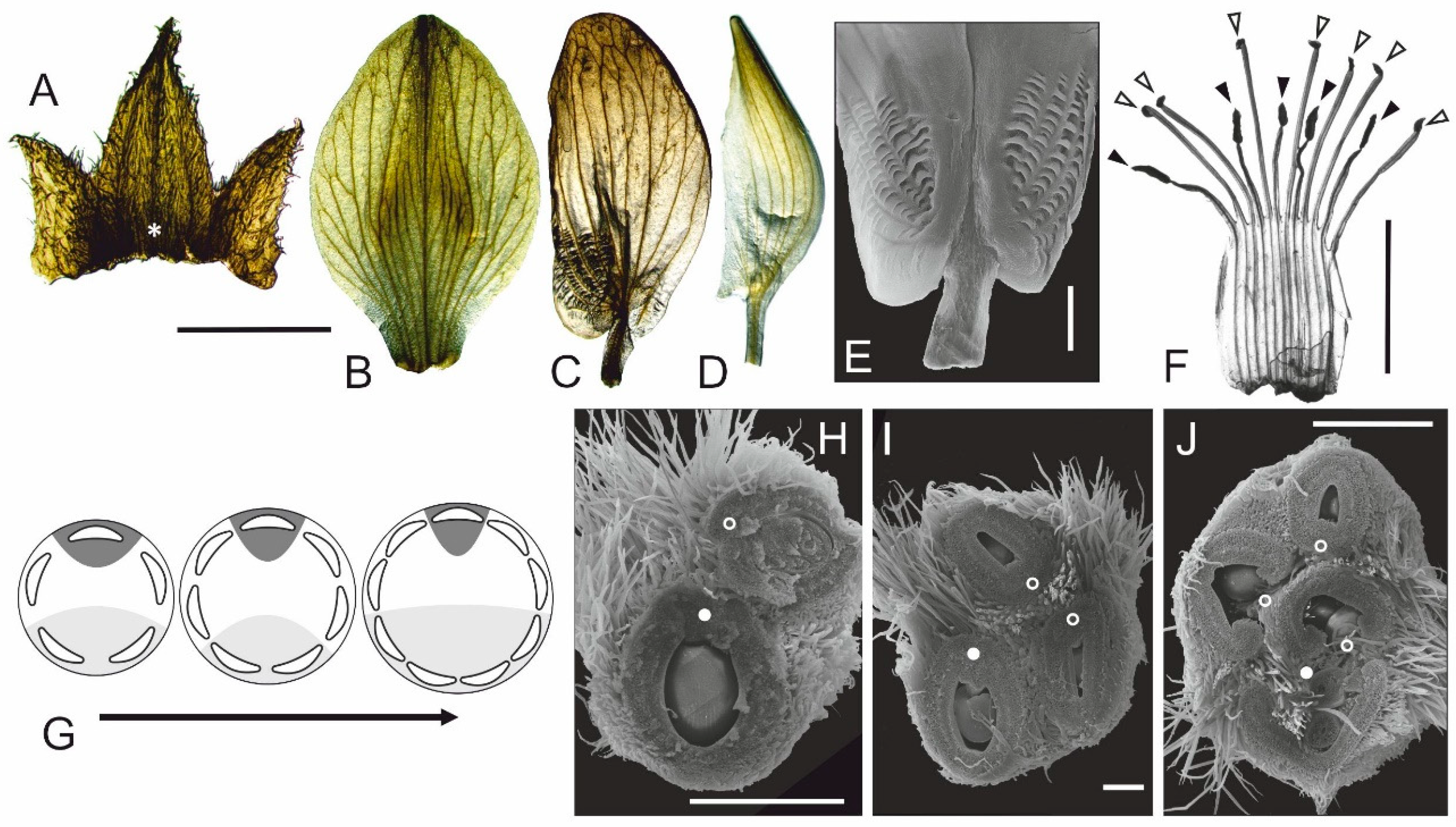
3.2. Normal Flower Development and Structure in Lupinus angustifolius
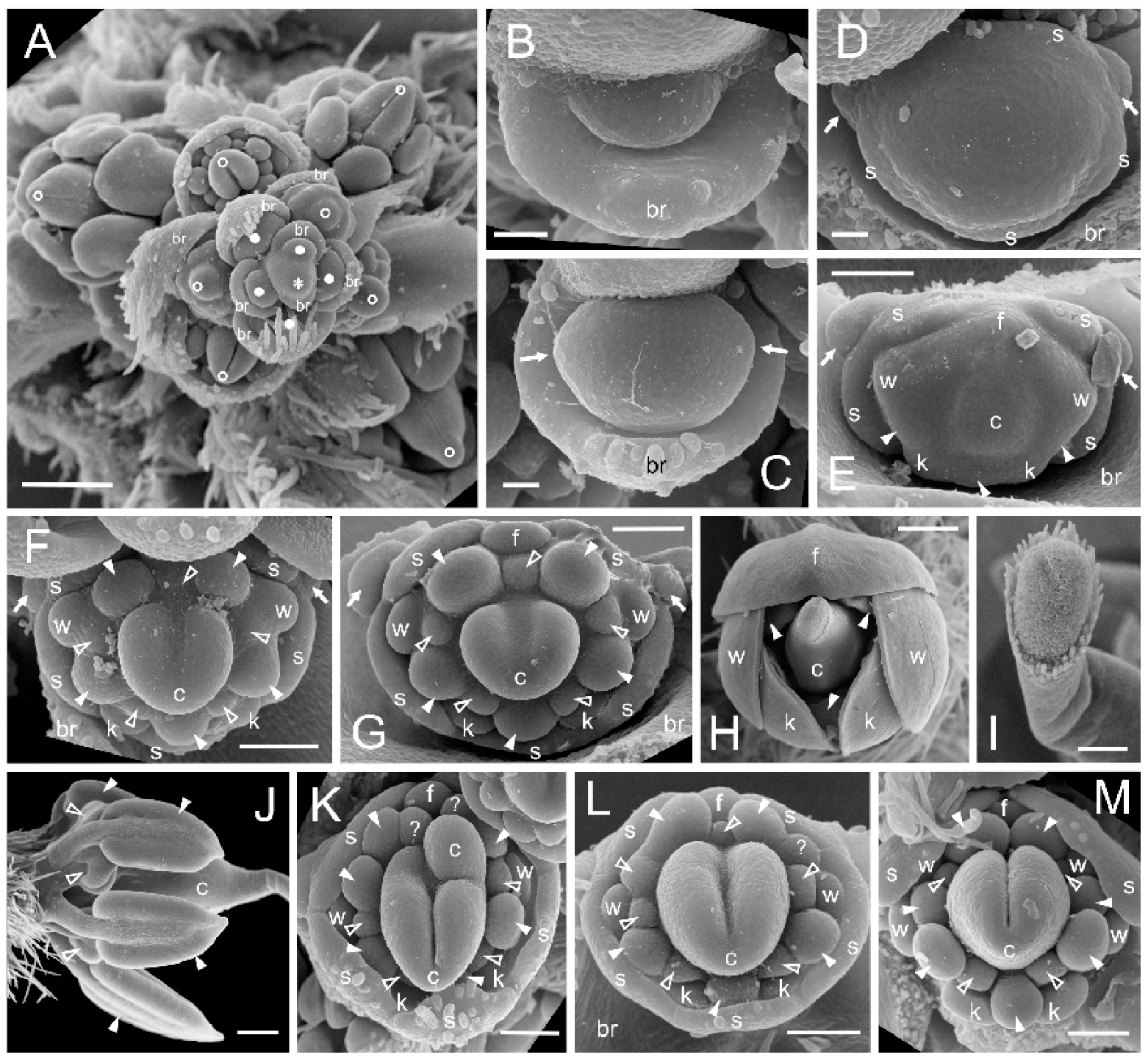
3.3. Floral Structure in Fasciated Mutant
4. Discussion
4.1. Phenotype of Fasciated Mutant of L. angustifolius Results from Superfluous Proliferation of Meristems
4.2. Different Floral Domains Are Affected with Unequal Frequencies
4.3. A Multicarpellate State in Fasciated Mutant Results from Proliferation of Floral Meristem (FM)
5. Conclusions
- The vegetative anomalies of fasciated mutant of L. angustifolius result from uncontrolled proliferation of SAM leading to initiation of superfluous structures, i.e., leaves and leaflets, asymmetric leaf arrangement on nodes and changes in phyllotaxis. As it was described by [2], the fasciated phenotype is caused by a mutation in a single gene. We hereby conclude that normal function of this gene is the negative regulation of SAM size. The causative mutation has a pleiotropic action leading both to SAM enlargement and to the increase of floral merism.
- Floral fasciation does not interfere with a control of monosymmetry, so in fasciated flowers all three types of petals differentiate properly.
- In fasciated flowers, different whorls are affected with unequal frequency. The least stable is the latest initiated inner whorl of the androecium.
- If judged by corolla, the lateral floral domains are the most susceptible to initiation of supernumerary organs with much higher frequency than the abaxial and adaxial domains. Thus, the petals positioned in (or close to) the medial plane of flower symmetry, are less prone to fluctuation. The stability of different corolla domains decreases in the following order: adaxial–abaxial–lateral.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sinjushin, A.A.; Karasyova, T.A. Stability of the floral structure in Leguminosae with flag versus non-flag blossom. Wulfenia 2017, 24, 1–10. [Google Scholar]
- Konovalov, Y.B.; Klochko, N.A.; Anikeyeva, N.F. Induced mutant of narrow-leaved lupine. Izvestiya TSKhA 1989, 6, 185–188. (In Russian) [Google Scholar]
- Sinjushin, A.A.; Tekdal, D.; Ciftci, C.; Cetiner, S. Floral development in Thermopsis turcica, an unusual multicarpellate papilionoid legume. Plant Syst. Evol. 2018, 304, 461–471. [Google Scholar] [CrossRef]
- Tucker, S.C. Origin of symmetry in flowers. In Contemporary Problems in Plant Anatomy; White, R.A., Dickinson, W.C., Eds.; Academic Press Inc.: New York, NY, USA, 1984; pp. 351–396. ISBN 0-12-746620-7. [Google Scholar]
- Paulino, J.V.; Mansano, V.F.; Prenner, G. Evidence for division of labor and division of function related to the pollen release in Papilionoideae (Leguminosae) with a heteromorphic androecium. Int. J. Plant Sci. 2016, 177, 590–607. [Google Scholar] [CrossRef]
- Prenner, G.; Bateman, R.M.; Rudall, P.J. Floral formulae updated for routine inclusion in formal taxonomic descriptions. Taxon 2010, 59, 241–250. [Google Scholar] [CrossRef]
- Sinjushin, A.A. Effects of stem fasciation on inflorescence and flower morphology in legumes. Wulfenia 2016, 23, 127–134. [Google Scholar]
- White, O.E. Fasciation. Bot. Rev. 1948, 14, 319–358. [Google Scholar] [CrossRef]
- Bowman, J.L.; Eshed, Y. Formation and maintenance of the shoot apical meristem. Trends Plant Sci. 2000, 5, 110–115. [Google Scholar] [CrossRef]
- Hagemann, W.; Gleissberg, S. Organogenetic capacity of leaves: The significance of marginal blastozones in angiosperms. Plant Syst. Evol. 1996, 199, 121–152. [Google Scholar] [CrossRef]
- Bykova, E.A.; Labunskaya, E.A.; Choob, V.V. Morphological changes in the structure of blastozones during fasciation of Pisum sativum L. and Arabidopsis thaliana (L.) Heynh. Biol. Bull. 2015, 42, 179–185. [Google Scholar] [CrossRef]
- Bykova, E.A.; Labunskaya, E.A.; Sinjushin, A.A.; Choob, V.V. Changes in the structure of a compound leaf in fasciated mutants of pea (Pisum sativum L.). In Proceedings of the 9th International Conference on Ecological Morphology of Plants Dedicated to the Memory of I.G. and T.I. Serebryakovy. V. 1, Moscow, Russia, 10–13 December 2014; pp. 101–104. [Google Scholar]
- Kajanus, B. Über einige vegetative Anomalien bei Trifolium pratense L. Zeitschrift für induktive Abstammungs- und Vererbungslehre 1913, 9, 111–133. [Google Scholar] [CrossRef]
- Snow, M.; Snow, G.R.S. A theory of the regulation of phyllotaxis based on Lupinus albus. Philos. Trans. R. Soc. Lond. B 1962, 244, 483–513. [Google Scholar] [CrossRef]
- Clark, S.E.; Running, M.P.; Meyerowitz, E.M. CLAVATA1, a regulator of meristem and flower development in Arabidopsis. Development 1993, 119, 397–418. [Google Scholar] [PubMed]
- Clark, S.E.; Running, M.P.; Meyerowitz, E.M. CLAVATA3 is a specific regulator of shoot and floral meristem development affecting the same processes as CLAVATA1. Development 1995, 121, 2057–2067. [Google Scholar]
- Suzaki, T.; Sato, M.; Ashikari, M.; Miyoshi, M.; Nagato, Y.; Hirano, H.-Y. The gene FLORAL ORGAN NUMBER1 regulates floral meristem size in rice and encodes a leucine-rich repeat receptor kinase orthologous to Arabidopsis CLAVATA1. Development 2004, 131, 5649–5657. [Google Scholar] [CrossRef] [PubMed]
- Bykova, E.A.; Chergintsev, D.A.; Vlasova, T.A.; Choob, V.V. Effect of the auxin polar transport inhibitor on the morphogenesis of leaves and generative structures during fasciation in Arabidopsis thaliana (L.) Heynh. Russ. J. Dev. Biol. 2016, 47, 207–215. [Google Scholar] [CrossRef]
- Sokoloff, D.; Rudall, P.J.; Remizowa, M. Flower-like terminal structures in racemose inflorescences: A tool in morphogenetic and evolutionary research. J. Exp. Bot. 2006, 57, 3517–3530. [Google Scholar] [CrossRef] [PubMed]
- Penin, A.A.; Choob, V.V.; Ezhova, T.A. Basic principles of terminal flower formation. Russ. J. Dev. Biol. 2005, 36, 65–69. [Google Scholar] [CrossRef]
- Tucker, S.C. Floral development in legumes. Plant Physiol. 2003, 131, 911–926. [Google Scholar] [CrossRef] [PubMed]
- Ronse Decraene, L.P.; Smets, E.F. The morphological variation and systematic value of stamen pairs in the Magnoliatae. Feddes Repert. 1996, 107, 1–17. [Google Scholar] [CrossRef]
- Zimmerman, E.; Prenner, G.; Bruneau, A. Floral ontogeny in Dialiinae (Caesalpinioideae: Cassieae), a study in organ loss and instability. S. Afr. J. Bot. 2013, 89, 188–209. [Google Scholar] [CrossRef]
- Wojciechowski, M.F.; Lavin, M.; Sanderson, M.J. A phylogeny of legumes (Leguminosae) based on analysis of the plastid matK gene resolves many well-supported subclades within the family. Am. J. Bot. 2004, 91, 1846–1862. [Google Scholar] [CrossRef] [PubMed]
- Arber, A. The interpretation of the flower: A study of some aspects of morphological thought. Biol. Rev. 1937, 12, 157–184. [Google Scholar] [CrossRef]
- Coen, E.S.; Meyerowitz, E.M. The war of the whorls: Genetic interactions controlling flower development. Nature 1991, 353, 31–37. [Google Scholar] [CrossRef] [PubMed]
- Paulino, J.V.; Prenner, G.; Mansano, V.F.; Teixeira, S.P. Comparative development of rare cases of a polycarpellate gynoecium in an otherwise monocarpellate family, Leguminosae. Am. J. Bot. 2014, 101, 572–586. [Google Scholar] [CrossRef] [PubMed]
| Type of Superfluous Organ(s) | Percent of Anomalous Flowers |
|---|---|
| Adaxial petal (flag) | 2.9% |
| Lateral petals (wings) | 68.6% |
| Abaxial petals (keel) | 5.7% |
| Outer staminal whorl | 54.3% |
| Inner staminal whorl | 85.7% |
| Gynoecium | 20.0% |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sinjushin, A.A.; Bykova, E.A.; Choob, V.V. Interaction between Floral Merism and Symmetry: Evidence from Fasciated Mutant of Lupinus angustifolius L. (Leguminosae). Symmetry 2019, 11, 321. https://doi.org/10.3390/sym11030321
Sinjushin AA, Bykova EA, Choob VV. Interaction between Floral Merism and Symmetry: Evidence from Fasciated Mutant of Lupinus angustifolius L. (Leguminosae). Symmetry. 2019; 11(3):321. https://doi.org/10.3390/sym11030321
Chicago/Turabian StyleSinjushin, Andrey A., Ekaterina A. Bykova, and Vladimir V. Choob. 2019. "Interaction between Floral Merism and Symmetry: Evidence from Fasciated Mutant of Lupinus angustifolius L. (Leguminosae)" Symmetry 11, no. 3: 321. https://doi.org/10.3390/sym11030321
APA StyleSinjushin, A. A., Bykova, E. A., & Choob, V. V. (2019). Interaction between Floral Merism and Symmetry: Evidence from Fasciated Mutant of Lupinus angustifolius L. (Leguminosae). Symmetry, 11(3), 321. https://doi.org/10.3390/sym11030321





