Patterns of Diversity of Floral Symmetry in Angiosperms: A Case Study of the Order Apiales
Abstract
1. Introduction
2. Background: The Concepts of Floral Actinomorphy and Zygomorphy from Geometrical and Ecological Viewpoints
3. Materials and Methods
4. Results
4.1. Polysymmetric Flowers
4.2. Disymmetric Flowers
4.3. Monosymmetric Flowers
4.4. Asymmetric Flowers
4.5. Minor Deviations from Polysymmetry in Particular Whorls of Flowers of Apiales
5. Discussion
5.1. Types of Floral Symmetry in Apiales: Applications of Various Approaches
5.2. The Impact of Gynoecium Orientation on Floral Symmetry in Apiales
5.3. The Impact of Merism and the Outline of the Polymerous Gynoecium on Floral Symmetry in Apiales
5.4. Orientation of Disymmetric and Monosymmetric Flowers Relative to the Inflorescence Axis in Apiales
5.5. Implications for the Evolution of Floral Symmetry in Asterids
6. Conclusions and Prospects
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Chase, M.W.; Christenhusz, M.J.; Fay, M.F.; Byng, J.W.; Judd, W.S.; Soltis, D.E.; Mabberley, D.J.; Sennikov, A.N.; Soltis, P.S.; Stevens, P.F. An update of the Angiosperm Phylogeny Group classification for the orders and families of flowering plants: APG IV. Bot. J. Linn. Soc. 2016, 181, 1–20. [Google Scholar]
- Nuraliev, M.S.; Oskolski, A.A.; Sokoloff, D.D.; Remizowa, M.V. Flowers of Araliaceae: Structural diversity, developmental and evolutionary aspects. Plant Divers. Evol. 2010, 128, 247–268. [Google Scholar] [CrossRef]
- Nuraliev, M.S.; Sokoloff, D.D.; Oskolski, A.A. Evolutionary Floral Morphology of Araliaceae: A Case Study of the Asian Schefflera; MAKS Press: Moscow, Russia, 2017; ISBN 978-5-317-05663-6. [Google Scholar]
- Bakker, K.; van Steenis, C.G.G.J. Pittosporaceae. In Flora Malesiana; van Steenis, C.G.G.J., Ed.; Series I: Spermatophyta; Noordhoff-Kolff N.V.: Jakarta, Indonesia, 1957; Volume 5, pp. 345–362. [Google Scholar]
- Kårehed, J. The family Pennantiaceae and its relationships to Apiales. Bot. J. Linn. Soc. 2003, 141, 1–24. [Google Scholar] [CrossRef]
- Sokoloff, D.D.; Karpunina, P.V.; Nuraliev, M.S.; Oskolski, A.A. Flower structure and development in Melanophylla (Torricelliaceae: Apiales): Lability in direction of corolla contortion and orientation of pseudomonomerous gynoecium in a campanulid eudicot. Bot. J. Linn. Soc. 2018, 187, 247–271. [Google Scholar]
- Neal, P.R.; Dafni, A.; Giurfa, M. Floral symmetry and its role in plant-pollinator systems: Terminology, distribution, and hypotheses. Annu. Rev. Ecol. Syst. 1998, 29, 345–373. [Google Scholar] [CrossRef]
- Endress, P.K. Symmetry in flowers: Diversity and evolution. Int. J. Plant Sci. 1999, 160, S3–S23. [Google Scholar] [CrossRef] [PubMed]
- Endress, P.K. Evolution of floral symmetry. Curr. Opin. Plant Biol. 2001, 4, 86–91. [Google Scholar] [CrossRef]
- Endress, P.K. Angiosperm floral evolution: Morphological developmental framework. In Advances in Botanical Research; Elsevier: Amsterdam, The Netherlands, 2006; Volume 44, pp. 1–61. ISBN 978-0-12-005944-7. [Google Scholar]
- Endress, P.K. The immense diversity of floral monosymmetry and asymmetry across angiosperms. Bot. Rev. 2012, 78, 345–397. [Google Scholar]
- Giurfa, M.; Dafni, A.; Neal, P.R. Floral symmetry and its role in plant-pollinator systems. Int. J. Plant Sci. 1999, 160, S41–S50. [Google Scholar] [CrossRef]
- Kalisz, S.; Ree, R.H.; Sargent, R.D. Linking floral symmetry genes to breeding system evolution. Trends Plant Sci. 2006, 11, 568–573. [Google Scholar] [CrossRef] [PubMed]
- Jabbour, F.; Nadot, S.; Damerval, C. Evolution of floral symmetry: A state of the art. C. R. Biol. 2009, 332, 219–231. [Google Scholar] [CrossRef] [PubMed]
- Citerne, H.; Jabbour, F.; Nadot, S.; Damerval, C. The evolution of floral symmetry. In Advances in Botanical Research; Elsevier: Amsterdam, The Netherlands, 2010; Volume 54, pp. 85–137. ISBN 978-0-12-380870-7. [Google Scholar]
- Leins, P.; Erbar, C. Flower and Fruit: Morphology, Ontogeny, Phylogeny, Function and Ecology; Schweizerbart: Stuttgart, Germany, 2010; ISBN 978-3-510-65261-7. [Google Scholar]
- Luo, D.; Carpenter, R.; Vincent, C.; Copsey, L.; Coen, E. Origin of floral asymmetry in Antirrhinum. Nature 1996, 383, 794–799. [Google Scholar] [CrossRef]
- Luo, D.; Carpenter, R.; Copsey, L.; Vincent, C.; Clark, J.; Coen, E. Control of organ asymmetry in flowers of Antirrhinum. Cell 1999, 99, 367–376. [Google Scholar] [CrossRef]
- Cubas, P.; Coen, E.; Zapater, J.M.M. Ancient asymmetries in the evolution of flowers. Curr. Biol. 2001, 11, 1050–1052. [Google Scholar] [CrossRef]
- Jabbour, F.; Damerval, C.; Nadot, S. Evolutionary trends in the flowers of Asteridae: Is polyandry an alternative to zygomorphy? Ann. Bot. 2008, 102, 153–165. [Google Scholar] [CrossRef]
- Fenster, C.B.; Armbruster, W.S.; Dudash, M.R. Specialization of flowers: Is floral orientation an overlooked first step? New Phytol. 2009, 183, 502–506. [Google Scholar] [CrossRef] [PubMed]
- Giurfa, M.; Eichmann, B.; Menzel, R. Symmetry perception in an insect. Nature 1996, 382, 458–461. [Google Scholar] [CrossRef] [PubMed]
- West, E.L.; Laverty, T.M. Effect of floral symmetry on flower choice and foraging behaviour of bumble bees. Can. J. Zool. 1998, 76, 730–739. [Google Scholar] [CrossRef]
- Reyes, E.; Sauquet, H.; Nadot, S. Perianth symmetry changed at least 199 times in angiosperm evolution. Taxon 2016, 65, 945–964. [Google Scholar] [CrossRef]
- Leppik, E.E. The form and function of numeral patterns in flowers. Am. J. Bot. 1956, 43, 445–455. [Google Scholar] [CrossRef]
- Leppik, E.E. A new system for classification of flower types. Taxon 1957, 6, 64–67. [Google Scholar] [CrossRef]
- Erbar, C.; Leins, P. Floral developmental studies in Aralia and Hedera (Araliaceae). Flora 1988, 180, 391–406. [Google Scholar] [CrossRef]
- Nuraliev, M.S.; Sokoloff, D.D.; Oskolski, A.A. Floral anatomy of Asian Schefflera (Araliaceae, Apiales): Comparing variation of flower groundplan and vascular patterns. Int. J. Plant Sci. 2011, 172, 735–762. [Google Scholar] [CrossRef]
- Ronse De Craene, L.P. Floral Diagrams: An Aid to Understanding Flower Morphology and Evolution; Cambridge University Press: Cambridge, UK, 2010; ISBN 978-0-521-49346-8. [Google Scholar]
- Philipson, W.R. Araliaceae. In Flora Malesiana; van Steenis, C.G.G.J., Ed.; Series I: Spermatophyta; Martinus Nijhoff: The Hague, The Netherlands, 1979; Volume 9, pp. 1–105. [Google Scholar]
- Nuraliev, M.S.; Degtjareva, G.V.; Sokoloff, D.D.; Oskolski, A.A.; Samigullin, T.H.; Valiejo-Roman, C.M. Flower morphology and relationships of Schefflera subintegra (Araliaceae, Apiales): An evolutionary step towards extreme floral polymery. Bot. J. Linn. Soc. 2014, 175, 553–597. [Google Scholar] [CrossRef]
- Sokoloff, D.D.; Oskolski, A.A.; Remizowa, M.V.; Nuraliev, M.S. Flower structure and development in Tupidanthus calyptratus (Araliaceae): An extreme case of polymery among asterids. Plant Syst. Evol. 2007, 268, 209–234. [Google Scholar] [CrossRef]
- Ajani, Y.; Bull-Hereñu, K.; Claßen-Bockhoff, R. Patterns of flower development in Apiaceae–Apioideae. Flora 2016, 221, 38–45. [Google Scholar] [CrossRef]
- Magin, N. Das Gynoeceum der Apiaceae—Modell und Ontogenie. Ber. Deutsch. Bot. Ges. 1977, 90, 53–66. [Google Scholar]
- Leins, P.; Erbar, C.; van Wyk, B.-E.; Tilney, P.M. Floral organ sequences in Apiales (Apiaceae, Araliaceae, Pittosporaceae). S. Afr. J. Bot. 2004, 70, 468–474. [Google Scholar] [CrossRef]
- Harms, H. Araliaceae. In Die naturlichen Pflanzenfamilien; Engler, A., Prantl, K., Eds.; W. Engelmann: Leipzig, Germany, 1898; Volume 3, pp. 1–62. [Google Scholar]
- Merrett, M.F. The breeding system of Raukaua anomalus, a small-leaved shrub from New Zealand. N. Z. J. Bot. 2005, 43, 205–210. [Google Scholar] [CrossRef]
- Oskolski, A.A.; Sokoloff, D.D.; Van Wyk, B.-E. False paracarpy in Seemannaralia (Araliaceae): From bilocular ovary to unilocular fruit. Ann. Bot. 2010, 106, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Lowry, P.P.; Plunkett, G.M.; Oskolski, A.A. Early lineages in Apiales: Insights from morphology, wood anatomy and molecular data. Edinb. J. Bot. 2001, 58, 207–220. [Google Scholar] [CrossRef]
- Froebe, H.A. Randmusterbildung und Synorganisation bei strahlenden Apiaceendolden. Plant Syst. Evol. 1980, 133, 223–237. [Google Scholar] [CrossRef]
- Hart, J.M.; Henwood, M.J. A revision of Australian Trachymene (Apiaceae: Hydrocotyloideae). Aust. Syst. Bot. 2006, 19, 11–57. [Google Scholar] [CrossRef]
- Liu, M.; Van Wyk, B.-E.; Tilney, P.M. Ontogeny of the fruits of two anomalous African woody genera, Polemanniopsis and Steganotaenia (Apiaceae), and their phylogenetic relationship. Edinb. J. Bot. 2003, 60, 249–257. [Google Scholar] [CrossRef]
- Baumann-Bodenheim, M.G. Ableitung und Bau bicarpellatmonospermer und pseudomonocarpellater Araliaceen- und Umbelliferen-Früchte. Ber. Schweiz. Bot. Ges. 1955, 65, 481–510. [Google Scholar]
- De Castro, O.; Colombo, P.; Gianguzzi, L.; Perrone, R. Flower and fruit structure of the endangered species Petagnaea gussonei (Sprengel) Rauschert (Saniculoideae, Apiaceae) and implications for its reproductive biology. Plant Biosyst. 2015, 149, 1042–1051. [Google Scholar] [CrossRef]
- Karpunina, P.V.; Oskolski, A.A.; Nuraliev, M.S.; Lowry, P.P.; Degtjareva, G.V.; Samigullin, T.H.; Valiejo-Roman, C.M.; Sokoloff, D.D. Gradual vs. abrupt reduction of carpels in syncarpous gynoecia: A case study from Polyscias subg. Arthrophyllum (Araliaceae: Apiales). Am. J. Bot. 2016, 103, 2028–2057. [Google Scholar] [PubMed]
- Wen, J. Generic delimitation of Aralia (Araliaceae). Brittonia 1993, 45, 47–55. [Google Scholar] [CrossRef]
- Wen, J.; Plunkett, G.M.; Mitchell, A.D.; Wagstaff, S.J. The evolution of Araliaceae: A phylogenetic analysis based on ITS sequences of nuclear ribosomal DNA. Syst. Bot. 2001, 26, 144–167. [Google Scholar]
- Shang, C.-B.; Lowry, P.P.I. Araliaceae. In Flora of China; Wu, Z., Raven, P.H., Hong, D.Y., Eds.; Science Press: Beijing, China; Missouri Botanical Garden: St. Louis, MO, USA, 2007; Volume 13, pp. 435–491. [Google Scholar]
- Drude, O. Umbelliferae. In Die naturlichen Pflanzenfamilien; Engler, A., Prantl, K., Eds.; W. Engelmann: Leipzig, Germany, 1898; Volume 3, pp. 63–250. [Google Scholar]
- Narayana, L.L.; Radhakrishnaiah, M. Floral anatomy of Pittosporaceae: Five species of Pittosporum. Can. J. Bot. 1982, 60, 1859–1867. [Google Scholar] [CrossRef]
- Zhou, Q.; Fu, D.; Jin, X. Floral morphology and anatomy of Pittosporum tobira (Pittosporaceae). Nord. J. Bot. 2005, 23, 345–352. [Google Scholar] [CrossRef]
- Erbar, C.; Leins, P. An analysis of the early floral development of Pittosporum tobira (Thunb.) Aiton and some remarks on the systematic position of the family Pittosporaceae. Feddes Repertorium 1995, 106, 463–473. [Google Scholar] [CrossRef]
- Cufodontis, G. Pittosporum in Aethiopien: Vorarbeit zu einer Revision der afrikanischen Arten. Österreichische Botanische Zeitschrift 1951, 98, 105–137. [Google Scholar] [CrossRef]
- Haas, J.E. The Pacific species of Pittosporum Banks ex Gaertn. (Pittosporaceae). Allertonia 1977, 1, 73–167. [Google Scholar]
- Friis, I. A Reconsideration of Pittosporum in Africa and Arabia. Kew Bull. 1987, 42, 319–335. [Google Scholar] [CrossRef]
- Judd, W.S. The Pittosporaceae in the southeastern United States. Harv. Pap. Bot. 1996, 1, 15–26. [Google Scholar]
- Prenner, G.; Bateman, R.M.; Rudall, P.J. Floral formulae updated for routine inclusion in formal taxonomic descriptions. Taxon 2010, 59, 241–250. [Google Scholar] [CrossRef]
- Schoute, J.C. On corolla aestivation and phyllotaxis of floral phyllomes. Verhandeling der Koninklijke Akademie van Wetenschappen te Amsterdam Afdeeling Natuurkunde 1935, 34, 1–77. [Google Scholar]
- Kaden, N.N.; Urmantzev, Y.A. Isomery in live nature. II. Results of investigations. Botanichesky Zhurnal 1971, 56, 161–174. [Google Scholar]
- Sokoloff, D.D.; Remizowa, M.V.; Timonin, A.C.; Oskolski, A.A.; Nuraliev, M.S. Types of organ fusion in Angiosperm flowers (with examples from Chloranthaceae, Araliaceae and monocots). Biol. Serb. 2018, 40, 16–46. [Google Scholar]
- Nuraliev, M.S.; Beer, A.S.; Oskolski, A.A. Vascular anatomy of flower of Tupidanthus and related species of Schefflera and the origin of floral polymery in Araliaceae. Botanichesky Zhurnal 2009, 94, 1–18. [Google Scholar]
- Henslow, G. On the vascular systems of floral organs, and their importance in the interpretation of the morphology of flowers. J. Linn. Soc. Lond. Bot. 1890, 28, 151–197. [Google Scholar] [CrossRef]
- Jackson, G. A study of the carpophore of the Umbelliferae. Am. J. Bot. 1933, 20, 121–144. [Google Scholar] [CrossRef]
- Theobald, W.L. Venation pattern and fruit development in Lomatium dasycarpum (Umbelliferae). Ann. Bot. 1967, 31, 255–262. [Google Scholar] [CrossRef]
- Magin, N. Eine blütenmorphologische Analyse der Lagoecieae (Apiaceae). Plant Syst. Evol. 1980, 133, 239–259. [Google Scholar] [CrossRef]
- Liu, M.; Plunkett, G.M.; Lowry, P.P.; Wyk, B.-E.V.; Tilney, P.M. The taxonomic value of fruit wing types in the order Apiales. Am. J. Bot. 2006, 93, 1357–1368. [Google Scholar] [CrossRef]
- Perkins, A.J. Hydrocotyle spinulifera and H. dimorphocarpa (Araliaceae), two new Western Australian species with dimorphic mericarps. Nuytsia 2018, 29, 57–65. [Google Scholar]
- Konstantinova, A.I. The systematic position of Uldinia ceratocarpa (Trachymene ceratocarpa) in the order Apiales based on the analysis of comparative carpological data. Bull. Mosc. Soc. Nat. Biol. Ser. 2015, 120, 38–48. [Google Scholar]
- Bukhari, G.; Zhang, J.; Stevens, P.F.; Zhang, W. Evolution of the process underlying floral zygomorphy development in pentapetalous angiosperms. Am. J. Bot. 2017, 104, 1846–1856. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, H.; Chang, H.; Turland, N.J. Pittosporaceae. In Flora of China; Wu, Z., Raven, P.H., Eds.; Science Press: Beijing, China; Missouri Botanical Garden: St. Louis, MO, USA, 2003; Volume 9, pp. 1–17. [Google Scholar]
- Karpunina, P.V.; Nuraliev, M.S.; Oskolski, A.A.; Sokoloff, D.D. Transference of positional information from bracteoles and sepals to petals in species with labile handedness of contort corolla: Mechanical forces or prepatterning? In Asymmetry in Plants: Biology of Handedness; Bahadur, B., Krishnamurthy, K.V., Ghose, M., Adams, S.J., Eds.; CRC Press: Boca Raton, FL, USA, 2019; pp. 285–300. ISBN 978-1-138-58794-6. [Google Scholar]
- Philipson, W.R.; Stone, B.C. The systematic position of Aralidium Miq.—A multidisciplinary study. 1. Introduction and floral and general anatomy. Taxon 1980, 29, 391–403. [Google Scholar] [CrossRef]
- Plunkett, G.M.; Wen, J.; Lowry, P.P., II. Infrafamilial classifications and characters in Araliaceae: Insights from the phylogenetic analysis of nuclear (ITS) and plastid (trnL-trnF) sequence data. Plant Syst. Evol. 2004, 245, 1–39. [Google Scholar] [CrossRef]
- Jebb, M.H.P. A revision of the genus Trevesia (Araliaceae). Glasra 1998, 3, 85–113. [Google Scholar]
- Erbar, C.; Leins, P. Different patterns of floral development in whorled flowers, exemplified by Apiaceae and Brassicaceae. Int. J. Plant Sci. 1997, 158, S49–S64. [Google Scholar] [CrossRef]
- Erbar, C.; Leins, P. Sympetaly in Apiales (Apiaceae, Araliaceae, Pittosporaceae). S. Afr. J. Bot. 2004, 70, 458–467. [Google Scholar] [CrossRef]
- Philipson, W.R. Constant and variable features of the Araliaceae. Bot. J. Linn. Soc. 1970, 63 (Suppl. 1), 87–100. [Google Scholar]
- Takhtajan, A. Flowering Plants; Springer: Dordrecht, The Netherlands, 2009; ISBN 978-1-4020-9608-2. [Google Scholar]
- Endress, P.K. Flower structure and trends of evolution in eudicots and their major subclades. Ann. Mo. Bot. Gard. 2010, 97, 541–583. [Google Scholar] [CrossRef]
- Ushimaru, A.; Hyodo, F. Why do bilaterally symmetrical flowers orient vertically? Flower orientation influences pollinator landing behaviour. Evol. Ecol. Res. 2005, 7, 151–160. [Google Scholar]
- Ushimaru, A.; Dohzono, I.; Takami, Y.; Hyodo, F. Flower orientation enhances pollen transfer in bilaterally symmetrical flowers. Oecologia 2009, 160, 667–674. [Google Scholar] [CrossRef] [PubMed]
- Alexeev, D.V.; Ezhova, T.A.; Kozlov, V.N.; Kudryavtsev, V.B.; Nosov, M.V.; Penin, A.A.; Skryabin, K.G.; Choob, V.V.; Shulga, O.A.; Shestakov, S.V. Spatial pattern formation in the flower of Arabidopsis thaliana: Mathematical modeling. Dokl. Biol. Sci. 2005, 401, 133–135. [Google Scholar] [CrossRef]
- Rudall, P.J. All in a spin: Centrifugal organ formation and floral patterning. Curr. Opin. Plant Biol. 2010, 13, 108–114. [Google Scholar] [CrossRef] [PubMed]
- Penin, A.A.; Logacheva, M.D. Correlation between number and position of floral organs in Arabidopsis. Ann. Bot. 2011, 108, 123–131. [Google Scholar] [CrossRef]
- Endress, P.K. Syncarpy and alternative modes of escaping disadvantages of apocarpy in primitive angiosperms. Taxon 1982, 31, 48–52. [Google Scholar] [CrossRef]
- Armbruster, W.S.; Debevec, E.M.; Willson, M.F. Evolution of syncarpy in angiosperms: Theoretical and phylogenetic analyses of the effects of carpel fusion on offspring quantity and quality: Evolution of syncarpy. J. Evol. Biol. 2002, 15, 657–672. [Google Scholar] [CrossRef]
- Endress, P.K. Multicarpellate gynoecia in angiosperms: Occurrence, development, organization and architectural constraints. Bot. J. Linn. Soc. 2014, 174, 1–43. [Google Scholar] [CrossRef]
- Ronse De Craene, L. Understanding the role of floral development in the evolution of angiosperm flowers: Clarifications from a historical and physico-dynamic perspective. J. Plant Res. 2018, 131, 367–393. [Google Scholar] [CrossRef]
- Tucker, S.C. Evolutionary lability of symmetry in early floral development. Int. J. Plant Sci. 1999, 160, S25–S39. [Google Scholar] [CrossRef]
- Faegri, K.; Van Der Pijl, L. The Principles of Pollination Ecology; Pergamon Press: Oxford, UK, 1966; ISBN 978-0-08-016421-2. [Google Scholar]
- Ronse De Craene, L.P.; Smets, E.F. Merosity in flowers: Definition, origin, and taxonomic significance. Plant Syst. Evol. 1994, 191, 83–104. [Google Scholar] [CrossRef]
- Sokoloff, D.D.; Nuraliev, M.S.; Oskolski, A.A.; Remizowa, M.V. Gynoecium evolution in angiosperms: Monomery, pseudomonomery, and mixomery. Mosc. Univ. Biol. Sci. Bull. 2017, 72, 97–108. [Google Scholar] [CrossRef]
- Zhang, J.; Stevens, P.F.; Zhang, W. Evolution of floral zygomorphy in androecium and corolla in Solanaceae. J. Syst. Evol. 2017, 55, 581–590. [Google Scholar] [CrossRef]
- Madrigal, Y.; Alzate, J.F.; González, F.; Pabón-Mora, N. Evolution of RADIALIS and DIVARICATA gene lineages in flowering plants with an expanded sampling in non-core eudicots. Am. J. Bot. 2019, 106, 334–351. [Google Scholar] [CrossRef]
- Clark, J.I.; Coen, E.S. The cycloidea gene can respond to a common dorsoventral prepattern in Antirrhinum. Plant J. 2002, 30, 639–648. [Google Scholar] [CrossRef] [PubMed]
- Damerval, C.; Guilloux, M.L.; Jager, M.; Charon, C. Diversity and evolution of CYCLOIDEA-like TCP genes in relation to flower development in Papaveraceae. Plant Physiol. 2007, 143, 759–772. [Google Scholar] [CrossRef] [PubMed]
- Sauquet, H.; von Balthazar, M.; Magallón, S.; Doyle, J.A.; Endress, P.K.; Bailes, E.J.; Barroso de Morais, E.; Bull-Hereñu, K.; Carrive, L.; Chartier, M.; et al. The ancestral flower of angiosperms and its early diversification. Nat. Commun. 2017, 8, 16047. [Google Scholar] [CrossRef] [PubMed]
- Sokoloff, D.D.; Remizowa, M.V.; Bateman, R.M.; Rudall, P.J. Was the ancestral angiosperm flower whorled throughout? Am. J. Bot. 2018, 105, 5–15. [Google Scholar] [CrossRef]
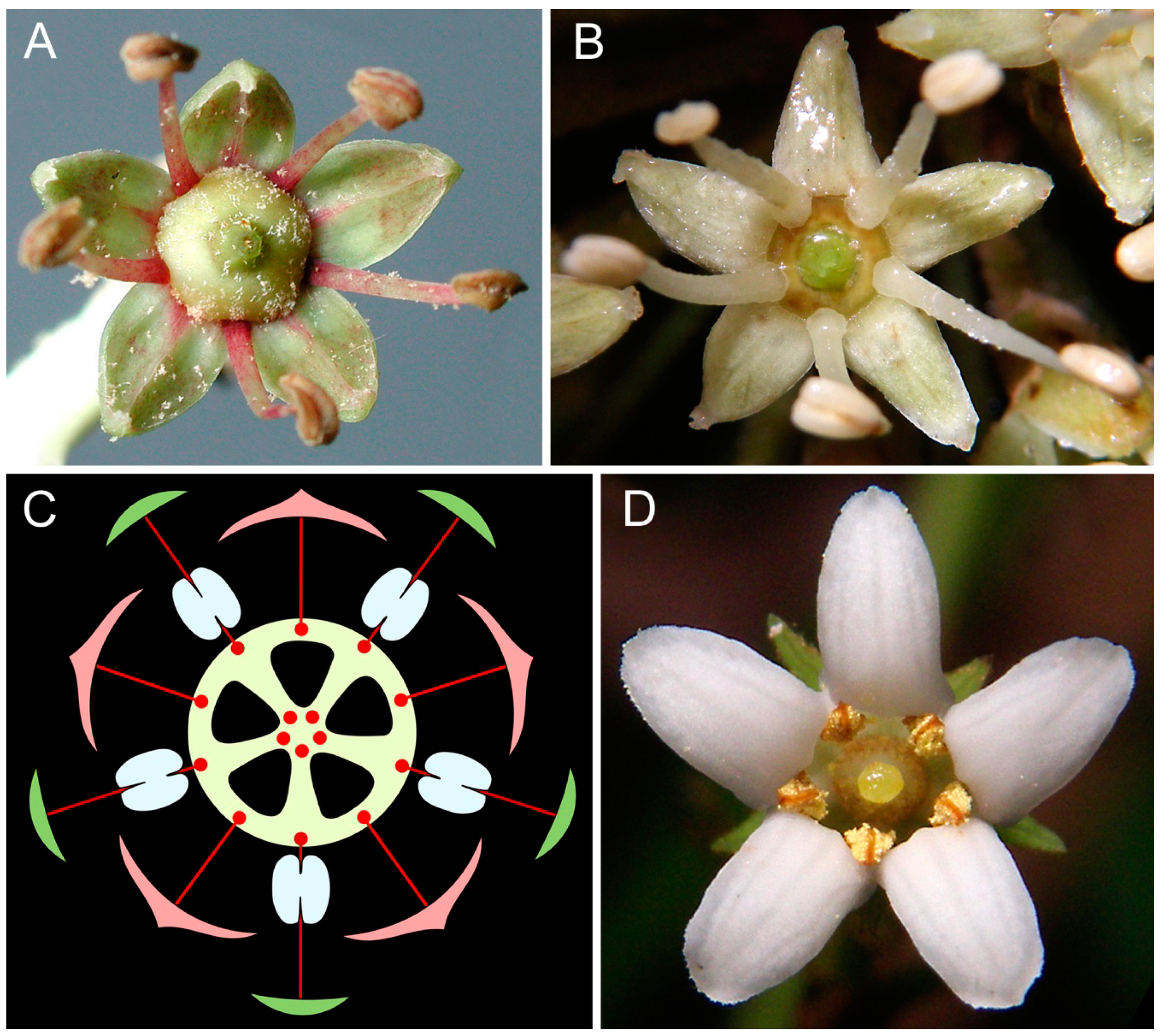


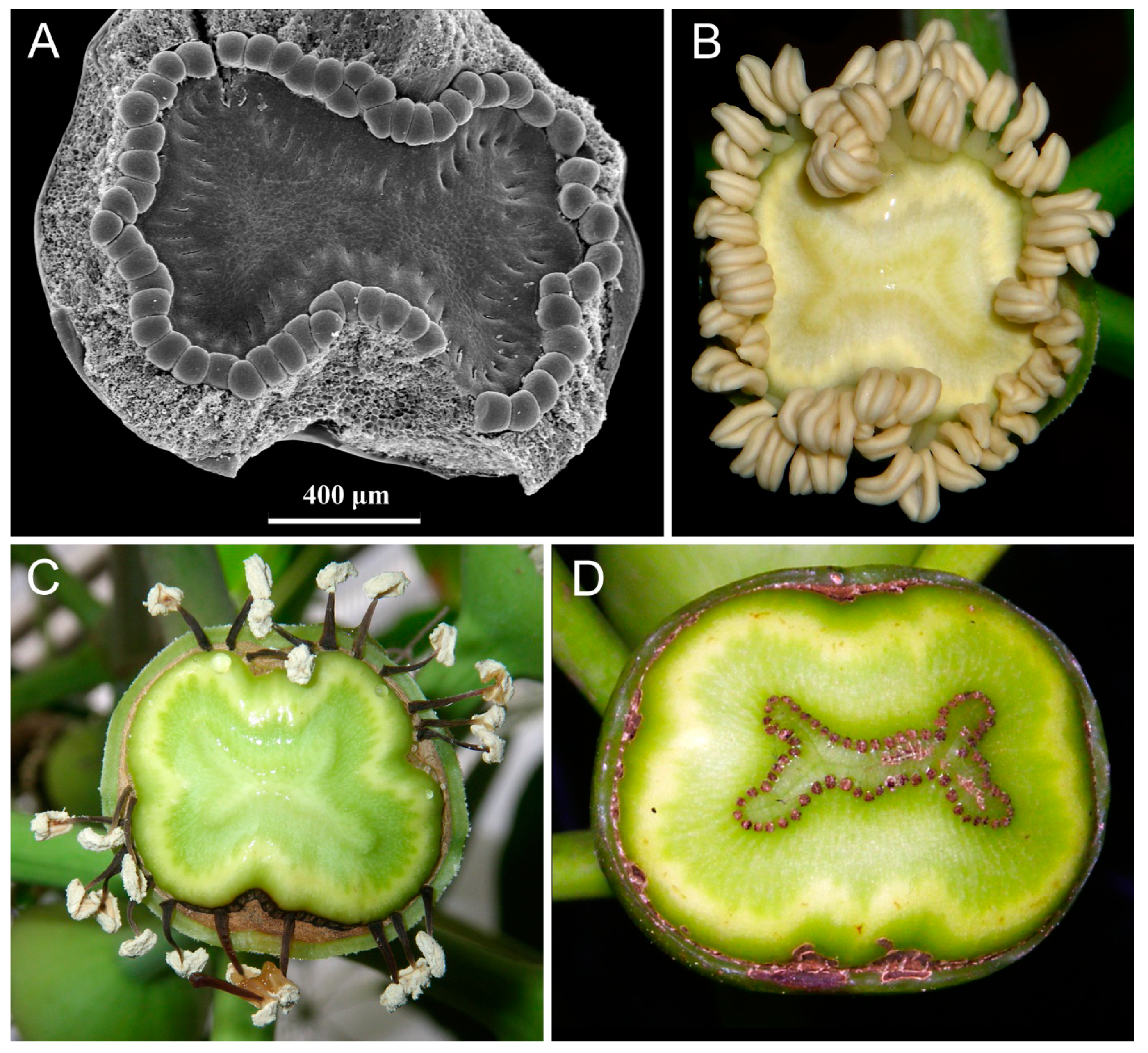

| Notation | Floral Formula | Important Features | Examples of Taxa and References |
|---|---|---|---|
| P1 | R * Kn * ↔ Cn * ↔ An * ↔ Gn * | Isomerous (mainly pentamerous) whorls, stable orientation of the gynoecium | Araliaceae: Aralia, Hedera [27], some Plerandra subg. Canacoschefflera (pers. obervations), some Asian Schefflera, Schefflera actinophylla (early stages) [2,3,28,29] |
| P2 | R * Kn * ↔ Cn * ↔ A∞ * Gn * | Isomerous (mainly pentamerous) perianth whorls and gynoecium, polymerous androecium, stable orientation of gynoecium | Araliaceae: some Plerandra subg. Plerandra, some Polyscias subg. Tetraplasandra [2] |
| P3 | R * Kn * ↔ Cn * A∞ * G∞ * | Isomerous (mainly pentamerous) perianth, polymerous androecium and gynoecium | Araliaceae: some Plerandra subg. Plerandra (pers. observations) |
| D1 | R * K4 * ↔ C4 * ↔ A4 * ↔ G2 ┼ | Stable orientation of the dimerous gynoecium, superimposed with tetramerous other whorls | Araliaceae: Tetrapanax (pers. observations) |
| D2 | R * K * ↔ Cn * ↔ An * ↔ Gn ┼ | Disymmetric gynoecium in its symplicate zone, with carpels in two opposite rows | Araliaceae: Schefflera actinophylla, S. brevipedicellata [3,28] |
| D3 | R ┼ ↔ K ┼ ↔ C ┼ ↔ A∞ ┼ ↔ G∞ ┼ | Disymmetric outline of the receptacle and whorls, two rows of carpels associated with polymery of the androecium and gynoecium | Araliaceae: some Osmoxylon [30], Schefflera subintegra, Tupidanthus calyptratus [3,31,32] |
| D4 | R * Kn * ↔ Cn * ↔ A∞ * G∞ ┼ | P3 + disymmetric gynoecium with two rows of carpels (at least in the symplicate zone) | Araliaceae: certain Plerandra subg. Plerandra [2] |
| M1 | R * K5 * ↔ C5 * ↔ A5 * ↔ G2 ┼ | Stable median orientation of a dimerous gynoecium superimposed with other pentamerous whorls | Most Apiaceae [29,33,34,35], Araliaceae: Cussonia, Harmsiopanax, Polyscias elegans, Polyscias subg. Polyscias [30,36], Raukaua [37], Seemannaralia [38], Myodocarpaceae [39] |
| M2 | R * K5 * ↔ C5 ↓ ↔ A5 * ↔ G2 ┼ | M1 + monosymmetric corolla | Apiaceae: Ammi, Artedia, Coriandrum, Daucus, certain Heracleum, Orlaya, Ormosciadium, Tordylium [40] (and pers. observations), Araliaceae: Trachymene pilosa [41] |
| M3 | R * K5 * ↔ C5 * ↔ A5 * ↔ G2 ↓ | M1 + monosymmetric heterocarpellate gynoecium | Apiaceae: Actinotus, Arctopus, Heteromorpha, Lagoecia, Petagnaea, Pollemanniopsis, Steganotaenia [34,42,43,44] |
| M4 | R * Kn * ↔ Cn * ↔ An * ↔ ≈Gn’ Ø | Variable orientation of a monosymmetric gynoecium (occasional coincidence of its symmetry plane with those of other whorls) | Araliaceae: Polyscias diversifolia, P. schultzei (n′ = 1) [45] |
| M5 | R * Kn *↔ Cn *↔ An * ↔ ≈G3 * | Variable orientation of a polysymmetric gynoecium (occasional coincidence of its symmetry plane with those of other whorls) | Araliaceae: Cheirodendron (pers. observations) |
| M6 | R * K5 * ↔ C5 * ↔ A5 * ↔ G3→ | Stable transverse orientation of a monosymmetric gynoecium | Torricelliaceae: Melanophylla [6] |
| M7 | R→ ↔ K→ ↔ C→ ↔ A∞→ ↔ G∞→ | Monosymmetric outline of the receptacle and the floral whorls | Araliaceae: Tupidanthus calyptratus [32] |
| A1 | R * K5 * ↔ C5 ∂ A5 * ↔ G5 * | P1 + imbricate petal aestivation | Araliaceae (buds): Aralia [30,46,47,48] |
| A2 | R * K5 * ↔ C5 ∂ A5 * ↔ G2 ┼ | M1 + imbricate petal aestivation | Some Apiaceae (buds) [49], Araliaceae (buds): Harmsiopanax, most Panax [30,36,47,48], Seemannaralia [38], Trachymene, Myodocarpaceae (buds): Delarbrea, Myodocarpus [39,47] |
| A3 | R * K5 * ↔ C5 ϟ A5 ϟ G3→ | Contort aestivation with the opposite direction of the contortion in the corolla and androecium | Torricelliaceae (buds): Melanophylla [6] |
| A4 | R * Kn * ↔ Cn * ↔ An * ≈ Gn’ Ø | Variable orientation of a monosymmetric gynoecium | Araliaceae: Polyscias diversifolia and P. schultzei (n′ = 1) [45] |
| A5 | R * Kn * ↔ Cn * ↔ An * ≈G3 * | Variable orientation of a polysymmetric gynoecium | Araliaceae: Cheirodendron (pers. observations), Pittosporaceae: many Pittosporum [50,51,52] |
| A6 | R * Kn ∂ Cn ∂ An * ≈G3 * | A5 + imbricate aestivation of petals and often also sepals | Pittosporaceae: many Pittosporum (buds) [4,50,51,53,54,55,56] |
| A7 | R ∂ K ∂ C ∂ A∞ ∂ G∞ ∂ | Asymmetric outline of the receptacle and the floral whorls | Araliaceae: Tupidanthus calyptratus [32] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nuraliev, M.S.; Sokoloff, D.D.; Karpunina, P.V.; Oskolski, A.A. Patterns of Diversity of Floral Symmetry in Angiosperms: A Case Study of the Order Apiales. Symmetry 2019, 11, 473. https://doi.org/10.3390/sym11040473
Nuraliev MS, Sokoloff DD, Karpunina PV, Oskolski AA. Patterns of Diversity of Floral Symmetry in Angiosperms: A Case Study of the Order Apiales. Symmetry. 2019; 11(4):473. https://doi.org/10.3390/sym11040473
Chicago/Turabian StyleNuraliev, Maxim S., Dmitry D. Sokoloff, Polina V. Karpunina, and Alexei A. Oskolski. 2019. "Patterns of Diversity of Floral Symmetry in Angiosperms: A Case Study of the Order Apiales" Symmetry 11, no. 4: 473. https://doi.org/10.3390/sym11040473
APA StyleNuraliev, M. S., Sokoloff, D. D., Karpunina, P. V., & Oskolski, A. A. (2019). Patterns of Diversity of Floral Symmetry in Angiosperms: A Case Study of the Order Apiales. Symmetry, 11(4), 473. https://doi.org/10.3390/sym11040473





