Chiral Interface of Amyloid Beta (Aβ): Relevance to Protein Aging, Aggregation and Neurodegeneration
Abstract
1. Introduction
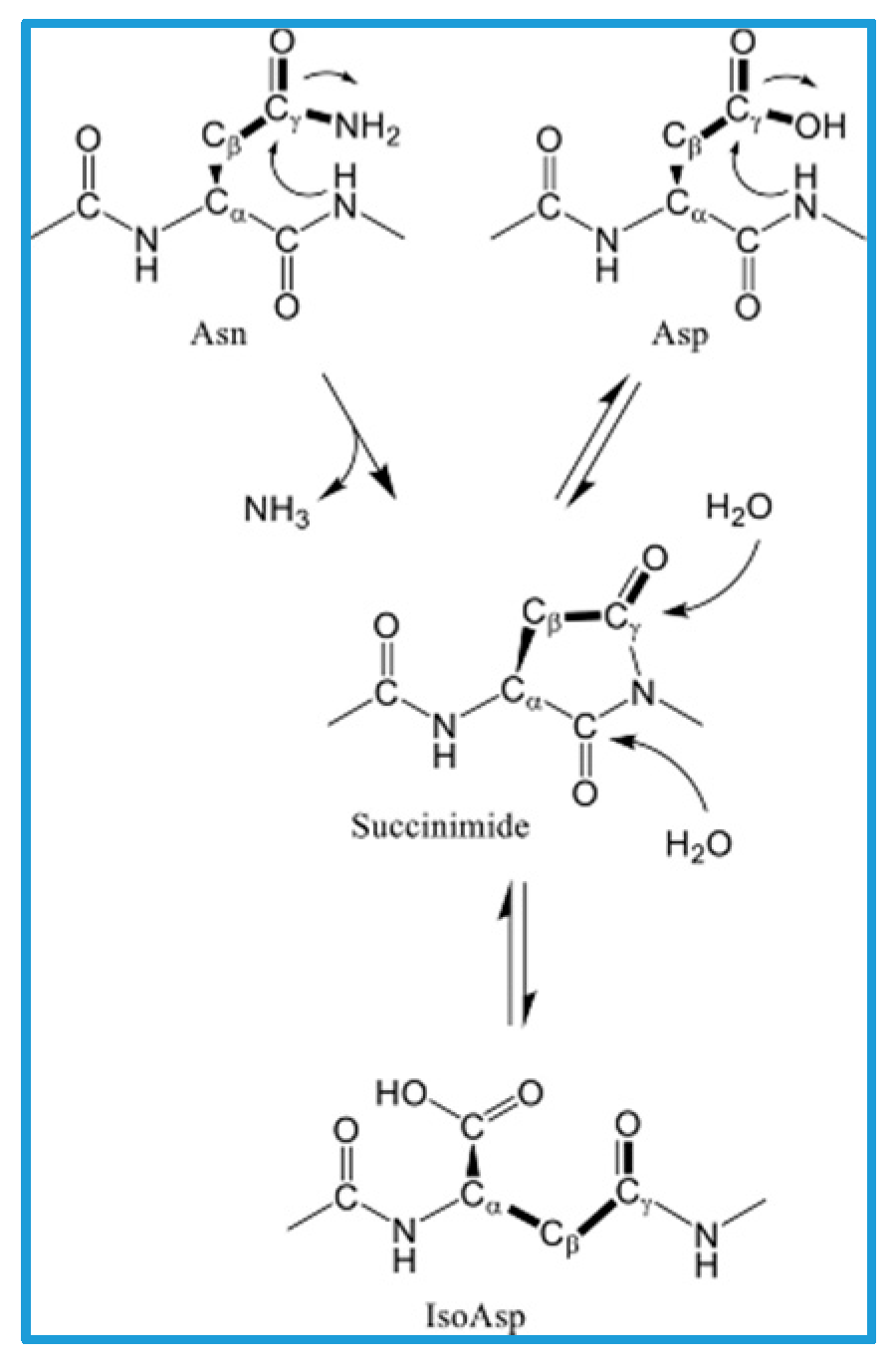
2. Racemization of the Aβ
3. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| ACH | Amyloid cascade hypothesis |
| PTMs | post-translational modifications |
| PTMs Enz | enzymic PTMs |
| PTMs Sp | spontaneous PTMs |
| PhTs NE | non-equilibrium phase transitions |
| Aβ | amyloid beta |
| RHPA | racemization hypothesis of protein aggregation |
| Ala | Alanine |
| Asn | Asparagine |
| Ser | Serine |
| Asp | aspartic acid |
| Glu | Glutamate |
| Ile | Isoleucine |
| Tyr | Tyrosine |
| Pro | Proline |
References
- Schreiner, E.; Trabuco, L.G.; Freddolino, P.L.; Schulten, K. Stereochemical errors and their implications for molecular dynamics simulations. BMC Bioinform. 2011, 12, 190. [Google Scholar] [CrossRef] [PubMed]
- Dunlop, D.S.; Neidle, A.; McHale, D.; Dunlop, D.M.; Lajtha, A. The presence of free D-aspartic acid in rodents and man. Biochem. Biophys. Res. Commun. 1986, 141, 27–32. [Google Scholar] [CrossRef]
- Jamasbi, E.; Separovic, F.; Hossain, M.A.; Ciccotosto, G.D. Phosphorylation of a full-length amyloid-β peptide modulates its amyloid aggregation, cell binding and neurotoxic properties. Mol. BioSyst. 2017, 13, 1545–1551. [Google Scholar] [CrossRef] [PubMed]
- Yokoyama, H.; Mizutani, R.; Noguchi, S.; Hayashida, N. Structural and biochemical basis of the formation of isoaspartate in the complementarity-determining region of antibody 64M-5 Fab. Sci. Rep. 2019, 9, 18494. [Google Scholar] [CrossRef]
- Moro, M.L.; Collins, M.J.; Cappellini, E. Alzheimer’s disease and amyloid β-peptide deposition in the brain: A matter of ‘aging’? Biochem. Soc. Trans. 2010, 38, 539–544. [Google Scholar] [CrossRef]
- Roher, A.E.; Kokjohn, T.A.; Clarke, S.G.; Sierks, M.R.; Maarouf, C.L.; Serrano, G.E.; Sabbagh, M.S.; Beach, T.G. APP/Aβ structural diversity and Alzheimer’s disease pathogenesis. Neurochem. Int. 2018, 110, 1–13. [Google Scholar] [CrossRef]
- McCudden, C.R.; Kraus, V.B. Biochemistry of amino acid racemization and clinical application to musculoskeletal disease. Clin. Biochem. 2006, 39, 1112–1130. [Google Scholar] [CrossRef]
- Radkiewicz, J.L.; Zipse, H.; Clarke, S.; Houk, K.N. Accelerated racemization of aspartic acid and asparagine residues via succinimide intermediates: An ab initio theoretical exploration of mechanism. J. Am. Chem. Soc. 1996, 118, 9148–9155. [Google Scholar] [CrossRef]
- Helfman, P.M.; Bada, J.L.; Shou, M.Y. Considerations on the role of aspartic acid racemization in the aging process. Gerontology 1977, 23, 419–425. [Google Scholar] [CrossRef]
- Takahashi, O.; Kirikoshi, R.; Manabe, N. Academic editor Mihai V. Putz. Racemization of the succinimide intermediate formed in proteins and peptides: A computational study of the mechanism catalyzed by dihydrogen phosphate ion. Int. J. Mol. Sci. 2016, 10, 1698. [Google Scholar] [CrossRef]
- Kubo, T.; Kumagae, Y.; Miller, C.A.; Kaneko, I. Beta-amyloid racemized at the Ser26 residue in the brains of patients with Alzheimer’s disease: Implications in the pathogenesis of Alzheimer’s disease. J. Neuropathol. Exp. Neurol. 2003, 62, 248–259. [Google Scholar] [CrossRef] [PubMed]
- Madeira, C.; Lourenco, M.V.; Vargas-Lopes, C.; Suemoto, C.K.; Brandão, C.O.; Reis, T.; Leite, R.E.P.; Laks, J.; Jacob-Filho, W.; Pasqualucci, C.A. D-serine levels in Alzheimer’s disease: Implications for novel biomarker development. Transl. Psychiatry 2015, 5, e561. [Google Scholar] [CrossRef] [PubMed]
- Ritz-Timme, S.; Collins, M.J. Racemization of aspartic acid in human proteins. Ageing Res. Rev. 2002, 1, 43–59. [Google Scholar] [CrossRef]
- Roher, A.E.; Yablenson, J.D.; Clarke, S.; Wolkow, C.; Wang, R.; Cotter, R.J.; Reardon, I.M.; Ziircher-Neely, H.A.; Heinrikson, R.L.; Ball, M.J.; et al. Structural alterations in the peptide backbone of β-amyloid core protein may account for its deposition and stability in Alzheimer’s disease. J. Biol. Chem. 1993, 268, 3072–3083. [Google Scholar]
- Kuo, Y.M.; Webster, S.; Emmerling, M.R.; de Lima, N.; Roher, A.E. Irreversible dimerization/tetramerization and post-translational modifications inhibit proteolytic degradation of Aβ peptides of Alzheimer’s disease. Biochim. Biophys. Acta. 1998, 1406, 291–298. [Google Scholar] [CrossRef]
- Kumar, S.; Walter, J. Phosphorylation of amyloid beta (Aβ) peptides—A trigger for formation of toxic aggregates in Alzheimer’s disease. Aging 2011, 3, 803–812. [Google Scholar] [CrossRef]
- Szendrei, G.I.; Fabian, H.; Mantsch, H.H.; Lovas, S.; Nyéki, O.; Schön, I.; Otvos, L. Aspartate-bond isomerization affects the major conformations of synthetic peptides. Eur. J. Biochem. FEBS 1994, 226, 917–924. [Google Scholar] [CrossRef]
- Xiao, Y.; Ma, B.; McElheny, D.; Parthasarathy, S.; Long, F.; Hoshi, M.; Nussinov, R.; Ishii, Y. Aβ (1–42) fibril structure illuminates self-recognition and replication of amyloid in Alzheimer’s disease. Nat. Struct. Mol. Biol. 2015, 22, 499–505. [Google Scholar] [CrossRef]
- Wildburger, N.C.; Esparza, T.J.; LeDuc, R.D.; Fellers, R.T.; Thomas, P.M.; Cairns, N.J.; Kelleher, N.L.; Bateman, R.J.; David, L.; Brody, D.L. Diversity of amyloid-beta proteoforms in the Alzheimer’s disease brain. Sci. Rep. 2017, 7, 9520. [Google Scholar] [CrossRef]
- Jiang, N.; Leithold, L.H.E.; Post, J.; Ziehm, T.; Mauler, J.; Gremer, L.; Cremer, M.; Schartmann, E.; Shah, N.J.; Kutzsche, J.; et al. Preclinical pharmacokinetic studies of the tritium labelled D-enantiomeric peptide D3 developed for the treatment of Alzheimer’s disease. PLoS ONE 2015, 10, e0128553. [Google Scholar]
- Malishev, R.; Arad, E.; Bhunia, S.K.; Shaham-Niv, S.; Kolusheva, S.; Gazit, E.; Jelinek, R. Chiral modulation of amyloid beta fibrillation and cytotoxicity by enantiomeric carbon dots. Chem. Commun. 2018, 54, 7762–7765. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Sun, Y.; Ma, Q.H.; Liu, Y. Alzheimer’s disease: Amyloid-based pathogenesis and potential therapies. Cell Stress. 2018, 2, 150–161. [Google Scholar] [CrossRef] [PubMed]
- Ravikirana, B.; Mahalakshmi, R. Unusual post-translational protein modifications: The benefits of sophistication. RSC Adv. 2014, 4, 33958–33974. [Google Scholar] [CrossRef]
- Osna, N.A.; Carter, W.G.; Ganesan, M.; Kirpich, I.A.; McClain, C.J.; Petersen, D.R.; Shearn, C.T.; Tomasi, M.L.; Kharbanda, K.K. Aberrant post-translational protein modifications in the pathogenesis of alcohol-induced liver injury. World J. Gastroenterol. 2016, 22, 6192–6200. [Google Scholar] [CrossRef] [PubMed]
- Grau, S.; Baldi, A.; Bussani, R.; Tian, X.; Stefanescu, R.; Przybylski, M.; Richards, P.; Jones, S.A.; Shridhar, V.; Tim Clausen, T.; et al. Implications of the serine protease HtrA1 in amyloid precursor protein processing. Proc. Natl. Acad. Sci. USA 2005, 102, 6021–6026. [Google Scholar] [CrossRef] [PubMed]
- Gieldon, A.; Zurawa-Janicka, D.; Jarzab, M.; Wenta, T.; Golik, P.; Dubin, G.; Lipinska, B.; Ciarkowski, J. Distinct 3D architecture and dynamics of the human Htra2(Omi) protease and its mutated variants. PLoS ONE 2016, 11, e0161526. [Google Scholar] [CrossRef] [PubMed]
- Goo, H.G.; Rhim, H.; Kang, S. Pathogenic role of serine protease HtrA2/Omi in neurodegenerative diseases. Curr. Protein Pept. Sci. 2017, 18, 746–757. [Google Scholar] [CrossRef]
- Singh, M.K.; Sharma, B.; Tiwari, P.C. The small heat shock protein Hsp27: Present understanding and future prospects. J. Therm. Biol. 2017, 69, 149–154. [Google Scholar] [CrossRef]
- Landry, J.; Lambert, H.; Zhou, M.; Lavoie, J.N.; Hickey, E.; Weber, L.A.; Anderson, C.W. Human HSP27 is phosphorylated at serines 78 and 82 by heat shock and mitogen-activated kinases that recognize the same amino acid motif as S6 kinase II. J. Biol. Chem. 1992, 267, 794–803. [Google Scholar]
- Katsogiannou, M.; Andrieu, C.; Rocchi, P. Heat shock protein 27 phosphorylation state is associated with cancer progression. Front. Genet. 2014. [Google Scholar] [CrossRef]
- Lowenson, J.D.; Clarke, S.; Roher, A.E. Chemical modifications of deposited amyloid-β peptides. Methods Enzym. 1999, 309, 89–105. [Google Scholar]
- Takahashi, O.; Kirikoshi, R.; Manabe, N. Racemization of serine residues catalyzed by dihydrogen phosphate Ion: A computational Study. Catalysts 2017, 7, 363. [Google Scholar] [CrossRef]
- Shapira, R.; Austin, G.E.; Mirra, S.S. Neuritic plaque amyloid in Alzheimer’s disease is highly racemized. J. Neurochem. 1988, 50, 69–74. [Google Scholar] [CrossRef] [PubMed]
- Kaneko, I.; Morimoto, K.; Kubo, T. Drastic neuronal loss in vivo by beta-amyloid racemized at Ser (26) residue: Conversion of non-toxic [D-Ser (26)] beta-amyloid 1–40 to toxic and proteinase-resistant fragments. Neuroscience 2001, 104, 1003–1011. [Google Scholar] [CrossRef]
- Tomiyama, T.; Asano, S.; Furiya, Y.; Shirasawa, T.; Endo, N.; Mori, H. Racemization of Asp23 residue affects the aggregation properties of Alzheimer amyloid beta protein analogues. J. Biol. Chem. 1994, 269, 10205–10208. [Google Scholar]
- Shimizu, T.; Fukuda, H.; Murayama, S.; Izumiyama, N.; Shirasawa, T. Iso-aspartate formation at position 23 of amyloid beta peptide enhanced fibril formation and deposited onto senile plaques and vascular amyloids in Alzheimer’s disease. J. Neurosci. Res. 2002, 70, 451–461. [Google Scholar] [CrossRef]
- Warmack, R.A.; Boyer, D.R.; Zee, C.T.; Richards, L.R.; Sawaya, M.R.; Cascio, D.; Gonen, T.; Eisenberg, D.S.; Clarke, S.G. Structure of A-β (20–34) with Alzheimer’s-associated isomerization at Asp23 reveals a distinct protofilament interface. Nat. Commun. 2019, 10, 3357. [Google Scholar] [CrossRef]
- Kumar, S.; Singh, S.; Hinze, D.; Josten, M.; Sahl, H.G.; Siepmann, M.; Walter, J. Phosphorylation of amyloid-β peptide at serine-8 attenuates its clearance via insulin-degrading and angiotensin-converting enzymes. J. Biol. Chem. 2012, 287, 8641–8651. [Google Scholar] [CrossRef]
- Barykin, E.P.; Petrushanko, I.Y.; Kozin, S.A.; Telegi, G.B.; Cherno, A.S.; Lopina, O.D.; Radko, S.P.; Mitkevich, V.A.; Makarov, A.M. Phosphorylation of the amyloid-beta peptide inhibits zinc-dependent aggregation, prevents Na,K-ATPase inhibition, and reduces cerebral plaque deposition. Front. Mol. Neurosci. 2018, 11, 302. [Google Scholar] [CrossRef]
- Rezaei-Ghaleh, N.; Amininasab, M.; Kumar, S.; Walter, J.; Zweckstetterc, M. Phosphorylation modifies the molecular stability of β-amyloid deposits. Nat. Commun. 2016, 7, 11359. [Google Scholar] [CrossRef]
- Milton, N.G. Phosphorylation of amyloid-beta at the serine 26 residue by human cdc2 kinase. Neuroreport 2001, 12, 3839–3844. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Wirths, O.; Stüber, K.; Wunderlich, P.; Koch, P.; Theil, S.; Rezaei-Ghaleh, N.; Zweckstetter, M.; Bayer, T.A.; Brüstle, O.; et al. Phosphorylation of the amyloid β-peptide at Ser26 stabilizes oligomeric assembly and increases neurotoxicity. Acta Neuropathol. 2016, 131, 525–537. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Wang, P.; Li, H.; Gao, Z. Nitration of Y10 in Aβ1–40: Is it a compensatory reaction against oxidative/nitrative stress and Aβ aggregation? Chem. Res. Toxicol. 2015, 28, 401–407. [Google Scholar] [CrossRef] [PubMed]
- Ribó, J.M.; Hochberg, D. Concept Paper. Chemical basis of biological homochirality during the abiotic evolution stages on Earth. Symmetry 2019, 11, 814. [Google Scholar] [CrossRef]
- Mori, H.; Ishii, K.; Tomiyama, T.; Furiya, Y.; Sahara, N.; Asano, S.; Endo, N.; Shirasawa, T.; Takio, K. Racemization: Its biological significance on neuropathogenesis of Alzheimer’s disease. Tohoku J. Exp. Med. 1994, 174, 251–262. [Google Scholar] [CrossRef]
- Kasim, J.K.; Kavianinia, I.; Harris, P.W.R.; Brimble, M.A. Three decades of amyloid beta synthesis: Challenges and advances. Front. Chem. 2019, 7, 472. [Google Scholar] [CrossRef]
- Dyakin, V.V.; Lucas, J. Non-equilibrium phase transition in biochemical—Systems. Chain of chirality transfer as Determinant of Brain Functional Laterality. Relevance to Alzheimer disease and cognitive psychology. In Proceedings of the Alzheimer’s Association International Conference (AAIC-2017), London, UK, 16–20 July 2017. [Google Scholar]
- Ornes, S. Core concept: How nonequilibrium thermodynamics speaks to the mystery of life. Proc. Natl. Acad. Sci. USA 2017, 114, 423–424. [Google Scholar] [CrossRef]
- Schrödinger, E. What is Life? The Physical Aspect of the Living Cell; Cambridge University Press: Cambridge, UK, 1994. [Google Scholar]
- Nansheng, Z. The role of homochirality in evolution. In Advances in BioChirality; Zucchi, C., Caglioti, L., Palyi, G., Eds.; Elsevier Science: Amsterdam, The Netherlands, 1999. [Google Scholar]
- Hsu, Y.H.; Chen, Y.-W.; Wu, M.-H.; Tu, L.H. Protein glycation by glyoxal promotes amyloid formation by Islet Amyloid polypeptide. Biophys. J. 2019, 116, 2304–2313. [Google Scholar] [CrossRef]
- Zhang, A.; Qi, W.; Good, T.A.; Fernandez, E.J. Structural differences between Aβ (1–40) intermediate oligomers and fibrils elucidated by proteolytic fragmentation and hydrogen/deuterium exchange. Biophys. J. 2009, 96, 1091–1104. [Google Scholar]
| The Frequency (f) of the AAs Appearance in A-beta (1–42) | |||||
|---|---|---|---|---|---|
| f | Amino Acids | ||||
| 6 | Gly | Val | |||
| 4 | Ala | ||||
| 3 | Asp | Glu | Phe | His | Ile |
| 2 | Lys | Leu | Ser | ||
| 1 | Met | Asn | Arg | Gln | Tyr |
| Peptide | Disease | Residue | PTMs | ||
|---|---|---|---|---|---|
| Rcm. | Ism. | Ph. | |||
| Spontaneous | Enzymic | ||||
| A-β (40–42) | AD | Ser-8 | [24, 35] | [3, 40, 41, 42] | |
| Ser-26 | [13, 35, 36] | [43. 44] | |||
| Asp-23 | [35, 37, 38] | [40] | |||
| A-β (20–34) | Asp-23 | [39] | |||
| A-Beta (1–42) | |||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N-Terminal | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | 19 | 20 | 21 | 22 | 23 | 24 | 25 | 26 | 27 | 28 | 29 | 30 | 31 | 32 | 33 | 34 | 35 | 36 | 37 | 38 | 39 | 40 | 41 | 42 | C-Terminal |
| Asp | Ala | Glu | Phe | Arg | His | Asp | Ser | Gly | Tyr | Glu | Val | His | His | Gln | Lys | Leu | Val | Phe | Phe | Ala | Glu | Asp | Val | Gly | Ser | Asn | Lys | Gly | Ala | Ile | Ile | Gly | Leu | Met | Val | Gly | Gly | Val | Val | Ile | Ala | ||
| D | A | E | F | R | H | D | S | G | Y | E | V | H | H | Q | K | L | V | F | F | A | E | D | V | G | S | N | K | G | A | I | I | G | L | M | V | G | G | V | V | I | A | ||
| * | * | ||||||||||||||||||||||||||||||||||||||||||
| ** | |||||||||||||||||||||||||||||||||||||||||||
| *** | *** | *** | |||||||||||||||||||||||||||||||||||||||||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dyakin, V.V.; Wisniewski, T.M.; Lajtha, A. Chiral Interface of Amyloid Beta (Aβ): Relevance to Protein Aging, Aggregation and Neurodegeneration. Symmetry 2020, 12, 585. https://doi.org/10.3390/sym12040585
Dyakin VV, Wisniewski TM, Lajtha A. Chiral Interface of Amyloid Beta (Aβ): Relevance to Protein Aging, Aggregation and Neurodegeneration. Symmetry. 2020; 12(4):585. https://doi.org/10.3390/sym12040585
Chicago/Turabian StyleDyakin, Victor V., Thomas M. Wisniewski, and Abel Lajtha. 2020. "Chiral Interface of Amyloid Beta (Aβ): Relevance to Protein Aging, Aggregation and Neurodegeneration" Symmetry 12, no. 4: 585. https://doi.org/10.3390/sym12040585
APA StyleDyakin, V. V., Wisniewski, T. M., & Lajtha, A. (2020). Chiral Interface of Amyloid Beta (Aβ): Relevance to Protein Aging, Aggregation and Neurodegeneration. Symmetry, 12(4), 585. https://doi.org/10.3390/sym12040585







