In-Situ Energy Dispersive X-ray Reflectivity Applied to Polyoxometalate Films: An Approach to Morphology and Interface Stability Issues in Organic Photovoltaics
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Layers Preparation
2.3. Methods
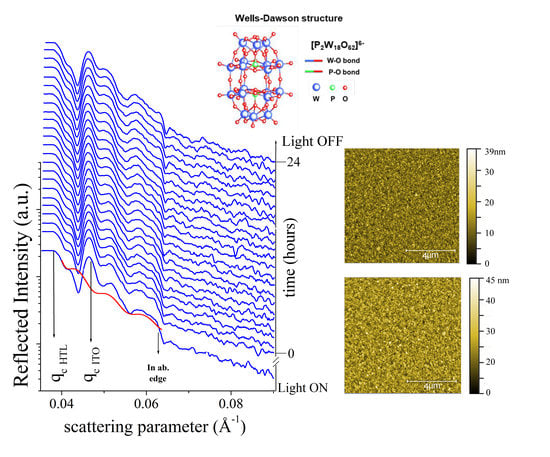
3. Results
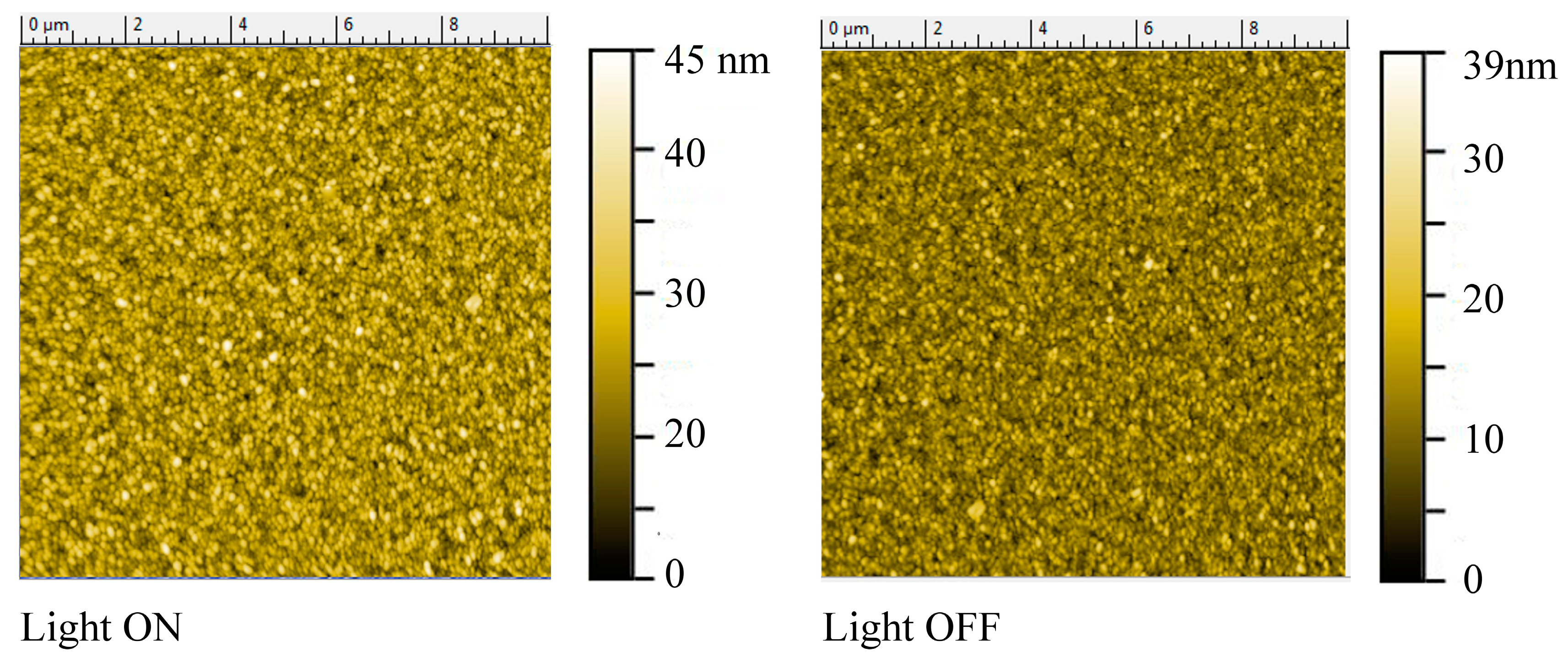
3.1. Ex-Situ Measurements
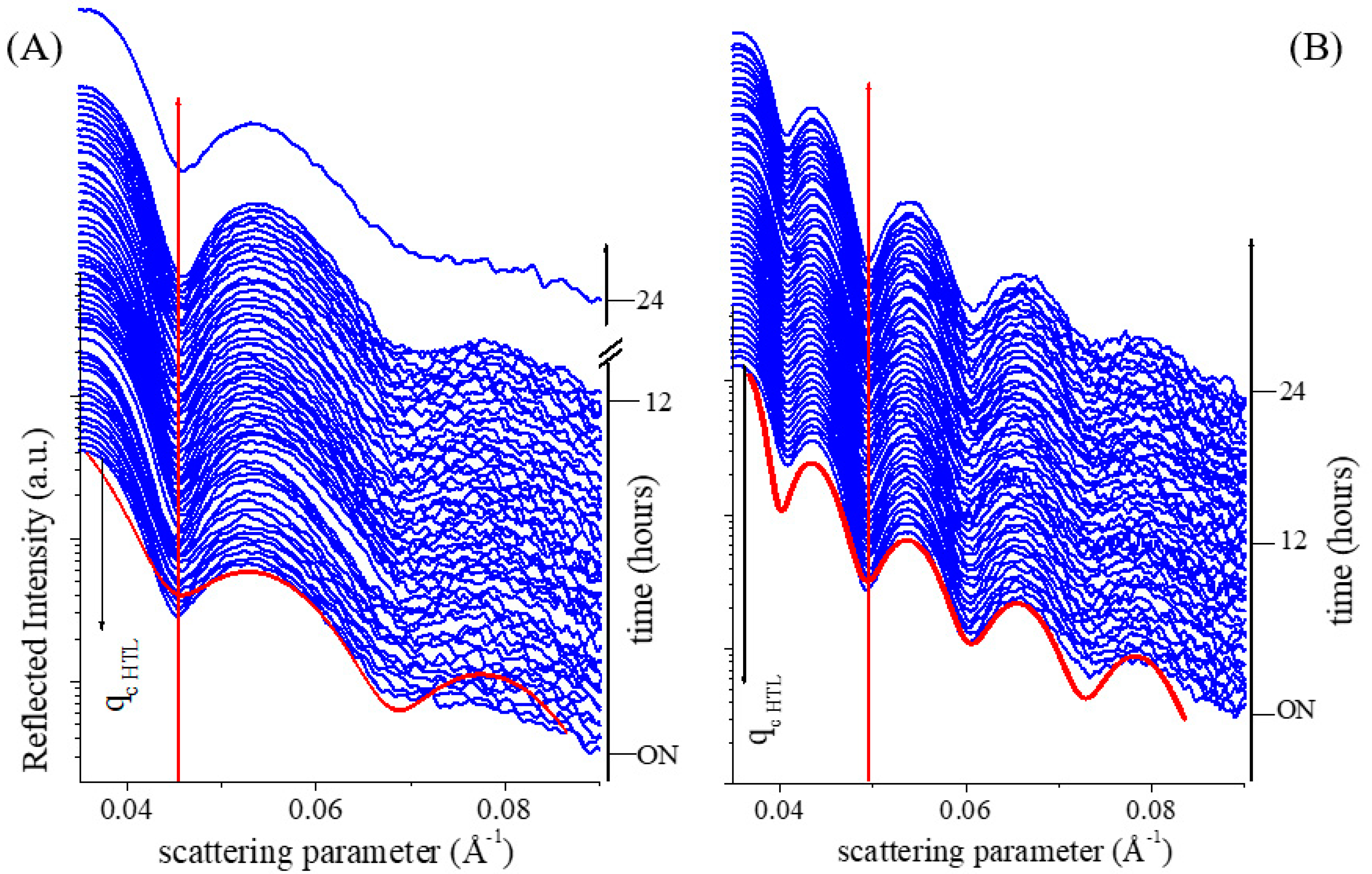
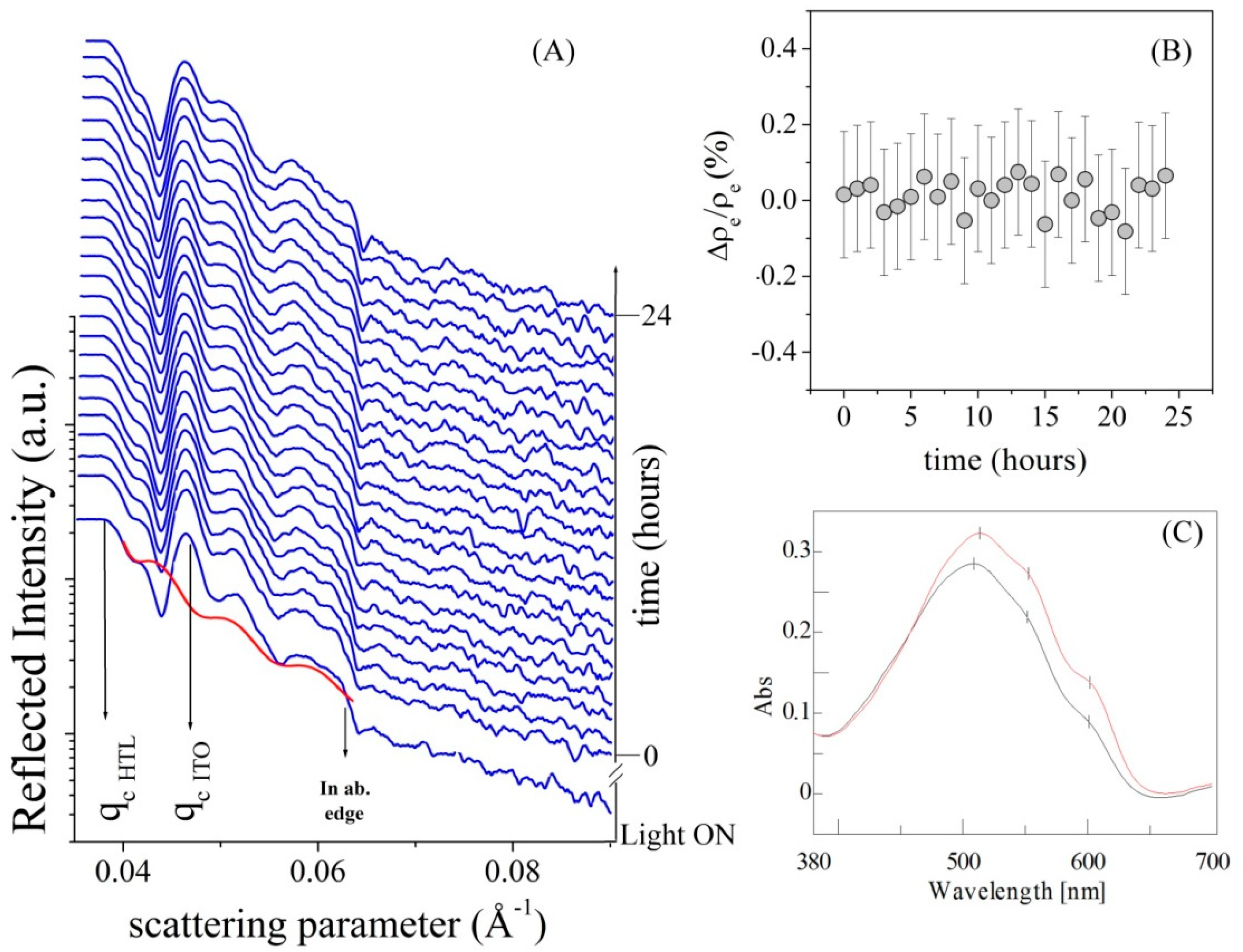
3.2. In-Situ Time-Resolved Measurements
3.3. Photovoltaic Characterization Data
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Cao, Q.; Kim, H.S.; Pimparkar, N.; Kulkarni, J.P.; Wang, C.; Shim, M.; Roy, K.; Alam, M.A.; Rogers, J.A. Medium-scale carbon nanotube thin-film integrated circuits on flexible plastic substrates. Nature 2008, 454, 495–500. [Google Scholar] [CrossRef]
- Ulanski, J.; Luszczynska, B.; Matyjaszewski, K. (Eds.) Solution-Processable Components for Organic Electronic Devices; Wiley-VCH: Weinheim, Germany, 2019. [Google Scholar]
- Lee, C.K.; Seo, J.G.; Kim, H.J.; Hong, S.J.; Song, G.; Ahn, C.; Lee, D.J.; Song, S.H. Versatile and Tunable Electrical Properties of Doped Nonoxidized Graphene Using Alkali Metal Chlorides. ACS Appl. Mater. Interfaces 2019, 11, 42520–42527. [Google Scholar] [CrossRef]
- Hammond, S.R.; Meyer, J.; Widjonarko, N.E.; Ndione, P.F.; Sigdel, A.K.; Garcia, A.; Miedaner, A.; Lloyd, M.T.; Kahn, A.; Ginley, D.S.; et al. Low-temperature, solution-processed molybdenum oxide hole-collection layer for organic photovoltaics. J. Mater. Chem. 2012, 22, 3249–3254. [Google Scholar] [CrossRef]
- Hösel, M.; Angmo, D.; Krebs, F.C. Handbook of Organic Materials for Optical and (Opto)Electronic Devices: Properties and Applications; Series in Electronic and Optical Materials; Woodhead Publishing: Sawston/Cambridge, UK, 2013; pp. 473–507. [Google Scholar]
- Available online: www.nrel.gov/pv/cell-efficiency.html (accessed on 3 July 2020).
- Zhan, L.; Li, S.; Lau, T.-K.; Cui, Y.; Lu, X.; Shi, M.; Li, C.-Z.; Li, H.; Hou, J.; Chen, H. Over 17% efficiency ternary organic solar cells enabled by two non-fullerene acceptors working in an alloy-like model. Energy Environ. Sci. 2020, 13, 635–645. [Google Scholar] [CrossRef]
- Basu, P.K.; Kumbhar, S.; Sreejith, K.P.; Yadav, T.S.; Kottantharayil, A.; Arora, B.M.; Narasimhan, K.L.; Sharma, A.K. Active area cell efficiency (19%) monocrystalline silicon solar cell fabrication using low-cost processing with small footprint laboratory tools. Bull. Mater. Sci. 2019, 42, 33. [Google Scholar] [CrossRef] [Green Version]
- Lucera, L.; Machui, F.; Kubis, P.; Schmidt, H.D.; Adams, J.; Strohm, S.; Ahmad, T.; Forberich, K.; Egelhaaf, H.-J.; Brabec, C.J. Highly efficient, large area, roll coated flexible and rigid OPV modules with geometric fill factors up to 98.5% processed with commercially available materials. Energy Environ. Sci. 2016, 9, 89–94. [Google Scholar]
- Taroni, P.J.; Hoces, I.; Stingelin, N.; Heeney, M.; Bilotti, E. Thermoelectric Materials: A Brief Historical Survey from Metal Junctions and Inorganic Semiconductors to Organic Polymers. IJC: Org. Electron. 2014, 54, 534–552. [Google Scholar]
- Zhu, Q.; Paci, B.; Generosi, A.; Renaudineau, S.; Gouzerh, P.; Liang, X.; Mathieu, C.; Rountree, C.; Izzet, G.; Proust, A.; et al. Conductivity via Thermally Induced Gap State in a Polyoxometalate Thin Layer. J. Phys. Chem. C 2019, 123, 1922–1930. [Google Scholar] [CrossRef] [Green Version]
- Pope, M.T.; Müller, A. Polyoxometalate Chemistry: An Old Field with New Dimensions in Several Disciplines. Angew. Chem. Int. Ed. Engl. 1991, 30, 34–48. [Google Scholar] [CrossRef]
- Sadakane, M.; Steckhan, E. Electrochemical Properties of Polyoxometalates as Electrocatalysts. Chem. Rev. 1998, 98, 219–238. [Google Scholar] [CrossRef]
- Papaconstantinou, E. Photochemistry of Polyoxometallates of Molybdenum and Tungsten and/or Vanadium. Chem. Soc. Rev. 1989, 18, 1–31. [Google Scholar] [CrossRef]
- Gómez-Romero, P. Polyoxometalates as photoelectrochemical models for quantum-sized colloidal semiconducting oxides. Solid State Ion. 1997, 101, 243–248. [Google Scholar] [CrossRef]
- Busche, C.; Vilà-Nadal, L.; Yan, J.; Miras, H.N.; Long, D.-L.; Georgiev, V.P.; Asenov, A.; Pedersen, R.H.; Gadegaard, N.; Mirza, M.M.; et al. Design and Fabrication of Memory Devices Based on Nanoscale Polyoxometalate Clusters. Nature 2014, 515, 545–549. [Google Scholar] [CrossRef] [PubMed]
- Vasilopoulou, M.; Polydorou, E.; Douvas, A.M.; Palilis, L.C.; Kennou, S.; Argitis, P. Annealing-Free Highly Crystalline Solution-Processed Molecular Metal Oxides for Efficient Single-Junction and Tandem Polymer Solar Cells. Energy Environ. Sci. 2015, 8, 2448–2463. [Google Scholar] [CrossRef]
- Jeevanandam, J.; Barhoum, A.; Chan, Y.S.; Dufresne, A.; Danquah, M.K. Review on nanoparticles and nanostructured materials: History, sources, toxicity and regulations. Beilstein J. Nanotechnol. 2018, 9, 1050–1074. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, W.; Nikiforov, M.P.; Darling, S.B. Morphology characterization in organic and hybrid solar cells. Energy Environ. Sci. 2012, 5, 8045–8074. [Google Scholar] [CrossRef]
- Paci, B.; Generosi, A.; Rossi Albertini, V.; Perfetti, P.; de Bettignies, R. The Role of C60 Barrier Layer in Improving the Performances of Efficient Polymer-Based Photovoltaic Devices: An AFM/EDXR Time-Resolved Study. J. Phys. Chem. C 2009, 113, 19740–19747. [Google Scholar] [CrossRef]
- Paci, B.; Generosi, A.; Rossi Albertini, V.; Perfetti, P.; de Bettignies, R.; Sentein, C. Photo-degradation and stabilization effects in operating organic photovoltaic devices by joint photo-current and morphological monitoring. Solar Energy Mater. Solar Cells 2008, 92, 799–804. [Google Scholar] [CrossRef]
- Guerrero, A.; Garcia-Belmonte, G. Recent Advances to Understand Morphology Stability of Organic Photovoltaics. Nano-Micro Lett. 2017, 9, 10. [Google Scholar] [CrossRef] [Green Version]
- McEvoy, A.; Castaner, L.; Markvart, T. (Eds.) Solar Cells: Materials, Manufacture and Operation, 2nd ed.; Academic Press: Cambridge, MA, USA, 2012. [Google Scholar]
- Felici, R. On the use of energy dispersive X-ray reflection to study the electronic density profile at surfaces and interfaces. Rigaku J. 1995, 12, 11–17. [Google Scholar]
- Daillant, J.; Gibaud, A. (Eds.) X-ray and Neutron Reflectivity Principles and Applications, 1st ed.; Springer: Berlin, Germany, 1999. [Google Scholar]
- Tanner, B.K. Grazing incidence X-ray reflectivity and scattering. In Handbook of Advanced Non-Destructive Evaluation; Ida, N., Meyendorf, N., Eds.; Springer: Cham, Switzerland, 2018. [Google Scholar]
- Chebil, S.M.; Vignaud, G.; Grohens, Y.; Konovalov, O.; Sanyal, M.K.; Beuvier, T.; Gibaud, A. In Situ X-ray Reflectivity Study of Polystyrene Ultrathin Films Swollen in Carbon Dioxide. Macromolecules 2012, 45, 6611–6617. [Google Scholar] [CrossRef]
- Orita, K.; Morimura, T.; Horiuchi, T.; Matsushige, K. In situ energy-dispersive X-ray reflectivity measurements of structural changes in thin films for organic electroluminescent devices. Synth. Met. 1997, 91, 155–158. [Google Scholar] [CrossRef]
- Rossi Albertini, V.; Paci, B.; Generosi, A. Energy dispersive X-ray reflectometry as a unique laboratory tool for investigating morphological properties of layered systems and devices. J. Phys. D Appl. Phys. 2006, 39, 461–486. [Google Scholar] [CrossRef]
- Generosi, A.; Rossi Albertini, V.; Rossi, G.; Paoletti, A.M.; Caminiti, R. Energy dispersive X-ray reflectometry of the NO2 interaction with ruthenium phthalocyanine films. J. Phys. Chem. B 2003, 107, 575–579. [Google Scholar] [CrossRef]
- Generosi, A.; Paci, B.; Rossi Albertini, V.; Perfetti, P.; de Bettignies, R.; Firon, M.; Leroy, J.; Sentein, C. In situ energy dispersive X-ray reflectometry measurements on organic solar cells upon working. Appl. Phys. Lett. 2005, 87, 194110–194112. [Google Scholar]
- Paci, B.; Generosi, A.; Rossi Albertini, V.; Spyropoulos, G.D.; Stratakis, E.; Kymakis, E. Enhancement of photo/thermal stability of organic bulk heterojunction photovoltaic devices via gold nanoparticles doping of the active layer. Nanoscale 2012, 4, 7452–7459. [Google Scholar] [CrossRef]
- Paci, B.; Generosi, A.; Rossi Albertini, V.; Perfetti, P.; de Bettignies, R.; Sentein, C. Time resolved morphological study of organic thin film solar cells based on calcium/aluminum cathode material. Chem. Phys. Lett. 2008, 461, 77–81. [Google Scholar] [CrossRef]
- Chason, E.; Mayer, T.M. Thin film and surface characterization by specular X-ray reflectivity. Crit. Rev. Solid State Mater. Sci. 1997, 22, 1–67. [Google Scholar] [CrossRef]
- Bontempi, E.; Depero, L.E.; Bergese, P. A simple solution to systematic errors in density determination by X-ray reflectivity: The XRR-density evaluation (XRR-DE). Appl. Surf. Sci. 2006, 253, 28–32. [Google Scholar]
- Neuhold, A.; Brandner, H.; Ausserlechner, S.J.; Lorbek, S.; Neuschitzer, M.; Zojer, E.; Teichert, C.; Resel, R. X-ray based tools for the investigation of buried interfaces in organic electronic devices. Org. Electron. 2013, 14, 479–487. [Google Scholar] [CrossRef] [Green Version]
- Sakurai, K. Exploring surfaces and buried interfaces of functional materials by advanced X-ray and neutron techniques. J. Phys. Condens. Matter 2010, 22, 470301. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kakavelakis, G.; Paradisanos, I.; Paci, B.; Generosi, A.; Papachatzakis, M.; Maksudov, T.; Del Rio Castillo, A.E.; Najafi, L.; Kioseoglou, G.; Stratakis, E.; et al. Large area-ambient stable Perovskite Solar Cells using MoS2 as hole transport interlayer. Adv. Energy Mater. 2018, 8, 170228. [Google Scholar]
- Paci, B.; Generosi, A.; Generosi, R.; Bailo, D.; Rossi Albertini, V. Joint time-resolved AFM/EDXR techniques for thin films morphological in situ studies. Chem. Phys. Lett. 2009, 483, 159–163. [Google Scholar] [CrossRef]
- Parratt, L.G. Surface Studies of Solids by Total Reflection of X-rays. Phys. Rev. 1954, 95, 359–369. [Google Scholar] [CrossRef]
- Ballirano, P.; Caminiti, R. Rietveld refinement on laboratory energy dispersive X-ray diffraction (EDXD) data. J. Appl. Crystallogr. 2001, 34, 757–762. [Google Scholar] [CrossRef] [Green Version]
- Fujii, Y. Analysis of surface roughness correlation function by X-ray reflectivity. Surf. Interface Anal. 2016, 48, 1136–1138. [Google Scholar] [CrossRef]



| Sample | Thickness d(Å) | Roughness σ(Å) |
|---|---|---|
| Glass/ITO | 1160(5) | 8(1) |
| Glass/ITO/HTL | 435(5) | 5(1) |
| Glass/ITO/HTL/BHJ | 1520(5) | 5(1) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Generosi, A.; Guaragno, M.; Zhu, Q.; Proust, A.; Barrett, N.T.; Tortech, L.; Paci, B. In-Situ Energy Dispersive X-ray Reflectivity Applied to Polyoxometalate Films: An Approach to Morphology and Interface Stability Issues in Organic Photovoltaics. Symmetry 2020, 12, 1240. https://doi.org/10.3390/sym12081240
Generosi A, Guaragno M, Zhu Q, Proust A, Barrett NT, Tortech L, Paci B. In-Situ Energy Dispersive X-ray Reflectivity Applied to Polyoxometalate Films: An Approach to Morphology and Interface Stability Issues in Organic Photovoltaics. Symmetry. 2020; 12(8):1240. https://doi.org/10.3390/sym12081240
Chicago/Turabian StyleGenerosi, Amanda, Marco Guaragno, Qirong Zhu, Anna Proust, Nicholas T. Barrett, Ludovic Tortech, and Barbara Paci. 2020. "In-Situ Energy Dispersive X-ray Reflectivity Applied to Polyoxometalate Films: An Approach to Morphology and Interface Stability Issues in Organic Photovoltaics" Symmetry 12, no. 8: 1240. https://doi.org/10.3390/sym12081240
APA StyleGenerosi, A., Guaragno, M., Zhu, Q., Proust, A., Barrett, N. T., Tortech, L., & Paci, B. (2020). In-Situ Energy Dispersive X-ray Reflectivity Applied to Polyoxometalate Films: An Approach to Morphology and Interface Stability Issues in Organic Photovoltaics. Symmetry, 12(8), 1240. https://doi.org/10.3390/sym12081240