Room Temperature Syntheses of ZnO and Their Structures
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Keimer, B.; Kivelson, S.A.; Norman, M.R.; Uchida, S.; Zaanen, J. From Quantum Matter to High-temperature Superconductivity in Copper Oxides. Nature 2015, 518, 179–186. [Google Scholar] [CrossRef]
- Rijckaert, H.; Pollefeyt, G.; Sieger, M.; Hänisch, J.; Bennewitz, J.; De Keukeleere, K.; De Roo, J.; Hühne, R.; Bäcker, M.; Paturi, P.; et al. Optimizing Nanocomposites through Nanocrystal Surface Chemistry: Superconducting YBa2Cu3O7 Thin Films via Low-Fluorine Metal Organic Deposition and Preformed Metal Oxide Nanocrystals. Chem. Mater. 2017, 29, 6104–6113. [Google Scholar] [CrossRef] [Green Version]
- Chakhalian, J.; Liu, X.; Fiete, G.A. Strongly Correlated and Topological States in Grown Transition Metal Oxide Thin Films and Heterostructures Editors-pick. APL Mater. 2020, 8, 050904. [Google Scholar] [CrossRef]
- Neri, G. First Fifty Years of Chemoresistive Gas Sensors. Chemosensors 2015, 3, 1–20. [Google Scholar] [CrossRef]
- Carbone, M. CQDs@NiO: An Efficient Tool for CH4 Sensing. Appl. Sci. 2020, 10, 6251. [Google Scholar] [CrossRef]
- Sturaro, M.; Della Gaspera, E.; Michieli, N.; Cantalini, C.; Emamjomeh, S.M.; Guglielmi, M.; Martucci, A. Degenerately Doped Metal Oxide Nanocrystals as Plasmonic and Chemoresistive Gas Sensors. ACS Appl. Mater. Interfaces 2016, 8, 30440–30448. [Google Scholar] [CrossRef]
- Lin, T.; Lv, X.; Hu, Z.; Xu, A.; Feng, C. Semiconductor Metal Oxides as Chemoresistive Sensors for Detecting Volatile Organic Compounds. Sensors 2019, 19, 233. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carbone, M.; Tagliatesta, P. NiO Grained-flowers and Nanoparticles for Ethanol Sensing. Materials 2020, 13, 1880. [Google Scholar] [CrossRef] [Green Version]
- Potyrailo, R.A.; Go, S.; Sexton, D.; Li, X.; Alkadi, N.; Kolmakov, A.; Amm, B.; St-Pierre, R.; Scherer, B.; Nayeri, M.; et al. Extraordinary Performance of Semiconducting Metal Oxide Gas Sensors Using Dielectric Excitation. Nat. Electron. 2020, 3, 80–289. [Google Scholar] [CrossRef]
- Lee, H.J. Linear Gas Sensing with Dielectric Excitation. Nat. Electron. 2020, 3, 239–240. [Google Scholar] [CrossRef]
- Yao, Y.; Sang, D.; Duan, S.; Wang, Q.; Liu, C. Review on the Properties of Boron-Doped Diamond and One-Dimensional-Metal-Oxide Based P-N Heterojunction. Molecules 2021, 26, 71. [Google Scholar] [CrossRef]
- Staerz, A.; Gao, X.; Cetmi, F.; Ming, Z.; Weimar, U.; Zhang, T.; Barsan, N. Dominant Role of Heterojunctions in Gas Sensing with Composite Materials. ACS Appl. Mater. Interfaces 2020, 12, 21127–21132. [Google Scholar] [CrossRef] [PubMed]
- Carbone, M. Zn Defective ZnCo2O4 Nanorods as High Capacity Anode for Lithium Ion Batteries. J. Electroanal. Chem. 2018, 815, 151–157. [Google Scholar] [CrossRef]
- Fang, S.; Bresser, D.; Passerini, S. Transition Metal Oxide Anodes for Electrochemical Energy Storage in Lithium- and Sodium-Ion Batteries. Adv. Energy Mater. 2020, 10, 1902485. [Google Scholar] [CrossRef]
- Li, Q.; Li, H.; Xia, Q.; Hu, Z.; Zhu, Y.; Yan, S.; Ge, C.; Zhang, Q.; Wang, X.; Shang, X.; et al. Extra Storage Capacity in Transition Metal Oxide Lithium-ion Batteries Revealed by in situ Magnetometry. Nat. Mater. 2021, 20, 76–83. [Google Scholar] [CrossRef]
- Carbone, M.; Nesticò, A.; Bellucci, N.; Micheli, L.; Palleschi, G. Enhanced Performances of Sensors Based on Screen Printed Electrodes Modified with Nanosized NiO Particles. Electrochim. Acta 2017, 246, 580–587. [Google Scholar] [CrossRef]
- Agnihotri, A.S.; Varghese, A.; Nidhin, M. Transition Metal Oxides in Electrochemical and Bio Sensing: A State-of-art Review. Appl. Surf. Sci. Adv. 2021, 4, 100072. [Google Scholar] [CrossRef]
- Valentini, F.; Roscioli, D.; Carbone, M.; Conte, V.; Floris, B.; Palleschi, G.; Flammini, R.; Bauer, E.M.; Nasillo, G.; Caponetti, E. Oxidized Graphene in Ionic Liquids for Assembling Chemically Modified Electrodes: A Structural and Electrochemical Characterization Study. Anal. Chem. 2012, 84, 5823–5831. [Google Scholar] [CrossRef] [Green Version]
- Stankic, S.; Suman, S.; Haque, F.; Vidic, J. Pure and Multi Metal Oxide Nanoparticles: Synthesis, Antibacterial and Cytotoxic Properties. J. Nanobiotechnol. 2016, 14, 73. [Google Scholar] [CrossRef] [Green Version]
- Valentini, F.; Roscioli, D.; Carbone, M.; Conte, V.; Floris, B.; Bauer, E.M.; Ditaranto, N.; Sabbatini, L.; Caponetti, E.; Chillura-Martino, D. Graphene and Ionic Liquids New Gel Paste Electrodes for Caffeic Acid Quantification. Sens. Actuator B Chem. 2015, 212, 248–255. [Google Scholar] [CrossRef]
- Zhang, W.; Li, J.; Zhang, J.; Sheng, J.; He, T.; Tian, M.; Zhao, Y.; Xie, C.; Mai, L.; Mu, S. Top-Down Strategy to Synthesize Mesoporous Dual Carbon Armored MnO Nanoparticles for Lithium-Ion Battery Anodes. ACS Appl. Mater. Interfaces 2017, 9, 12680–12686. [Google Scholar] [CrossRef]
- Casaletto, M.P.; Zanoni, R.; Carbone, M.; Piancastelli, M.N.; Aballe, L.; Weiss, K.; Horn, K. Ethylene Adsorption on Si(100)2 × 1: A High-resolution Photoemission Study. Phys. Rev. B 2000, 62, 17128–17133. [Google Scholar] [CrossRef]
- Li, F.; Ran, J.; Jaroniec, M.; Qiao, S.Z. Solution Combustion Synthesis of Metal Oxide Nanomaterials for Energy Storage and Conversion. Nanoscale 2015, 7, 17590–17610. [Google Scholar] [CrossRef]
- Carbone, M.; Caminiti, R. Adsorption States and Site Conversions of Phenylacetylene on Si(100)2 × 1 Calculated by DFT. J. Theor. Comput. Chem. 2012, 11, 1089–1099. [Google Scholar] [CrossRef]
- Ortega, S.; Ibáñez, M.; Liu, Y.; Zhang, Y.; Kovalenko, M.V.; Cadavid, D.; Cabot, A. Bottom-up Engineering of Thermoelectric Nanomaterials and Devices from Solution-processed Nanoparticle Building Blocks. Chem. Soc. Rev. 2017, 46, 3510–3528. [Google Scholar] [CrossRef] [Green Version]
- Carbone, M.; Piancastelli, M.N.; Casaletto, M.P.; Zanoni, R.; Besnard-Ramage, M.J.; Comtet, G.; Dujardin, G.; Hellner, L. Phenol Adsorption on Si (111)7 × 7 Studied by Synchrotron Radiation Photoemission and Photodesorption. Surf. Sci. 1999, 419, 114–119. [Google Scholar] [CrossRef]
- de Oliveira, P.F.M.; Torresi, R.M.; Emmerling, F.; Camargo, P.H.C. Challenges and Opportunities in the Bottom-up Mechanochemical Synthesis of Noble Metal Nanoparticles. J. Mater. Chem. A 2020, 8, 16114–16141. [Google Scholar] [CrossRef]
- Gontrani, L.; Tagliatesta, P.; Agresti, A.; Pescetelli, S.; Carbone, M. New Insights into the Structure of Glycols and Derivatives: A Comparative X-ray Diffraction, Raman and Molecular Dynamics Study of Ethane-1,2-diol, 2-methoxyethan-1-ol and 1,2-dimethoxy Ethane. Crystals 2020, 10, 1011. [Google Scholar] [CrossRef]
- Mondal, K. Recent Advances in the Synthesis of Metal Oxide Nanofibers and Their Environmental Remediation Applications. Inventions 2017, 2, 9. [Google Scholar] [CrossRef]
- Carbone, M. Cu-Zn-Co Nanosized Mixed Oxides Prepared from Hydroxycarbonate Precursors. J. Alloys Compd. 2016, 688, 202–209. [Google Scholar] [CrossRef]
- Chen, Y.; Lu, W.; Guo, Y.; Zhu, Y.; Lu, H.; Song, Y. Synthesis, Characterization and Photocatalytic Activity of Nanocrystalline First Transition-Metal (Ti, Mn, Co, Ni and Zn) Oxisde Nanofibers by Electrospinning. Appl. Sci. 2019, 9, 8. [Google Scholar] [CrossRef] [Green Version]
- Carbone, M.; Bauer, E.M.; Micheli, L.; Missori, M. NiO Morphology Dependent Optical and Electrochemical Properties. Colloid Surf. A 2017, 532, 178–182. [Google Scholar] [CrossRef]
- Atchison, J.S.; Zeiger, M.; Tolosa, A.; Funke, L.M.; Jackel, N.; Presser, V. Electrospinning of Ultrafine Metal Oxide/Carbon and Metal Carbide/Carbon Nanocomposite Fibers. RSC Adv. 2015, 5, 35683–35692. [Google Scholar] [CrossRef] [Green Version]
- Carbone, M.; Missori, M.; Micheli, L.; Tagliatesta, P.; Bauer, E.M. NiO Pseudocapacitance and Optical Properties: Does the Shape Win? Materials 2020, 13, 1417. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xia, X.; Zhang, Y.; Chao, D.; Guan, C.; Zhang, Y.; Li, L.; Ge, X.; Mínguez Bacho, I.; Tu, J.; Fan, H.J. Solution Synthesis of Metal Oxides for Electrochemical Energy Storage Applications. Nanoscale 2014, 6, 5008–5048. [Google Scholar] [CrossRef]
- Djurišić, A.B.; Chen, X.; Leung, Y.H.; Ching Ng, A.M. ZnO Nanostructures: Growth, Properties and Applications. J. Mater. Chem. 2012, 22, 6526–6535. [Google Scholar] [CrossRef]
- Wang, J.; Chen, R.; Xiang, L.; Komarneni, S. Synthesis, Properties and Applications of ZnO Nanomaterials with Oxygen Vacancies: A review. Ceram. Int. 2018, 44, 7357–7377. [Google Scholar] [CrossRef]
- Son, D.-Y.; Im, J.-H.; Kim, H.-S.; Park, N.-G. Efficient Perovskite Solar Cell Based on ZnO Nanorods: An Effective Charge Collection System. J. Phys. Chem. C 2014, 118, 16567–16573. [Google Scholar] [CrossRef]
- Wang, X.; Sun, F.; Duan, Y.; Yin, Z.; Luo, W.; Huang, Y.; Chen, J. Highly Sensitive, Temperature-dependent Gas Sensor Based on Hierarchical ZnO Nanorod Arrays. J. Mater. Chem. C 2015, 3, 11397–11405. [Google Scholar] [CrossRef]
- Lee, K.M.; Lai, C.W.; Ngai, K.S.; Juan, J.C. Recent Developments of Zinc Oxide Based Photocatalyst in Water Treatment Technology: A Review. Water Res. 2016, 88, 428–448. [Google Scholar] [CrossRef]
- He, Y.-B.; Li, G.-R.; Wang, Z.-L.; Su, C.-Y.; Tong, Y.-X. Single-crystal ZnO Nanorod/Amorphous and Nanoporous Metal Oxide Shell Composites: Controllable Electrochemical Synthesis and Enhanced Supercapacitor Performances. Energy Environ. Sci. 2011, 4, 1288–1292. [Google Scholar] [CrossRef]
- Ong, C.B.; Ng, L.Y.; Mohammad, A.W. A Review of ZnO Nanoparticles as Solar Photocatalysts: Synthesis, Mechanisms and Applications. Renew. Sustain. Energy Rev. 2018, 81, 536–551. [Google Scholar] [CrossRef]
- Ammar, S.H.; Abdulnabi, W.A.; Abdul Kader, H.D. Synthesis, Characterization and Environmental Remediation Applications of Polyoxometalates-based Magnetic Zinc Oxide Nanocomposites (Fe3O4@ZnO/PMOs). Environ. Nanotechnol. Monit. Manag. 2020, 13, 100289. [Google Scholar] [CrossRef]
- Donia, D.T.; Carbone, M. Fate of the Nanoparticles in Environmental Cycles. Int. J. Environ. Sci. Technol. 2019, 16, 583–600. [Google Scholar] [CrossRef]
- Carbone, M.; Briancesco, R.; Bonadonna, L. Antimicrobial Power of Cu/Zn Mixed Oxide Nanoparticles to Escherichia coli. Environ. Nanotechnol. Monit. Manag. 2017, 7, 97–102. [Google Scholar] [CrossRef]
- Sheoran, P.; Grewal, S.; Kumari, S.; Goel, S. Enhancement of Growth and Yield, Leaching Reduction in Triticum aestivum Using Biogenic Synthesized Zinc Oxide Fertilizer. Biocatal. Agric. Biotechnol. 2021, 32, 101938. [Google Scholar] [CrossRef]
- Oliveira, A.P.A.; Hochepied, J.-F.; Grillon, F.; Berger, M.-H. Controlled Precipitation of Zinc Oxide Particles at Room Temperature. Chem. Mater. 2003, 15, 3202–3207. [Google Scholar] [CrossRef]
- Cao, H.L.; Qian, X.F.; Gong, Q.; Du, W.M.; Ma, X.D.; Zhu, Z.K. Shape- and Size-controlled Synthesis of Nanometre ZnO from a Simple Solution Route at Room Temperature. Nanotechnology 2006, 17, 3632–3636. [Google Scholar] [CrossRef]
- Stahl, R.; Jung, C.; Lutz, H.D.; Kockelmann, W.; Jacobs, H. Kristallstrukturen und Wasserstoffbrückenbindungen bei β-Be(OH)2 und ϵ-Zn(OH)2. Z. Anorg. Allg. Chem. 1998, 624, 1130–1136. [Google Scholar] [CrossRef]
- Wang, M.; Zhang, Y.; Zhou, Y.; Yang, F.; Kim, E.J.; Hahn, S.H.; Seong, S.G. Rapid Room-temperature Synthesis of Nanosheet Assembled ZnO Mesocrystals with Excellent Photocatalytic Activity. CrystEngComm 2013, 15, 754–763. [Google Scholar] [CrossRef]
- Wahab, R.; Ansari, S.G.; Kim, Y.-S.; Seo, H.-K.; Shin, H.-S. Room Temperature Synthesis of Needle-shaped ZnO Nanorods via Sonochemical Method. App. Surf. Sci. 2007, 253, 7622–7626. [Google Scholar] [CrossRef]
- Jaber, B.; Laânab, L. One Step Synthesis of ZnO Nanoparticles in Free Organic Medium: Structural and Optical Characterizations. Mater. Sci. Semicon. Proc. 2014, 27, 446–451. [Google Scholar] [CrossRef]
- Li, S.-M.; Zhang, L.-X.; Zhu, M.-Y.; Ji, G.-J.; Zhao, L.-X.; Yin, J.; Bie, L.-J. Acetone Sensing of ZnO Nanosheets Synthesized Using Room-temperature Precipitation. Sens. Actuator B Chem. 2017, 249, 611–623. [Google Scholar] [CrossRef]
- Kumar, V.R.; Wariar, P.R.S.; Prasad, V.S.; Koshy, J. A Novel Approach for the Synthesis of Nanocrystalline Zinc Oxide Powders by Room Temperature Co-precipitation Method. Mater. Lett. 2011, 65, 2059–2061. [Google Scholar] [CrossRef]
- Liu, Y.; Gao, W. Growth Process, Crystal Size and Alignment of ZnO Nanorods Synthesized under Neutral and Acid Conditions. J. Alloys Compd. 2015, 629, 84–91. [Google Scholar] [CrossRef]
- Toby, B.H.; Von Dreele, R.B. GSAS-II: The Genesis of a Modern Open-source All Purpose Crystallography Software Package. J. Appl. Cryst. 2013, 46, 544–549. [Google Scholar] [CrossRef]
- Xu, Y.N.; Ching, W.Y. Electronic, Optical, and Structural Properties of Some Wurtzite Crystals. Phys. Rev. B 1993, 48, 4335–4351. [Google Scholar] [CrossRef]
- Downs, R.T.; Hall-Wallace, M. The American Mineralogist Crystal Structure Database. Am. Mineral. 2003, 88, 247–250. [Google Scholar]
- Finger, L.W.; Cox, D.E.; Jephcoat, A.P. A Correction for Powder Diffraction Peak Asymmetry Due to Axial Divergence. J. Appl. Cryst. 1994, 27, 892–900. [Google Scholar] [CrossRef]
- Warren, B.E. X-ray Diffraction; Dover Publications, Inc.: New York, NY, USA, 1990. [Google Scholar]
- Campetella, M.; Mariani, A.; Sadun, C.; Wu, B.; Castner, E.W., Jr.; Gontrani, L. Structure and Dynamics of Propylammonium Nitrate-acetonitrile Mixtures: An Intricate Multi-scale System Probed with Experimental and Theoretical Techniques. J. Chem. Phys. 2018, 148, 134507. [Google Scholar] [CrossRef]
- Haynes, W.M. (Ed.) CRC Handbook of Chemistry and Physics, 94th ed.; CRC Press: Boca Raton, FL, USA, 2014; pp. 4–100. [Google Scholar]
- Kubelka, P.; Munk, F. Ein Beitrag Zur Optik Der Farbanstriche. Z. Tech. Phys. 1931, 12, 593–601. [Google Scholar]
- Missori, M.; Pulci, O.; Teodonio, L.; Violante, C.; Kupchak, I.; Bagniuk, J.; Łojewska, J.; Mosca Conte, A. Optical Response of Strongly Absorbing Inhomogeneous Materials: Application to paper degradation. Phys. Rev. B 2014, 89, 054201. [Google Scholar] [CrossRef]
- Missori, M. Optical Spectroscopy of Ancient Paper and Textiles. Nuovo Cim. C 2016, 39, 293. [Google Scholar]
- Smith, R.A. Semiconductors, 2nd ed.; Cambridge University Press: Cambridge, UK, 1978. [Google Scholar]
- Brus, L.E. Electron-electron and Electron-hole Interactions in Small Semiconductor Crystallites: The Size Dependence of the Lowest Excited Electronic State. J. Chem. Phys. 1984, 80, 4403–4409. [Google Scholar] [CrossRef] [Green Version]
- Sayari, A. Characterization of Nanocrystalline ZnO Flakes Synthesized by a Simple Reaction Process. KONA Powder Part. J. 2013, 30, 119–124. [Google Scholar] [CrossRef] [Green Version]
- Repp, S.; Erdem, E. Controlling the Exciton Energy of Zinc Oxide (ZnO) Quantum Dots by Changing the Confinement Conditions. Spectrochim. Acta A 2016, 152, 637–644. [Google Scholar] [CrossRef]
- Studenikin, S.A.; Golego, N.; Cocivera, M. Fabrication of Green and Orange Photoluminescent, Undoped ZnO Films Using Spray Pyrolysis. J. Appl. Phys. 1998, 84, 2287. [Google Scholar] [CrossRef]
- Debanath, M.K.; Karmakar, S. Study of Blue Shift of Optical Band Gap in Zinc Oxide (ZnO) Nanoparticles Prepared by Low-temperature Wet Chemical Method. Mater. Lett. 2013, 111, 116–119. [Google Scholar] [CrossRef]
- Lin, K.-F.; Cheng, H.-M.; Hsu, H.-C.; Lin, L.-J.; Hsieh, W.-F. Band Gap Variation of Size-controlled ZnO Quantum Dots Synthesized by Sol–gel Method. Chem. Phys. Lett. 2005, 409, 208–211. [Google Scholar] [CrossRef]
- Davis, K.; Yarbrough, R.; Froeschle, M.; White, J.; Rathnayake, H. Band Gap Engineered Zinc Oxide Nanostructures via a Sol–gel Synthesis of Solvent Driven Shapecontrolled Crystal Growth. RSC Adv. 2019, 9, 14638–14648. [Google Scholar] [CrossRef] [Green Version]
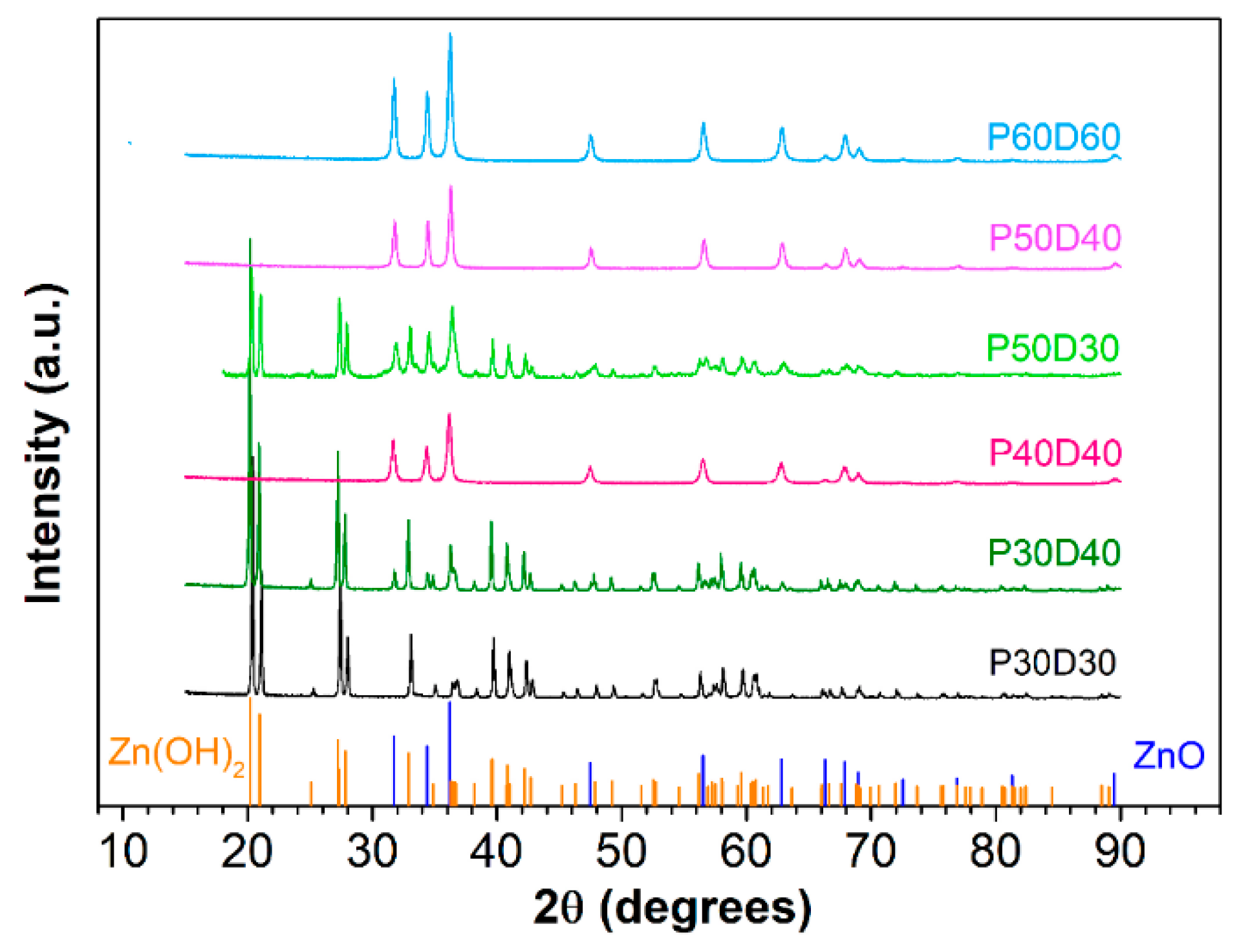
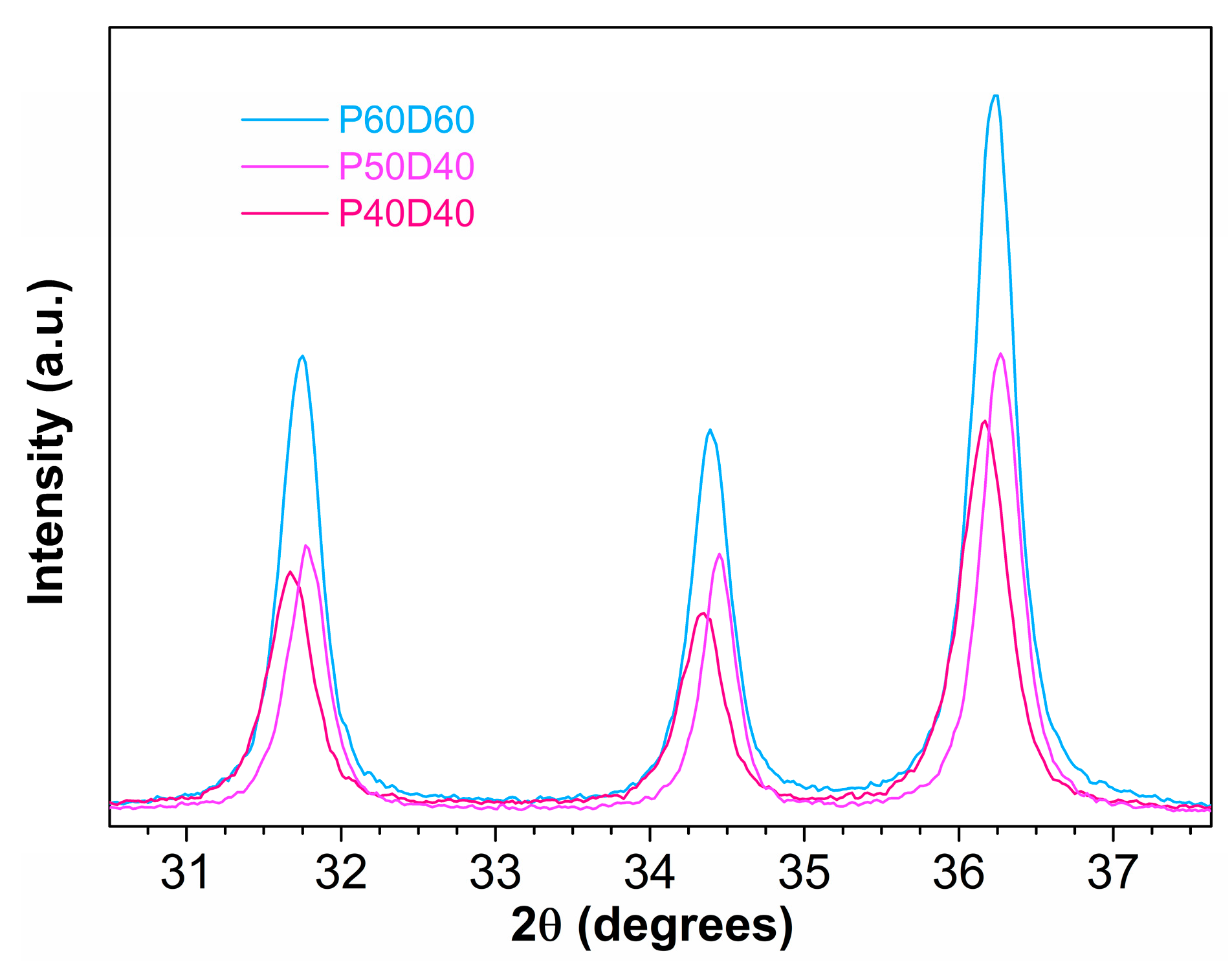
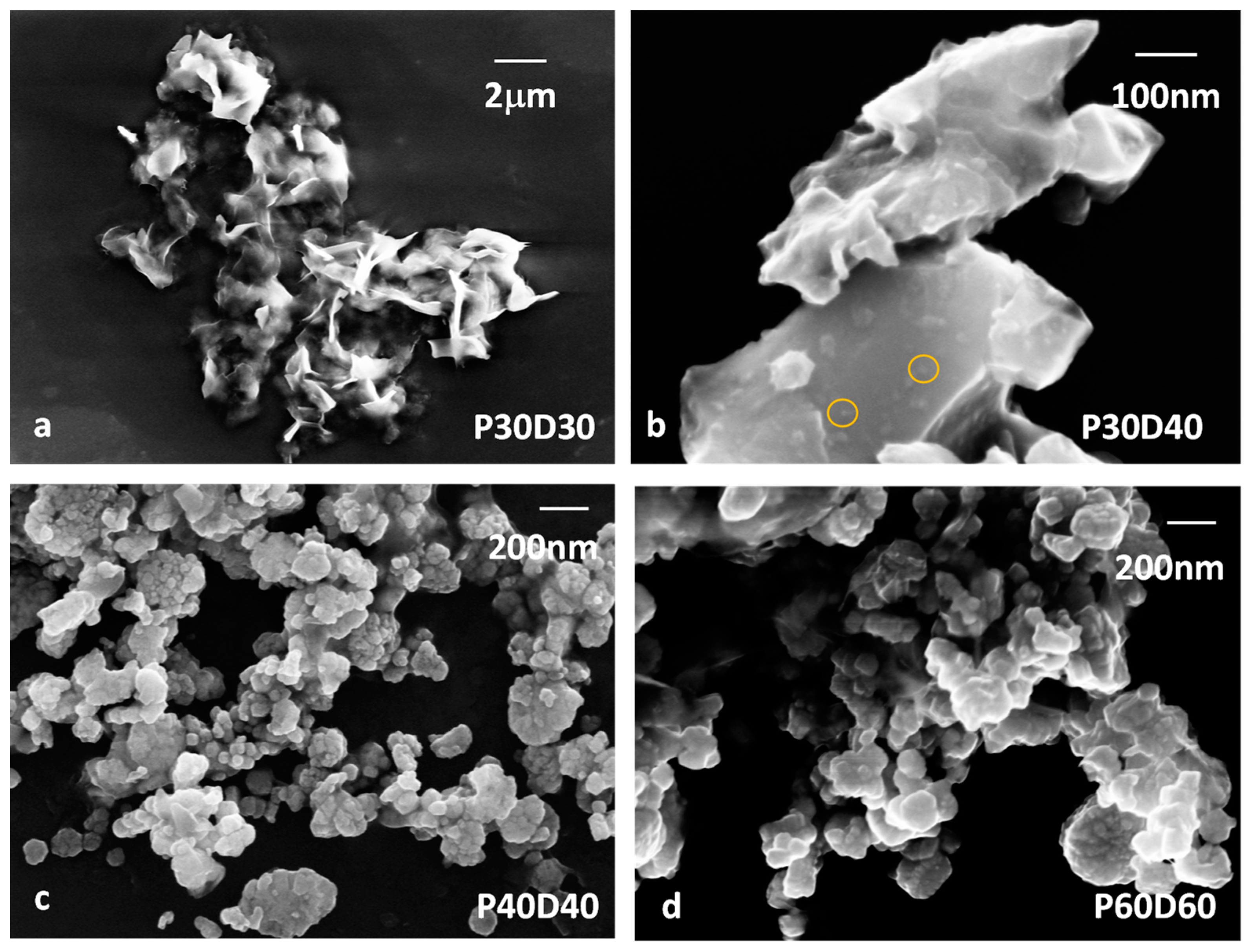
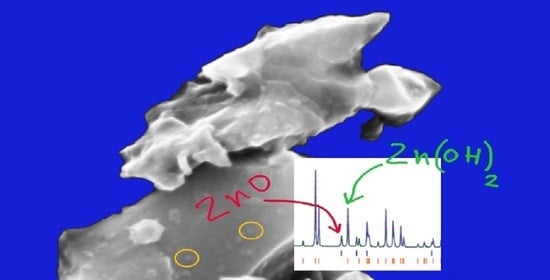
| Sample | Reaction Temperature | Drying Temperature |
|---|---|---|
| P30D30 | 30 °C | 30 °C |
| P30D40 | 30 °C | 40 °C |
| P40D40 | 40 °C | 40 °C |
| P50D30 | 50 °C | 30 °C |
| P50D50 | 50 °C | 50 °C |
| P60D60 | 60 °C | 60 °C |
| Sample | a (Å) | b (Å) | c (Å) | Volume (Å3) | Density (gcm−3) | Cryst. Size (nm) |
| P40D40 | 3.32 | 3.32 | 5.22 | 49.70 | 5.44 | 42 |
| P50D40 | 3.25 | 3.25 | 5.21 | 47.56 | 5.68 | 76 |
| P60D60 | 3.27 | 3.27 | 5.21 | 48.17 | 5.58 | 71 |
| Fractional Coordinates | X (Zn) | Y (Zn) | Z (Zn) | X (O) | Y (O) | Z (O) |
| P40D40 | 0.33 | 0.67 | −0.23 | 0.33 | 0.67 | 0.37 |
| P50D40 | 0.33 | 0.67 | −0.05 | 0.33 | 0.67 | 0.35 |
| P60D60 | 0.33 | 0.67 | −0.02 | 0.33 | 0.67 | 0.37 |
| Sample/Phase | a (Å) | b (Å) | c (Å) | Volume (Å3) | Density (gcm−3) | Cryst. Size (nm) | Fraction | ||
| P30D40 | |||||||||
| ZnO | 3.26 | 3.26 | 5.21 | 47.88 | 5.65 | 90 | 22.46% | ||
| Zn(OH)2 | 4.91 | 5.14 | 8.47 | 213.84 | 3.09 | 243 | 77.54% | ||
| Fractional Coordinates | X(Zn) | Y (Zn) | Z (Zn) | X(O1) | Y (O1) | Z (O1) | X(O2) | Y (O2) | Z (O2) |
| ZnO | 0.33 | 0.67 | −0.02 | 0.33 | 0.67 | 0.36 | |||
| Zn(OH)2 | 0.07 | 0.65 | 0.62 | 0.13 | 0.13 | 0.08 | 0.19 | 0.31 | 0.73 |
| Sample/Phase | a (Å) | b (Å) | c (Å) | Volume (Å3) | Density (gcm−3) | Dom. Size (nm) | Fraction | ||
| P30D30 | |||||||||
| Zn(OH)2 | 4.67 | 5.46 | 8.36 | 212.99 | 3.10 | 261 | ≈100% | ||
| Fractional Coordinates | X(Zn) | Y (Zn) | Z (Zn) | X(O1) | Y (O1) | Z (O1) | X(O2) | Y (O2) | Z (O2) |
| Zn(OH)2 | 0.07 | 0.65 | 0.62 | 0.11 | 0.13 | 0.08 | 0.19 | 0.31 | 0.73 |
| Sample/Phase | a (Å) | b (Å) | c (Å) | Volume (Å3) | Density (gcm−3) | Dom. Size (nm) | Fraction | ||
| P50D30 | |||||||||
| ZnO | 3.24 | 3.24 | 5.20 | 47.45 | 5.70 | 36 | 68.98% | ||
| Zn(OH)2 | 4.90 | 5.13 | 8.46 | 212.56 | 3.11 | 900 | 31.02% | ||
| Fractional Coordinates | X(Zn) | Y (Zn) | Z (Zn) | X(O1) | Y (O1) | Z (O1) | X(O2) | Y (O2) | Z (O2) |
| ZnO | 0.33 | 0.67 | −0.02 | 0.33 | 0.67 | 0.37 | |||
| Zn(OH)2 | 0.07 | 0.64 | 0.63 | 0.13 | 0.12 | 0.09 | 0.16 | 0.36 | 0.72 |
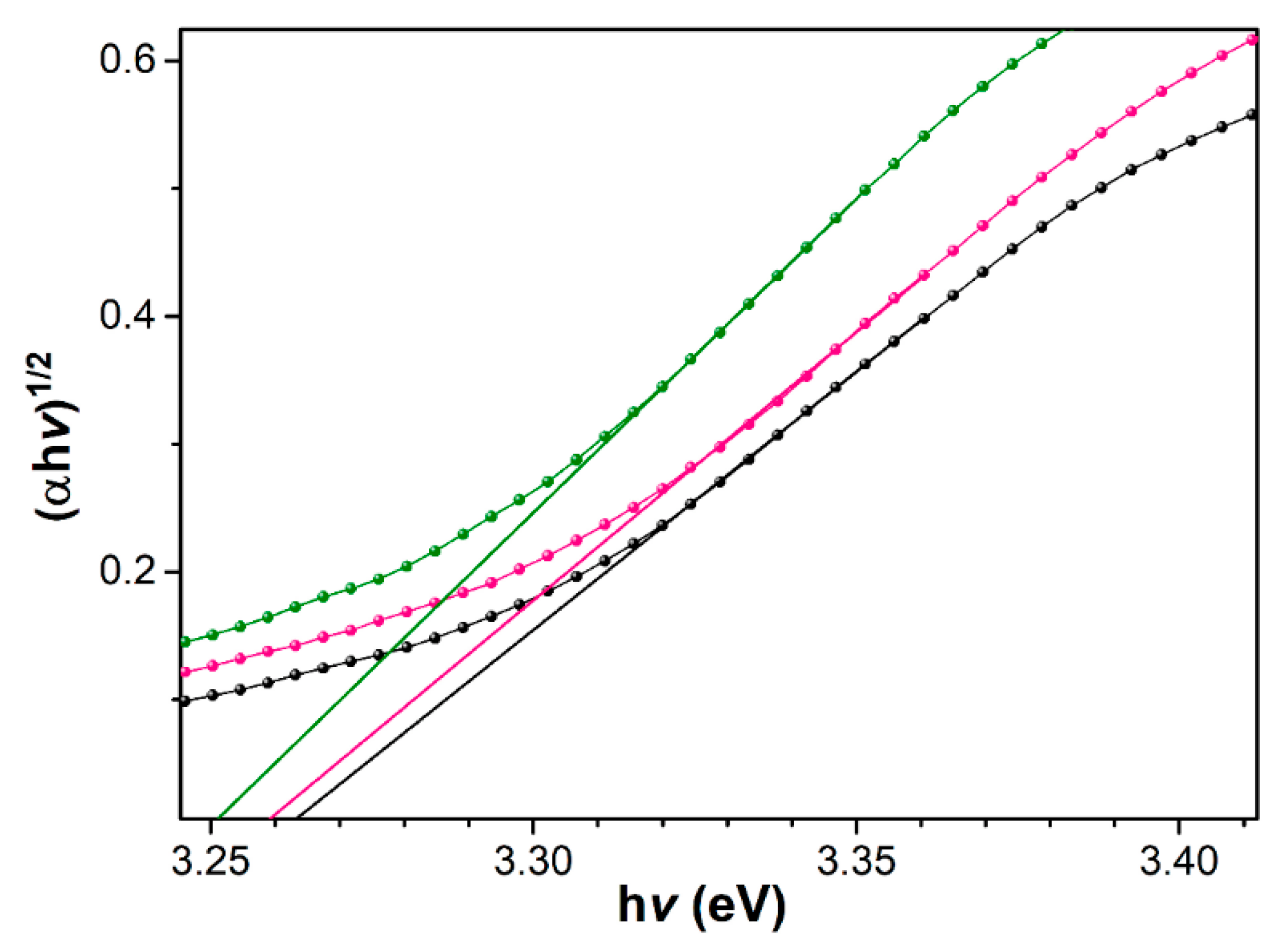
| Sample | Band Gap (eV) |
|---|---|
| P40D40 | 3.25 (0) |
| P50D40 | 3.25 (8) |
| P60D60 | 3.26 (2) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Donia, D.T.; Bauer, E.M.; Missori, M.; Roselli, L.; Cecchetti, D.; Tagliatesta, P.; Gontrani, L.; Carbone, M. Room Temperature Syntheses of ZnO and Their Structures. Symmetry 2021, 13, 733. https://doi.org/10.3390/sym13040733
Donia DT, Bauer EM, Missori M, Roselli L, Cecchetti D, Tagliatesta P, Gontrani L, Carbone M. Room Temperature Syntheses of ZnO and Their Structures. Symmetry. 2021; 13(4):733. https://doi.org/10.3390/sym13040733
Chicago/Turabian StyleDonia, Domenica Tommasa, Elvira Maria Bauer, Mauro Missori, Ludovica Roselli, Daniele Cecchetti, Pietro Tagliatesta, Lorenzo Gontrani, and Marilena Carbone. 2021. "Room Temperature Syntheses of ZnO and Their Structures" Symmetry 13, no. 4: 733. https://doi.org/10.3390/sym13040733
APA StyleDonia, D. T., Bauer, E. M., Missori, M., Roselli, L., Cecchetti, D., Tagliatesta, P., Gontrani, L., & Carbone, M. (2021). Room Temperature Syntheses of ZnO and Their Structures. Symmetry, 13(4), 733. https://doi.org/10.3390/sym13040733