Electrochemical Comparison on New (Z)-5-(Azulen-1-Ylmethylene)-2-Thioxo-Thiazolidin-4-Ones
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussions
3.1. Studies by DPV
3.2. Studies by CV
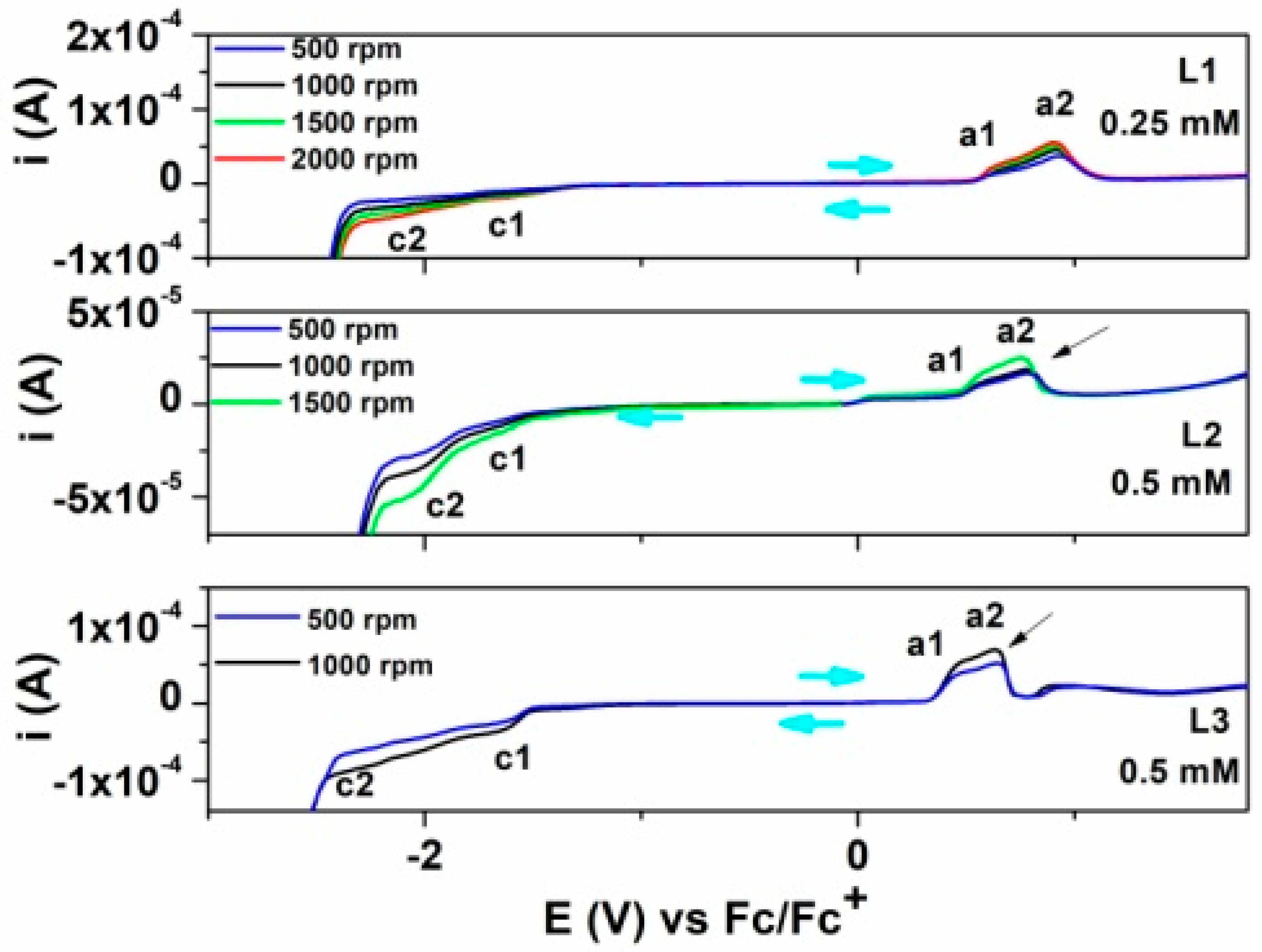
3.3. Studies by RDE
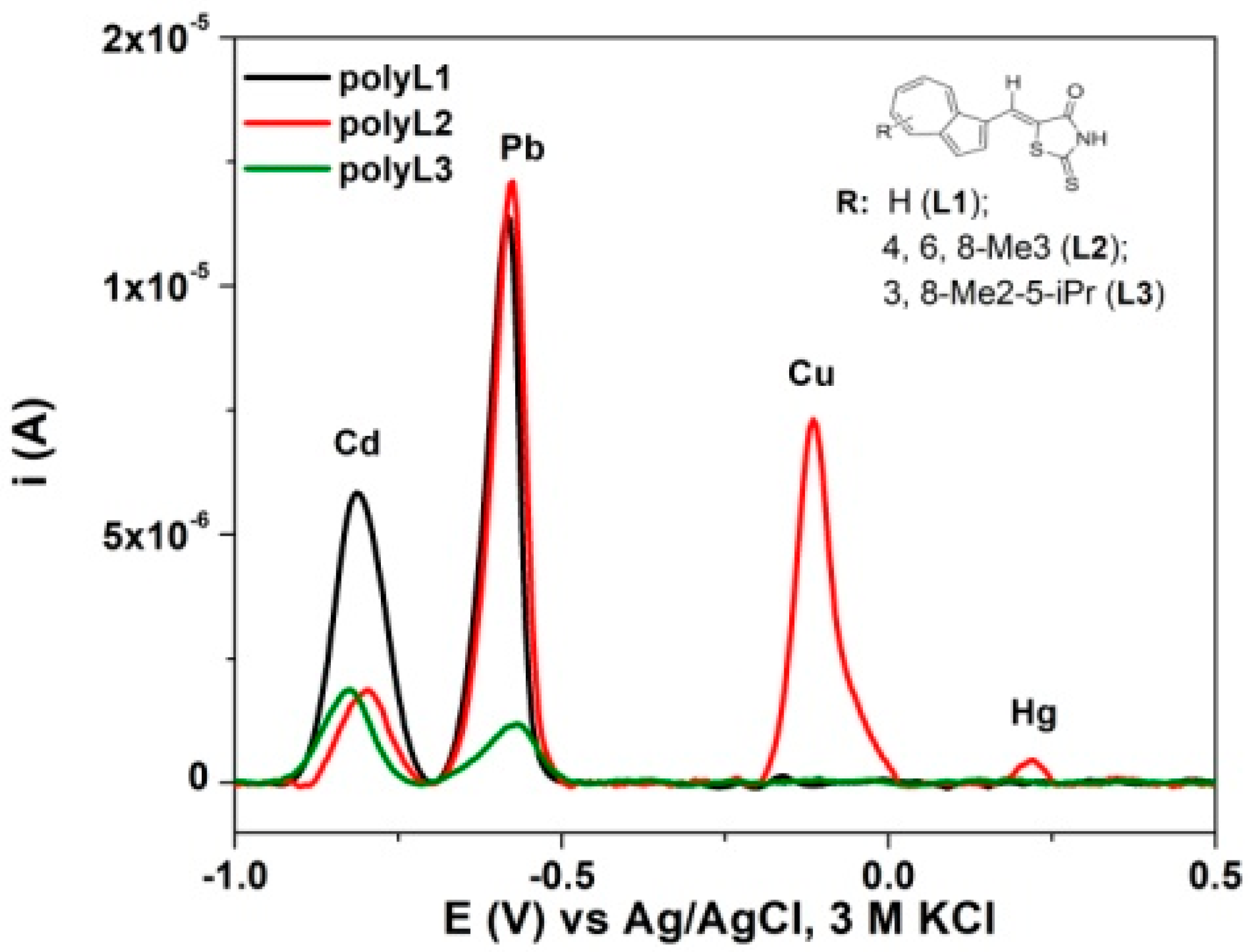
3.4. Modified Electrodes
3.5. Metal Binding Properties
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Dong, J.-X.; Zhang, H.-L. Azulene-based organic functional molecules for optoelectronics. Rev. Chin. Chem. Lett. 2016, 27, 1097–1104. [Google Scholar] [CrossRef]
- Xin, H.; Gao, X. Application of Azulene in Constructing Organic Optoelectronic Materials: New Tricks for an Old Dog. Chem. Plus. Chem. 2017, 82, 945–956. [Google Scholar] [CrossRef] [PubMed]
- Bakun, P.; Czarczynska-Goslinska, B.; Goslinski, T.; Lijewski, S. In vitro and in vivo biological activities of azulene derivatives with potential applications in medicine. Med. Chem. Res. 2021, 30, 834–846. [Google Scholar] [CrossRef]
- Chen, Z.; Droste, J.; Zhai, G.; Zhu, J.; Yang, J.; Hansen, M.R.; Zhuang, X. Sulfur-anchored azulene as a cathode material for Li–S batteries. Chem.Commun. 2019, 55, 9047–9050. [Google Scholar] [CrossRef]
- Suominen, M.; Lehtimäki, S.; Yewale, R.; Damlin, P.; Tuukkanen, S.; Kvarnström, C. Electropolymerized polyazulene as active material in flexible supercapacitors. J. Power Sources 2017, 356, 181–190. [Google Scholar] [CrossRef]
- Yang, L.; Zhu, Y.; Liu, J.; Chen, Y.; Wu, J.; Pang, Z.; Lu, Z.; Zhao, S.; Huang, Y. Marked effects of azulenyl vs. naphthyl groups on donor-pi-acceptor-pi-donor small molecules for organic photovoltaic cells. Dyes Pigment. 2021, 187, 109079. [Google Scholar] [CrossRef]
- Murfin, L.C.; Lewis, S.E. Azulene-A bright core for sensing and imaging. Molecules 2021, 26, 353. [Google Scholar] [CrossRef] [PubMed]
- Dutta, S.; Lakshmi, S.; Pati, S.K. Comparative study of electron conduction in azulene and naphthalene. Bull. Mater. Sci. 2008, 31, 353–358. [Google Scholar] [CrossRef]
- Ungureanu, E.-M.; Amarandei, C.A.; Caval, D.I.; Buica, G.-O.; Razus, A.C.; Birzan, L. Electrochemical synthesis of azo azulene films. J. Optoelectron. Adv. Mater. 2010, 12, 1805–1810. [Google Scholar]
- Buica, G.-O.; Lazar, I.-G.; Birzan, L.; Lete, C.; Prodana, M.; Enachescu, M.; Tecuceanu, V.; Stoian, A.B.; Ungureanu, E.-M. Azulene-ethylenediaminetetraacetic acid: A versatile molecule for colorimetric and electrochemical sensors for metal ions. Electrochim. Acta 2018, 263, 382–390. [Google Scholar] [CrossRef]
- Buica, G.-O.; Birzan, L.; Tecuceanu, V.; Razus, A.C.; Arnold, G.-L.; Ungureanu, E.-M. Modified Electrodes Based on Poly[(2E)-2-(Azulen-1-ylmethylidene)hydrazinecarbothioamide] for Heavy Metal Ions Complexation. Electroanalysis 2017, 29, 93–102. [Google Scholar] [CrossRef]
- Amarandei, C.-A.; Buica, G.-O.; Inel, G.A.; Birzan, L.; Ungureanu, E.-M. Study of the complexation of diethyl 2-[(E)-3-azulen-1-ylprop-2-enylidene]propanedioate with lanthanide cations. Acta Chim. Slov. 2014, 61, 894–899. [Google Scholar]
- Bargon, J.; Mohmand, S.; Waltman, R.J. Polyazulene, A Member of a New Class of Polymers. Mol. Cryst. Liq. Cryst. 1983, 93, 279–291. [Google Scholar] [CrossRef]
- Buica, G.-O.; Ivanov, A.A.; Lazar, I.-G.; Tatu (Arnold), G.-L.; Omocea, C.; Birzan, L.; Ungureanu, E.-M.J. Colorimetric and voltammetricsensing of mercury ions using 2,2′-(ethane-1,2-diylbis((2-(azulen-2-ylamino)-2-oxoethyl)azanediyl))diacetic acid. J. Electroanal. Chem. 2019, 849, 113351. [Google Scholar] [CrossRef]
- Buica, G.-O.; Ungureanu, E.-M.; Birzan, L.; Razus, A.C.; Mandoc, L.-R. Voltammetric sensing of lead and cadmium using poly(4-azulen-1-yl-2,6-bis(2-thienyl)pyridine) complexing films. J. Electroanal. Chem. 2013, 693, 67–72. [Google Scholar] [CrossRef]
- Birzan, L.; Cristea, M.; Draghici, C.C.; Tecuceanu, V.; Maganu, M.; Hanganu, A.; Arnold, G.-L.; Ungureanu, E.-M.; Razus, A.C. 1-vinylazulenes—Potential host molecules in ligands for metal ions detectors. Tetrahedron 2016, 72, 2316–2326. [Google Scholar] [CrossRef]
- Arnold, G.-L.; Lazar, I.G.; Buica, G.-O.; Ungureanu, E.-M.; Birzan, L. New Azulene Modified Electrodes for Heavy Metal Ions Recongnition. Bulg. Chem. Commun. 2017, 49, 205–210. [Google Scholar]
- Mousavi, S.M.; Zarei, M.; Hashemi, S.A.; Babapoor, A.; Amani, A.M. A conceptual review of rhodanine: Current applications of antiviral drugs, anticancer and antimicrobial activities. Artif. Cells Nanomed. Biotechnol. 2019, 47, 1132–1148. [Google Scholar] [CrossRef]
- Kshirsagar, V.; Gandhem, S.; Gautam, M.D. Electrochemical studies on p-dimethylaminobenzylidine rhodanine and its application as amperometric reagent. Rasāyan J. Chem. 2010, 3, 772–776. [Google Scholar]
- Thamaraiselvi, P.; Duraipandy, N.; Kiran, M.S.; Easwaramoorthi, S. Triarylamine rhodanine derivatives as red emissive sensor for discriminative detection of Ag+ and Hg2+ ions in buffer-free aqueous solutions. ACS Sustain. Chem. Eng. 2019, 7, 9865–9874. [Google Scholar] [CrossRef]
- Wang, F.; Lai, Y.H.; Han, M.Y. Stimuli-Responsive Conjugated Copolymers Having Electro-Active Azulene and Bithiophene Units in the Polymer Skeleton: Effect of Protonation and p-Doping on Conducting Properties. Macromolecules 2004, 37, 3222–3230. [Google Scholar] [CrossRef]
- Lash, T.D.; Colby, D.A.; Graham, S.R.; Ferrence, G.M.; Szczpura, L.F. Organometallic chemistry of azuliporphyrins: Synthesis, spectroscopy, electrochemistry and structural characterization of nickel(II), palladium(II) and platinum(II) complexes of azuliporphyrins. Inorg. Chem. 2003, 42, 7326–7338. [Google Scholar] [CrossRef] [PubMed]
- Ungureanu, E.-M.; Razus, A.C.; Birzan, L.; Cretu, M.-S.; Buica, G.-O. Electrochemistry of Functionalized Azo Azulenes. Electrochim. Acta 2008, 53, 7089–7099. [Google Scholar] [CrossRef]
- Wakabayashi, S.; Uchida, M.; Tanaka, R.; Habata, Y.; Shimizu, M. Synthesis of Azulene Derivatives That Have an Azathiacrown Ether Moiety and Their Selective Color Reaction Towards Silver Ions. Asian J. Org. Chem. 2013, 2, 786–791. [Google Scholar] [CrossRef]
- Arnold, G.-L. Sensors Based on Azulene Modified Electrodes for Testing Metals in Waters. Ph.D. Thesis, University Politehnica of Bucharest, Bucharest, Romania, 2017. [Google Scholar]
- Birzan, L.; Cristea, M.; Draghici, C.C.; Tecuceanu, V.; Maganu, M.; Hanganu, A.; Razus, A.C.; Buica, G.O.; Ungureanu, E.M. Vinylazulenes chromophores: Synthesis and characterization. Dyes Pigment. 2016, 131, 246–255. [Google Scholar] [CrossRef]
- Diacu, E.; Buica, G.-O.; Chilibon, I.; Birzan, L.; Arnold, G.-L.; Ungureanu, E.-M. Chemically Modified Electrodes Based on 5-(Azulen-1-yl)methylene)-2-thioxothiazolidin-4-one. J. Solut. Chem. 2016, 45, 1588–1597. [Google Scholar] [CrossRef]
- García-Miranda Ferrari, A.; Foster, C.W.; Kelly, P.J.; Brownson, D.A.C.; Banks, C.E. Determination of the electrochemical area of screen-printed electrochemical sensing platforms. Biosensors 2018, 8, 53. [Google Scholar] [CrossRef]
- Balram, D.; Lian, K.Y.; Sebastian, N.; Rasana, N. Ultrasensitive detection of cytotoxic food preservative tert-butylhydroquinone using 3D cupric oxide nanoflowers embedded functionalized carbon nanotubes. J. Hazard. Mater. 2021, 406, 124792. [Google Scholar] [CrossRef]
- Jampasa, S.; Siangproh, W.; Duangmal, K.; Chailapakul, O. Electrochemically reduced graphene oxide-modified screen-printed carbon electrodes for a simple and highly sensitive electrochemical detection of synthetic colorants in beverages. Talanta 2016, 160, 113–124. [Google Scholar] [CrossRef] [PubMed]
- Lazar, I.G.; Diacu, E.; Arnold, G.-L.; Ungureanu, E.-M.; Buica, G.-O.; Birzan, L. Synthesis and characterization of poly(azulene-thiophene vinyl pyrylium) salt. Bulg. Chem. Commun. 2017, 49, 227–232. [Google Scholar]
- Pop, M.D.; Anicai, L.; Cristea, M.; Ungureanu, E.-M.; Enachescu, M. Impedance studies on chemically modified electrodes based on 5-[(azulen-1-yl) methylene]-2-thioxothiazolidin-4-one. U.P.B. Sci. Bull. Series B 2017, 79, 3–12. [Google Scholar]
- Pop, M.D.; Brincoveanu, O.; Cristea, M.; Buica, G.O.; Enachescu, M. AFM and SEM characterization of chemically modified electrodes based on 5-[(azulen-1-yl) methylene]-2-thioxothiazolidin-4-one. Rev. Chim. 2017, 68, 2799–2803. [Google Scholar] [CrossRef]
- Nefedov, V.A.; Obshch, K.Z. Substitution reactions of copper salts. Chem. Abs. 1969, 71, 50159. [Google Scholar]









| Peak | L1 [23] | L2 [15] | L3 | Assessed Process | |||
|---|---|---|---|---|---|---|---|
| Method | Method | Method | |||||
| DPV | CV | DPV | CV | DPV | CV | ||
| O2/O2.− | −1.202 | −1.316 (i) * | - | - | - | - | Oxygen reduction |
| c1 | −1.528 | - | −1.541 | - | −1.543 | −1.610 (i) * | Low peak current process (negligible) due to reduction of the protonated ligand |
| c2 | −1.801 | −1.842 (q) * | −2.216 | −2.112 (r) * | - | −1.991 (q) * | Reduction of C=C bond |
| c3 | −2.431 | −2.482 (q) * | - | - | −2.191 | −2.430 (q) * | Low peak current process (negligible) due to the reduction of a fragment (probably as a result of the decomposition of the azulene |
| c4 | −2.721 | −2.794 (q) * | - | - | −2.748 | −2.831 (q) * | Reduction of the C–S bond |
| a1 | 0.541 | 0.578 (i) * | 0.473 | 0.493 (i) * | 0.373 | 0.421 (i) * | Radical cation formation |
| a2 | 1.021 | 1.143 (i) * | 0.942 | 0.847 (i) * | 0.673 | 0.721 (i) * | Oligomer oxidation |
| a3 | - | - | - | 1.571 (i) * | - | 1.490 (i) * | - |
| a4 | - | - | - | - | - | 1.785 (i) * | - |
| ipa1 vs. v1/2 | ipc1 vs. v1/2 | |
|---|---|---|
| L1 | ipa1 = −3.80 × 10−6 + 44.67 × 10−6 × v1/2, R2 = 0.998 | Not recorded |
| L2 | ipa1 = −6.58 × 10−6 + 40.2 × 10−6 × v1/2, R2 = 0.935 | ipc1 = −1.25 × 10−6 − 24.6 × 10−6 × v1/2, R2 = 0.971 |
| L3 | ipa1= −16.66 × 10−6 + 122.07 × 10−6 × v1/2 R2 = 0.995 | ipc1 = 1.58 × 10−6 − 56.44 × 10−6 × v1/2, R2 = 0.998 |
| Compound | L1 | L2 | L3 |
|---|---|---|---|
| 105 × D (cm2 s−1) | 2.2 | 1.8 * | 16.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ungureanu, E.-M.; Popescu, M.; Tatu, G.-L.; Birzan, L.; Isopescu, R.; Stanciu, G.; Buica, G.-O. Electrochemical Comparison on New (Z)-5-(Azulen-1-Ylmethylene)-2-Thioxo-Thiazolidin-4-Ones. Symmetry 2021, 13, 588. https://doi.org/10.3390/sym13040588
Ungureanu E-M, Popescu M, Tatu G-L, Birzan L, Isopescu R, Stanciu G, Buica G-O. Electrochemical Comparison on New (Z)-5-(Azulen-1-Ylmethylene)-2-Thioxo-Thiazolidin-4-Ones. Symmetry. 2021; 13(4):588. https://doi.org/10.3390/sym13040588
Chicago/Turabian StyleUngureanu, Eleonora-Mihaela, Mariana Popescu (Apostoiu), Georgiana-Luiza Tatu (Arnold), Liviu Birzan, Raluca Isopescu, Gabriela Stanciu, and George-Octavian Buica. 2021. "Electrochemical Comparison on New (Z)-5-(Azulen-1-Ylmethylene)-2-Thioxo-Thiazolidin-4-Ones" Symmetry 13, no. 4: 588. https://doi.org/10.3390/sym13040588
APA StyleUngureanu, E.-M., Popescu, M., Tatu, G.-L., Birzan, L., Isopescu, R., Stanciu, G., & Buica, G.-O. (2021). Electrochemical Comparison on New (Z)-5-(Azulen-1-Ylmethylene)-2-Thioxo-Thiazolidin-4-Ones. Symmetry, 13(4), 588. https://doi.org/10.3390/sym13040588






