The Symmetric 3D Organization of Connective Tissue around Implant Abutment: A Key-Issue to Prevent Bone Resorption
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample description and Ethical Committee Approval
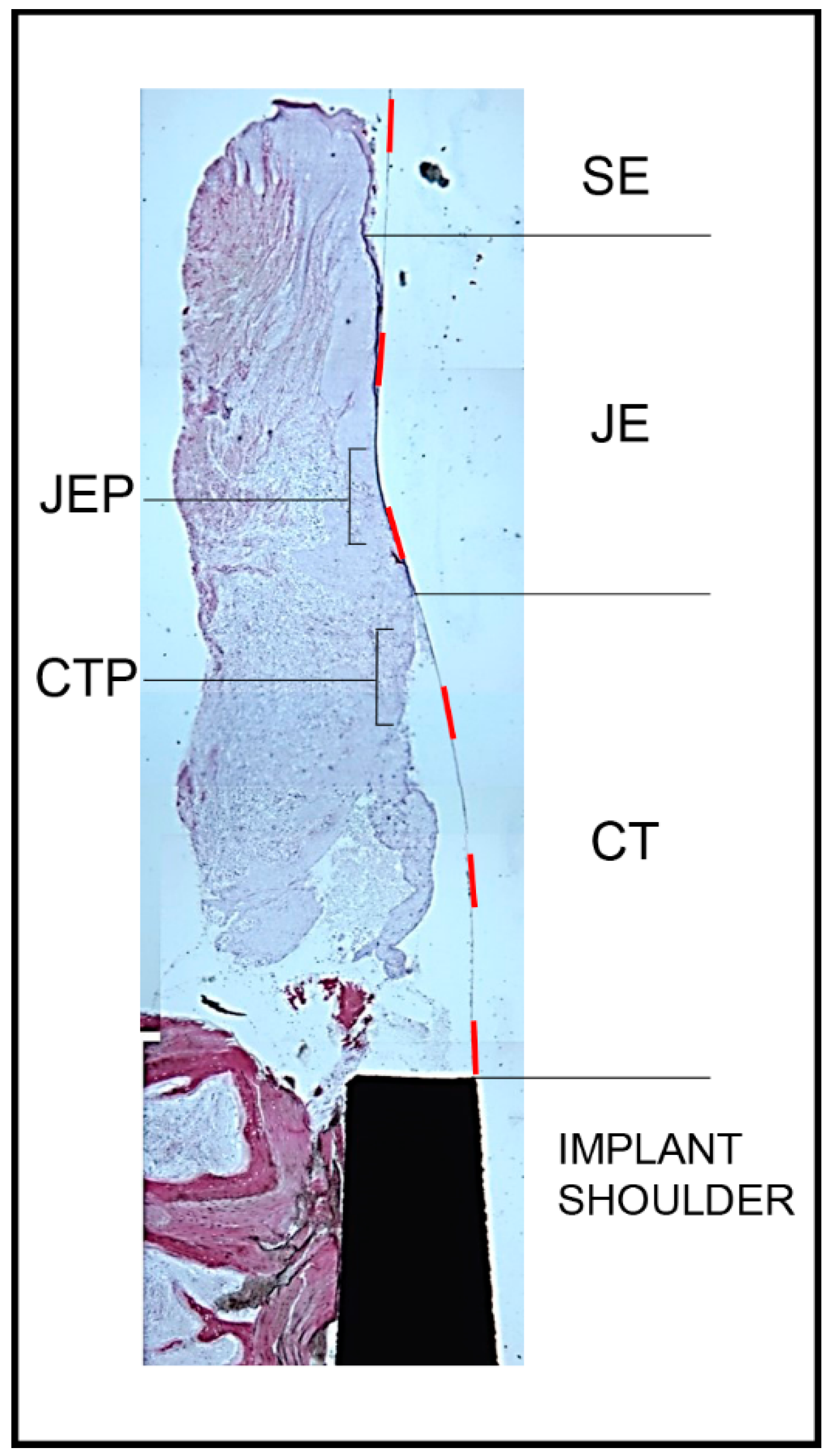
2.2. Histology
2.3. Polarized Light
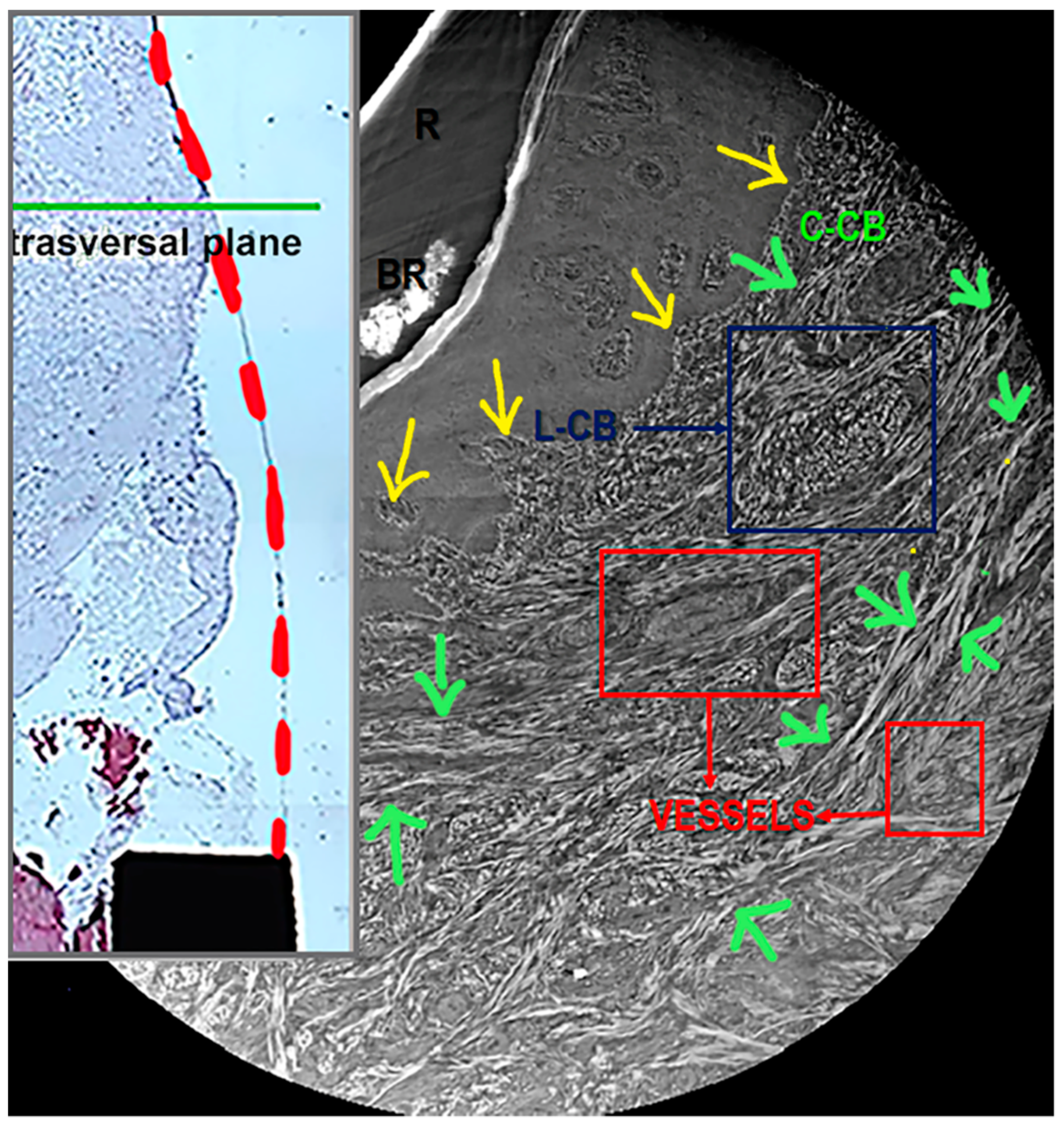
2.4. Synchrotron Radiation-Based Phase-Contrast Microtomography
3. Results
3.1. Histology
3.2. Polarized Light
3.3. Synchrotron Radiation-Based Phase-Contrast Microtomography
- Although the number of samples analyzed was comparable, the standard deviation of data obtained for Transversal_CB was always higher than for Longitudinal_CB and the standard deviation of data obtained for Longitudinal_CB-outer was always higher than for Longitudinal_CB-inner;
- The anisotropy degree (DA) in Transversal_CB was always lower (higher DA values) than for Longitudinal_CB;
- The connectivity density (Conn. D) and the 3D fractal dimension were lower in Transversal_CB than in Longitudinal_CB.
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lindhe, J.; Meyle, J.; on behalf of Group D of the European Workshop on Periodontology. Peri-implant diseases: Consensus Report of the Sixth European Workshop on Periodontology. J. Clin. Periodontol. 2008, 35, 282–285. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Berglundh, T.; Lindhe, J.; Jonsson, K.; Ericsson, I. The topography of the vascular systems in the periodontal and peri-implant tissues in the dog. J. Clin. Periodontol. 1994, 21, 189–193. [Google Scholar] [CrossRef] [PubMed]
- Moon, I.-S.; Berglundh, T.; Abrahamsson, I.; Linder, E.; Lindhe, J. The barrier between the keratinized mucosa and the dental implant. J. Clin. Periodontol. 1999, 26, 658–663. [Google Scholar] [CrossRef] [PubMed]
- Piattelli, A.; Scarano, A.; Bertolai, R.; Panzoni, E.; Piattelli, M. Histologic Aspects of the Bone and Soft Tissues Surrounding Three Titanium Non-Submerged Plasma-Sprayed Implants Retrieved at Autopsy: A Case Report. J. Periodontol. 1997, 68, 694–700. [Google Scholar] [CrossRef]
- Schierano, G.; Ramieri, G.; Cortese, M.; Aimetti, M.; Preti, G. Organization of the connective tissue barrier around long-term loaded implant abutments in man. Clin. Oral Implant. Res. 2002, 13, 460–464. [Google Scholar] [CrossRef]
- Degidi, M.; Iezzi, G.; Scarano, A.; Piattelli, A. Immediately loaded titanium implant with a tissue-stabilizing/maintaining design (‘beyond platform switch’) retrieved from man after 4 weeks: A histological and histomorphometrical evaluation. A case report. Clin. Oral Implant. Res. 2008, 19, 276–282. [Google Scholar] [CrossRef]
- Degidi, M.; Perrotti, V.; Shibli, J.A.; Novaes, A.B.; Piattelli, A.; Iezzi, G. Equicrestal and Subcrestal Dental Implants: A Histologic and Histomorphometric Evaluation of Nine Retrieved Human Implants. J. Periodontol. 2011, 82, 708–715. [Google Scholar] [CrossRef]
- Degidi, M.; Piattelli, A.; Scarano, A.; Shibli, J.A.; Iezzi, G. Peri-implant collagen fibers around human cone Morse connection implants under polarized light: A report of three cases. Int. J. Periodontics Restor. Dent. 2012, 32, 323–328. [Google Scholar]
- Rodríguez, X.; Navajas, A.; Vela, X.; Fortuño, A.; Jimenez, J.; Nevins, M. Arrangement of Peri-implant Connective Tissue Fibers Around Platform-Switching Implants with Conical Abutments and Its Relationship to the Underlying Bone: A Human Histologic Study. Int. J. Periodontics Restor. Dent. 2016, 36, 533–540. [Google Scholar]
- Romanos, E.G.; Schröter-Kermani, C.; Weingart, D.; Strub, J.R. Health human periodontal versus peri-implant gingival tissues: An immunohistochemical differentiation of the extracellular matrix. Int. J. Oral Maxillofac. Implant. 1995, 10, 750–758. [Google Scholar]
- Rompen, E.; Domken, O.; Degidi, M.; Pontes, A.E.F.; Piattelli, A. The effect of material characteristics, of surface topography and of implant components and connections on soft tissue integration: A literature review. Clin. Oral Implant. Res. 2006, 17, 55–67. [Google Scholar] [CrossRef]
- Rugger, A.; Franchi, M.; Marini, N.; Trisi, P.; Piattelli, A. Supracrestal circular collagen fiber network around osseointegrated nonsubmerged titanium implants. Clin. Oral Implant. Res. 1992, 3, 169–175. [Google Scholar] [CrossRef]
- Rodríguez, X.; Vela, X.; Calvo-Guirado, J.L.; Nart, J.; Stappert, C.F.J. Effect of platform switching on collagen fiber orientation and bone resorption around dental implants: A preliminary histologic animal study. Int. J. Oral Maxillofac. Implant. 2012, 27, 1116–1122. [Google Scholar]
- Sculean, A.; Gruber, R.; Bosshardt, D.D. Soft tissue wound healing around teeth and dental implants. J. Clin. Periodontol. 2014, 41, S6–S22. [Google Scholar] [CrossRef] [Green Version]
- Abrahamsson, I.; Berglundh, T.; Glantz, P.-O.; Lindhe, J. The mucosal attachment at different abutments. An experimental study in dogs. J. Clin. Periodontol. 1998, 25, 721–727. [Google Scholar] [CrossRef]
- Nevins, M.; Camelo, M.; Nevins, M.L.; Schupbach, P.; Kim, D.M. Connective tissue attachment to laser-microgrooved abut-ments: A human histologic case report. Int. J. Periodontics Restor. Dent. 2012, 32, 385–392. [Google Scholar]
- Romanos, G.E.; Traini, T.; Johansson, C.B.; Piattelli, A. Biologic Width and Morphologic Characteristics of Soft Tissues Around Immediately Loaded Implants: Studies Performed on Human Autopsy Specimens. J. Periodontol. 2010, 81, 70–78. [Google Scholar] [CrossRef] [Green Version]
- Giuliani, A.; Cedola, A. Advanced high-resolution tomography in regenerative Medicine. In Three-Dimensional Exploration into the Interactions between Tissues, Cells, and Biomaterials, 1st ed.; Springer: Cham, Switzerland, 2018. [Google Scholar]
- Albertini, G.; Giuliani, A.; Komlev, V.; Moroncini, F.; Pugnaloni, A.; Pennesi, G.; Belicchi, M.; Rubini, C.; Rustichelli, F.; Tasso, R.; et al. Organization of Extracellular Matrix Fibers Within Polyglycolic Acid–Polylactic Acid Scaffolds Analyzed Using X-Ray Synchrotron-Radiation Phase-Contrast Micro Computed Tomography. Tissue Eng. Part C Methods 2009, 15, 403–411. [Google Scholar] [CrossRef]
- Giuliani, A.; Moroncini, F.; Mazzoni, S.; Belicchi, M.L.; Villa, C.; Erratico, S.; Colombo, E.; Calcaterra, F.; Brambilla, L.; Torrente, Y.; et al. Polyglycolic Acid-Polylactic Acid Scaffold Response to Different Progenitor Cell In Vitro Cultures: A Demonstrative and Comparative X-Ray Synchrotron Radiation Phase-Contrast Microtomography Study. Tissue Eng. Part C Methods 2014, 20, 308–316. [Google Scholar] [CrossRef] [Green Version]
- Giuliani, A.; Greco, S.; Pacilè, S.; Zannotti, A.; Carpini, G.D.; Tromba, G.; Giannubilo, S.R.; Ciavattini, A.; Ciarmela, P. Advanced 3D Imaging of Uterine Leiomyoma’s Morphology by Propagation-based Phase-Contrast Microtomography. Sci. Rep. 2019, 9, 10580. [Google Scholar] [CrossRef]
- Bravin, A.; Coan, P.; Suortti, P. X-ray phase-contrast imaging: From pre-clinical applications towards clinics. Phys. Med. Biol. 2012, 58, R1–R35. [Google Scholar] [CrossRef]
- Brun, F.; Massimi, L.; Fratini, M.; Dreossi, D.; Billé, F.; Accardo, A.; Pugliese, R.; Cedola, A. SYRMEP Tomo Project: A graphical user interface for customizing CT reconstruction workflows. Adv. Struct. Chem. Imaging 2017, 3, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Paganin, D.; Mayo, S.C.; Gureyev, T.E.; Miller, P.R.; Wilkins, S.W. Simultaneous phase and amplitude extraction from a single defocused image of a homogeneous object. J. Microsc. 2002, 206, 33–40. [Google Scholar] [CrossRef]
- Drezner, M.K.; Glorieux, F.H.; Kanis, J.A.; Malluche, H.; Meunier, P.J.; Ott, S.M.; Recker, R.R. Bone histomorphometry: Standardization of nomenclature, symbols, and units: Report of the asbmr histomorphometry nomenclature committee. J. Bone Min. Res. 1987, 2, 595–610. [Google Scholar] [CrossRef]
- Fazzalari, N.L.; Parkinson, I.H. Fractal dimension and architecture of trabecular bone. J. Pathol. 1996, 178, 100–105. [Google Scholar] [CrossRef]
- Bolle, C.; Felice, P.; Barausse, C.; Pistilli, V.; Trullenque-Eriksson, A.; Esposito, M. Four mm long vs longer implants in aug-mented bone in posterior atrophic jaws: 1-year post-loading results from a multicentre randomised controlled trial. Eur. J. Oral Implant. 2018, 11, 31–47. [Google Scholar]
- Brauer, E.; Lippens, E.; Klein, O.; Nebrich, G.; Schreivogel, S.; Korus, G.; Duda, G.N.; Petersen, A. Collagen Fibrils Mechanically Contribute to Tissue Contraction in an In Vitro Wound Healing Scenario. Adv. Sci. 2019, 6, 1801780. [Google Scholar] [CrossRef] [PubMed]
- Alberich-Bayarri, A.; Marti-Bonmati, L.; Angeles Pérez, M.; Sanz-Requena, R.; Lerma-Garrido, J.J.; García-Martí, G.; Moratal, D. Assessment of 2D and 3D fractal dimension measurements of trabecular bone from high-spatial resolution magnetic reso-nance images at 3 T. Med. Phys. 2010, 37, 4930–4937. [Google Scholar] [CrossRef] [PubMed]
- Meyer, M. Processing of collagen based biomaterials and the resulting materials properties. Biomed. Eng. Online 2019, 18, 1–74. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parry, D.A. The molecular fibrillar structure of collagen and its relationship to the mechanical properties of connective tissue. Biophys. Chem. 1988, 29, 195–209. [Google Scholar] [CrossRef]
- Kayed, H.R.; Kirby, N.; Hawley, A.; Mudie, S.T.; Haverkamp, R.G. Collagen fibril strain, recruitment and orientation for pericardium under tension and the effect of cross links. RSC Adv. 2015, 5, 103703–103712. [Google Scholar] [CrossRef]
- Hinz, B. The myofibroblast: Paradigm for a mechanically active cell. J. Biomech. 2010, 43, 146–155. [Google Scholar] [CrossRef]
- Mangano, C.; Piattelli, A.; Mangano, F.; Rustichelli, F.; Shibli, J.A.; Iezzi, G.; Giuliani, A. Histological and Synchrotron Radia-tion-Based Computed Microtomography Study of 2 Human-Retrieved Direct Laser Metal Formed Titanium Implants. Implant Dent. 2013, 22, 175–181. [Google Scholar] [CrossRef]
- Manescu, A.; Giuliani, A.; Mohammadi, S.; Tromba, G.; Mazzoni, S.; Diomede, F.; Zini, N.; Piattelli, A.; Trubiani, O. Osteogenic potential of dualblocks cultured with human periodontal ligament stem cells: In vitro and synchrotron microtomography study. J. Periodontal Res. 2016, 51, 112–124. [Google Scholar] [CrossRef]
- Mazzoni, S.; Mohammadi, S.; Tromba, G.; Diomede, F.; Piattelli, A.; Trubiani, O.; Giuliani, A. Role of cortico-cancellous het-erologous bone in human periodontal ligament stem cell xeno-free culture studied by Synchrotron radiation phase-contrast microtomography. Int. J. Mol. Sci. 2017, 18, 364. [Google Scholar] [CrossRef] [Green Version]


| Transversal CB mTh: 300 µm | Longitudinal CB—Inner mTh: 250 µm | Longitudinal CB—Outer mTh: 250 µm | |
|---|---|---|---|
| CollV/TV (%) | 35 ± 25 | 38 ± 6 | 51 ± 10 |
| CollTh (µm) | 33 ± 15 | 36 ± 6 | 47 ± 11 |
| CollNr (mm−1) | 9 ± 2 | 10 ± 1 | 10 ± 2 |
| CollSp (µm) | 78 ± 38 | 64 ± 10 | 52 ± 16 |
| DA | 0.70 ± 0.20 | 0.61 ± 0.04 | 0.67 ± 0.05 |
| Conn.D (×10−6) (px−3) | 10.4 ± 5.3 | 13.4 ± 3.3 | 13.3 ± 4.4 |
| 3D Fractal Dimension | 2.35 ± 0.07 | 2.39 ± 0.02 | 2.42 ± 0.07 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iezzi, G.; Di Lillo, F.; Furlani, M.; Degidi, M.; Piattelli, A.; Giuliani, A. The Symmetric 3D Organization of Connective Tissue around Implant Abutment: A Key-Issue to Prevent Bone Resorption. Symmetry 2021, 13, 1126. https://doi.org/10.3390/sym13071126
Iezzi G, Di Lillo F, Furlani M, Degidi M, Piattelli A, Giuliani A. The Symmetric 3D Organization of Connective Tissue around Implant Abutment: A Key-Issue to Prevent Bone Resorption. Symmetry. 2021; 13(7):1126. https://doi.org/10.3390/sym13071126
Chicago/Turabian StyleIezzi, Giovanna, Francesca Di Lillo, Michele Furlani, Marco Degidi, Adriano Piattelli, and Alessandra Giuliani. 2021. "The Symmetric 3D Organization of Connective Tissue around Implant Abutment: A Key-Issue to Prevent Bone Resorption" Symmetry 13, no. 7: 1126. https://doi.org/10.3390/sym13071126
APA StyleIezzi, G., Di Lillo, F., Furlani, M., Degidi, M., Piattelli, A., & Giuliani, A. (2021). The Symmetric 3D Organization of Connective Tissue around Implant Abutment: A Key-Issue to Prevent Bone Resorption. Symmetry, 13(7), 1126. https://doi.org/10.3390/sym13071126










