Base Catalysis of Sodium Salts of [Ta6−xNbxO19]8− Mixed-Oxide Clusters
Abstract
:1. Introduction
2. Materials and Methods
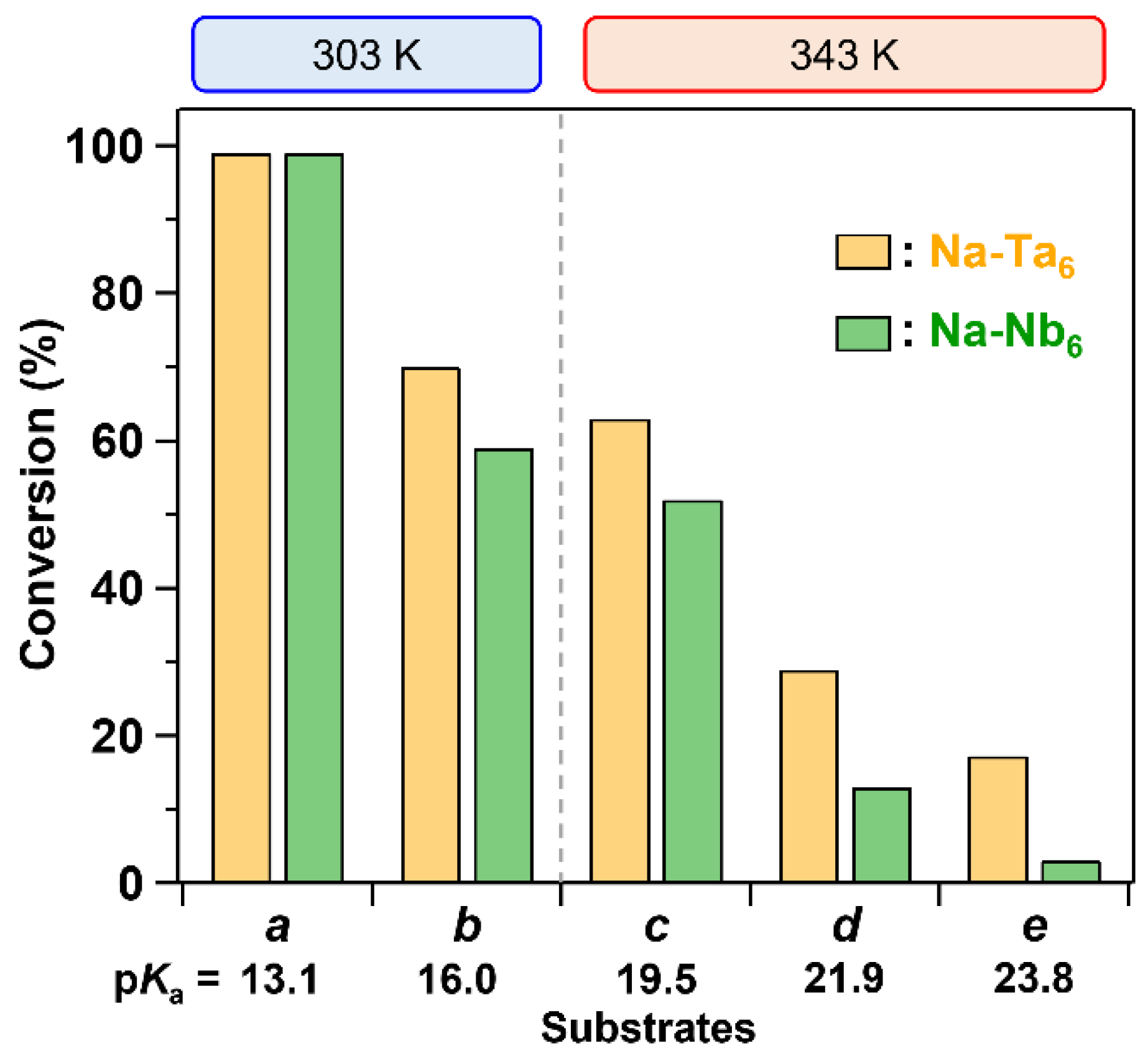
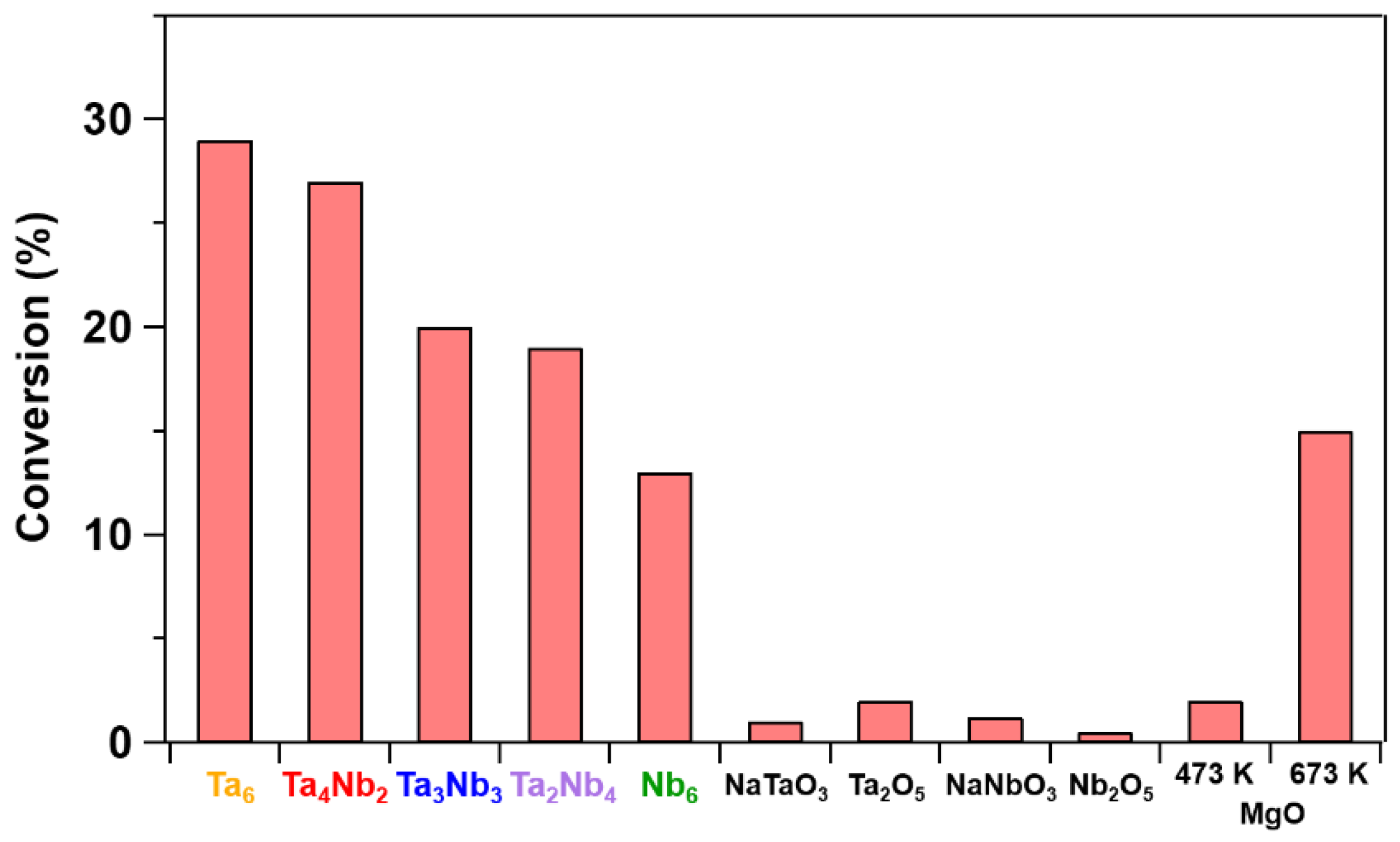
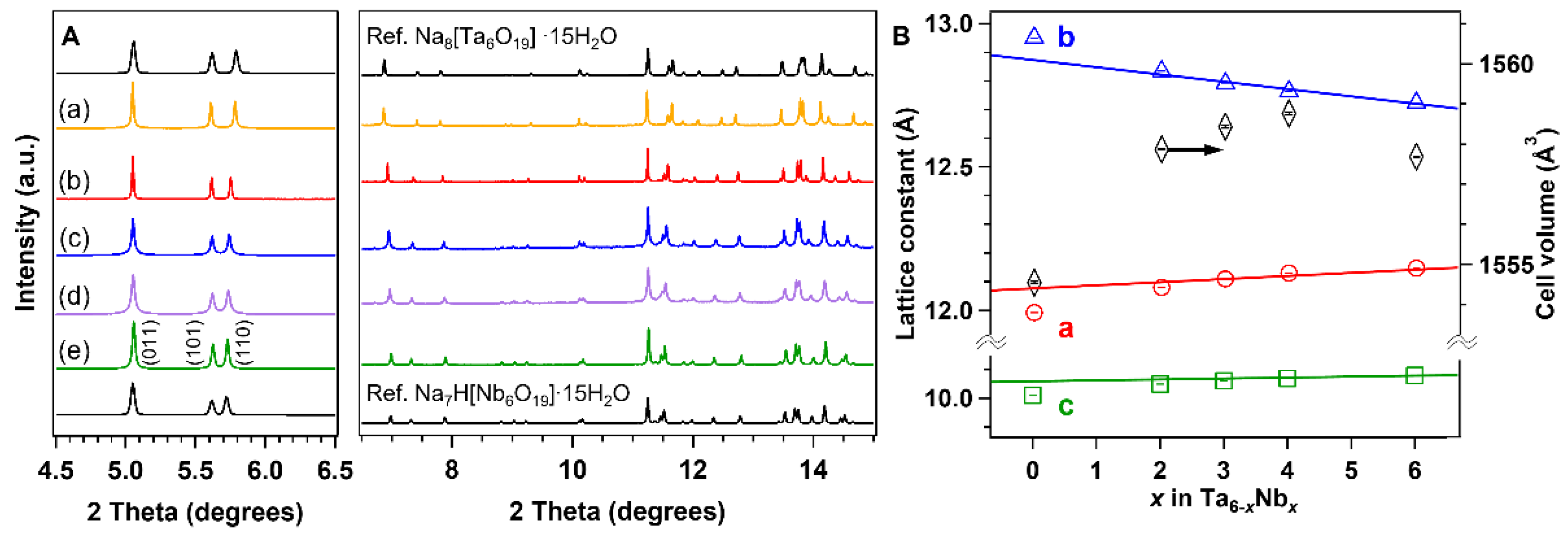
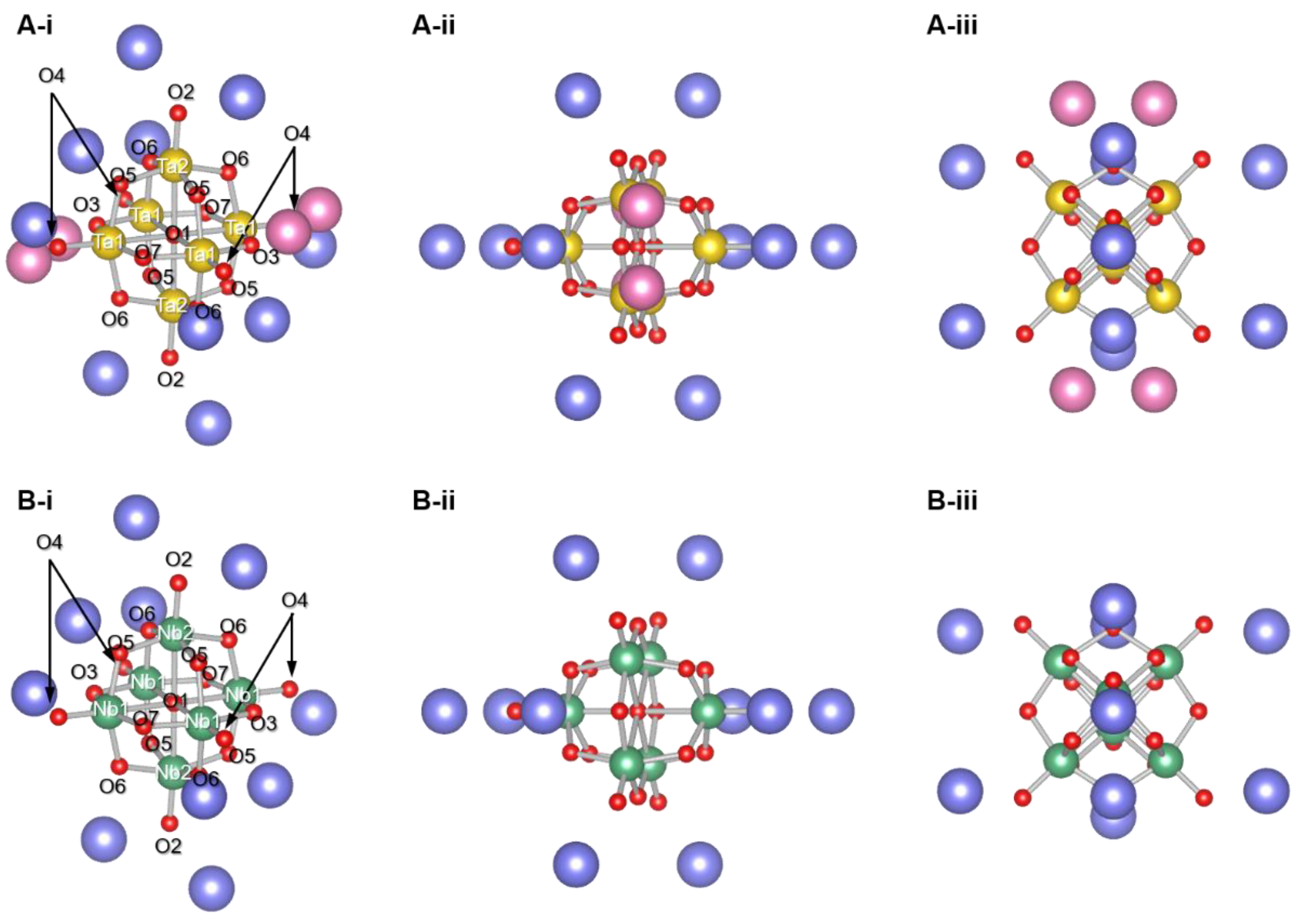
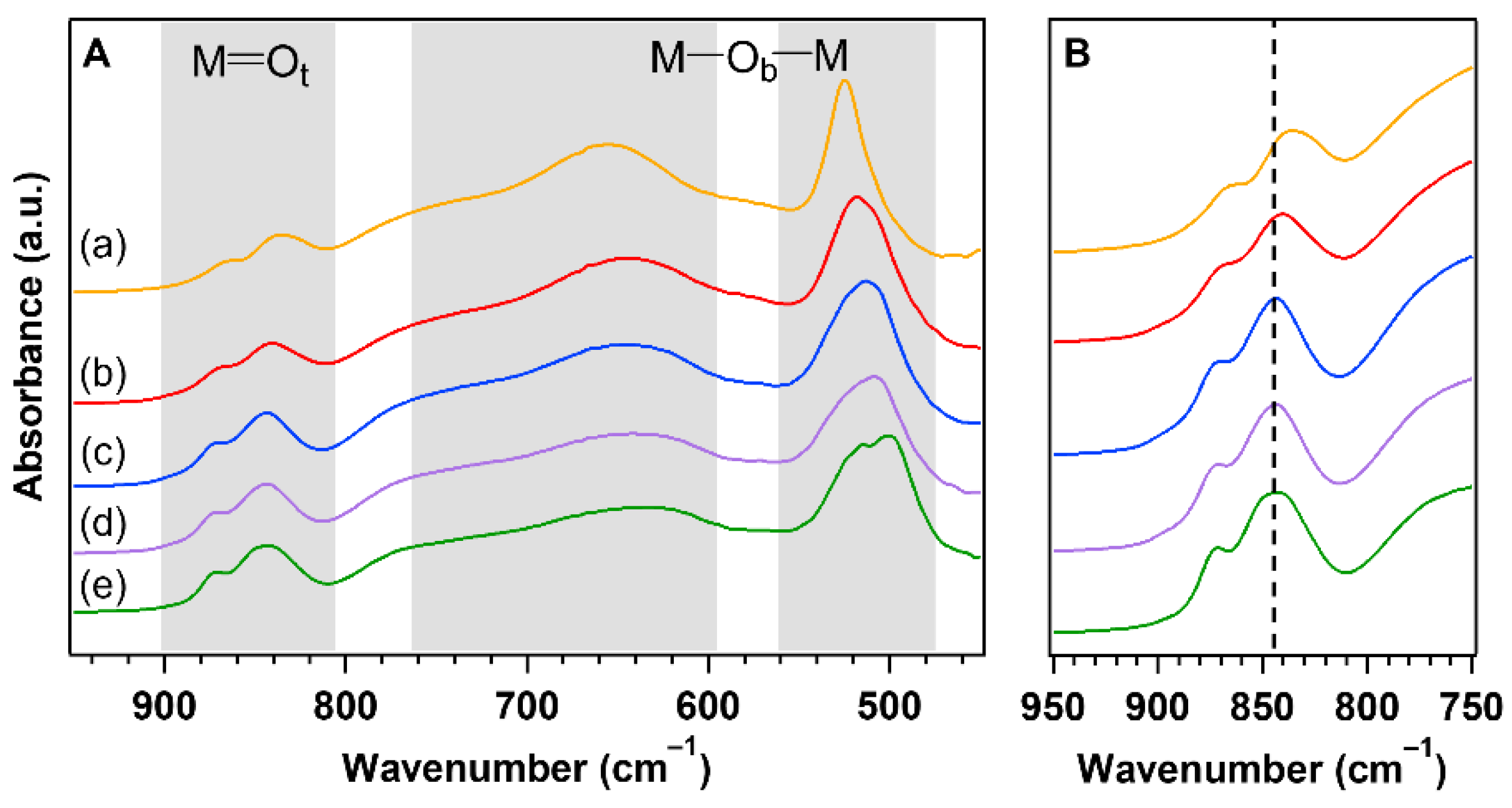
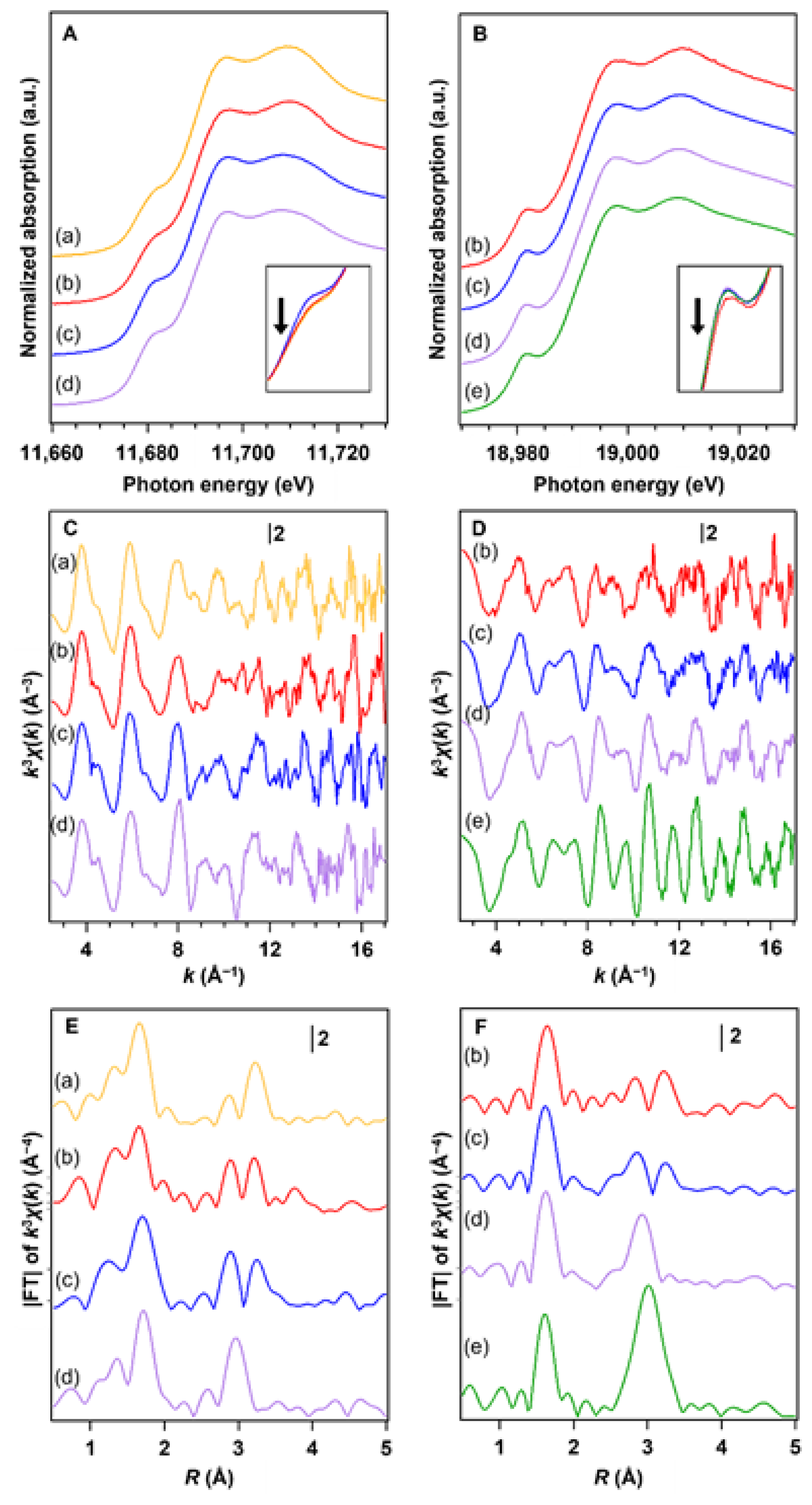
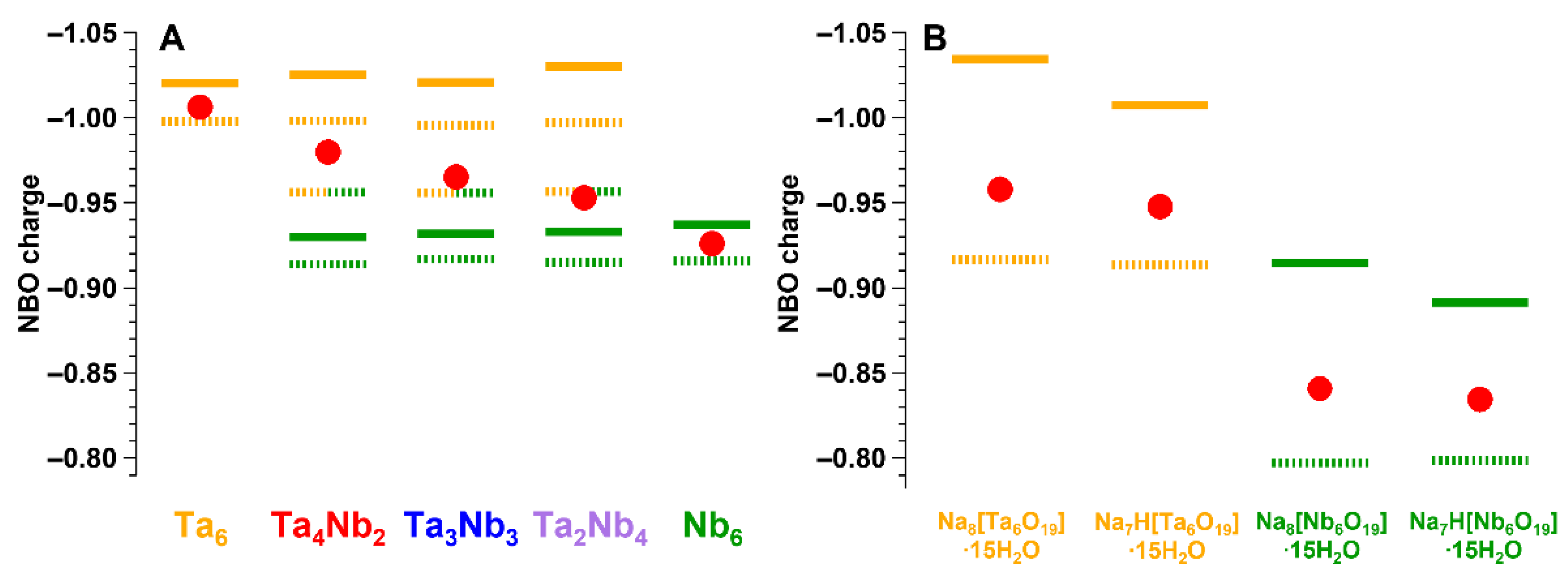
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Olah, G.A.; Sommer, J.; Namanworth, E. Stable carbonium ions. XLI: Protonated aliphatic alcohols and their cleavage to carbonium ions. J. Am. Chem. Soc. 1967, 89, 3576–3581. [Google Scholar] [CrossRef]
- Birchall, T.; Gillespie, R. Nuclear magnetic resonance studies of the protonation of weak bases in fluorosulfuric acid: V. Ketones, carboxylic acids, and some other oxygen bases. Can. J. Chem. 1965, 43, 1045–1051. [Google Scholar] [CrossRef]
- Mizuno, N.; Misono, M. Heterogeneous catalysis. Chem. Rev. 1998, 98, 199–218. [Google Scholar] [CrossRef]
- Kozhevnikov, I.V. Catalysis by heteropoly acids and multicomponent polyoxometalates in liquid-phase reactions. Chem. Rev. 1998, 98, 171–198. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, K.; Mizuno, N.; Yamaguchi, K. Polyoxometalate photocatalysis for liquid-phase selective organic functional group transformations. ACS Catal. 2018, 8, 10809–10825. [Google Scholar] [CrossRef]
- Iwase, Y.; Tomita, O.; Naito, H.; Higashi, M.; Abe, R. Molybdenum-substituted polyoxometalate as stable shuttle redox mediator for visible light driven Z-scheme water splitting system. J. Photochem. Photobiol. A Chem. 2018, 356, 347–354. [Google Scholar] [CrossRef]
- Sadakane, M.; Steckhan, E. Electrochemical properties of polyoxometalates as electrocatalysts. Chem. Rev. 1998, 98, 219–238. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.S.; Yang, G.Y. Recent advances in polyoxometalate–catalyzed reactions. Chem. Rev. 2015, 115, 4893–4962. [Google Scholar] [CrossRef] [PubMed]
- Albert, J.; Mehler, J.; Tucher, J.; Kastner, K.; Streb, C. One-step synthesizable Lindqvist−isopolyoxometalates as promising new catalysts for selective conversion of glucose as a model substrate for lignocellulosic biomass to formic acid. ChemistrySelect 2016, 1, 2889–2894. [Google Scholar] [CrossRef]
- Cheng, F.; Wang, H.; Rogers, R.D. Oxygen enhances polyoxometalate-based catalytic dissolution and delignification of woody biomass in ionic liquids. ACS Sustain. Chem. Eng. 2014, 2, 2859–2865. [Google Scholar] [CrossRef]
- Kimura, T.; Kamata, K.; Mizuno, N. A bifunctional tungstate catalyst for chemical fixation of CO2 at atmospheric pressure. Angew. Chem. Int. Ed. Engl. 2012, 51, 6700–6703. [Google Scholar] [CrossRef]
- Kimura, T.; Sunaba, H.; Kamata, K.; Mizuno, N. Efficient [WO4]2−-catalyzed chemical fixation of carbon dioxide with 2-aminobenzonitriles to quinazoline-2,4(1H,3H)-diones. Inorg. Chem. 2012, 51, 13001–13008. [Google Scholar] [CrossRef] [PubMed]
- Sugahara, K.; Kimura, T.; Kamata, K.; Yamaguchi, K.; Mizuno, N. A highly negatively charged gamma-Keggin germanodecatungstate efficient for Knoevenagel condensation. Chem. Commun. 2012, 48, 8422–8424. [Google Scholar] [CrossRef]
- Hayashi, S.; Sasaki, N.; Yamazoe, S.; Tsukuda, T. Superior base catalysis of group 5 hexametalates [M6O19]8– (M = Ta, Nb) over group 6 hexametalates [M6O19]2– (M = Mo, W). J. Phys. Chem. C 2018, 122, 29398–29404. [Google Scholar] [CrossRef]
- Xu, Q.; Niu, Y.; Wang, G.; Li, Y.; Zhao, Y.; Singh, V.; Niu, J.; Wang, J. Polyoxoniobates as a superior Lewis base efficiently catalyzed Knoevenagel condensation. Mol. Catal. 2018, 453, 93–99. [Google Scholar] [CrossRef]
- Hayashi, S.; Yamazoe, S.; Koyasu, K.; Tsukuda, T. Application of group V polyoxometalate as an efficient base catalyst: A case study of decaniobate clusters. RSC Adv. 2016, 6, 16239–16242. [Google Scholar] [CrossRef] [Green Version]
- Hayashi, S.; Yamazoe, S.; Koyasu, K.; Tsukuda, T. Lewis base catalytic properties of [Nb10O28]6− for CO2 fixation to epoxide: Kinetic and theoretical studies. Chem. Asian J. 2017, 12, 1635–1640. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, S.; Yamazoe, S.; Tsukuda, T. Base catalytic activity of [Nb10O28]6–: Effect of countercations. J. Phys. Chem. C 2020, 124, 10975–10980. [Google Scholar] [CrossRef]
- Gutierrez, L.F.; Nope, E.; Rojas, H.A.; Cubillos, J.A.; Sathicq, A.G.; Romanelli, G.P.; Martínez, J.J. New application of decaniobate salt as basic solid in the synthesis of 4H-pyrans by microwave assisted multicomponent reactions. Res. Chem. Intermed. 2018, 44, 5559–5568. [Google Scholar] [CrossRef]
- Ge, W.; Wang, X.; Zhang, L.; Du, L.; Zhou, Y.; Wang, J. Fully-occupied Keggin type polyoxometalate as solid base for catalyzing CO2 cycloaddition and Knoevenagel condensation. Catal. Sci. Technol. 2016, 6, 460–467. [Google Scholar] [CrossRef]
- Zhao, S.; Chen, Y.; Song, Y.-F. Tri-lacunary polyoxometalates of Na8H[PW9O34] as heterogeneous Lewis base catalysts for Knoevenagel condensation, cyanosilylation and the synthesis of benzoxazole derivatives. Appl. Catal. A 2014, 475, 140–146. [Google Scholar] [CrossRef]
- Simms, C.; Kondinski, A.; Parac-Vogt, T.N. Metal-addenda substitution in plenary polyoxometalates and in their modular transition metal analogues. Eur. J. Inorg. Chem. 2020, 2020, 2559–2572. [Google Scholar] [CrossRef]
- Anderson, T.M.; Rodriguez, M.A.; Stewart, T.A.; Bixler, J.N.; Xu, W.; Parise, J.B.; Nyman, M. Controlled sssembly of [Nb6–xWxO19](8–x)– (x = 0–4) Lindqvist ions with (amine)copper Complexes. Eur. J. Inorg. Chem. 2008, 2008, 3286–3294. [Google Scholar] [CrossRef]
- Isobe, K.; Hoshi, T.; Suzuki, T.; Hagiwara, H. Knoevenagel reaction in water catalyzed by amine supported on silica gel. Mol. Divers. 2005, 9, 317–320. [Google Scholar] [CrossRef] [PubMed]
- Tran, U.P.N.; Le, K.K.A.; Phan, N.T.S. Expanding applications of metal−organic frameworks: Zeolite imidazolate framework ZIF-8 as an efficient heterogeneous catalyst for the Knoevenagel reaction. ACS Catal. 2011, 1, 120–127. [Google Scholar] [CrossRef]
- Yamazoe, S.; Shibata, K.; Kato, K.; Wada, T. Needle-like NaNbO3 synthesis via Nb6O198− cluster using Na3NbO4 precursor by dissolution–precipitation method. Chem. Lett. 2013, 42, 380–382. [Google Scholar] [CrossRef] [Green Version]
- Fukada, M.; Shibata, K.; Imai, T.; Yamazoe, S.; Hosokawa, S.; Wada, T. Fabrication of lead-free piezoelectric NaNbO3 ceramics at low temperature using NaNbO3 nanoparticles synthesized by solvothermal method. J. Ceram. Soc. Jpn. 2013, 121, 116–119. [Google Scholar] [CrossRef] [Green Version]
- Izumi, F.; Momma, K. Three-dimensional visualization in powder diffraction. Solid State Phenom. 2007, 130, 15–20. [Google Scholar] [CrossRef]
- Momma, K.; Izumi, F. VESTA 3 for three-dimensional visualization of crystal, volumetric and morphology data. J. Appl. Crystallogr. 2011, 44, 1272–1276. [Google Scholar] [CrossRef]
- Asakura, H.; Yamazoe, S.; Misumi, T.; Fujita, A.; Tsukuda, T.; Tanaka, T. xTunes: A new XAS processing tool for detailed and on-the-fly analysis. Radiat. Phys. Chem. 2020, 175, 108270–108273. [Google Scholar] [CrossRef]
- Matsuhashi, H.; Nagashima, K.; Naijo, N.; Aritani, H. Surface base sites of MgO covered with Al2O3: XANES analysis of Al and Mg K-edges. Top. Catal. 2010, 53, 659–663. [Google Scholar] [CrossRef]
- Anderson, T.M.; Rodriguez, M.A.; Bonhomme, F.; Bixler, J.N.; Alam, T.M.; Nyman, M. An aqueous route to [Ta6O19]8− and solid-state studies of isostructural niobium and tantalum oxide complexes. Dalton Trans. 2007, 4517–4522. [Google Scholar] [CrossRef]
- Ma, P.T.; Chen, G.; Wang, G.; Wang, J.P. Cobalt–sandwiched Lindqvist hexaniobate dimer [Co(III)H5(Nb6O19)2]8−. Russ. J. Coord. Chem. 2011, 37, 772–775. [Google Scholar] [CrossRef]
- Besserguenev, A.V.; Dickman, M.H.; Pope, M.T. Robust, alkali-stable, triscarbonyl metal derivatives of hexametalate anions, [M6O19 {M’(CO)3}n](8−n)−(M = Nb, Ta; M’ = Mn, Re; n= 1, 2). Inorg. Chem. 2001, 40, 2582–2586. [Google Scholar] [CrossRef] [PubMed]
- Farges, F.; Brown, G.E.; Rehr, J. Ti K-edge XANES studies of Ti coordination and disorder in oxide compounds: Comparison between theory and experiment. Phys. Rev. B 1997, 56, 1809–1819. [Google Scholar] [CrossRef]
- Yamazoe, S.; Hitomi, Y.; Shishido, T.; Tanaka, T. XAFS study of tungsten L1-and L3-edges: Structural analysis of WO3 species loaded on TiO2 as a catalyst for photo-oxidation of NH3. J. Phys. Chem. C 2008, 112, 6869–6879. [Google Scholar] [CrossRef]
- Asakura, H.; Shishido, T.; Yamazoe, S.; Teramura, K.; Tanaka, T. Structural analysis of group V, VI, and VII metal compounds by XAFS. J. Phys. Chem. C 2011, 115, 23653–23663. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kikkawa, S.; Tsukada, M.; Shibata, K.; Fujiki, Y.; Shibusawa, K.; Hirayama, J.; Nakatani, N.; Yamamoto, T.; Yamazoe, S. Base Catalysis of Sodium Salts of [Ta6−xNbxO19]8− Mixed-Oxide Clusters. Symmetry 2021, 13, 1267. https://doi.org/10.3390/sym13071267
Kikkawa S, Tsukada M, Shibata K, Fujiki Y, Shibusawa K, Hirayama J, Nakatani N, Yamamoto T, Yamazoe S. Base Catalysis of Sodium Salts of [Ta6−xNbxO19]8− Mixed-Oxide Clusters. Symmetry. 2021; 13(7):1267. https://doi.org/10.3390/sym13071267
Chicago/Turabian StyleKikkawa, Soichi, Mio Tsukada, Kanako Shibata, Yu Fujiki, Kazuki Shibusawa, Jun Hirayama, Naoki Nakatani, Takafumi Yamamoto, and Seiji Yamazoe. 2021. "Base Catalysis of Sodium Salts of [Ta6−xNbxO19]8− Mixed-Oxide Clusters" Symmetry 13, no. 7: 1267. https://doi.org/10.3390/sym13071267





