Stability Analysis of a Mathematical Model of Hepatitis B Virus with Unbounded Memory Control on the Immune System in the Neighborhood of the Equilibrium Free Point
Abstract
:1. Introduction
2. Description of the Model
3. Relation between the Convergence Rate of System (1) and (3)
4. Exponential Stability of System (3) with Unbounded Memory Control Function
5. Exponential Stability of System (3) with Delay in Upper Limit of Control Function
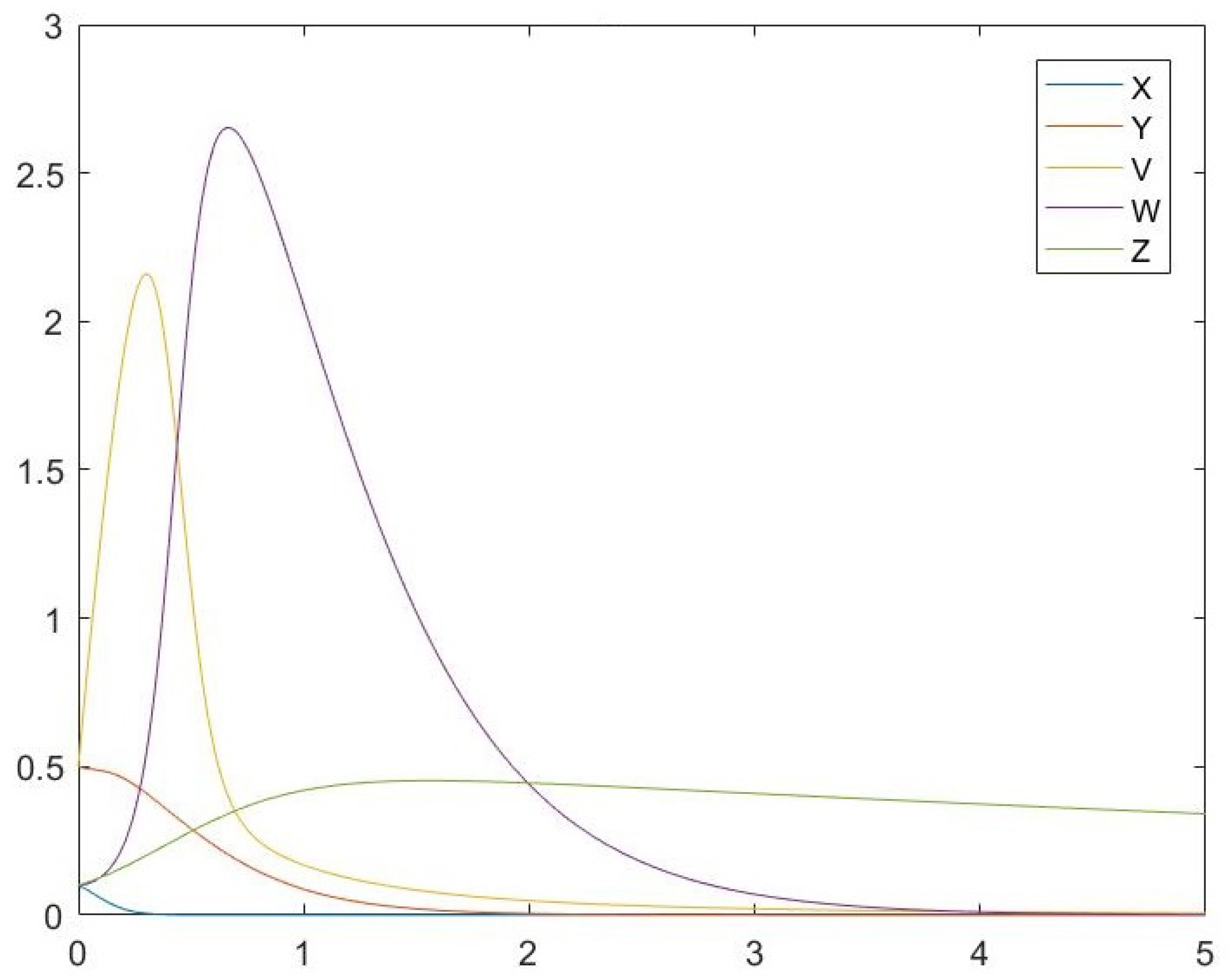
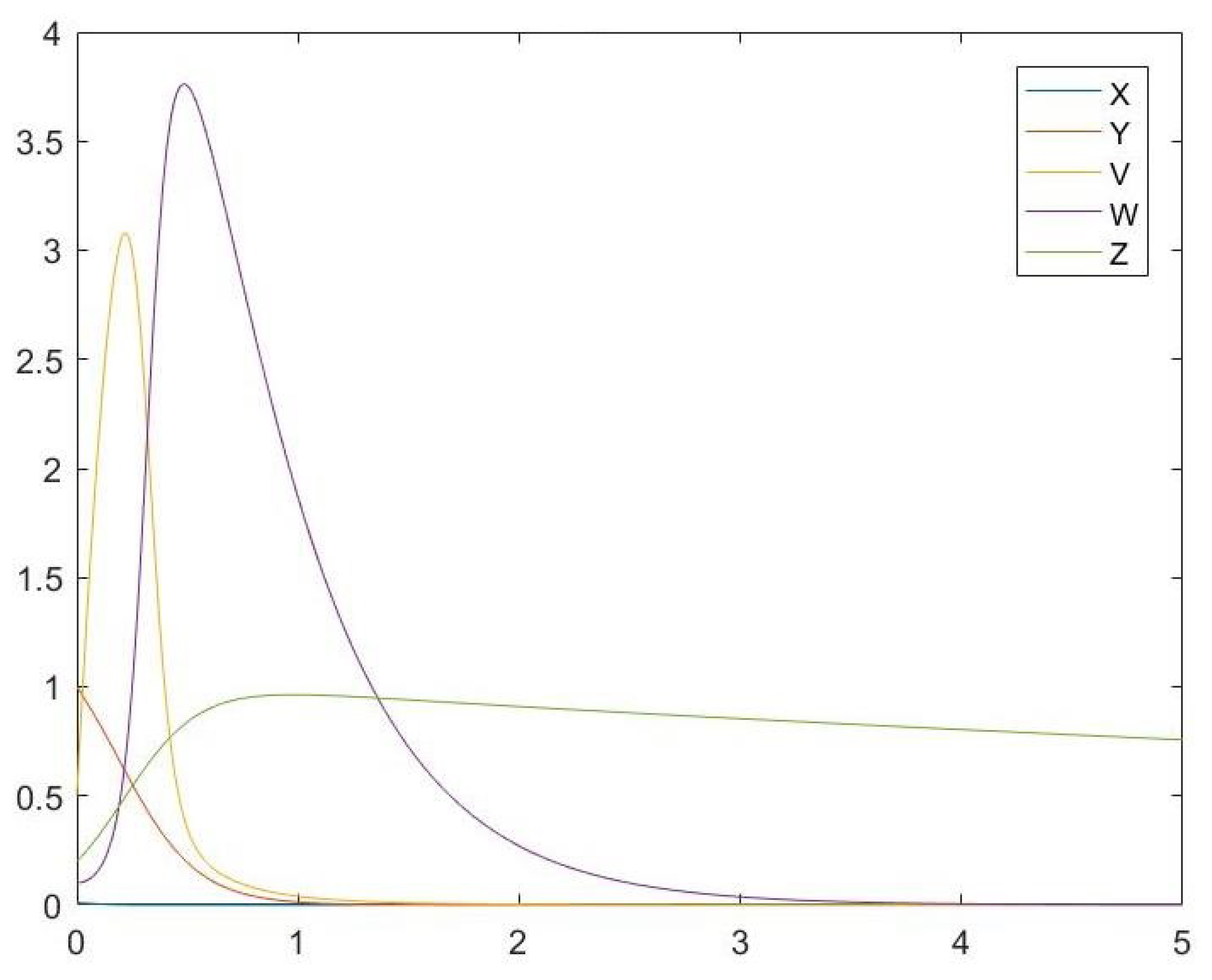
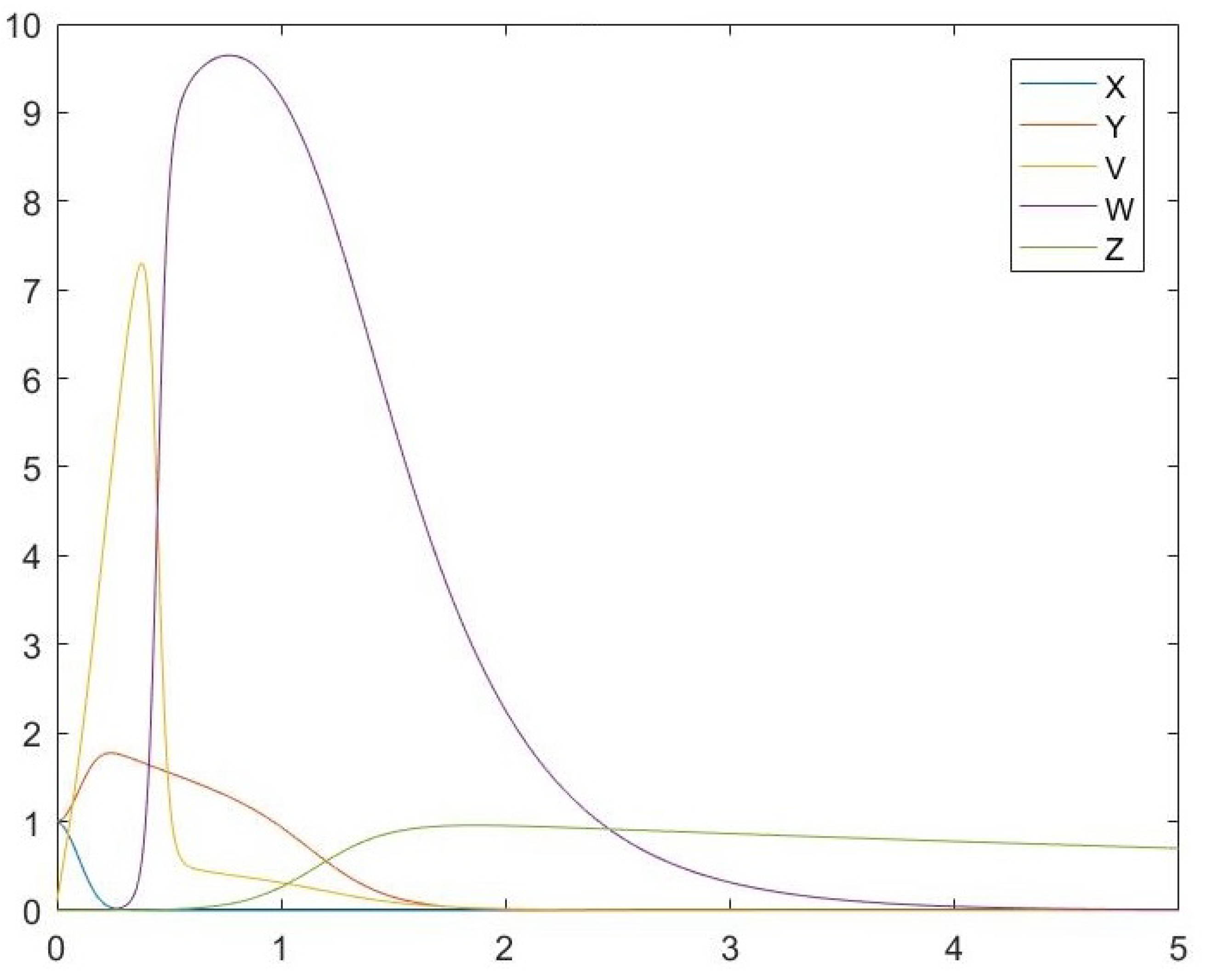
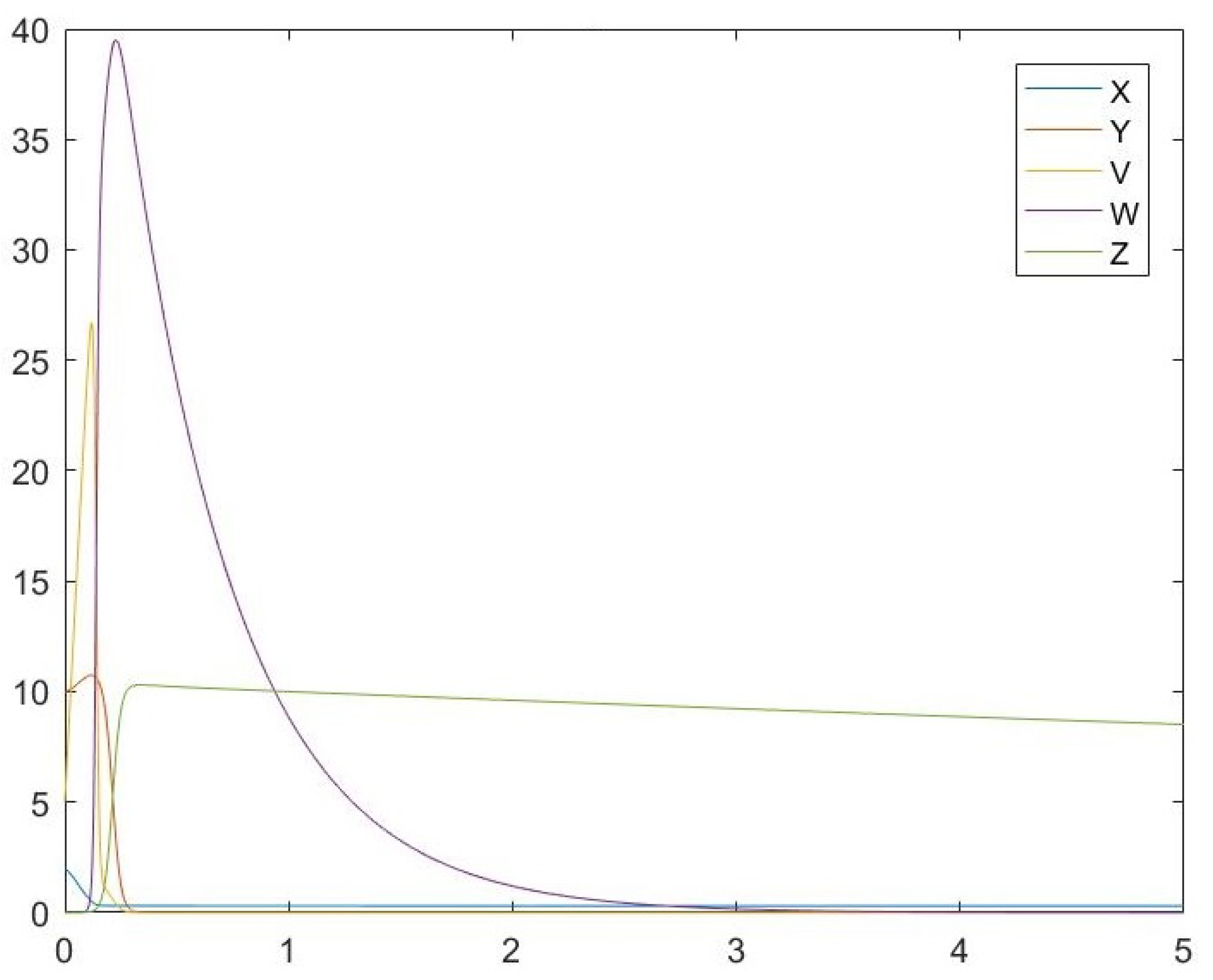
6. Simulations
7. Conclusions
Funding
Informed Consent Statement
Conflicts of Interest
References
- Tang, L.S.Y.; Covert, E.; Wilson, E.; Kottilil, S. Chronic Hepatitis B Infection: A Review. JAMA 2018, 319, 1802–1813. [Google Scholar] [CrossRef] [PubMed]
- Long, C.; Qi, H.; Huang, S.H. Mathematical modeling of cytotoxic lymphocyte-mediated immune response to hepatitis B virus infection. J. Biomed. Biotechnol. 2008, 2008, 743690. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Poh, Z.; Goh, B.B.; Chang, P.E.; Tan, C.K. Rates of cirrhosis and hepatocellular carcinoma in chronic hepatitis B and the role of surveillance: A 10-year follow-up of 673 patients. Eur. J. Gastroenterol. Hepatol. 2015, 27, 638–643. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parkin, D.M. The global health burden of infection-associated cancers in the year 2002. Int. J. Cancer 2006, 118, 3030–3044. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- European Association for the Study of the Liver. EASL 2017 Clinical Practice Guidelines on the management of hepatitis B virus infection. J. Hepatol. 2017, 67, 370–398. [Google Scholar] [CrossRef] [Green Version]
- Berezansky, L.; Bunimovich-Mendrazitsky, S.; Schklar, B. Stability and controllability issues in mathematical modeling of the intensive treatment of leukemia. J. Optim. Theory Appl. 2015, 167, 326–341. [Google Scholar] [CrossRef]
- Berezansky, L.; Bunimovich-Mendrazitsky, S.; Domoshnitsky, A. A mathematical model with time-varying delays in the combined treatment of chronic myeloid leukemia. Adv. Differ. Equ. 2012, 217, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Bershadsky, M.; Chirkov, M.; Domoshnitsky, A.; Rusakov, S.; Volinsky, I. Distributed Control and the Lyapunov Characteristic Exponents in the Model of Infectious Diseases. Complexity 2019, 2019, 5234854. [Google Scholar] [CrossRef]
- Bunimovich-Mendrazitsky, S.; Kronik, N.; Vainstein, V. Optimization of interferon-alpha and imatinib combination therapy for chronic myeloid leukemia: A modeling approach. Adv. Theory Simul. 2019, 2, 1800081. [Google Scholar] [CrossRef]
- Bunimovich-Mendrazitsky, S.; Goltser, Y. Use of quasi-normal form to examine stability of tumor-free equilibrium in a mathematical model of BCG treatment of bladder cancer. Math. Biosci. Eng. 2011, 8, 529–547. [Google Scholar] [CrossRef] [PubMed]
- Domoshnitsky, A.; Volinsky, I.; Bershadsky, M. Around the Model of Infection Disease: The Cauchy Matrix and Its Properties. Symmetry 2019, 11, 1016. [Google Scholar] [CrossRef] [Green Version]
- Domoshnitsky, A.; Volinsky, I.; Pinhasov, O. Some developments in the model of testosterone regulation. AIP Conf. Proc. 2019, 2159, 030010. [Google Scholar] [CrossRef]
- Domoshnitsky, A.; Volinsky, I.; Pinhasov, O.; Bershadsky, M. Questions of Stability of Functional Differential Systems around the Model of Testosterone Regulation. Bound. Value Probl. 2019, 1, 1–13. [Google Scholar] [CrossRef]
- Colombatto, P.; Civitano, L.; Bizzarri, R.; Oliveri, F.; Choudhury, S.; Gieschke, R.; Bonino, F.; Brunetto, M.R.; Peginterferon Alfa-2a HBeAg-Negative Chronic Hepatitis B Study Group. A multiphase model of the dynamics of HBV infection in HBeAg-negative patients during pegylated interferon-α, lamivudine and combination therapy. Antivir. Ther. 2006, 11, 197–212. [Google Scholar] [PubMed]
- Nowak, M.A.; Bonhoeffer, S.; Hill, A.M.; Boehme, R.; Thomas, H.C.; McDade, H. Viral dynamics in hepatitis B virus infection. Proc. Natl. Acad. Sci. USA 1996, 93, 4398–4402. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Perelson, A.S.; Ribeiro, R.M. Hepatitis B virus kinetics and mathematical modeling. Semin. Liver Dis. 2004, 24, 11–16. [Google Scholar] [CrossRef] [PubMed]
- Wain-Hobson, S. Virus Dynamics: Mathematical Principles of Immunology and Virology. Nat. Med. 2001, 7, 525–526. [Google Scholar] [CrossRef]
- Yousdi, N.; Hattaf, K.; Rachik, M. Analysis of a HCV model with CTL and antibody responses. Appl. Math. Sci. 2009, 3, 2835–2845. [Google Scholar]
- Volinsky, I.; Lombardo, S.D.; Cheredman, P. Stability Analysis and Cauchy Matrix of a Mathematical Model of Hepatitis B Virus with Control on Immune System near Neighborhood of Equilibrium Free Point. Symmetry 2021, 13, 166. [Google Scholar] [CrossRef]
- Chenar, F.F.; Kyrychko, Y.N.; Blyuss, K.B. Mathematical model of immune response to hepatitis B. J. Theor. Biol. 2018, 447, 98–110. [Google Scholar] [CrossRef] [PubMed] [Green Version]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Volinsky, I. Stability Analysis of a Mathematical Model of Hepatitis B Virus with Unbounded Memory Control on the Immune System in the Neighborhood of the Equilibrium Free Point. Symmetry 2021, 13, 1437. https://doi.org/10.3390/sym13081437
Volinsky I. Stability Analysis of a Mathematical Model of Hepatitis B Virus with Unbounded Memory Control on the Immune System in the Neighborhood of the Equilibrium Free Point. Symmetry. 2021; 13(8):1437. https://doi.org/10.3390/sym13081437
Chicago/Turabian StyleVolinsky, Irina. 2021. "Stability Analysis of a Mathematical Model of Hepatitis B Virus with Unbounded Memory Control on the Immune System in the Neighborhood of the Equilibrium Free Point" Symmetry 13, no. 8: 1437. https://doi.org/10.3390/sym13081437




