Developmental Brain Asymmetry. The Good and the Bad Sides
Abstract
:1. Introduction
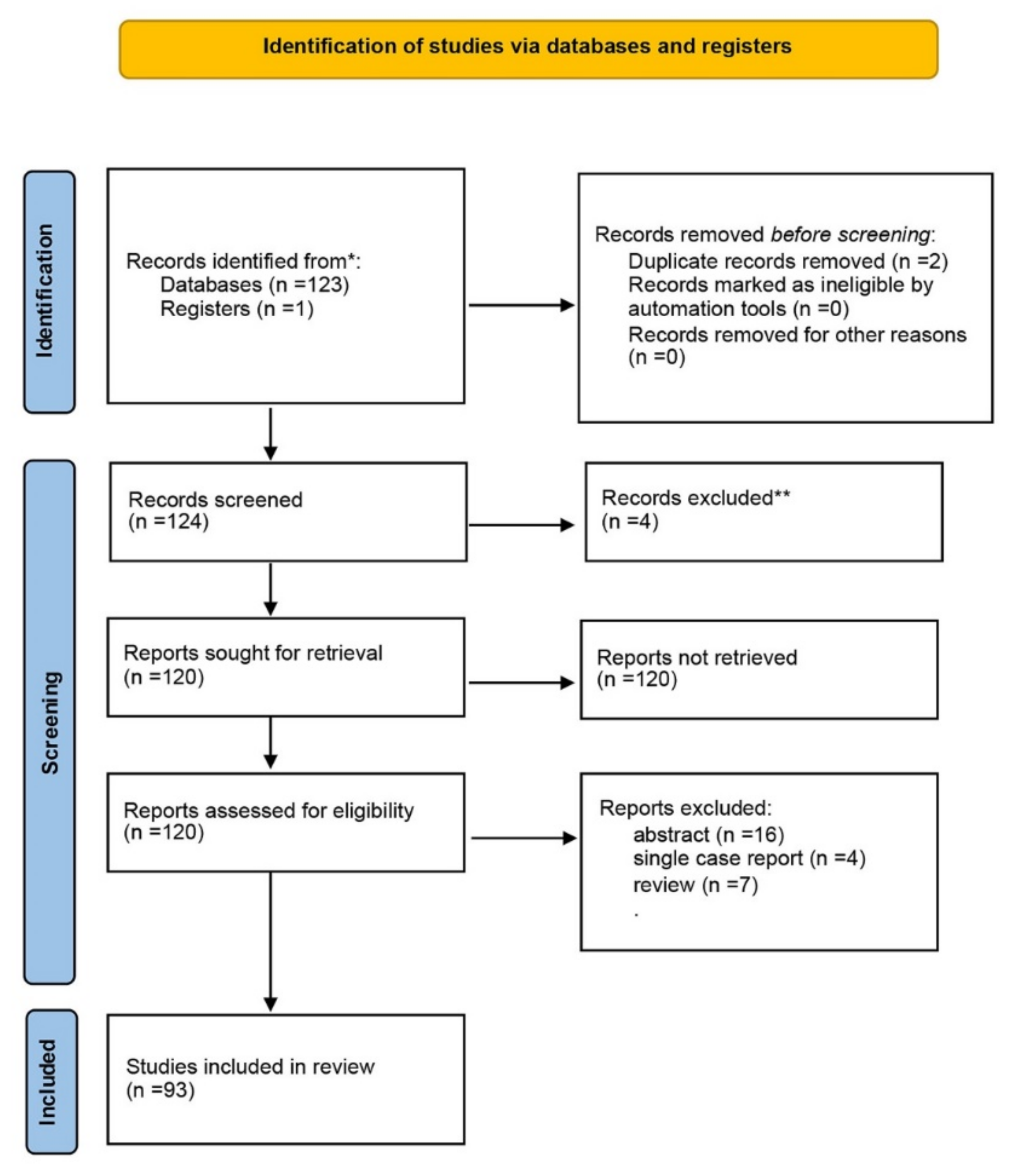
2. Materials and Methods
3. Results
3.1. “Good” Developmental Brain Asymmetry from Gene to Phenotype
3.1.1. Structural and Functional Asymmetry Lesson learned from In Vivo Studies
3.1.2. Genetic Clock in Normal and Pathological Brain Development
3.1.3. Epigenomic and Transcriptomic Asymmetry as Clue for Normal Brain Phenotypic Features
- neurotransmitter signaling pathways such as glutamate and γ-aminobutyric acid (GABA) signaling pathways that regulate the interaction between different areas of the developing brain and are essential for normal brain development. The GABA signaling pathway is also involved in neural plasticity later in adulthood [55,57,58];
- the dopaminergic pathway is involved in the normal function and behavior of the brain [55]. Here, some major dopaminergic pathways were described: mesocorticolimbic and nigrostriatal pathways that connect the middle brain region with the striatum and prefrontal cortex associated with cognition and motor functions. Dysregulation and disruptions of these pathways are involved in addiction, schizophrenia, ADHD, chorea, and Parkinson’s diseases later in life [55,59]. However, if this pattern is asymmetrical, being established during the prenatal period due to complex interaction with genetic or environmental factors, it remains to be elucidated.
3.2. Hallmark of “Bad” Developmental Brain Asymmetry
Bridge between Neurodevelopmental Brain Signature and Pathological Phenotype
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Kasprian, G.; Langs, G.; Brugger, P.C.; Bittner, M.; Weber, M.; Arantes, M.; Prayer, D. The prenatal origin of hemispheric asymmetry: An in utero neuroimaging study. Cereb. Cortex 2011, 21, 1076–1083. [Google Scholar] [CrossRef] [PubMed]
- Andescavage, N.N.; Du Plessis, A.; McCarter, R.; Serag, A.; Evangelou, I.; Vezina, G.; Robertson, R.; Limperopoulos, C. Complex Trajectories of Brain Development in the Healthy Human Fetus. Cereb. Cortex 2017, 27, 5274–5283. [Google Scholar] [CrossRef] [PubMed]
- Sha, Z.; Schijven, D.; Francks, C. Patterns of brain asymmetry associated with polygenic risks for autism and schizophrenia implicate language and executive functions but not brain masculinization. Mol. Psychiatry 2021. [Google Scholar] [CrossRef] [PubMed]
- Vingerhoets, G. Phenotypes in hemispheric functional segregation? Perspectives and challenges. Phys. Life Rev. 2019, 30, 1–18. [Google Scholar] [CrossRef]
- Goel, V. Hemispheric asymmetry in the prefrontal cortex for complex cognition. Handb. Clin. Neurol. 2019, 163, 179–196. [Google Scholar] [CrossRef] [PubMed]
- Nelson, K.B.; Blair, E. Prenatal Factors in Singletons with Cerebral Palsy Born at or near Term. N. Engl. J. Med. 2015, 373, 946–953. [Google Scholar] [CrossRef] [PubMed]
- Olulade, O.A.; Seydell-Greenwald, A.; Chambers, C.E.; Turkeltaub, P.E.; Dromerick, A.W.; Berl, M.M.; Gaillard, W.D.; Newport, E.L. The neural basis of language development: Changes in lateralization over age. Proc. Natl. Acad. Sci. USA 2020, 117, 23477–23483. [Google Scholar] [CrossRef] [PubMed]
- De Kovel, C.G.F.; Lisgo, S.N.; Fisher, S.E.; Francks, C. Subtle left-right asymmetry of gene expression profiles in embryonic and foetal human brains. Sci. Rep. 2018, 8, 12606. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ocklenburg, S.; Schmitz, J.; Moinfar, Z.; Moser, D.; Klose, R.; Lor, S.; Kunz, G.; Tegenthoff, M.; Faustmann, P.; Francks, C.; et al. Epigenetic regulation of lateralized fetal spinal gene expression underlies hemispheric asymmetries. Elife 2017, 6. [Google Scholar] [CrossRef] [PubMed]
- Miller, S.L.; Huppi, P.S.; Mallard, C. The consequences of fetal growth restriction on brain structure and neurodevelopmental outcome. J. Physiol. 2016, 594, 807–823. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Damoiseaux, J.S. Effects of aging on functional and structural brain connectivity. Neuroimage 2017, 160, 32–40. [Google Scholar] [CrossRef] [PubMed]
- Roe, J.M.; Vidal-Piñeiro, D.; Sørensen, Ø.; Brandmaier, A.M.; Düzel, S.; Gonzalez, H.A.; Kievit, R.A.; Knights, E.; Kühn, S.; Lindenberger, U.; et al. Asymmetric thinning of the cerebral cortex across the adult lifespan is accelerated in Alzheimer’s disease. Nat. Commun. 2021, 12, 721. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Robles, A.; Hopkins, W.D.; Sherwood, C.C. Increased morphological asymmetry, evolvability and plasticity in human brain evolution. Proc. Biol. Sci. 2013, 280, 20130575. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gómez-Robles, A.; Hopkins, W.D.; Schapiro, S.J.; Sherwood, C.C. The heritability of chimpanzee and human brain asymmetry. Proc. Biol. Sci. 2016, 283. [Google Scholar] [CrossRef] [Green Version]
- Neubauer, S.; Gunz, P.; Scott, N.A.; Hublin, J.J.; Mitteroecker, P. Evolution of brain lateralization: A shared hominid pattern of endocranial asymmetry is much more variable in humans than in great apes. Sci. Adv. 2020, 6, eaax9935. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheng, L.; Zhang, Y.; Li, G.; Wang, J.; Sherwood, C.; Gong, G.; Fan, L.; Jiang, T. Connectional asymmetry of the inferior parietal lobule shapes hemispheric specialization in humans, chimpanzees, and rhesus macaques. Elife 2021, 10. [Google Scholar] [CrossRef] [PubMed]
- Cochella, L.; Hobert, O. Embryonic priming of a miRNA locus predetermines postmitotic neuronal left/right asymmetry in C. elegans. Cell 2012, 151, 1229–1242. [Google Scholar] [CrossRef] [Green Version]
- Chou, M.Y.; Amo, R.; Kinoshita, M.; Cherng, B.W.; Shimazaki, H.; Agetsuma, M.; Shiraki, T.; Aoki, T.; Takahoko, M.; Yamazaki, M.; et al. Social conflict resolution regulated by two dorsal habenular subregions in zebrafish. Science 2016, 352, 87–90. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rathee, S.; Joshi, P.; Kelkar, A.; Seth, N. Fetal MRI: A pictorial essay. Indian J. Radiol. Imaging 2016, 26, 52–62. [Google Scholar] [CrossRef]
- Paladini, D.; Quarantelli, M.; Sglavo, G.; Pastore, G.; Cavallaro, A.; D’Armiento, M.R.; Salvatore, M.; Nappi, C. Accuracy of neurosonography and MRI in clinical management of fetuses referred with central nervous system abnormalities. Ultrasound Obstet. Gynecol. 2014, 44, 188–196. [Google Scholar] [CrossRef] [PubMed]
- Pfeifer, C.M.; Willard, S.D.; Cornejo, P. MRI depiction of fetal brain abnormalities. Acta Radiol. Open 2019, 8, 2058460119894987. [Google Scholar] [CrossRef]
- Iliescu, D.; Tudorache, S.; Comanescu, A.; Antsaklis, P.; Cotarcea, S.; Novac, L.; Cernea, N.; Antsaklis, A. Improved detection rate of structural abnormalities in the first trimester using an extended examination protocol. Ultrasound Obstet. Gynecol. 2013, 42, 300–309. [Google Scholar] [CrossRef] [PubMed]
- Griffiths, P.D.; Bradburn, M.; Campbell, M.J.; Cooper, C.L.; Embleton, N.; Graham, R.; Hart, A.R.; Jarvis, D.; Kilby, M.D.; Lie, M.; et al. MRI in the diagnosis of fetal developmental brain abnormalities: The MERIDIAN diagnostic accuracy study. Health Technol. Assess 2019, 23, 1–144. [Google Scholar] [CrossRef] [PubMed]
- Hart, A.R.; Embleton, N.D.; Bradburn, M.; Connolly, D.J.A.; Mandefield, L.; Mooney, C.; Griffiths, P.D. Accuracy of in-utero MRI to detect fetal brain abnormalities and prognosticate developmental outcome: Postnatal follow-up of the MERIDIAN cohort. Lancet Child Adolesc. Health 2020, 4, 131–140. [Google Scholar] [CrossRef] [Green Version]
- Khawam, M.; De Dumast, P.; Deman, P.; Kebiri, H.; Yu, T.; Tourbier, S.; Lajous, H.; Hagmann, P.; Maeder, P.; Thiran, J.P.; et al. Fetal Brain Biometric Measurements on 3D Super-Resolution Reconstructed T2-Weighted MRI: An Intra- and Inter-observer Agreement Study. Front. Pediatr. 2021, 9, 639746. [Google Scholar] [CrossRef] [PubMed]
- Schmidbauer, V.U.; Dovjak, G.O.; Yildirim, M.S.; Mayr-Geisl, G.; Weber, M.; Diogo, M.C.; Gruber, G.M.; Prayer, F.; Milos, R.I.; Stuempflen, M.; et al. Mapping Human Fetal Brain Maturation In Vivo Using Quantitative MRI. AJNR Am. J. Neuroradiol. 2021. [Google Scholar] [CrossRef] [PubMed]
- Sun, T.; Patoine, C.; Abu-Khalil, A.; Visvader, J.; Sum, E.; Cherry, T.J.; Orkin, S.H.; Geschwind, D.H.; Walsh, C.A. Early asymmetry of gene transcription in embryonic human left and right cerebral cortex. Science 2005, 308, 1794–1798. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, T.; Collura, R.V.; Ruvolo, M.; Walsh, C.A. Genomic and evolutionary analyses of asymmetrically expressed genes in human fetal left and right cerebral cortex. Cereb. Cortex 2006, 16 (Suppl. S1), i18–i25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hering-Hanit, R.; Achiron, R.; Lipitz, S.; Achiron, A. Asymmetry of fetal cerebral hemispheres: In utero ultrasound study. Arch Dis. Child Fetal Neonatal. Ed. 2001, 85, F194–F196. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kong, X.Z.; Mathias, S.R.; Guadalupe, T.; Glahn, D.C.; Franke, B.; Crivello, F.; Tzourio-Mazoyer, N.; Fisher, S.E.; Thompson, P.M.; Francks, C.; et al. Mapping cortical brain asymmetry in 17,141 healthy individuals worldwide via the ENIGMA Consortium. Proc. Natl. Acad. Sci. USA 2018, 115, E5154–E5163. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kong, X.Z.; Postema, M.; Schijven, D.; Castillo, A.C.; Pepe, A.; Crivello, F.; Joliot, M.; Mazoyer, B.; Fisher, S.E.; Francks, C. Large-Scale Phenomic and Genomic Analysis of Brain Asymmetrical Skew. Cereb. Cortex 2021, 31, 4151–4168. [Google Scholar] [CrossRef]
- Habas, P.A.; Scott, J.A.; Roosta, A.; Rajagopalan, V.; Kim, K.; Rousseau, F.; Barkovich, A.J.; Glenn, O.A.; Studholme, C. Early folding patterns and asymmetries of the normal human brain detected from in utero MRI. Cereb. Cortex 2012, 22, 13–25. [Google Scholar] [CrossRef]
- Lehtola, S.J.; Tuulari, J.J.; Karlsson, L.; Parkkola, R.; Merisaari, H.; Saunavaara, J.; Lähdesmäki, T.; Scheinin, N.M.; Karlsson, H. Associations of age and sex with brain volumes and asymmetry in 2–5-week-old infants. Brain Struct. Funct. 2019, 224, 501–513. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Song, J.W.; Mitchell, P.D.; Kolasinski, J.; Ellen Grant, P.; Galaburda, A.M.; Takahashi, E. Asymmetry of White Matter Pathways in Developing Human Brains. Cereb. Cortex 2015, 25, 2883–2893. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thomason, M.E. Structured Spontaneity: Building Circuits in the Human Prenatal Brain. Trends Neurosci. 2018, 41, 1–3. [Google Scholar] [CrossRef]
- Turk, E.; Van den Heuvel, M.I.; Benders, M.J.; De Heus, R.; Franx, A.; Manning, J.H.; Hect, J.L.; Hernandez-Andrade, E.; Hassan, S.S.; Romero, R.; et al. Functional Connectome of the Fetal Brain. J. Neurosci. 2019, 39, 9716–9724. [Google Scholar] [CrossRef] [PubMed]
- De Asis-Cruz, J.; Barnett, S.D.; Kim, J.H.; Limperopoulos, C. Functional Connectivity-Derived Optimal Gestational-Age Cut Points for Fetal Brain Network Maturity. Brain Sci. 2021, 11, 921. [Google Scholar] [CrossRef]
- Abu-Rustum, R.S.; Ziade, M.F.; Abu-Rustum, S.E. Reference values for the right and left fetal choroid plexus at 11 to 13 weeks: An early sign of “developmental” laterality? J. Ultrasound Med. 2013, 32, 1623–1629. [Google Scholar] [CrossRef] [PubMed]
- Stiles, J.; Jernigan, T.L. The basics of brain development. Neuropsychol. Rev. 2010, 20, 327–348. [Google Scholar] [CrossRef] [Green Version]
- Corballis, M.C. Early signs of brain asymmetry. Trends Cogn. Sci. 2013, 17, 554–555. [Google Scholar] [CrossRef] [PubMed]
- Muntané, G.; Santpere, G.; Verendeev, A.; Seeley, W.W.; Jacobs, B.; Hopkins, W.D.; Navarro, A.; Sherwood, C.C. Interhemispheric gene expression differences in the cerebral cortex of humans and macaque monkeys. Brain Struct. Funct. 2017, 222, 3241–3254. [Google Scholar] [CrossRef]
- Bono, H.; Kasukawa, T.; Furuno, M.; Hayashizaki, Y.; Okazaki, Y. FANTOM DB: Database of Functional Annotation of RIKEN Mouse cDNA Clones. Nucleic Acids Res. 2002, 30, 116–118. [Google Scholar] [CrossRef]
- Liu, T.; Ortiz, J.A.; Taing, L.; Meyer, C.A.; Lee, B.; Zhang, Y.; Shin, H.; Wong, S.S.; Ma, J.; Lei, Y.; et al. Cistrome: An integrative platform for transcriptional regulation studies. Genome Biol. 2011, 12, R83. [Google Scholar] [CrossRef] [Green Version]
- Xin, Y.; Chanrion, B.; O’Donnell, A.H.; Milekic, M.; Costa, R.; Ge, Y.; Haghighi, F.G. MethylomeDB: A database of DNA methylation profiles of the brain. Nucleic Acids Res. 2012, 40, D1245–D1249. [Google Scholar] [CrossRef] [Green Version]
- Cho, S.Y.; Chai, J.C.; Park, S.J.; Seo, H.; Sohn, C.B.; Lee, Y.S. EPITRANS: A database that integrates epigenome and transcriptome data. Mol. Cells 2013, 36, 472–475. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, H.; Zhu, R.; Lv, J.; He, H.; Yang, L.; Huang, Z.; Su, J.; Zhang, Y.; Yu, S.; Wu, Q. DevMouse, the mouse developmental methylome database and analysis tools. Database 2014, 2014, bat084. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Medvedeva, Y.A.; Lennartsson, A.; Ehsani, R.; Kulakovskiy, I.V.; Vorontsov, I.E.; Panahandeh, P.; Khimulya, G.; Kasukawa, T.; Drabløs, F.; Consortium, F. EpiFactors: A comprehensive database of human epigenetic factors and complexes. Database 2015, 2015, bav067. [Google Scholar] [CrossRef] [PubMed]
- Pomper, N.; Liu, Y.; Hoye, M.L.; Dougherty, J.D.; Miller, T.M. CNS microRNA profiles: A database for cell type enriched microRNA expression across the mouse central nervous system. Sci. Rep. 2020, 10, 4921. [Google Scholar] [CrossRef] [Green Version]
- Hoye, M.L.; Koval, E.D.; Wegener, A.J.; Hyman, T.S.; Yang, C.; O’Brien, D.R.; Miller, R.L.; Cole, T.; Schoch, K.M.; Shen, T.; et al. MicroRNA Profiling Reveals Marker of Motor Neuron Disease in ALS Models. J. Neurosci. 2017, 37, 5574–5586. [Google Scholar] [CrossRef]
- He, M.; Liu, Y.; Wang, X.; Zhang, M.Q.; Hannon, G.J.; Huang, Z.J. Cell-type-based analysis of microRNA profiles in the mouse brain. Neuron 2012, 73, 35–48. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, P.; Williams, B.A.; Trout, D.; Marinov, G.K.; Amrhein, H.; Berghella, L.; Goh, S.T.; Plajzer-Frick, I.; Afzal, V.; Pennacchio, L.A.; et al. The changing mouse embryo transcriptome at whole tissue and single-cell resolution. Nature 2020, 583, 760–767. [Google Scholar] [CrossRef]
- Bale, T.L. Epigenetic and transgenerational reprogramming of brain development. Nat. Rev. Neurosci. 2015, 16, 332–344. [Google Scholar] [CrossRef] [PubMed]
- Kozomara, A.; Birgaoanu, M.; Griffiths-Jones, S. miRBase: From microRNA sequences to function. Nucleic Acids Res. 2019, 47, D155–D162. [Google Scholar] [CrossRef]
- Miao, N.; Lai, X.; Zeng, Z.; Cai, W.; Chen, W.; Sun, T. Differential expression of microRNAs in the human fetal left and right cerebral cortex. Mol. Biol. Rep. 2020, 47, 6573–6586. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.W.; Kim, K.T. Expression of Genes Involved in Axon Guidance: How Much Have We Learned? Int. J. Mol. Sci. 2020, 21, 3566. [Google Scholar] [CrossRef] [PubMed]
- Behuet, S.; Cremer, J.N.; Cremer, M.; Palomero-Gallagher, N.; Zilles, K.; Amunts, K. Developmental Changes of Glutamate and GABA Receptor Densities in Wistar Rats. Front. Neuroanat. 2019, 13, 100. [Google Scholar] [CrossRef] [PubMed]
- Luhmann, H.J.; Fukuda, A.; Kilb, W. Control of cortical neuronal migration by glutamate and GABA. Front. Cell Neurosci. 2015, 9, 4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yamaguchi, T.; Wang, H.L.; Li, X.; Ng, T.H.; Morales, M. Mesocorticolimbic glutamatergic pathway. J. Neurosci. 2011, 31, 8476–8490. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Petzold, A.; Stiefel, D.; Copp, A.J. Amniotic fluid brain-specific proteins are biomarkers for spinal cord injury in experimental myelomeningocele. J. Neurochem. 2005, 95, 594–598. [Google Scholar] [CrossRef] [Green Version]
- Lehtinen, M.K.; Zappaterra, M.W.; Chen, X.; Yang, Y.J.; Hill, A.D.; Lun, M.; Maynard, T.; Gonzalez, D.; Kim, S.; Ye, P.; et al. The cerebrospinal fluid provides a proliferative niche for neural progenitor cells. Neuron 2011, 69, 893–905. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chau, K.F.; Springel, M.W.; Broadbelt, K.G.; Park, H.Y.; Topal, S.; Lun, M.P.; Mullan, H.; Maynard, T.; Steen, H.; LaMantia, A.S.; et al. Progressive Differentiation and Instructive Capacities of Amniotic Fluid and Cerebrospinal Fluid Proteomes following Neural Tube Closure. Dev. Cell 2015, 35, 789–802. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, T.; Li, W.; Li, T.; Ling, S. microRNA Profiling of Amniotic Fluid: Evidence of Synergy of microRNAs in Fetal Development. PLoS ONE 2016, 11, e0153950. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hui, L.; Slonim, D.K.; Wick, H.C.; Johnson, K.L.; Bianchi, D.W. The amniotic fluid transcriptome: A source of novel information about human fetal development. Obstet. Gynecol. 2012, 119, 111–118. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tarca, A.L.; Romero, R.; Pique-Regi, R.; Pacora, P.; Done, B.; Kacerovsky, M.; Bhatti, G.; Jaiman, S.; Hassan, S.S.; Hsu, C.D.; et al. Amniotic fluid cell-free transcriptome: A glimpse into fetal development and placental cellular dynamics during normal pregnancy. BMC Med. Genom. 2020, 13, 25. [Google Scholar] [CrossRef] [Green Version]
- Vizitiu, A.C.; Stambouli, D.; Pavel, A.G.; Muresan, M.C.; Anastasiu, D.M.; Bejinar, C.; Alexa, A.; Marian, C.; Sirbu, I.O.; Sima, L. Mature miR-99a Upregulation in the Amniotic Fluid Samples from Female Fetus Down Syndrome Pregnancies: A Pilot Study. Medicina 2019, 55, 728. [Google Scholar] [CrossRef] [Green Version]
- Buczyńska, A.; Sidorkiewicz, I.; Trochimiuk, A.; Ławicki, S.; Krętowski, A.J.; Zbucka-Krętowska, M. Novel Approaches to an Integrated Route for Trisomy 21 Evaluation. Biomolecules 2021, 11, 1328. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.H.; Park, H.J.; Jung, Y.W.; Shim, S.H.; Sung, S.R.; Park, J.E.; Cha, D.H.; Ahn, E.H. Comparative Transcriptome Analysis of Cell-Free Fetal RNA from Amniotic Fluid and RNA from Amniocytes in Uncomplicated Pregnancies. PLoS ONE 2015, 10, e0132955. [Google Scholar] [CrossRef] [PubMed]
- Magnin, E. Neurodevelopmental and Neurodegenerative Similarities and Interactions: A Point of View About Lifelong Neurocognitive Trajectories. J. Alzheimer’s Dis. 2021, 79, 1397–1407. [Google Scholar] [CrossRef] [PubMed]
- Lewis, E.M.; Kroll, K.L. Development and disease in a dish: The epigenetics of neurodevelopmental disorders. Epigenomics 2018, 10, 219–231. [Google Scholar] [CrossRef]
- Loke, Y.J.; Hannan, A.J.; Craig, J.M. The Role of Epigenetic Change in Autism Spectrum Disorders. Front. Neurol. 2015, 6, 107. [Google Scholar] [CrossRef] [Green Version]
- Li, M.; Santpere, G.; Imamura Kawasawa, Y.; Evgrafov, O.V.; Gulden, F.O.; Pochareddy, S.; Sunkin, S.M.; Li, Z.; Shin, Y.; Zhu, Y.; et al. Integrative functional genomic analysis of human brain development and neuropsychiatric risks. Science 2018, 362. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, Y.; Sousa, A.M.M.; Gao, T.; Skarica, M.; Li, M.; Santpere, G.; Esteller-Cucala, P.; Juan, D.; Ferrández-Peral, L.; Gulden, F.O.; et al. Spatiotemporal transcriptomic divergence across human and macaque brain development. Science 2018, 362. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Posey, J.E.; Rosenfeld, J.A.; James, R.A.; Bainbridge, M.; Niu, Z.; Wang, X.; Dhar, S.; Wiszniewski, W.; Akdemir, Z.H.; Gambin, T.; et al. Molecular diagnostic experience of whole-exome sequencing in adult patients. Genet. Med. 2016, 18, 678–685. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, H.; Duyzend, M.H.; Coe, B.P.; Baker, C.; Hoekzema, K.; Gerdts, J.; Turner, T.N.; Zody, M.C.; Beighley, J.S.; Murali, S.C.; et al. Genome sequencing identifies multiple deleterious variants in autism patients with more severe phenotypes. Genet. Med. 2019, 21, 1611–1620. [Google Scholar] [CrossRef] [PubMed]
- Won, H.; De la Torre-Ubieta, L.; Stein, J.L.; Parikshak, N.N.; Huang, J.; Opland, C.K.; Gandal, M.J.; Sutton, G.J.; Hormozdiari, F.; Lu, D.; et al. Chromosome conformation elucidates regulatory relationships in developing human brain. Nature 2016, 538, 523–527. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lamb, A.N.; Rosenfeld, J.A.; Neill, N.J.; Talkowski, M.E.; Blumenthal, I.; Girirajan, S.; Keelean-Fuller, D.; Fan, Z.; Pouncey, J.; Stevens, C.; et al. Haploinsufficiency of SOX5 at 12p12.1 is associated with developmental delays with prominent language delay, behavior problems, and mild dysmorphic features. Hum. Mutat. 2012, 33, 728–740. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tolchin, D.; Yeager, J.P.; Prasad, P.; Dorrani, N.; Russi, A.S.; Martinez-Agosto, J.A.; Haseeb, A.; Angelozzi, M.; Santen, G.W.E.; Ruivenkamp, C.; et al. De Novo SOX6 Variants Cause a Neurodevelopmental Syndrome Associated with ADHD, Craniosynostosis, and Osteochondromas. Am. J. Hum. Genet. 2020, 106, 830–845. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, S.; Desai, S.; Cohen, J.; Smith-Hicks, C.; Barañano, K.; Fatemi, A.; Naidu, S. Monogenic disorders that mimic the phenotype of Rett syndrome. Neurogenetics 2018, 19, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Turro, E.; Astle, W.J.; Megy, K.; Gräf, S.; Greene, D.; Shamardina, O.; Allen, H.L.; Sanchis-Juan, A.; Frontini, M.; Thys, C.; et al. Whole-genome sequencing of patients with rare diseases in a national health system. Nature 2020, 583, 96–102. [Google Scholar] [CrossRef] [PubMed]
- Forrest, M.; Chapman, R.M.; Doyle, A.M.; Tinsley, C.L.; Waite, A.; Blake, D.J. Functional analysis of TCF4 missense mutations that cause Pitt-Hopkins syndrome. Hum. Mutat. 2012, 33, 1676–1686. [Google Scholar] [CrossRef] [PubMed]
- Seltzer, L.E.; Ma, M.; Ahmed, S.; Bertrand, M.; Dobyns, W.B.; Wheless, J.; Paciorkowski, A.R. Epilepsy and outcome in FOXG1-related disorders. Epilepsia 2014, 55, 1292–1300. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mariani, J.; Coppola, G.; Zhang, P.; Abyzov, A.; Provini, L.; Tomasini, L.; Amenduni, M.; Szekely, A.; Palejev, D.; Wilson, M.; et al. FOXG1-Dependent Dysregulation of GABA/Glutamate Neuron Differentiation in Autism Spectrum Disorders. Cell 2015, 162, 375–390. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lam, M.; Hill, W.D.; Trampush, J.W.; Yu, J.; Knowles, E.; Davies, G.; Stahl, E.; Huckins, L.; Liewald, D.C.; Djurovic, S.; et al. Pleiotropic Meta-Analysis of Cognition, Education, and Schizophrenia Differentiates Roles of Early Neurodevelopmental and Adult Synaptic Pathways. Am. J. Hum. Genet. 2019, 105, 334–350. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sollis, E.; Deriziotis, P.; Saitsu, H.; Miyake, N.; Matsumoto, N.; Hoffer, M.J.V.; Ruivenkamp, C.A.L.; Alders, M.; Okamoto, N.; Bijlsma, E.K.; et al. Equivalent missense variant in the FOXP2 and FOXP1 transcription factors causes distinct neurodevelopmental disorders. Hum. Mutat. 2017, 38, 1542–1554. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trelles, M.P.; Levy, T.; Lerman, B.; Siper, P.; Lozano, R.; Halpern, D.; Walker, H.; Zweifach, J.; Frank, Y.; Foss-Feig, J.; et al. Individuals with FOXP1 syndrome present with a complex neurobehavioral profile with high rates of ADHD, anxiety, repetitive behaviors, and sensory symptoms. Mol. Autism 2021, 12, 61. [Google Scholar] [CrossRef]
- Chatron, N.; Møller, R.S.; Champaigne, N.L.; Schneider, A.L.; Kuechler, A.; Labalme, A.; Simonet, T.; Baggett, L.; Bardel, C.; Kamsteeg, E.J.; et al. The epilepsy phenotypic spectrum associated with a recurrent CUX2 variant. Ann. Neurol. 2018, 83, 926–934. [Google Scholar] [CrossRef] [PubMed]
- Magno, L.; Asgarian, Z.; Pendolino, V.; Velona, T.; Mackintosh, A.; Lee, F.; Stryjewska, A.; Zimmer, C.; Guillemot, F.; Farrant, M.; et al. Transient developmental imbalance of cortical interneuron subtypes presages long-term changes in behavior. Cell Rep. 2021, 35, 109249. [Google Scholar] [CrossRef]
- Bruel, A.L.; Nambot, S.; Quéré, V.; Vitobello, A.; Thevenon, J.; Assoum, M.; Moutton, S.; Houcinat, N.; Lehalle, D.; Jean-Marçais, N.; et al. Increased diagnostic and new genes identification outcome using research reanalysis of singleton exome sequencing. Eur. J. Hum. Genet. 2019, 27, 1519–1531. [Google Scholar] [CrossRef] [PubMed]
- Den Hoed, J.; Sollis, E.; Venselaar, H.; Estruch, S.B.; Deriziotis, P.; Fisher, S.E. Functional characterization of TBR1 variants in neurodevelopmental disorder. Sci. Rep. 2018, 8, 14279. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Kovel, C.G.F.; Lisgo, S.N.; Francks, C. Transcriptomic analysis of left-right differences in human embryonic forebrain and midbrain. Sci. Data 2018, 5, 180164. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ribolsi, M.; Daskalakis, Z.J.; Siracusano, A.; Koch, G. Abnormal asymmetry of brain connectivity in schizophrenia. Front. Hum. Neurosci. 2014, 8, 1010. [Google Scholar] [CrossRef] [Green Version]
- Floris, D.L.; Barber, A.D.; Nebel, M.B.; Martinelli, M.; Lai, M.C.; Crocetti, D.; Baron-Cohen, S.; Suckling, J.; Pekar, J.J.; Mostofsky, S.H. Atypical lateralization of motor circuit functional connectivity in children with autism is associated with motor deficits. Mol. Autism 2016, 7, 35. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abdolmaleky, H.M.; Gower, A.C.; Wong, C.K.; Cox, J.W.; Zhang, X.; Thiagalingam, A.; Shafa, R.; Sivaraman, V.; Zhou, J.R.; Thiagalingam, S. Aberrant transcriptomes and DNA methylomes define pathways that drive pathogenesis and loss of brain laterality/asymmetry in schizophrenia and bipolar disorder. Am. J. Med. Genet. B Neuropsychiatr. Genet 2019, 180, 138–149. [Google Scholar] [CrossRef]


| Pipelines | Organism | Registry | Reference |
|---|---|---|---|
| FANTOM | RIKE Mice | cDNA clone functional annotation | FANTOM3 cDNA Annotation (2 October 2021); (http://fantom.gsc.riken.go.jp/db [43] |
| CISTROME | Humans Mus musculus | Transcription factors functional annotation or histone modification | Chromatin Remodeling data (CR Cistrome) (2 October 2021); (http://cistrome.org) accessed in 2 october 2021 [44] |
| METHYLOME | Human Mouse brain | DNA methylation | MethDB –brain methilation data (4 October 2021) (http://epigenomics.columbia.edu/methylomedb); [45] |
| EPITRANS | Humans | DNA methylation and histone modification | Human brain NGS data (4 October 2021) (http://epitrans.org); [46] |
| DEVMOUSE | Mice | Developmental methylome | Brain methylome (4 October 2021) (http://www.devmouse.org); [47] |
| EpiFactors | Humans | DNA and histone modification | Fetal brain expression data (4 October 2021) (http://epifactors.autosome.ru); [48] |
| miRNA | Mice | Central nervous system miRNAs | miRNA (miRNA.wustl.edu); [49,50,51] |
| SCREEN | Humans Mice | cis-regulatory elements (cCREs) | Registry of cCREs (4 October 2021) (https://screen.encodeproject.org); [52] |
| Genes (Function of Encoded Protein) | Genomic Locus (Variant Type/Subtype) | Process/Signaling Pathway | Diseases Associated Phenotype | Ref. |
|---|---|---|---|---|
| MEF2C (transcription activator) | chr5:88,717,117-88,904,257 CNV/deletion | Normal neuronal development, neurogenesis and in the cortical development Developmental biology pathway | Mental retardation, stereotypic movements, epilepsy, and/or cerebral malformations | [72,74,75] |
| NR4A2 (protein coding/transcription factor) | chr2:156,324,432-156,342,348 | Neuronal differentiation and self-renewal of dopaminergic neurons during development Nuclear receptor transcription pathway (NRT pathway) | NDS, dopaminergic dysfunction, including Parkinson disease, schizophrenia, and maniac depression | [72,75] |
| CNV/mutation | ||||
| SOX2 (transcription factor) | chr3:181,711,925-181,714,436 | Embryonic development; embryonic stem cell pluripotency; neural cell fate; CNS stem-cell self- renewal Wnt signaling pathway | Anophthalmia/microphthalmia-esophageal atresia syndrome; schizophrenia | [76] |
| CNV/mutation/deletion | ||||
| SOX5 (transcription factor) | chr12:23,682,438-24,715,425 CNV/mutation | Embryonic development and the neuronal cell fate (neuronal and glial differentiation). Wnt signaling pathway | Lamb-Shaffer syndrome; schizophrenia; neuroticism | [72,77] |
| SOX6 (transcription factor) | chr11:15,966,449-16,739,591 | Normal development of the central nervous system | NDS; Tolchin-Le Caignec syndrome | [78] |
| CNV/mutation | Wnt signaling pathway | |||
| TCF4 (transcription factor) | chr18:55,222,185-55,664,787 CNV/deletion | Nervous system development Wnt signaling pathway | Pitt-Hopkins syndrome; schizophrenia; MDD | [72,79,80,81] |
| FOXG1 (transcriptional repressor) | chr14:28,766,787-28,770,277 CNV/deletion | Brain development TGF signaling pathway; FGF signaling pathway | Rett syndrome, congenital variant; FOXG1 syndrome; ASD; schizophrenia | [76,82,83] |
| EMX1 (transcription factor) | chr2:72,910,949-72,936,691 CNV/deletion | Embryonic development; brain development; neuronal cell fate | Schizophrenia, schizoaffective disorders | [76,84] |
| FOXP1 (transcriptional repressor) | chr3:70,954,708-71,583,989 CNV/deletion | Brain development; pluripotent stem cell regulation WNT/Hedgehog/Notch signaling pathway | Foxp1 syndrome | [85,86] |
| CUX2 (transcription factor) | chr12:111,034,165-111,350,556 CNV/deletion/mutation | Brain development; neuronal cells proliferation and differentiation | Developmental and epileptic encephalopathy | [87,88] |
| TBR1 (transcriptional repressor) | chr2:162,272,808-162,282,381 CNV/deletion/mutation | Brain development | intellectual disability; ASD, language deficits; cortical malformation | [89,90] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cara, M.L.; Streata, I.; Buga, A.M.; Iliescu, D.G. Developmental Brain Asymmetry. The Good and the Bad Sides. Symmetry 2022, 14, 128. https://doi.org/10.3390/sym14010128
Cara ML, Streata I, Buga AM, Iliescu DG. Developmental Brain Asymmetry. The Good and the Bad Sides. Symmetry. 2022; 14(1):128. https://doi.org/10.3390/sym14010128
Chicago/Turabian StyleCara, Monica Laura, Ioana Streata, Ana Maria Buga, and Dominic Gabriel Iliescu. 2022. "Developmental Brain Asymmetry. The Good and the Bad Sides" Symmetry 14, no. 1: 128. https://doi.org/10.3390/sym14010128
APA StyleCara, M. L., Streata, I., Buga, A. M., & Iliescu, D. G. (2022). Developmental Brain Asymmetry. The Good and the Bad Sides. Symmetry, 14(1), 128. https://doi.org/10.3390/sym14010128








