A Study of the Optical and Structural Properties of SnO2 Nanoparticles Synthesized with Tilia cordata Applied in Methylene Blue Degradation
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Tilia Cordata Extract Preparation
2.3. Green Synthesis of SnO2 NPs
2.4. Characterization Techniques of the NPs
2.5. Photocatalytic Activity
3. Results and Discussion
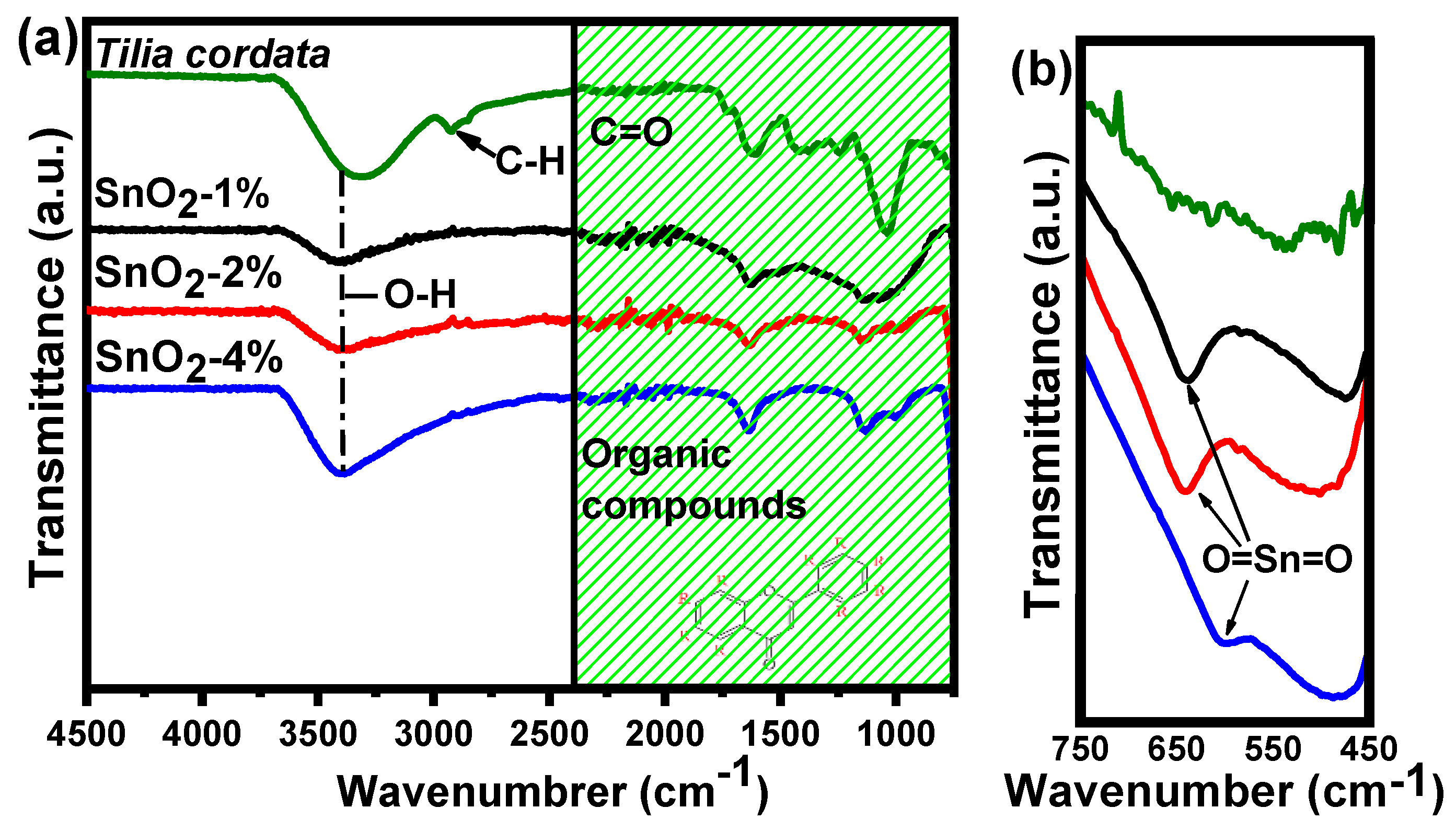
3.1. Fourier Transform Infrared Spectroscopy Analysis
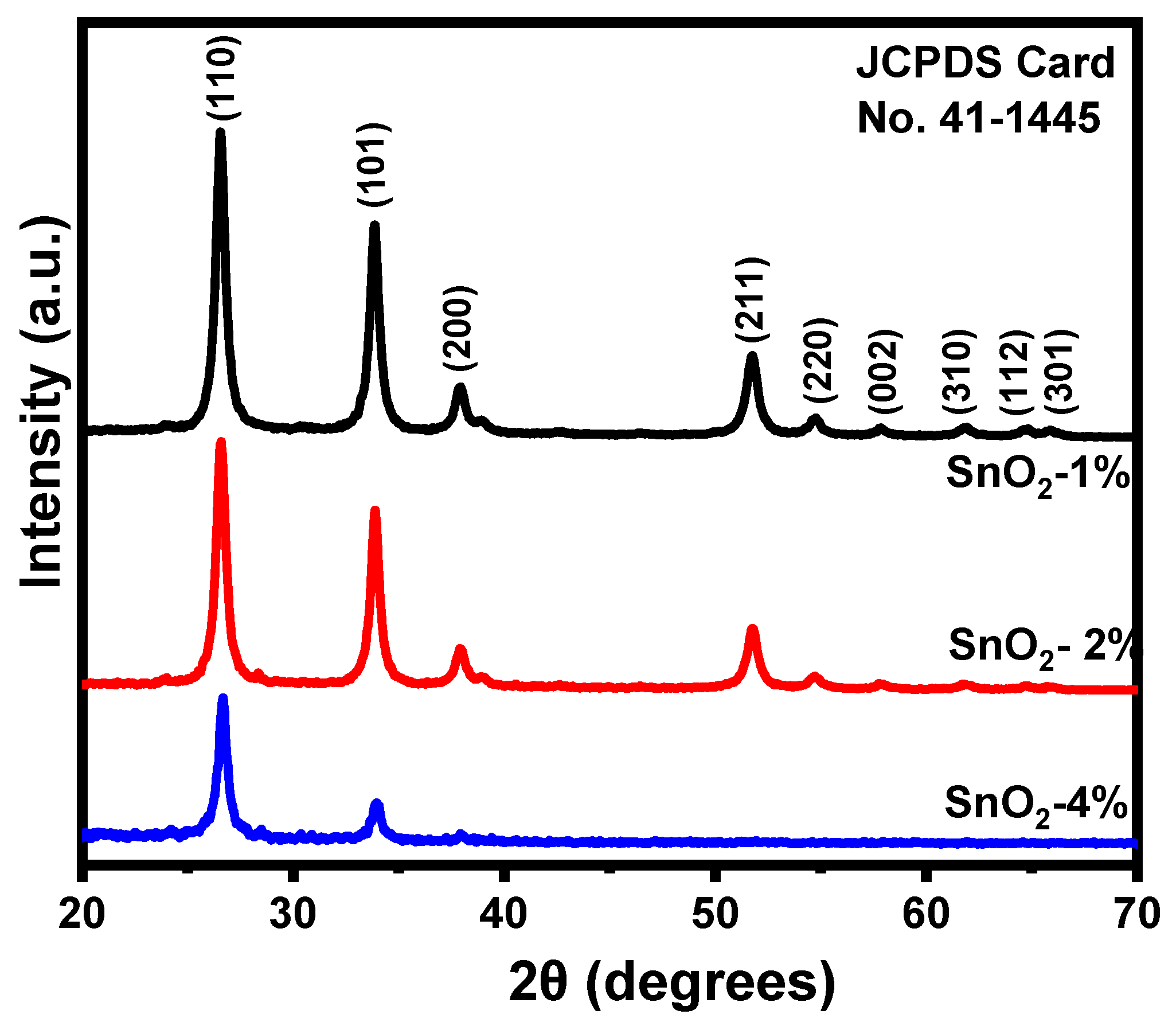
3.2. X-ray Diffraction
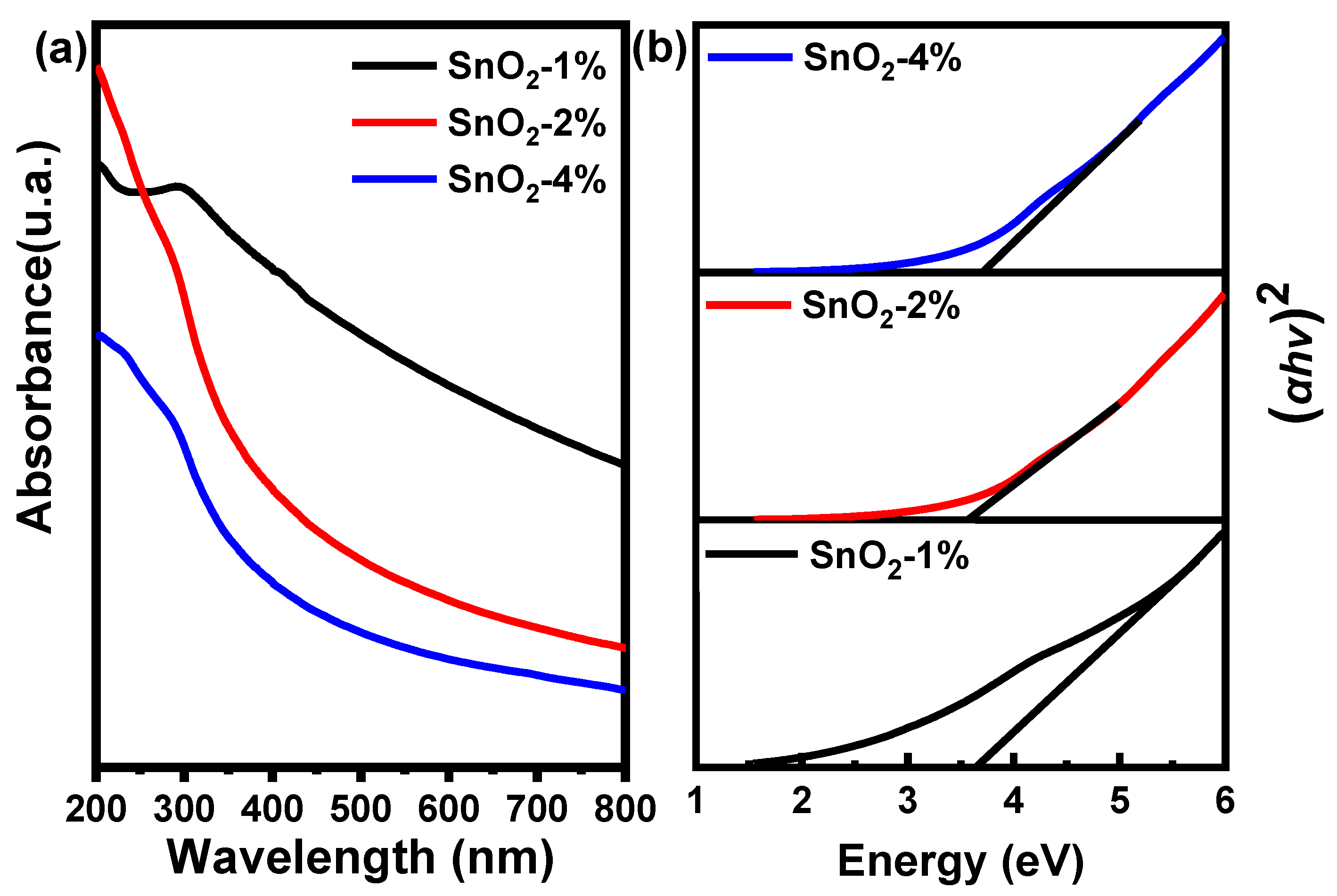
3.3. Optical Properties
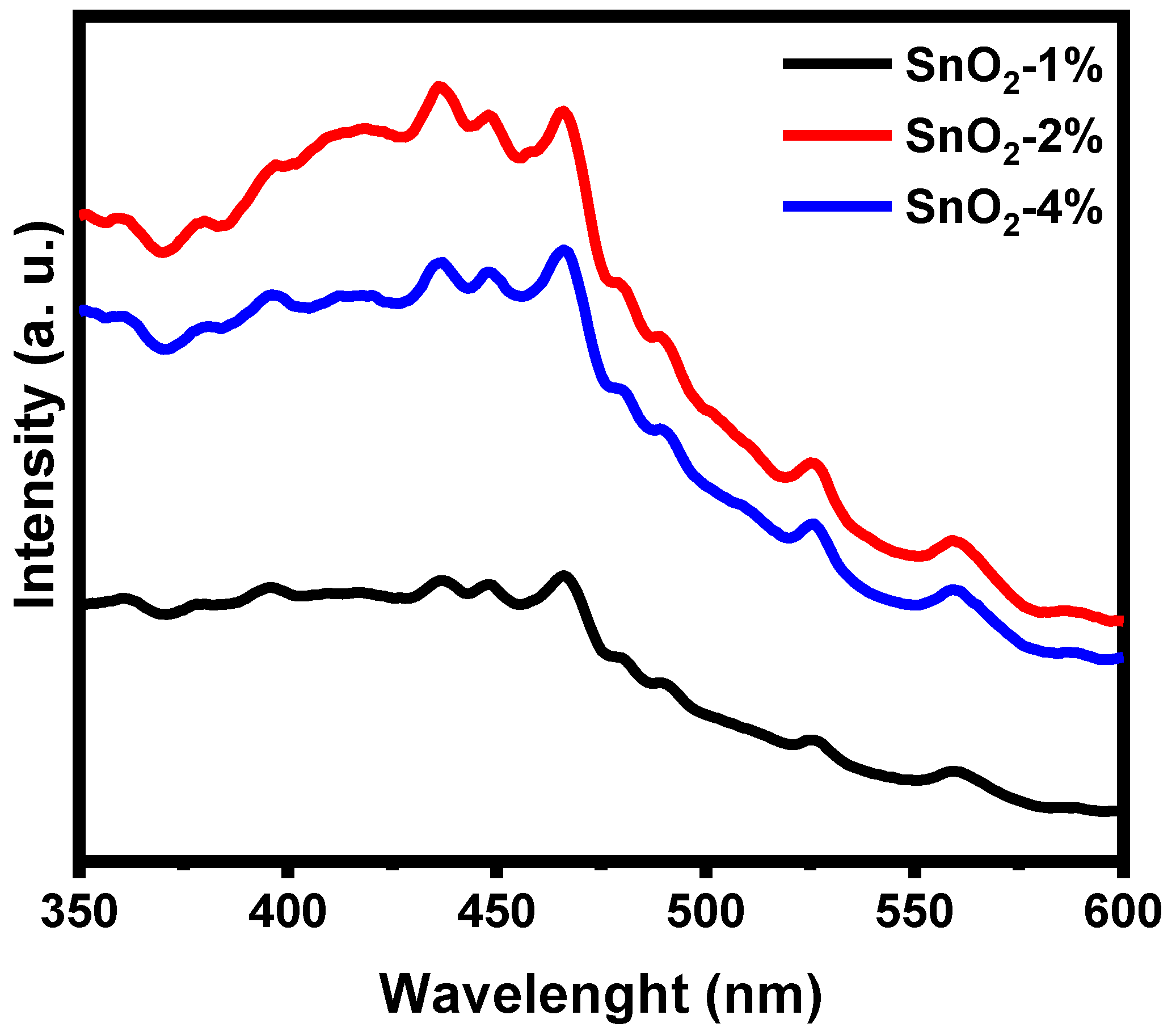
3.4. Photoluminescence
3.5. Surface Morphology
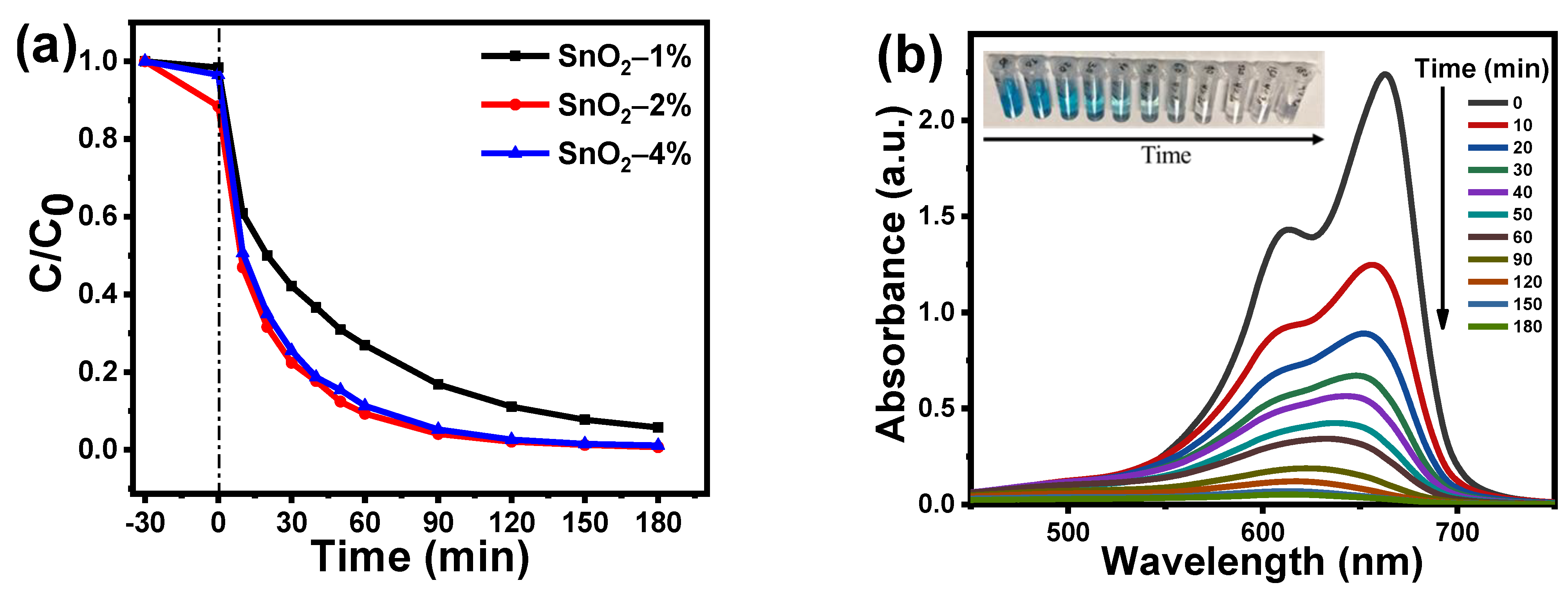
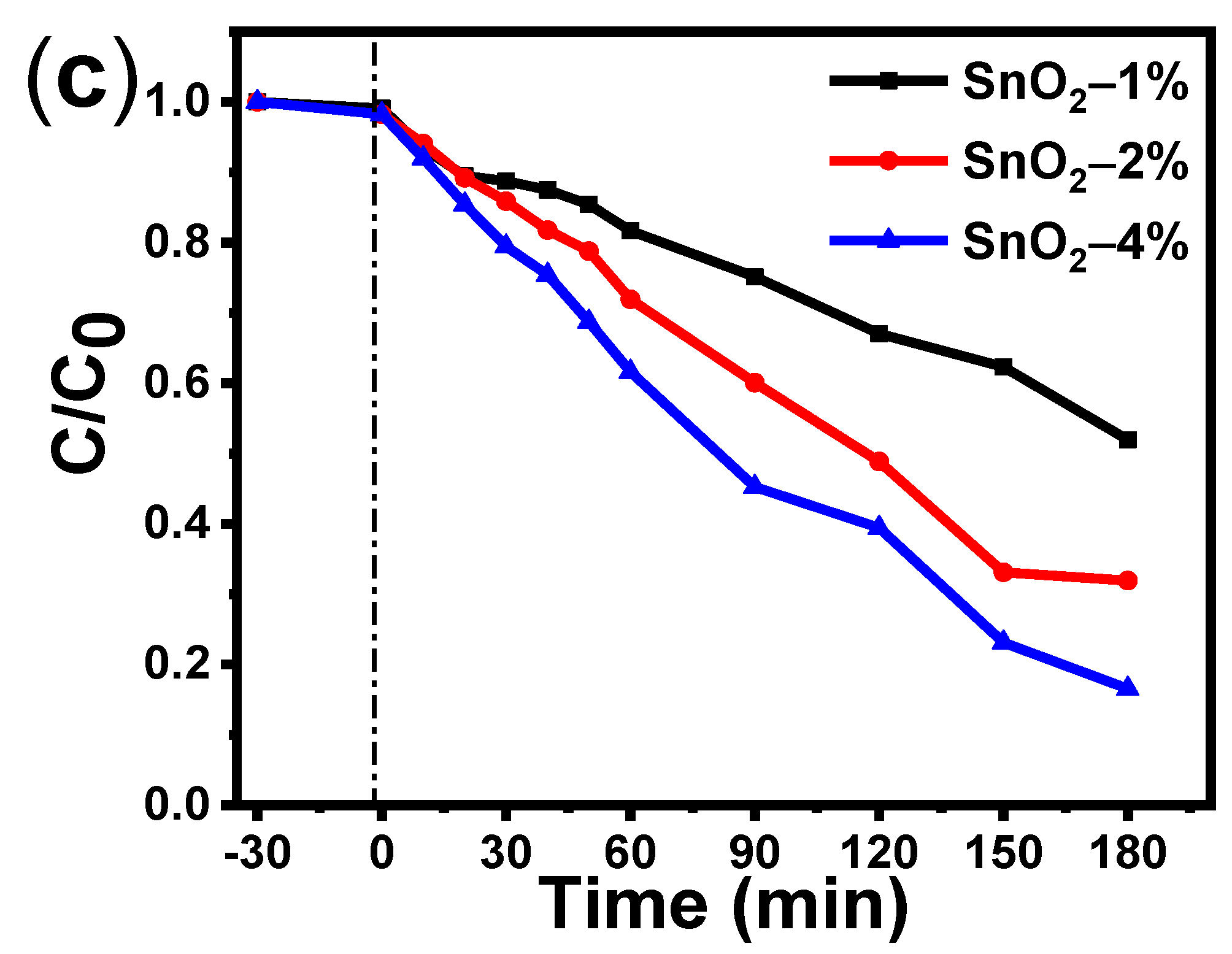
3.6. Photocatalytic Activiy
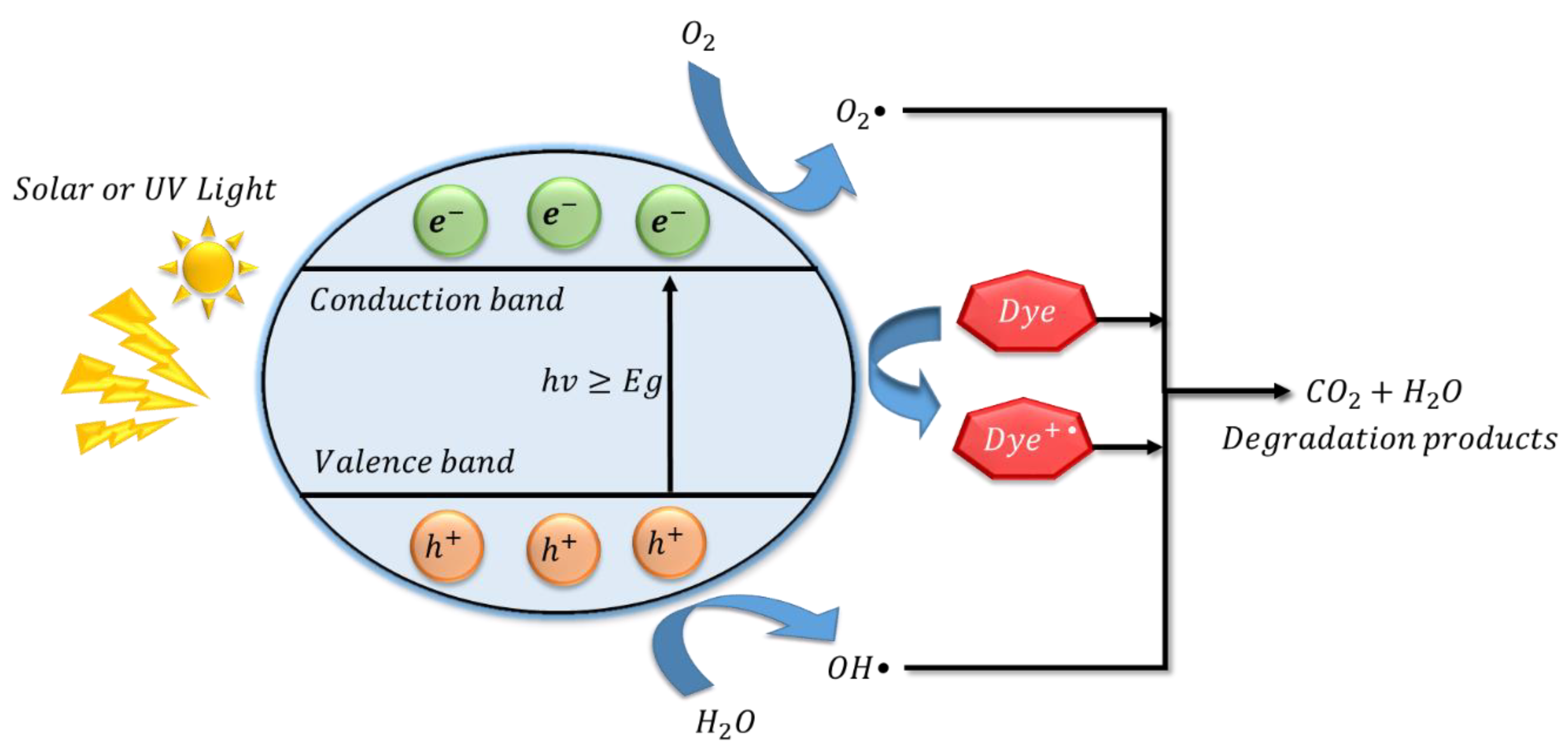
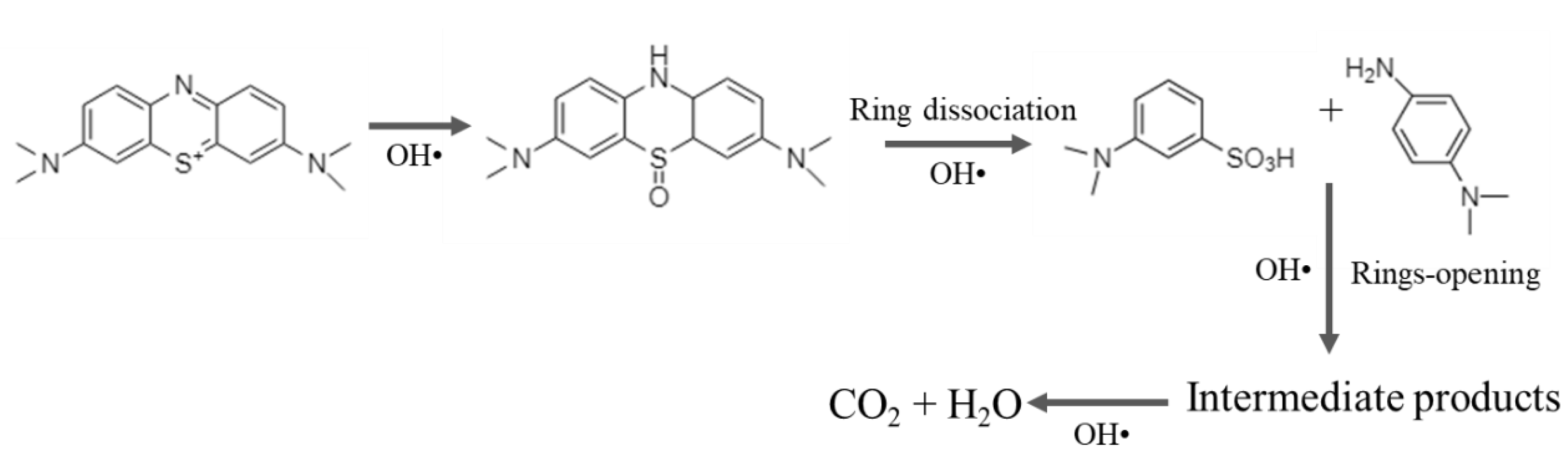
3.7. Degradation Mechanism
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Danish, M.S.S.; Estrella, L.L.; Alemaida, I.M.A.; Lisin, A.; Moiseev, N.; Ahmadi, M.; Nazari, M.; Wali, M.; Zaheb, H.; Senjyu, T. Photocatalytic Applications of Metal Oxides for Sustainable Environmental Remediation. Metals 2021, 11, 80. [Google Scholar] [CrossRef]
- Routoula, E.; Patwardhan, S. v Degradation of Anthraquinone Dyes from Effluents: A Review Focusing on Enzymatic Dye Degradation with Industrial Potential. Environ. Sci. Technol. 2020, 54, 647–664. [Google Scholar] [CrossRef] [PubMed]
- Al-Tohamy, R.; Ali, S.S.; Li, F.; Okasha, K.M.; Mahmoud, Y.A.-G.; Elsamahy, T.; Jiao, H.; Fu, Y.; Sun, J. A critical review on the treatment of dye-containing wastewater: Ecotoxicological and health concerns of textile dyes and possible remediation approaches for environmental safety. Ecotoxicol. Environ. Saf. 2022, 231, 113160. [Google Scholar] [CrossRef] [PubMed]
- Topaç, F.O.; Dindar, E.; Uçaroğlu, S.; Başkaya, H.S. Effect of a sulfonated azo dye and sulfanilic acid on nitrogen transformation processes in soil. J. Hazard. Mater. 2009, 170, 1006–1013. [Google Scholar] [CrossRef] [PubMed]
- Schneider, K.; Hafner, C.; Jäger, I. Mutagenicity of Textile Dye Products. J. Appl. Toxicol. Int. J. 2004, 24, 83–91. [Google Scholar] [CrossRef] [PubMed]
- Arshad, H.; Imran, M.; Ashraf, M. Toxic effects of Red-S3B dye on soil microbial activities, wheat yield, and their alleviation by pressmud application. Ecotoxicol. Environ. Saf. 2020, 204, 111030. [Google Scholar] [CrossRef]
- Kishor, R.; Bharagava, R.N.; Saxena, G. Industrial Wastewaters: The Major Sources of Dye Contamination in the Environment, Ecotoxicological Effects, and Bioremediation Approaches. In Recent Advances in Environmental Management; CRC Press: Boca Raton, FL, USA, 2018; pp. 1–25. ISBN 1351011251. [Google Scholar]
- Zhou, Y.; Lu, J.; Zhou, Y.; Liu, Y. Recent Advances for Dyes Removal Using Novel Adsorbents: A Review. Environ. Pollut. 2019, 252, 352–365. [Google Scholar] [CrossRef]
- Dotto, J.; Fagundes-Klen, M.R.; Veit, M.T.; Palácio, S.M.; Bergamasco, R. Performance of different coagulants in the coagulation/flocculation process of textile wastewater. J. Clean. Prod. 2018, 208, 656–665. [Google Scholar] [CrossRef]
- Le, T.M.H.; Nuisin, R.; Mongkolnavin, R.; Painmanakul, P.; Sairiam, S. Enhancing dye wastewater treatment efficiency in ozonation membrane contactors by chloro- and fluoro-organosilanes’ functionality on hydrophobic PVDF membrane modification. Sep. Purif. Technol. 2022, 288, 120711. [Google Scholar] [CrossRef]
- Shoukat, R.; Khan, S.J.; Jamal, Y. Hybrid anaerobic-aerobic biological treatment for real textile wastewater. J. Water Process Eng. 2019, 29, 100804. [Google Scholar] [CrossRef]
- Fujishima, A.; Honda, K. Electrochemical Photolysis of Water at a Semiconductor Electrode. Nature 1972, 238, 37–38. [Google Scholar] [CrossRef] [PubMed]
- Saravanan, A.; Kumar, P.S.; Vo, D.-V.N.; Yaashikaa, P.R.; Karishma, S.; Jeevanantham, S.; Gayathri, B.; Bharathi, V.D. Photocatalysis for removal of environmental pollutants and fuel production: A review. Environ. Chem. Lett. 2020, 19, 441–463. [Google Scholar] [CrossRef]
- Malato, S.; Maldonado, I.M.; Fernández-Ibáñez, P.; Oller, I.; Polo, I.; Sánchez-Moreno, R. Decontamination and Disinfection of Water by Solar Photocatalysis: The Pilot Plants of the Plataforma Solar de Almeria. Mater Sci. Semicond. Process 2016, 42 Pt 1, 15–23. [Google Scholar] [CrossRef]
- Enesca, A. The Influence of Photocatalytic Reactors Design and Operating Parameters on the Wastewater Organic Pollutants Removal—A Mini-Review. Catalysts 2021, 11, 556. [Google Scholar] [CrossRef]
- Youssef, Z.; Colombeau, L.; Yesmurzayeva, N.; Baros, F.; Vanderesse, R.; Hamieh, T.; Toufaily, J.; Frochot, C.; Roques-Carmes, T.; Acherar, S. Dye-sensitized nanoparticles for heterogeneous photocatalysis: Cases studies with TiO2, ZnO, fullerene and graphene for water purification. Dye. Pigment. 2018, 159, 49–71. [Google Scholar] [CrossRef]
- El-Belely, E.F.; Farag, M.M.S.; Said, H.A.; Amin, A.S.; Azab, E.; Gobouri, A.A.; Fouda, A. Green Synthesis of Zinc Oxide Nanoparticles (ZnO-NPs) Using Arthrospira platensis (Class: Cyanophyceae) and Evaluation of their Biomedical Activities. Nanomaterials 2021, 11, 95. [Google Scholar] [CrossRef] [PubMed]
- Yousefi, S.R.; Alshamsi, H.A.; Amiri, O.; Salavati-Niasari, M. Synthesis, characterization and application of Co/Co3O4 nanocomposites as an effective photocatalyst for discoloration of organic dye contaminants in wastewater and antibacterial properties. J. Mol. Liq. 2021, 337, 116405. [Google Scholar] [CrossRef]
- Osman, N.S.; Sulaiman, S.N.; Muhamad, E.N.; Mukhair, H.; Tan, S.T.; Abdullah, A.H. Synthesis of an Ag3PO4/Nb2O5 Photocatalyst for the Degradation of Dye. Catalysts 2021, 11, 458. [Google Scholar] [CrossRef]
- Mallikarjunaswamy, C.; Pramila, S.; Nagaraju, G.; Ramu, R.; Ranganatha, V.L. Green Synthesis and Evaluation of Antiangiogenic, Photocatalytic, and Electrochemical Activities of BiVO4 Nanoparticles. J. Mater. Sci. Mater. Electron. 2021, 32, 14028–14046. [Google Scholar] [CrossRef]
- Mahdi, M.A.; Yousefi, S.R.; Jasim, L.S.; Salavati-Niasari, M. Green Synthesis of DyBa2Fe3O7.988/DyFeO3 Nanocomposites Using Almond Extract with Dual Eco-Friendly Applications: Photocatalytic and Antibacterial Activities. Int. J. Hydrogen Energy 2022, 47, 14319–14330. [Google Scholar] [CrossRef]
- Buniyamin, I.; Akhir, R.; Asli, N.A.; Khusaimi, Z.; Mahmood, M.R. Biosynthesis of SnO2 nanoparticles by aqueous leaves extract of Aquilaria malaccensis (agarwood). IOP Conf. Ser. Mater. Sci. Eng. 2021, 1092, 012070. [Google Scholar] [CrossRef]
- Maruthupandy, M.; Muneeswaran, T.; Chackaravarthi, G.; Vennila, T.; Anand, M.; Cho, W.-S.; Quero, F. Synthesis of chitosan/SnO2 nanocomposites by chemical precipitation for enhanced visible light photocatalytic degradation efficiency of congo red and rhodamine-B dye molecules. J. Photochem. Photobiol. A Chem. 2022, 430, 113972. [Google Scholar] [CrossRef]
- Gomathi, E.; Jayapriya, M.; Arulmozhi, M. Environmental benign synthesis of tin oxide (SnO2) nanoparticles using Actinidia deliciosa (Kiwi) peel extract with enhanced catalytic properties. Inorg. Chem. Commun. 2021, 130, 108670. [Google Scholar] [CrossRef]
- Luque, P.; Chinchillas-Chinchillas, M.; Nava, O.; Lugo-Medina, E.; Martínez-Rosas, M.; Carrillo-Castillo, A.; Vilchis-Nestor, A.; Madrigal-Muñoz, L.; Garrafa-Gálvez, H. Green synthesis of tin dioxide nanoparticles using Camellia sinensis and its application in photocatalytic degradation of textile dyes. Optik 2021, 229, 166259. [Google Scholar] [CrossRef]
- Garrafa-Galvez, H.; Nava, O.; Soto-Robles, C.; Vilchis-Nestor, A.; Castro-Beltrán, A.; Luque, P. Green synthesis of SnO2 nanoparticle using Lycopersicon esculentum peel extract. J. Mol. Struct. 2019, 1197, 354–360. [Google Scholar] [CrossRef]
- Sagadevan, S.; Lett, J.A.; Fatimah, I.; Lokanathan, Y.; Léonard, E.; Oh, W.C.; Hossain, M.A.M.; Johan, M.R. Current Trends in the Green Syntheses of Tin Oxide Nanoparticles and Their Biomedical Applications. Mater. Res. Express 2021, 8, 082001. [Google Scholar] [CrossRef]
- Yadav, A.; Kon, K.; Kratošová, G.; Durán, N.; Ingle, A.P.; Rai, M. Fungi as an efficient mycosystem for the synthesis of metal nanoparticles: Progress and key aspects of research. Biotechnol. Lett. 2015, 37, 2099–2120. [Google Scholar] [CrossRef]
- Al-Enazi, N.M.; Alwakeel, S.; Alhomaidi, E. Photocatalytic and biological activities of green synthesized SnO2 nanoparticles using Chlorella vulgaris. J. Appl. Microbiol. 2022. [Google Scholar] [CrossRef]
- Gour, A.; Jain, N.K. Advances in green synthesis of nanoparticles. Artif. Cells Nanomed. Biotechnol. 2019, 47, 844–851. [Google Scholar] [CrossRef]
- Suresh, K.C.; Surendhiran, S.; Kumar, P.M.; Kumar, E.R.; Khadar, Y.A.S.; Balamurugan, A. Green synthesis of SnO2 nanoparticles using Delonix elata leaf extract: Evaluation of its structural, optical, morphological and photocatalytic properties. SN Appl. Sci. 2020, 2, 1735. [Google Scholar] [CrossRef]
- Karthik, K.; Revathi, V.; Tatarchuk, T. Microwave-assisted green synthesis of SnO2 nanoparticles and their optical and photocatalytic properties. Mol. Cryst. Liq. Cryst. 2018, 671, 17–23. [Google Scholar] [CrossRef]
- Rathinabala, R.; Thamizselvi, R.; Padmanabhan, D.; Alshahateet, S.F.; Fatimah, I.; Sibhatu, A.K.; Weldegebrieal, G.K.; Razak, S.I.A.; Sagadevan, S. Sun light-assisted enhanced photocatalytic activity and cytotoxicity of green synthesized SnO2 nanoparticles. Inorg. Chem. Commun. 2022, 143, 109783. [Google Scholar] [CrossRef]
- D’Ambrosio, U. Tilia Platyphyllos y Tilia Cordata; Pardo De Santayana, M., Morales, R., Tardío, J., Molina, M., Eds.; Inventario; Ministerio De Agricultura y Pesca, Alimentación y Medio Ambiente: Madrid, Spain, 2018. [Google Scholar]
- Cittan, M.; Altuntaş, E.; Çelik, A. Evaluation of Antioxidant Capacities and Phenolic Profiles in Tilia cordata Fruit Extracts: A Comparative Study to Determine the Efficiency of Traditional Hot Water Infusion Method. Ind. Crops Prod. 2018, 122, 553–558. [Google Scholar] [CrossRef]
- Saygi, K.O.; Cacan, E. Antioxidant and cytotoxic activities of silver nanoparticles synthesized using Tilia cordata flowers extract. Mater. Today Commun. 2021, 27, 102316. [Google Scholar] [CrossRef]
- Beevi, A.F.; Sreekala, G.; Beena, B. Synthesis, characterization and photocatalytic activity of SnO2, ZnO nanoparticles against congo red: A comparative study. Mater. Today Proc. 2021, 45, 4045–4051. [Google Scholar] [CrossRef]
- Van, K.N.; Huu, H.T.; Thi, V.N.N.; le Thi, T.L.; Truong, D.H.; Truong, T.T.; Dao, N.N.; Vo, V.; Vasseghian, Y. Facile Construction of S-Scheme SnO2/g-C3N4 Photocatalyst for Improved Photoactivity. Chemosphere 2022, 289, 133120. [Google Scholar] [CrossRef]
- Mahakal, M.; Kutumbale, A.; Mehta, D.; Mehta, B.K. Green synthesis of zno nanoparticles from the methanolic extract of embelia ribes and their characterization by uv-visible, ftir, xrd and sem analysis. Int. J. Pharm. Biol. Sci. 2018, 8, 402–409. [Google Scholar]
- Ramesh, P.; Saravanana, K.; Manogar, P.; Johnson, J.; Vinoth, E.; Mayakannan, M. Green synthesis and characterization of biocompatible zinc oxide nanoparticles and evaluation of its antibacterial potential. Sens. Bio-Sens. Res. 2021, 31, 100399. [Google Scholar] [CrossRef]
- Ben Soltan, W.; Ammar, S.; Olivier, C.; Toupance, T. Influence of zinc doping on the photocatalytic activity of nanocrystalline SnO2 particles synthesized by the polyol method for enhanced degradation of organic dyes. J. Alloys Compd. 2017, 729, 638–647. [Google Scholar] [CrossRef]
- Wan, W.; Li, Y.; Ren, X.; Zhao, Y.; Gao, F.; Zhao, H. 2D SnO2 Nanosheets: Synthesis, Characterization, Structures, and Excellent Sensing Performance to Ethylene Glycol. Nanomaterials 2018, 8, 112. [Google Scholar] [CrossRef]
- Mustapha, F.; Jalil, A.; Mohamed, M.; Triwahyono, S.; Hassan, N.; Khusnun, N.; Hitam, C.; Rahman, A.F.A.; Firmanshah, L.; Zolkifli, A. New insight into self-modified surfaces with defect-rich rutile TiO2 as a visible-light-driven photocatalyst. J. Clean. Prod. 2017, 168, 1150–1162. [Google Scholar] [CrossRef]
- Mahmood, H.; Khan, M.; Mohuddin, B.; Iqbal, T. Solution-phase growth of tin oxide (SnO2) nanostructures: Structural, optical and photocatalytic properties. Mater. Sci. Eng. B 2020, 258, 114568. [Google Scholar] [CrossRef]
- Bhuvaneswari, K.; Nguyen, B.-S.; Nguyen, V.-H.; Nguyen, V.-Q.; Nguyen, Q.-H.; Palanisamy, G.; Sivashanmugan, K.; Pazhanivel, T. Enhanced photocatalytic activity of ethylenediamine-assisted tin oxide (SnO2) nanorods for methylene blue dye degradation. Mater. Lett. 2020, 276, 128173. [Google Scholar] [CrossRef]
- Tauc, J. Optical properties and electronic structure of amorphous Ge and Si. Mater. Res. Bull. 1968, 3, 37–46. [Google Scholar] [CrossRef]
- Yubero, F.; Jiménez, V.; Gonzalez-Elipe, A. Optical properties and electronic transitions of SnO2 thin films by reflection electron energy loss spectroscopy. Surf. Sci. 1998, 400, 116–126. [Google Scholar] [CrossRef]
- Abdelkader, E.; Nadjia, L.; Naceur, B.; Noureddine, B. SnO2 foam grain-shaped nanoparticles: Synthesis, characterization and UVA light induced photocatalysis. J. Alloys Compd. 2016, 679, 408–419. [Google Scholar] [CrossRef]
- Sadeghzadeh-Attar, A. Efficient photocatalytic degradation of methylene blue dye by SnO2 nanotubes synthesized at different calcination temperatures. Sol. Energy Mater. Sol. Cells 2018, 183, 16–24. [Google Scholar] [CrossRef]
- Wenderich, K.; Mul, G. Methods, Mechanism, and Applications of Photodeposition in Photocatalysis: A Review. Chem. Rev. 2016, 116, 14587–14619. [Google Scholar] [CrossRef]
- Aly, B. Controlled optoelectronic properties and abrupt change in photosensitivity by suppressing density of oxygen vacancies in SnO2 nanocrystalline thin films prepared at various spray-deposition temperatures. Phys. Scr. 2020, 95, 065807. [Google Scholar] [CrossRef]
- Oskam, G. Metal oxide nanoparticles: Synthesis, characterization and application. J. Sol-Gel Sci. Technol. 2006, 37, 161–164. [Google Scholar] [CrossRef]
- Jolivet, J.-P.; Henry, M.; Livage, J. Metal Oxide Chemistry and Synthesis: From Solution to Solid State; Wiley-Blackwell: New York, NY, USA, 2000; ISBN 0471970565. [Google Scholar]
- Jadoun, S.; Arif, R.; Jangid, N.K.; Meena, R.K. Green synthesis of nanoparticles using plant extracts: A review. Environ. Chem. Lett. 2020, 19, 355–374. [Google Scholar] [CrossRef]
- Yang, L.; Huang, J.; Liu, H.; Li, S.; Han, Y.; Qi, G.; Lv, M.; Shang, Y.; Ye, J. SnO2−x/Sb2O3 composites synthesized by mechanical milling method with excellent photocatalytic properties for isopropyl alcohol oxidation. J. Mater. Sci. Mater. Electron. 2020, 31, 8564–8577. [Google Scholar] [CrossRef]
- Nakibli, Y.; Mazal, Y.; Dubi, Y.; Wächtler, M.; Amirav, L. Size Matters: Cocatalyst Size Effect on Charge Transfer and Photocatalytic Activity. Nano Lett. 2017, 18, 357–364. [Google Scholar] [CrossRef] [PubMed]
- Rodwihok, C.; Wongratanaphisan, D.; Ngo, Y.L.T.; Khandelwal, M.; Hur, S.H.; Chung, J.S. Effect of GO Additive in ZnO/rGO Nanocomposites with Enhanced Photosensitivity and Photocatalytic Activity. Nanomaterials 2019, 9, 1441. [Google Scholar] [CrossRef] [PubMed]
- Osuntokun, J.; Onwudiwe, D.C.; Ebenso, E.E. Biosynthesis and Photocatalytic Properties of SnO2 Nanoparticles Prepared Using Aqueous Extract of Cauliflower. J. Clust. Sci. 2017, 28, 1883–1896. [Google Scholar] [CrossRef]
- Honarmand, M.; Golmohammadi, M.; Naeimi, A. Biosynthesis of tin oxide (SnO2) nanoparticles using jujube fruit for photocatalytic degradation of organic dyes. Adv. Powder Technol. 2019, 30, 1551–1557. [Google Scholar] [CrossRef]
- Elango, G.; Roopan, S.M. Efficacy of SnO2 nanoparticles toward photocatalytic degradation of methylene blue dye. J. Photochem. Photobiol. B Biol. 2016, 155, 34–38. [Google Scholar] [CrossRef]
- Ramamoorthy, M.; Ragupathy, S.; Sakthi, D.; Arun, V.; Kannadasan, N. Synthesis of SnO2 loaded on corn cob activated carbon for enhancing the photodegradation of methylene blue under sunlight irradiation. J. Environ. Chem. Eng. 2020, 8, 104331. [Google Scholar] [CrossRef]
- Bhattacharjee, A.; Ahmaruzzaman, M. A green and novel approach for the synthesis of SnO2 nanoparticles and its exploitation as a catalyst in the degradation of methylene blue under solar radiation. Mater. Lett. 2015, 145, 74–78. [Google Scholar] [CrossRef]
- Kim, S.P.; Choi, M.Y.; Choi, H.C. Photocatalytic activity of SnO2 nanoparticles in methylene blue degradation. Mater. Res. Bull. 2016, 74, 85–89. [Google Scholar] [CrossRef]
- Sujatha, K.; Seethalakshmi, T.; Sudha, A.; Shanmugasundaram, O. Photocatalytic activity of pure, Zn doped and surfactants assisted Zn doped SnO2 nanoparticles for degradation of cationic dye. Nano-Struct. Nano-Objects 2019, 18, 100305. [Google Scholar] [CrossRef]
- Sagadevan, S.; Lett, J.A.; Alshahateet, S.F.; Fatimah, I.; Weldegebrieal, G.K.; Le, M.-V.; Leonard, E.; Paiman, S.; Soga, T. Photocatalytic degradation of methylene blue dye under direct sunlight irradiation using SnO2 nanoparticles. Inorg. Chem. Commun. 2022, 141, 109547. [Google Scholar] [CrossRef]
- Yang, X.; Wang, D. Photocatalysis: From Fundamental Principles to Materials and Applications. ACS Appl. Energy Mater. 2018, 1, 6657–6693. [Google Scholar] [CrossRef]
- Rosaline, D.R.; Suganthi, A.; Vinodhkumar, G.; Inbanathan, S.S.R.; Umar, A.; Ameen, S.; Akhtar, M.S.; LuizFoletto, E. Enhanced Sunlight-Driven Photocatalytic Activity of SnO2-Sb2O3 Composite towards Emerging Contaminant Degradation in Water. J. Alloys Compd. 2022, 897, 162935. [Google Scholar] [CrossRef]
- Bhattacharjee, A.; Ahmaruzzaman, M.; Devi, T.B.; Nath, J. Photodegradation of Methyl Violet 6B and Methylene Blue Using Tin-Oxide Nanoparticles (Synthesized via a Green Route). J. Photochem. Photobiol. A Chem. 2016, 325, 116–124. [Google Scholar] [CrossRef]

| % C | % O | % Sn | |
|---|---|---|---|
| SnO2-1% | 8.96 | 26.24 | 64.8 |
| SnO2-2% | 10.64 | 26.58 | 62.78 |
| SnO2-4% | 18.21 | 28.96 | 52.83 |
| Method of Synthesis | Reducing/ Stabilizing Agent | Radiation | NPs Dose (mg) | Time (min) | Degradation (%) | Ref. |
|---|---|---|---|---|---|---|
| Plant extract | Brassica oleracea L. var. botrytis | UV | 20 | 180 | 88.23–91.89 | [58] |
| Plant extract | Jujube | Solar | 8 | 300 | 90 | [59] |
| Plant extract | Cyphomandra betacea | UV | 25 | 70 | >99 | [60] |
| Plant extract | Zea Maiz activated carbon | Solar | 20 | 120 | 71.92 | [61] |
| Microwave | Arginine | Solar | 10 | 240 | 96.4 | [62] |
| Precipitation | H2O2, KOH | UV | 4 | 180 | 79 | [63] |
| Coprecipitation | Ammonia, surfactants | UV | 100 | 120 | 53 | [64] |
| Hydrotermal | NaOH, etOH | Solar | 20, 40, 60 | 120 | 83, 97, 95 | [65] |
| Plant extract | Tilia cordata | UV—Solar | 50 | 60 | 90 | This work |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
González, E.Q.; Medina, E.L.; Robles, R.V.Q.; Gálvez, H.E.G.; Lopez, Y.A.B.; Viveros, E.V.; Molina, F.A.; Nestor, A.R.V.; Morales, P.A.L. A Study of the Optical and Structural Properties of SnO2 Nanoparticles Synthesized with Tilia cordata Applied in Methylene Blue Degradation. Symmetry 2022, 14, 2231. https://doi.org/10.3390/sym14112231
González EQ, Medina EL, Robles RVQ, Gálvez HEG, Lopez YAB, Viveros EV, Molina FA, Nestor ARV, Morales PAL. A Study of the Optical and Structural Properties of SnO2 Nanoparticles Synthesized with Tilia cordata Applied in Methylene Blue Degradation. Symmetry. 2022; 14(11):2231. https://doi.org/10.3390/sym14112231
Chicago/Turabian StyleGonzález, Eduardo Quintero, Eder Lugo Medina, Reina Vianey Quevedo Robles, Horacio Edgardo Garrafa Gálvez, Yolanda Angelica Baez Lopez, Eunice Vargas Viveros, Ferdinanda Aguilera Molina, Alfredo Rafael Vilchis Nestor, and Priscy Alfredo Luque Morales. 2022. "A Study of the Optical and Structural Properties of SnO2 Nanoparticles Synthesized with Tilia cordata Applied in Methylene Blue Degradation" Symmetry 14, no. 11: 2231. https://doi.org/10.3390/sym14112231
APA StyleGonzález, E. Q., Medina, E. L., Robles, R. V. Q., Gálvez, H. E. G., Lopez, Y. A. B., Viveros, E. V., Molina, F. A., Nestor, A. R. V., & Morales, P. A. L. (2022). A Study of the Optical and Structural Properties of SnO2 Nanoparticles Synthesized with Tilia cordata Applied in Methylene Blue Degradation. Symmetry, 14(11), 2231. https://doi.org/10.3390/sym14112231










