Surface Terminations of MXene: Synthesis, Characterization, and Properties
Abstract
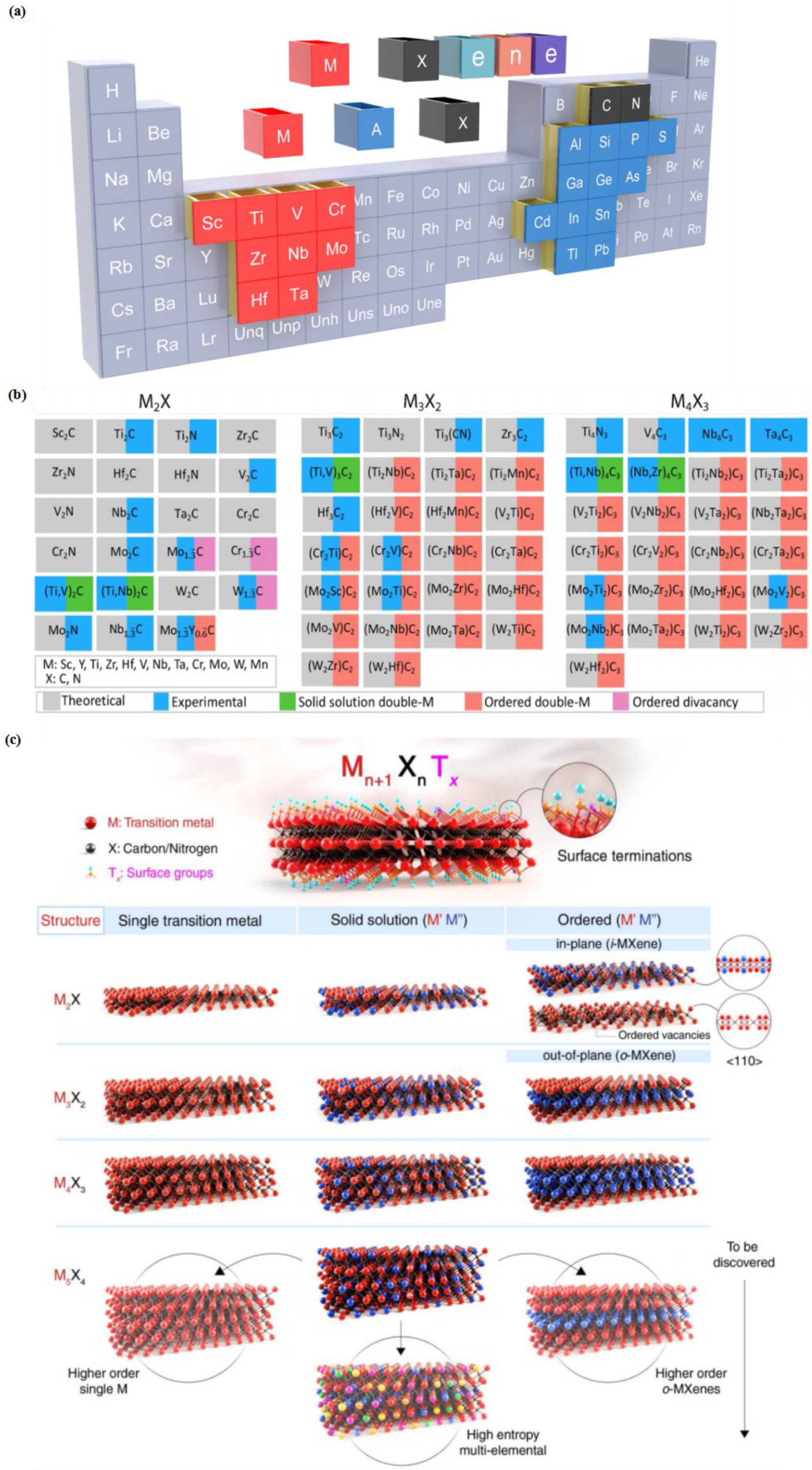
1. Introduction
2. Surface Termination Regulation
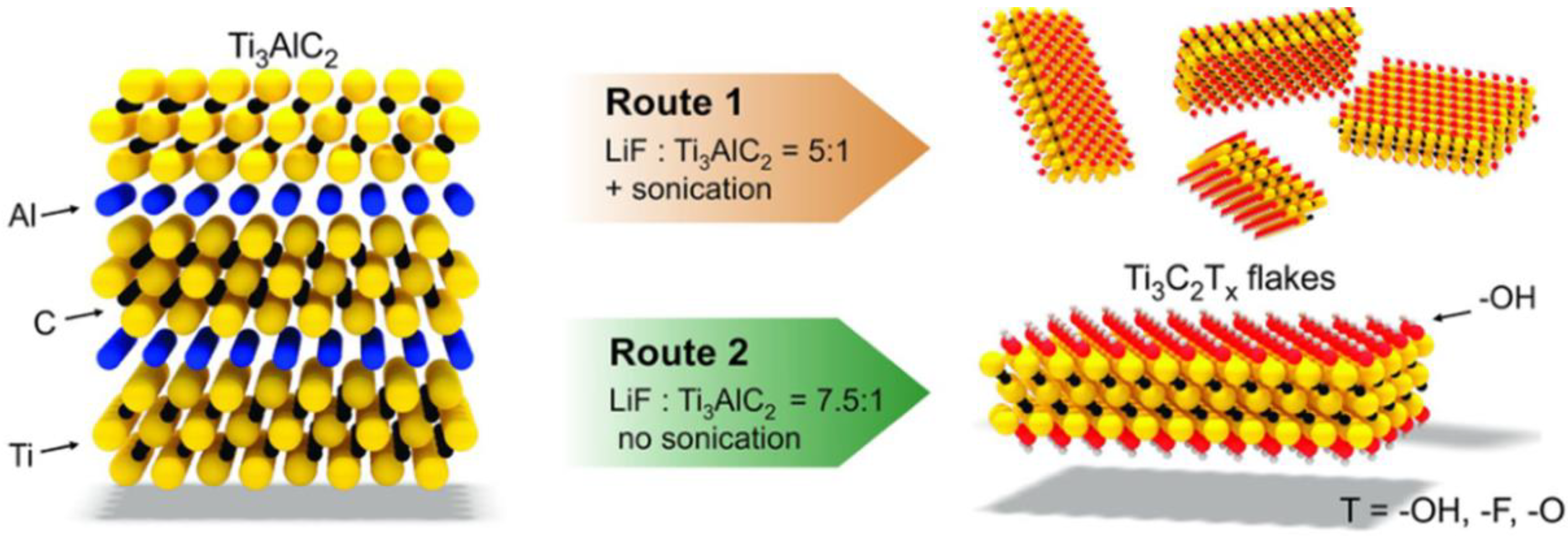
2.1. HF/Fluoride Etching

2.2. Molten Salt Etching Method
2.3. Ammoniation
2.4. Displacement and Esterification Method
2.5. Phase Transfer Method
3. Characterization of MXene’s Terminations
3.1. XRD
3.2. SEM and EDS
3.3. XPS
3.4. TEM
3.5. Raman and FTIR
- Qualitative analysis: Different substances have different characteristic spectra, so qualitative analysis can be carried out by spectra.
- Structure analysis: The analysis of spectral bands is the basis of material structure analysis.
- Quantitative analysis: According to the absorbance characteristics of the spectrum of substances, one can have a good ability to analyze the amount of substances.
3.6. Others
4. Properties Depended on Surface
4.1. Electrical Conductivity
4.2. Magnetic Properties
4.3. Optical Properties
4.4. Solubility and Dispersion
4.5. Mechanical and Tribological Properties
4.6. Water Purification
4.7. Biological Characteristics
4.8. Catalytic Performance
4.9. Others
5. Conclusions and Perspective
- (I)
- How to observe these surface terminations of MXene at the atomic level? Employing spherical aberration-corrected electron microscopy combined with advanced spectral characterization techniques may offer new sights for MXene’s surface studies. Through cross-section sample preparation, the interlayer of MXene can be observed.
- (II)
- How to prepare the bare Mxene without surface terminations? It is required for understanding the detailed bonding nature between surface species of bare MXene, the regulation rule between surface metal species and the surface functional groups, and the interfacial interaction of MXene and hybrid phases.
- (III)
- And how to construct the unsymmetrical MXene surface terminations on both sides of the single MXene nanosheets? There are key issues to improve to study the physical properties of MXene and support an accurate model for theoretical calculation and simulation.
- (IV)
- Developing MXene with a new organo-functional group and analyzing the interface between MXene and other hybrids.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Hu, M.; Zhang, H.; Hu, T.; Fan, B.; Wang, X.; Li, Z. Emerging 2D MXenes for Supercapacitors: Status, Challenges and Prospects. Chem. Soc. Rev. 2020, 49, 6666–6693. [Google Scholar] [CrossRef] [PubMed]
- Naguib, M.; Kurtoglu, M.; Presser, V.; Lu, J.; Niu, J.; Heon, M.; Hultman, L.; Gogotsi, Y.; Barsoum, M.W. Two-Dimensional Nanocrystals Produced by Exfoliation of Ti3AlC2. Adv. Mater. 2011, 23, 4248–4253. [Google Scholar] [CrossRef] [PubMed]
- Mendoza-Sánchez, B.; Gogotsi, Y. Synthesis of Two-Dimensional Materials for Capacitive Energy Storage. Adv. Mater. 2016, 28, 6104–6135. [Google Scholar] [CrossRef]
- Wan, Y.J.; Rajavel, K.; Li, X.M.; Wang, X.Y.; Liao, S.Y.; Lin, Z.Q.; Zhu, P.L.; Sun, R.; Wong, C.P. Electromagnetic Interference Shielding of Ti3C2Tx MXene Modified by Ionic Liquid for High Chemical Stability and Excellent Mechanical Strength. Chem. Eng. J. 2021, 408, 127303. [Google Scholar] [CrossRef]
- Wu, X.; Cui, X.; Wu, W.; Wang, J.; Li, Y.; Jiang, Z. Elucidating Ultrafast Molecular Permeation through Well-Defined 2D Nanochannels of Lamellar Membranes. Angew. Chem. Int. Ed. 2019, 131, 18695–18700. [Google Scholar] [CrossRef]
- Chen, J.; Huang, Q.; Huang, H.; Mao, L.; Liu, M.; Zhang, X.; Wei, Y. Recent Progress and Advances in the Environmental Applications of MXene Related Materials. Nanoscale 2020, 12, 3574–3592. [Google Scholar] [CrossRef]
- Magnuson, M.; Halim, J.; Näslund, L. Chemical Bonding in Carbide MXene Nanosheets. J. Electron. Spectrosc. Relat. Phenom. 2018, 224, 27–32. [Google Scholar] [CrossRef]
- Han, X.; Huang, J.; Lin, H.; Wang, Z.; Li, P.; Chen, Y. 2D Ultrathin MXene-Based Drug-Delivery Nanoplatform for Synergistic Photothermal Ablation and Chemotherapy of Cancer. Adv. Healthc. Mater. 2018, 7, 1701394. [Google Scholar] [CrossRef]
- Ouyang, H.; Zhou, M.; Guo, Y.; He, M.; Huang, H.; Ye, X.; Feng, Y.; Zhou, X.; Yang, S. Metabolites Profiling of Pulsatilla Saponin D in Rat by Ultra Performance Liquid Chromatography—Quadrupole Time-of-Flight Mass Spectrometry (UPLC/Q-TOF-MS/MS). Fitoterapia 2014, 96, 152–158. [Google Scholar] [CrossRef]
- Zhang, X.; Li, J.; Xie, B.; Wu, B.; Lei, S.; Yao, Y.; He, M.; Ouyang, H.; Feng, Y.; Xu, W.; et al. Comparative Metabolomics Analysis of Cervicitis in Human Patients and a Phenol Mucilage-Induced Rat Model Using Liquid Chromatography Tandem Mass Spectrometry. Front. Pharmacol. 2018, 9, 00282. [Google Scholar] [CrossRef]
- Zhang, X.; He, M.; Lei, S.; Wu, B.; Tan, T.; Ouyang, H.; Xu, W.; Feng, Y. An Integrative Investigation of the Therapeutic Mechanism of Ainsliaea Fragrans Champ. in Cervicitis Using Liquid Chromatography Tandem Mass Spectrometry Based on a Rat Plasma Metabolomics Strategy. J. Pharm. Biomed. Anal. 2018, 156, 221–231. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Naguib, M.; Mochalin, V.N.; Barsoum, M.W.; Gogotsi, Y.; Yu, X.; Nam, K.W.; Yang, X.Q.; Kolesnikov, A.I.; Kent, P.R.C. Role of Surface Structure on Li-Ion Energy Storage Capacity of Two-Dimensional Transition-Metal Carbides. J. Am. Chem. Soc. 2014, 136, 6385–6394. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Ma, X.; Zhou, J.; Du, F.; Teng, C. Bioinspired, High-Strength, and Flexible MXene/Aramid Fiber for Electromagnetic Interference Shielding Papers with Joule Heating Performance. ACS Nano 2022, 16, 6700–6711. [Google Scholar] [CrossRef] [PubMed]
- Parra-Muñoz, N.; Soler, M.; Rosenkranz, A. Covalent Functionalization of MXenes for Tribological Purposes—A Critical Review. Adv. Colloid Interface Sci. 2022, 309, 102792. [Google Scholar] [CrossRef]
- VahidMohammadi, A.; Rosen, J.; Gogotsi, Y. The World of Two-Dimensional Carbides and Nitrides (MXenes). Science 2021, 372, eabf1581. [Google Scholar] [CrossRef]
- Gogotsi, Y.; Anasori, B. The Rise of MXenes. ACS Nano 2019, 13, 8491–8494. [Google Scholar] [CrossRef]
- Tang, J.; Mathis, T.S.; Kurra, N.; Sarycheva, A.; Xiao, X.; Hedhili, M.N.; Jiang, Q.; Alshareef, H.N.; Xu, B.; Pan, F.; et al. Tuning the Electrochemical Performance of Titanium Carbide MXene by Controllable in Situ Anodic Oxidation. Angew. Chem. Int. Ed. 2019, 58, 17849–17855. [Google Scholar] [CrossRef]
- Li, K.; Liang, M.; Wang, H.; Wang, X.; Huang, Y.; Coelho, J.; Pinilla, S.; Zhang, Y.; Qi, F.; Nicolosi, V.; et al. 3D MXene Architectures for Efficient Energy Storage and Conversion. Adv. Funct. Mater. 2020, 30, 2000842. [Google Scholar] [CrossRef]
- Luo, Y.; Que, W.; Bin, X.; Xia, C.; Kong, B.; Gao, B.; Kong, L.B. Flexible MXene—Based Composite Films: Synthesis, Modification, and Applications as Electrodes of Supercapacitors. Small 2022, 18, 2201290. [Google Scholar] [CrossRef]
- Malaki, M.; Varma, R.S. Mechanotribological Aspects of MXene-Reinforced Nanocomposites. Adv. Mater. 2020, 32, 2003154. [Google Scholar] [CrossRef]
- Wu, Z.; Liu, X.; Shang, T.; Deng, Y.; Wang, N.; Dong, X.; Zhao, J.; Chen, D.; Tao, Y.; Yang, Q. Reassembly of MXene Hydrogels into Flexible Films towards Compact and Ultrafast Supercapacitors. Adv. Funct. Mater. 2021, 31, 2102874. [Google Scholar] [CrossRef]
- Yang, X.; Yao, Y.; Wang, Q.; Zhu, K.; Ye, K.; Wang, G.; Cao, D.; Yan, J. 3D Macroporous Oxidation-Resistant Ti3C2Tx MXene Hybrid Hydrogels for Enhanced Supercapacitive Performances with Ultralong Cycle Life. Adv. Funct. Mater. 2022, 32, 2109479. [Google Scholar]
- Zhang, X.; Lv, R.; Wang, A.; Guo, W.; Liu, X.; Luo, J. MXene Aerogel Scaffolds for High-Rate Lithium Metal Anodes. Angew. Chem. Int. Ed. 2018, 57, 15028–15033. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Feng, W.; Chen, Y. Chemistry of Two-Dimensional MXene Nanosheets in Theranostic Nanomedicine. Chin. Chem. Lett. 2020, 31, 937–946. [Google Scholar] [CrossRef]
- Shahzad, A.; Rasool, K.; Nawaz, M.; Miran, W.; Jang, J.; Moztahida, M.; Mahmoud, K.A.; Lee, D.S. Heterostructural TiO2/Ti3C2Tx (MXene) for Photocatalytic Degradation of Antiepileptic Drug Carbamazepine. Chem. Eng. J. 2018, 349, 748–755. [Google Scholar] [CrossRef]
- Sang, X.; Xie, Y.; Lin, M.W.; Alhabeb, M.; van Aken, K.L.; Gogotsi, Y.; Kent, P.R.C.; Xiao, K.; Unocic, R.R. Atomic Defects in Monolayer Titanium Carbide (Ti3C2Tx) MXene. ACS Nano 2016, 10, 9193–9200. [Google Scholar] [CrossRef]
- Li, M.; Lu, J.; Luo, K.; Li, Y.; Chang, K.; Chen, K.; Zhou, J.; Rosen, J.; Hultman, L.; Eklund, P.; et al. Element Replacement Approach by Reaction with Lewis Acidic Molten Salts to Synthesize Nanolaminated MAX Phases and MXenes. J. Am. Chem. Soc. 2019, 141, 4730–4737. [Google Scholar] [CrossRef]
- Li, M.; Li, X.; Qin, G.; Luo, K.; Lu, J.; Li, Y.; Liang, G.; Huang, Z.; Zhou, J.; Hultman, L.; et al. Halogenated Ti3C2 MXenes with Electrochemically Active Terminals for High-Performance Zinc Ion Batteries. ACS Nano 2021, 15, 1077–1085. [Google Scholar] [CrossRef]
- Kamysbayev, V.; Filatov, A.S.; Hu, H.; Rui, X.; Lagunas, F.; Wang, D.; Klie, R.F.; Talapin, D.V. Covalent Surface Modifications and Superconductivity of Two-Dimensional Metal Carbide MXenes. Science 2020, 369, 979–983. [Google Scholar] [CrossRef]
- Kim, D.; Ko, T.Y.; Kim, H.; Lee, G.H.; Cho, S.; Koo, C.M. Nonpolar Organic Dispersion of 2D Ti3C2Tx MXene Flakes via Simultaneous Interfacial Chemical Grafting and Phase Transfer Method. ACS Nano 2019, 13, 13818–13828. [Google Scholar] [CrossRef]
- Zhang, S.; Zhuo, H.; Li, S.; Bao, Z.; Deng, S.; Zhuang, G.; Zhong, X.; Wei, Z.; Yao, Z.; Wang, J. Effects of Surface Functionalization of Mxene-Based Nanocatalysts on Hydrogen Evolution Reaction Performance. Catal. Today 2021, 368, 187–195. [Google Scholar] [CrossRef]
- Wang, S.; Liu, Y.; Lü, Q.F.; Zhuang, H. Facile Preparation of Biosurfactant-Functionalized Ti2CTx MXene Nanosheets with an Enhanced Adsorption Performance for Pb(II) Ions. J. Mol. Liq. 2020, 297, 111810. [Google Scholar] [CrossRef]
- Rajan, A.C.; Mishra, A.; Satsangi, S.; Vaish, R.; Mizuseki, H.; Lee, K.R.; Singh, A.K. Machine-Learning-Assisted Accurate Band Gap Predictions of Functionalized Mxene. Chem. Mater. 2018, 30, 4031–4038. [Google Scholar] [CrossRef]
- Ghidiu, M.; Lukatskaya, M.R.; Zhao, M.Q.; Gogotsi, Y.; Barsoum, M.W. Conductive Two-Dimensional Titanium Carbide “clay” with High Volumetric Capacitance. Nature 2015, 516, 78–81. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Naguib, M.; Ghidiu, M.; Pan, L.M.; Gu, J.; Nanda, J.; Halim, J.; Gogotsi, Y.; Barsoum, M.W. Two-Dimensional Nb-Based M4C3 Solid Solutions (MXenes). J. Am. Ceram. Soc. 2016, 99, 660–666. [Google Scholar] [CrossRef]
- Lipatov, A.; Alhabeb, M.; Lukatskaya, M.R.; Boson, A.; Gogotsi, Y.; Sinitskii, A. Effect of Synthesis on Quality, Electronic Properties and Environmental Stability of Individual Monolayer Ti3C2 MXene Flakes. Adv. Electron. Mater. 2016, 2, 1600255. [Google Scholar]
- Liu, F.; Zhou, A.; Chen, J.; Jia, J.; Zhou, W.; Wang, L.; Hu, Q. Preparation of Ti3C2 and Ti2C MXenes by Fluoride Salts Etching and Methane Adsorptive Properties. Appl. Surf. Sci. 2017, 416, 781–789. [Google Scholar] [CrossRef]
- Lee, J.T.; Wyatt, B.C.; Davis, G.A.; Masterson, A.N.; Pagan, A.L.; Shah, A.; Anasori, B.; Sardar, R. Covalent Surface Modification of Ti3C2Tx MXene with Chemically Active Polymeric Ligands Producing Highly Conductive and Ordered Microstructure Films. ACS Nano 2021, 15, 19600–19612. [Google Scholar] [CrossRef]
- Guo, M.; Geng, W.C.; Liu, C.; Gu, J.; Zhang, Z.; Tang, Y. Ultrahigh Areal Capacitance of Flexible MXene Electrodes: Electrostatic and Steric Effects of Terminations. Chem. Mater. 2020, 32, 8257–8265. [Google Scholar] [CrossRef]
- Zou, X.; Liu, H.; Xu, H.; Wu, X.; Han, X.; Kang, J.; Reddy, K.M. A Simple Approach to Synthesis Cr2CTx MXene for Efficient Hydrogen Evolution Reaction. Mater. Today Energy 2021, 20, 100668. [Google Scholar] [CrossRef]
- Qiu, S.Y.; Wang, C.; Jiang, Z.X.; Zhang, L.S.; Gu, L.L.; Wang, K.X.; Gao, J.; Zhu, X.D.; Wu, G. Rational Design of MXene@TiO2 nanoarray Enabling Dual Lithium Polysulfide Chemisorption towards High-Performance Lithium-Sulfur Batteries. Nanoscale 2020, 12, 16678–16684. [Google Scholar] [CrossRef] [PubMed]
- Biggin, S.; Enderby, J.E. The Structure of Molten Zinc Chloride. J. Phys. C Solid State Phys. 1981, 14, 3129–3136. [Google Scholar] [CrossRef]
- Hefeng, L.; Kunquan, L.; Zhonghua, W.; Jun, D. EXAFS Studies of Molten EXAFS Studies of Molten ZnCl2, RbCl and Rb2ZnCl4. J. Phys. Condens. Matter 1994, 6, 3629. [Google Scholar] [CrossRef]
- Shen, M.; Jiang, W.; Liang, K.; Zhao, S.; Tang, R.; Zhang, L.; Wang, J. One-Pot Green Process to Synthesize MXene with Controllable Surface Terminations Using Molten Salts. Angew. Chem. Int. Ed. 2021, 60, 27013–27018. [Google Scholar] [CrossRef]
- Xiao, X.; Song, H.; Lin, S.; Zhou, Y.; Zhan, X.; Hu, Z.; Zhang, Q.; Sun, J.; Yang, B.; Li, T.; et al. Scalable Salt-Templated Synthesis of Two-Dimensional Transition Metal Oxides. Nat. Commun. 2016, 7, 11296. [Google Scholar] [CrossRef]
- Khaledialidusti, R.; Khazaei, M.V.; Wang, N.; Miao, C.; Si, J.; Wang, J.; Wang, J. Exploring structural, electronic, and mechanical properties of 2D hexagonal MBenes. J. Phys. Condens. Matter 2021, 33, 155503. [Google Scholar] [CrossRef]
- Weng, W.; Jiang, B.; Wang, Z.; Xiao, W. In Situ Electrochemical Conversion of CO2 in Molten Salts to Advanced Energy Materials with Reduced Carbon Emissions. Sci. Adv. 2020, 6, eaay9278. [Google Scholar] [CrossRef]
- Liang, X.; Xiao, J.; Weng, W.; Xiao, W. Electrochemical Reduction of Carbon Dioxide and Iron Oxide in Molten Salts to Fe/Fe3C Modified Carbon for Electrocatalytic Oxygen Evolution. Angew. Chem. Int. Ed. 2021, 60, 2120–2124. [Google Scholar] [CrossRef]
- Peng, M.; Dong, M.; Wei, W.; Xu, H.; Liu, C.; Shen, C. The Introduction of Amino Termination on Ti3C2 MXene Surface for Its Flexible Film with Excellent Property. Carbon 2021, 179, 400–407. [Google Scholar] [CrossRef]
- Nair, V.G.; Birowska, M.; Bury, D.; Jakubczak, M.; Rosenkranz, A.; Jastrzębska, A.M. 2D MBenes: A Novel Member in the Flatland. Adv. Mater. 2022, 34, 2108840. [Google Scholar] [CrossRef]
- Lu, C.; Yang, L.; Yan, B.; Sun, L.; Zhang, P.; Zhang, W.; Sun, Z.M. Nitrogen-Doped Ti3C2 MXene: Mechanism Investigation and Electrochemical Analysis. Adv. Funct. Mater. 2020, 30, 2000852. [Google Scholar] [CrossRef]
- Michałowski, P.P.; Anayee, M.; Mathis, T.S.; Kozdra, S.; Wójcik, A.; Hantanasirisakul, K.; Jóźwik, I.; Piątkowska, A.; Możdżonek, M.; Malinowska, A.; et al. Oxycarbide MXenes and MAX Phases Identification Using Monoatomic Layer-by-Layer Analysis with Ultralow-Energy Secondary-Ion Mass Spectrometry. Nat. Nanotechnol. 2022. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, K.; Li, Y.; Lan, J.; Yan, B.; Shi, L.; Ran, R. High-Strength, Self-Healable, Temperature-Sensitive, MXene-Containing Composite Hydrogel as a Smart Compression Sensor. ACS Appl. Mater. Inter. 2019, 11, 47350–47357. [Google Scholar] [CrossRef]
- Chao, M.; He, L.; Gong, M.; Li, N.; Li, X.; Peng, L.; Shi, F.; Zhang, L.; Wan, P. Breathable Ti3C2Tx MXene/Protein Nanocomposites for Ultrasensitive Medical Pressure Sensor with Degradability in Solvents. ACS Nano 2021, 15, 9746–9758. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Li, N.; Wang, Y.; Xu, Y.; Wu, J.; Jia, G.; Ji, F.; Fang, X.; Chen, F.; Cui, X. Ultrasensitive and Selective Determination of Carcinoembryonic Antigen Using Multifunctional Ultrathin Amino-Functionalized Ti3C2-MXene Nanosheets. Anal. Chem. 2020, 92, 3354–3360. [Google Scholar] [CrossRef] [PubMed]
- Halim, J.; Cook, K.M.; Naguib, M.; Eklund, P.; Gogotsi, Y.; Rosen, J.; Barsoum, M.W. X-Ray Photoelectron Spectroscopy of Select Multi-Layered Transition Metal Carbides (MXenes). Appl. Surf. Sci. 2016, 362, 406–417. [Google Scholar] [CrossRef]
- Schier, V.; Michel, H.-J.; Halbritter, J. ARXPS-Analysis of Sputtered TiC, SiC and Ti0.5Si0.5C Layers. Fresenius J. Anal. Chem. 1993, 346, 227–232. [Google Scholar] [CrossRef]
- Magni, E.; Somorjai, G. Preparation of a Model Ziegler-Natta Catalyst: Electron Irradiation Induced Titanium Chloride Deposition on Magnesium Chloride Thin Films Grown on Gold. Surf. Sci. 1996, 345, 1–16. [Google Scholar] [CrossRef]
- Mousty-Desbuquoit, C.; Riga, J.; Verbist, J.J. Solid State Effects in the Electronic Structure of TiCl4 Studied by XPS. J. Chem. Phys. 1983, 79, 26–32. [Google Scholar] [CrossRef]
- Saha, N.C.; Tompkins, H.G. Titanium Nitride Oxidation Chemistry: An x-ray Photoelectron Spectroscopy Study. J. Appl. Phys. 1992, 72, 3072–3079. [Google Scholar] [CrossRef]
- Tong, X.; Liu, S.; Zhao, Y.; Huang, L.; Crittenden, J.; Chen, Y. MXene Composite Membranes with Enhanced Ion Transport and Regulated Ion Selectivity. Environ. Sci. Technol. 2022, 56, 8964–8974. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Huang, C.; Sun, Y.; Yu, H. Amino-Functionalized Niobium-Carbide MXene Serving as Electron Transport Layer and Perovskite Additive for the Preparation of High-Performance and Stable Methylammonium-Free Perovskite Solar Cells. Adv. Funct. Mater. 2022, 32, 2113367. [Google Scholar] [CrossRef]
- Miao, N.; Wang, J.; Gong, Y.; Wu, J.; Niu, H.; Wang, S.; Li, K.; Oganov, A.R.; Tada, T.; Hosono, H. Computational Prediction of Boron-Based MAX Phases and MXene Derivatives. Chem. Mater. 2020, 32, 6947. [Google Scholar] [CrossRef]
- Karlsson, L.H.; Birch, J.; Halim, J.; Barsoum, M.W.; Persson, P.O.A. Atomically Resolved Structural and Chemical Investigation of Single MXene Sheets. Nano Lett. 2015, 15, 4955–4960. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Kent, P.R.C. Hybrid Density Functional Study of Structural and Electronic Properties of Functionalized Tin+1Xn (X=C, N) Monolayers. Phys. Rev. B Matter Mater. Phys. 2013, 87, 235441. [Google Scholar] [CrossRef]
- Kim, H.; Wang, Z.; Alshareef, H.N. MXetronics: Electronic and Photonic Applications of MXenes. Nano Energy 2019, 60, 179–197. [Google Scholar] [CrossRef]
- Khazaei, M.; Arai, M.; Sasaki, T.; Chung, C.Y.; Venkataramanan, N.S.; Estili, M.; Sakka, Y.; Kawazoe, Y. Novel Electronic and Magnetic Properties of Two-Dimensional Transition Metal Carbides and Nitrides. Adv. Funct. Mater. 2012, 23, 2185–2192. [Google Scholar] [CrossRef]
- Kumar, S.; Schwingenschlögl, U. Thermoelectric Performance of Functionalized Sc2C MXenes. Phys. Rev. B 2016, 94, 035405. [Google Scholar] [CrossRef]
- Lee, Y.; Hwang, Y.; Chung, Y.C. Achieving Type-I, II, and III Heterojunction Using Functionalized MXene. ACS Appl. Mater. Inter. 2015, 7, 7163–7169. [Google Scholar] [CrossRef]
- Liu, J.H.; Kan, X.; Amin, B.; Gan, L.Y.; Zhao, Y. Theoretical Exploration of the Potential Applications of Sc-Based MXenes. Phys. Chem. Chem. Phys. 2017, 19, 32253–32261. [Google Scholar] [CrossRef]
- Chandrasekaran, A.; Mishra, A.; Singh, A.K. Ferroelectricity, Antiferroelectricity, and Ultrathin 2D Electron/Hole Gas in Multifunctional Monolayer MXene. Nano Lett. 2017, 17, 3290–3296. [Google Scholar] [CrossRef] [PubMed]
- Khazaei, M.; Arai, M.; Sasaki, T.; Estili, M.; Sakka, Y. Two-Dimensional Molybdenum Carbides: Potential Thermoelectric Materials of the MXene Family. Phys. Chem. Chem. Phys. 2014, 16, 7841–7849. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.; Hu, Z.; Zhao, X.; Tang, Q.; Wu, D.; Zhou, Z.; Zhang, L. Tunable Band Structures of Heterostructured Bilayers with Transition-Metal Dichalcogenide and MXene Monolayer. J. Phys. Chem. C 2014, 118, 5593–5599. [Google Scholar] [CrossRef]
- Guo, J.; Sun, Y.; Liu, B.; Zhang, Q.; Peng, Q. Two-Dimensional Scandium-Based Carbides (MXene): Band Gap Modulation and Optical Properties. J. Alloys Compd. 2017, 712, 752–759. [Google Scholar] [CrossRef]
- Gandi, A.N.; Alshareef, H.N.; Schwingenschlögl, U. Thermoelectric Performance of the MXenes M2CO2 (M=Ti, Zr, or Hf). Chem. Mater. 2016, 28, 1647–1652. [Google Scholar] [CrossRef]
- Gao, G.; O’Mullane, A.P.; Du, A. 2D MXenes: A New Family of Promising Catalysts for the Hydrogen Evolution Reaction. ACS Catal. 2017, 7, 494–500. [Google Scholar] [CrossRef]
- Zhang, H.; Yang, G.; Zuo, X.; Tang, H.; Yang, Q.; Li, G. Computational Studies on the Structural, Electronic and Optical Properties of Graphene-like MXenes (M2CT2, M=Ti, Zr, Hf; T=O, F, OH) and Their Potential Applications as Visible-Light Driven Photocatalysts. J. Mater. Chem. A 2016, 4, 12913–12920. [Google Scholar] [CrossRef]
- Zha, X.H.; Huang, Q.; He, J.; He, H.; Zhai, J.; Francisco, J.S.; Du, S. The Thermal and Electrical Properties of the Promising Semiconductor MXene Hf2CO2. Sci. Rep. 2016, 6, 27971. [Google Scholar] [CrossRef]
- Yang, J.; Zhou, X.; Luo, X.; Zhang, S.; Chen, L. Tunable Electronic and Magnetic Properties of Cr2M′C2T2 (M’=Ti or V.; T=O, OH or F). Appl. Phys. Lett. 2016, 109, 203109. [Google Scholar] [CrossRef]
- Hu, J.; Xu, B.; Ouyang, C.; Yang, S.A.; Yao, Y. Investigations on V2C and V2CX2 (X=F, OH) Monolayer as a Promising Anode Material for Li Ion Batteries from First-Principles Calculations. J. Phys. Chem. C 2014, 118, 24274–24281. [Google Scholar] [CrossRef]
- Si, C.; Zhou, J.; Sun, Z. Half-Metallic Ferromagnetism and Surface Functionalization-Induced Metal-Insulator Transition in Graphene-like Two-Dimensional Cr2C Crystals. ACS Appl. Mater. Inter. 2015, 7, 17510–17515. [Google Scholar] [CrossRef] [PubMed]
- Weng, H.; Ranjbar, A.; Liang, Y.; Song, Z.; Khazaei, M.; Yunoki, S.; Arai, M.; Kawazoe, Y.; Fang, Z.; Dai, X. Large-Gap Two-Dimensional Topological Insulator in Oxygen Functionalized MXene. Phys. Rev. B 2015, 92, 075436. [Google Scholar] [CrossRef]
- Khazaei, M.; Wang, V.; Sevik, C.; Ranjbar, A.; Arai, M.; Yunoki, S. Electronic Structures of IMAX Phases and Their Two-Dimensional Derivatives: A Family of Piezoelectric Materials. Phys. Rev. Mater. 2018, 2, 074002. [Google Scholar] [CrossRef]
- Lind, H.; Halim, J.; Simak, S.I.; Rosen, J. Investigation of Vacancy-Ordered Mo1.33C MXene from First Principles and X-ray Photoelectron Spectroscopy. Phys. Rev. Mater. 2017, 1, 044002. [Google Scholar] [CrossRef]
- Zha, X.H.; Zhou, J.; Luo, K.; Lang, J.; Huang, Q.; Zhou, X.; Francisco, J.S.; He, J.; Du, S. Controllable Magnitude and Anisotropy of the Electrical Conductivity of Hf3C2O2 MXene. J. Phys. Condens. Matter 2017, 29, 165701. [Google Scholar] [CrossRef]
- Dong, L.; Kumar, H.; Anasori, B.; Gogotsi, Y.; Shenoy, V.B. Rational Design of Two-Dimensional Metallic and Semiconducting Spintronic Materials Based on Ordered Double-Transition-Metal MXenes. J. Phys. Chem. Lett. 2017, 8, 422–428. [Google Scholar] [CrossRef]
- Zhang, J.J.; Lin, L.; Zhang, Y.; Wu, M.; Yakobson, B.I.; Dong, S. Type-II Multiferroic Hf2VC2F2 MXene Monolayer with High Transition Temperature. J. Am. Chem. Soc. 2018, 140, 9768–9773. [Google Scholar] [CrossRef]
- Khazaei, M.; Ranjbar, A.; Arai, M.; Yunoki, S. Topological Insulators in the Ordered Double Transition Metals M2′M″C2 MXenes (M’= Mo, W.; M”=Ti, Zr, Hf). Phys. Rev. B 2016, 94, 125152. [Google Scholar] [CrossRef]
- Si, C.; Jin, K.H.; Zhou, J.; Sun, Z.; Liu, F. Large-Gap Quantum Spin Hall State in MXenes: D-Band Topological Order in a Triangular Lattice. Nano Lett. 2016, 16, 6584–6591. [Google Scholar] [CrossRef]
- Wang, H.; Yuan, H.; Yu, J.; Zhang, C.; Li, K.; You, M.; Li, W.; Shao, J.; Wei, J.; Zhang, X.; et al. Boosting the Efficiency of NiOx—Based Perovskite Light-Emitting Diodes by Interface Engineering. ACS Appl. Mater. Inter. 2020, 12, 53528–53536. [Google Scholar] [CrossRef]
- Yin, L.; Li, Y.; Yao, X.; Wang, Y.; Jia, L.; Liu, Q.; Li, J.; Li, Y.; He, D. MXenes for Solar Cells. Nano-Micro Lett. 2021, 13, 78. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Meng, X.; Dall’Agnese, Y.; Dall’Agnese, C.; Duan, S.; Gao, Y.; Chen, G.; Wang, X.F. 2D MXenes as Co-Catalysts in Photocatalysis: Synthetic Methods. Nano-Micro Lett. 2019, 11, 79. [Google Scholar] [CrossRef] [PubMed]
- Khazaei, M.; Mishra, A.; Venkataramanan, N.S.; Singh, A.K.; Yunoki, S. Recent Advances in MXenes: From Fundamentals to Applications. Curr. Opin. Solid St. M. 2019, 23, 164–178. [Google Scholar] [CrossRef]
- Naguib, M.; Mochalin, V.N.; Barsoum, M.W.; Gogotsi, Y. 25th Anniversary Article: MXenes: A New Family of Two-Dimensional Materials. Adv. Mater. 2014, 26, 992–1005. [Google Scholar] [CrossRef] [PubMed]
- Khazaei, M.; Ranjbar, A.; Arai, M.; Sasaki, T.; Yunoki, S. Electronic Properties and Applications of MXenes: A Theoretical Review. J. Mater. Chem. C 2017, 5, 2488–2503. [Google Scholar] [CrossRef]
- Wang, X.; Huang, S.; Deng, L.; Luo, H.; Li, C.; Xu, Y.; Yan, Y.; Tang, Z. Enhanced Optical Absorption of Fe-, Co- and Ni- Decorated Ti3C2 MXene: A First-Principles Investigation. Phys. E 2021, 127, 114565. [Google Scholar] [CrossRef]
- Zhang, J.; Kong, N.; Uzun, S.; Levitt, A.; Seyedin, S.; Lynch, P.A.; Qin, S.; Han, M.; Yang, W.; Liu, J.; et al. Scalable Manufacturing of Free-Standing, Strong Ti3C2Tx MXene Films with Outstanding Conductivity. Adv. Mater. 2020, 32, 2001093. [Google Scholar]
- Moltved, K.A.; Kepp, K.P. The Chemical Bond between Transition Metals and Oxygen: Electronegativity, d-Orbital Effects, and Oxophilicity as Descriptors of Metal-Oxygen Interactions. J. Phys. Chem. C 2019, 123, 18432–18444. [Google Scholar] [CrossRef]
- Kazemi, S.A.; Wang, Y. Super Strong 2D Titanium Carbide MXene-Based Materials: A Theoretical Prediction. J. Phys.-Condens. Mat. 2019, 32, 11LT01. [Google Scholar] [CrossRef]
- Fu, Z.; Zhang, H.; Si, C.; Legut, D.; Germann, T.C.; Zhang, Q.; Du, S.; Francisco, J.S.; Zhang, R. Mechanistic Quantification of Thermodynamic Stability and Mechanical Strength for Two-Dimensional Transition-Metal Carbides. J. Phys. Chem. C 2018, 122, 4710–4722. [Google Scholar] [CrossRef]
- Hu, T.; Yang, J.; Li, W.; Wang, X.; Li, C.M. Quantifying the Rigidity of 2D Carbides (MXenes). Phys. Chem. Chem. Phys. 2020, 22, 2115–2121. [Google Scholar] [CrossRef] [PubMed]
- Wyatt, B.C.; Rosenkranz, A.; Anasori, B. 2D MXenes: Tunable Mechanical and Tribological Properties. Adv. Mater. 2021, 33, 2007973. [Google Scholar] [CrossRef] [PubMed]
- Yan, H.; Cai, M.; Li, W.; Fan, X.; Zhu, M. Amino-Functionalized Ti3C2Tx with Anti-Corrosive/Wear Function for Waterborne Epoxy Coating. J. Mater. Sci. Technol. 2020, 54, 144–159. [Google Scholar] [CrossRef]
- Karahan, H.E.; Goh, K.; Zhang, C.; Yang, E.; Yıldırım, C.; Chuah, C.Y.; Ahunbay, M.G.; Lee, J.; Tantekin-Ersolmaz, B.; Chen, Y.; et al. MXene Materials for Designing Advanced Separation Membranes. Adv. Mater. 2020, 32, 1906697. [Google Scholar] [CrossRef]
- Yu, F.; Zhang, X.; Yang, Z.; Yang, P.; Ma, J. Environmental Applications of Two-Dimensional Transition Metal Carbides and Nitrides for Water Purification: A Review. Environ. Chem. Lett. 2022, 20, 633–660. [Google Scholar] [CrossRef]
- Jakubczak, M.; Szuplewska, A.; Rozmysłowska-Wojciechowska, A.; Rosenkranz, A.; Jastrzębska, A.M. Novel 2D MBenes—Synthesis, Structure, and Biotechnological Potential. Adv. Funct. Mater. 2021, 31, 2103048. [Google Scholar] [CrossRef]
- Wu, L.; Lu, X.; Dhanjai; Wu, Z.S.; Dong, Y.; Wang, X.; Zheng, S.; Chen, J. 2D Transition Metal Carbide MXene as a Robust Biosensing Platform for Enzyme Immobilization and Ultrasensitive Detection of Phenol. Biosens. Bioelectron. 2018, 107, 69–75. [Google Scholar] [CrossRef]
- Rozmysłowska-Wojciechowska, A.; Wojciechowski, T.; Ziemkowska, W.; Chlubny, L.; Olszyna, A.; Jastrzębska, A.M. Surface Interactions between 2D Ti3C2/Ti2C MXenes and Lysozyme. Appl. Surf. Sci. 2019, 473, 409–418. [Google Scholar] [CrossRef]
- Rozmysłowska-Wojciechowska, A.; Szuplewska, A.; Wojciechowski, T.; Poźniak, S.; Mitrzak, J.; Chudy, M.; Ziemkowska, W.; Chlubny, L.; Olszyna, A.; Jastrzębska, A. A Simple, Low-Cost and Green Method for Controlling the Cytotoxicity of MXenes. Mater. Sci. Eng. C 2020, 111, 110790. [Google Scholar] [CrossRef]
- Liu, G.; Zou, J.; Tang, Q.; Yang, X.; Zhang, Y.; Zhang, Q.; Huang, W.; Chen, P.; Shao, J.; Dong, X. Surface Modified Ti3C2 MXene Nanosheets for Tumor Targeting Photothermal/Photodynamic/Chemo Synergistic Therapy. ACS Appl. Mater. Interfaces 2017, 9, 40077–40086. [Google Scholar] [CrossRef]
- Szuplewska, A.; Kulpińska, D.; Dybko, A.; Jastrzębska, A.M.; Wojciechowski, T.; Rozmysłowska, A.; Chudy, M.; Grabowska-Jadach, I.; Ziemkowska, W.; Brzózka, Z.; et al. 2D Ti2C (MXene) as a Novel Highly Efficient and Selective Agent for Photothermal Therapy. Mater. Sci. Eng. C 2019, 98, 874–886. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.; Wang, Y.; Gao, S.; Chen, Y.; Shi, J. Theranostic 2D Tantalum Carbide (MXene). Adv. Mater. 2017, 30, 1703284. [Google Scholar]
- Lin, H.; Gao, S.; Dai, C.; Chen, Y.; Shi, J. A Two-Dimensional Biodegradable Niobium Carbide (MXene) for Photothermal Tumor Eradication in NIR-I and NIR-II Biowindows. J. Am. Chem. Soc. 2017, 139, 16235–16247. [Google Scholar] [CrossRef]
- Geim, A.K.; Grigorieva, I.V. Van Der Waals Heterostructures. Nature 2013, 499, 419–425. [Google Scholar] [CrossRef]
- Gan, L.Y.; Zhao, Y.J.; Huang, D.; Schwingenschlögl, U. First-Principles Analysis of MoS2/Ti2C and MoS2/Ti2CY2 (Y=F and OH) All-2D Semiconductor/Metal Contacts. Phys. Rev. B 2013, 87, 245307. [Google Scholar] [CrossRef]
- Paul, J.T.; Singh, A.; Dong, Z.; Zhuang, H.; Revard, B.C.; Rijal, B.; Ashton, M.; Linscheid, A.; Blonsky, M.; Gluhovic, D.; et al. Computational Methods for 2D Materials: Discovery, Property Characterization, and Application Design. J. Phys. Condens. Matter 2017, 29, 473001. [Google Scholar] [CrossRef] [PubMed]
- Jeon, J.; Park, Y.; Choi, S.; Lee, J.; Lim, S.S.; Lee, B.H.; Song, Y.J.; Cho, J.H.; Jang, Y.H.; Lee, S. Epitaxial Synthesis of Molybdenum Carbide and Formation of a Mo2C/MoS2 Hybrid Structure via Chemical Conversion of Molybdenum Disulfide. ACS Nano 2018, 12, 338–346. [Google Scholar] [CrossRef]
- Sun, Y.; Jin, D.; Sun, Y.; Meng, X.; Gao, Y.; Dall’Agnese, Y.; Chen, G.; Wang, X.F. G-C3N4/Ti3C2Tx (MXenes) Composite with Oxidized Surface Groups for Efficient Photocatalytic Hydrogen Evolution. J. Mater. Chem. A Mater. 2018, 6, 9124–9131. [Google Scholar] [CrossRef]
- Zhou, S.; Yang, X.; Pei, W.; Liu, N.; Zhao, J. Heterostructures of MXenes and N-Doped Graphene as Highly Active Bifunctional Electrocatalysts. Nanoscale 2018, 10, 10876–10883. [Google Scholar] [CrossRef]
- Cao, S.; Shen, B.; Tong, T.; Fu, J.; Yu, J. 2D/2D Heterojunction of Ultrathin MXene/Bi2WO6 Nanosheets for Improved Photocatalytic CO2 Reduction. Adv. Funct. Mater. 2018, 28, 1800136. [Google Scholar] [CrossRef]
- Nosaka, Y.; Nosaka, A.Y. Generation and Detection of Reactive Oxygen Species in Photocatalysis. Chem. Rev. 2017, 117, 11302–11336. [Google Scholar] [CrossRef] [PubMed]
- Ramírez-Sánchez, I.M.; Tuberty, S.; Hambourger, M.; Bandala, E.R. Resource Efficiency Analysis for Photocatalytic Degradation and Mineralization of Estriol Using TiO2 Nanoparticles. Chemosphere 2017, 184, 1270–1285. [Google Scholar] [CrossRef] [PubMed]
- Ribao, P.; Corredor, J.; Rivero, M.; Ortiz, I. Role of Reactive Oxygen Species on the Activity of Noble Metal-Doped TiO2 Photocatalysts. J. Hazard Mater. 2019, 372, 45–51. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Wang, Z.; Yang, Q.; Tan, L.; Dong, L.; Dong, M. Ultrathin Ti3C2Tx (MXene) Nanosheet-Wrapped NiSe2 Octahedral Crystal for Enhanced Supercapacitor Performance and Synergetic Electrocatalytic Water Splitting. Nanomicro Lett. 2019, 11, 31. [Google Scholar] [CrossRef] [PubMed]
- Rosales, M.; Zoltan, T.; Yadarola, C.; Mosquera, E.; Gracia, F.; García, A. The Influence of the Morphology of 1D TiO2 Nanostructures on Photogeneration of Reactive Oxygen Species and Enhanced Photocatalytic Activity. J. Mol. Liq. 2019, 281, 59–69. [Google Scholar] [CrossRef]
- Zoltan, T.; Rosales, M.; Yadarola, C. Reactive Oxygen Species Quantification and Their Correlation with the Photocatalytic Activity of TiO2 (Anatase and Rutile) Sensitized with Asymmetric Porphyrins. J. Environ. Chem. Eng. 2016, 4, 3967–3980. [Google Scholar] [CrossRef]
- Zhang, J.; Nosaka, Y. Mechanism of the OH Radical Generation in Photocatalysis with TiO2 of Different Crystalline Types. J. Phys. Chem. C 2014, 118, 10824–10832. [Google Scholar] [CrossRef]
- Tariq, A.; Ali, S.I.; Akinwande, D.; Rizwan, S. Efficient Visible-Light Photocatalysis of 2D-MXene Nanohybrids with Gd3+- and Sn4+-Codoped Bismuth Ferrite. ACS Omega 2018, 3, 13828–13836. [Google Scholar] [CrossRef]
- Ye, S.; Yan, M.; Tan, X.; Liang, J.; Zeng, G.; Wu, H.; Song, B.; Zhou, C.; Yang, Y.; Wang, H. Facile Assembled Biochar-Based Nanocomposite with Improved Graphitization for Efficient Photocatalytic Activity Driven by Visible Light. Appl. Catal. B Environ. 2019, 250, 78–88. [Google Scholar] [CrossRef]
- Rosales, M.; Garcia, A.; Fuenzalida, V.M.; Espinoza-González, R.; Song, G.; Wang, B.; Yu, J.; Gracia, F.; Rosenkranz, A. Unprecedented Arsenic Photo-Oxidation Behavior of Few- and Multi-Layer Ti3C2Tx Nano-Sheets. Appl. Mater. Today 2020, 20, 100769. [Google Scholar] [CrossRef]
- Mashtalir, O.; Cook, K.M.; Mochalin, V.N.; Crowe, M.; Barsoum, M.W.; Gogotsi, Y. Dye Adsorption and Decomposition on Two-Dimensional Titanium Carbide in Aqueous Media. J. Mater. Chem. A 2014, 2, 14334–14338. [Google Scholar] [CrossRef]
- Gao, Y.; Wang, L.; Zhou, A.; Li, Z.; Chen, J.; Bala, H.; Hu, Q.; Cao, X. Hydrothermal Synthesis of TiO2/Ti3C2 Nanocomposites with Enhanced Photocatalytic Activity. Mater Lett. 2015, 150, 62–64. [Google Scholar] [CrossRef]
- Chae, Y.; Kim, S.J.; Cho, S.Y.; Choi, J.; Maleski, K.; Lee, B.J.; Jung, H.T.; Gogotsi, Y.; Lee, Y.; Ahn, C.W. An Investigation into the Factors Governing the Oxidation of Two-Dimensional Ti3C2 MXene. Nanoscale 2019, 11, 8387–8393. [Google Scholar] [CrossRef] [PubMed]
- Pang, J.; Mendes, R.G.; Bachmatiuk, A.; Zhao, L.; Ta, H.Q.; Gemming, T.; Liu, H.; Liu, Z.; Rummeli, M.H. Applications of 2D MXenes in Energy Conversion and Storage Systems. Chem. Soc. Rev. 2019, 48, 72–133. [Google Scholar] [CrossRef]
- Kumar, S.; Lei, Y.; Alshareef, N.H.; Quevedo-Lopez, M.A.; Salama, K.N. Biofunctionalized Two-Dimensional Ti3C2 MXenes for Ultrasensitive Detection of Cancer Biomarker. Biosens. Bioelectron. 2018, 121, 243–249. [Google Scholar] [CrossRef]
- Lim, S.; Park, H.; Yang, J.; Kwak, C.; Lee, J. Stable Colloidal Dispersion of Octylated Ti3C2-MXenes in a Nonpolar Solvent. Colloids. Surf. A Physicochem. Eng. Asp. 2019, 579, 123648. [Google Scholar] [CrossRef]
- McDaniel, R.M.; Carey, M.S.; Wilson, O.R.; Barsoum, M.W.; Magenau, A.J.D. Well-Dispersed Nanocomposites Using Covalently Modified, Multilayer, 2D Titanium Carbide (MXene) and In-Situ “Click” Polymerization. Chem. Mater. 2021, 33, 1648–1656. [Google Scholar] [CrossRef]
- Bian, R.; Xiang, S.; Cai, D. Fast Treatment of MXene Films with Isocyanate to Give Enhanced Stability. ChemNanoMat 2020, 6, 64–67. [Google Scholar] [CrossRef]
- Guo, Q.; Zhang, X.; Zhao, F.; Song, Q.; Su, G.; Tan, Y.; Tao, Q.; Zhou, T.; Yu, Y.; Zhou, Z.; et al. Protein-Inspired Self-Healable Ti3C2 MXenes/Rubber-Based Supramolecular Elastomer for Intelligent Sensing. ACS Nano 2020, 14, 2788–2797. [Google Scholar] [CrossRef]
- Mayorga-Burrezo, P.; Muñoz, J.; Zaoralová, D.; Otyepka, M.; Pumera, M. Multiresponsive 2D Ti3C2Tx MXene via Implanting Molecular Properties. ACS Nano 2021, 15, 10067–10075. [Google Scholar] [CrossRef]
- Riazi, H.; Taghizadeh, G.; Soroush, M. MXene-Based Nanocomposite Sensors. ACS Omega 2021, 6, 11103–11112. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhang, X.; Zhang, W.; Ge, X.; Wang, Y.; Zou, X.; Zhou, X.; Zheng, W. MXene-based Quantum Dots Optimize Hydrogen Production via Spontaneous Evolution of Cl- to O-terminated Surface Groups. Energy Environ. Mater. 2022. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, W.; Zheng, W. Quantum Dots Compete at the Acme of MXene Family for the Optimal Catalysis. Nano-Micro Lett. 2022, 14, 158. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Liu, M.; Wang, Z.; Gu, Q.; Liu, B.; Zhao, C.; Zhang, J.; Xu, S.; Lu, M.; Li, H.; et al. Interlayer Environment Engineered MXene: Pre-Intercalated Zn2+ Ions as Intercalants Renders the Modulated Li Storage. J. Energy Chem. 2022, 68, 306–313. [Google Scholar] [CrossRef]
- Liu, M.; Qu, X.; Wang, Y.; Zhang, Y.; Zhao, C.; Liu, B.; Xue, X.; Zhang, J.; Wang, Z.; Li, J.; et al. Inspired by Philosophizing of “Point to Area”: Ion Pre-Intercalation Induces the Reconstitution of Interlayer Environment of MXene for Lithium Storage. Chem. Eng. J. 2023, 451, 139015. [Google Scholar] [CrossRef]
- Tan, S.; Zhao, Q.; Geng, Y.; Yin, J.; Zhou, C.; Zhang, P.; Chu, X.; Xu, S.; Lu, M.; Wang, L.; et al. Enhanced Lithium Storage Capacitance of Layered CoFe2O4&V2CTx Hybrid Anode Material Synthesized by In-Situ Hydrothermal Method. J. Alloys Compd. 2022, 918, 165778. [Google Scholar]
- Li, J.; Zhang, W.; Ge, X.; Lu, M.; Xue, X.; Wang, Z.; Yue, N.; Zhang, J.; Lang, X.; Jiang, Q.; et al. Etching-Courtesy NH4+ Pre-Intercalation Enables Highly-Efficient Li+ Storage of MXenes via the Renaissance of Interlayer Redox. J. Energy Chem. 2022, 72, 26–32. [Google Scholar] [CrossRef]






















| Samples | Molar Ratio |
|---|---|
| Ti3C2Cl2 | Ti3AlC2/CuCl2 = 1/4 |
| Ti3C2Br2 | Ti3AlC2/CuBr2 = 1/4 |
| Ti3C2I2 | Ti3AlC2/CuI = 1/6 |
| Ti3C2(ClBr) | Ti3AlC2/CuCl2/CuBr2 = 1/1/4 |
| Ti3C2(ClI) | Ti3AlC2/CuCl2/CuI = 1/1/6 |
| Ti3C2(BrI) | Ti3AlC2/CuBr2/CuI = 1/1/6 |
| Ti3C2(ClBrI) | Ti3AlC2/CuCl2//CuBr2/CuI = 1/1/1/6 |
| MXene | Termination | PBE (eV) | HSE06 (eV) |
|---|---|---|---|
| Sc2C | O | 1.8 [67], 1.84 [68], 1.86 [69] | 2.90 [70], 2.92 [71], 3.01 [69] |
| F | 1.0 [69,72], 1.03 [67,68], 1.05 [73] | 1.64 [73], 1.84 [70], 1.88 [69] | |
| OH | 0.34 [69], 0.44 [68], 0.45 [67], 0.71 [72] | 0.71 [69], 0.74 [70] | |
| Cl | 0.88 [70] | 1.64 [70] | |
| Ti2C | O | 0.17 [72], 0.24 [74], 0.33 [75] | 0.78 [76], 0.88 [74], 0.92 [77] |
| Zr2C | O | 0.66 [72], 0.88 [67], 0.95 [75] | 1.54 [77] |
| Hf2C | O | 0.8 [72], 1.00 [67,75] | 1.657 [78,79], 1.75 [77] |
| V2C | F | 0.56 [80] | |
| OH | 0.44 [80] | ||
| Cr2C | O | ||
| F | 0.22 [72] | 3.15 [79], 3.49 [81] | |
| OH | 0.03 [72] | 1.39 [79], 1.76 [81] | |
| Cl | 0.15 [72] | 2.56 [81] | |
| Mo2C | O | ||
| F | 0.25 [72] | ||
| OH | 0.1 [72] | ||
| Cl | 0.15 [72] | ||
| W2C | O | 0.194 [82] | 0.472 [82] |
| (Mo2/3Sc1/3)2C | O | 0.04 [83] | 0.58 [83] |
| (Mo2/3Y1/3)2C | O | 0.45 [83] | 1.23 [83] |
| (W2/3Sc1/3)2C | O | 0.675 [83] | 1.3 [83] |
| (W2/3Y1/3)2C | O | 0.625 [83] | 1.3 [83] |
| Mo1.33C | O2/3F1/3 | 0.5 [84] | |
| Hf3C2 | O | 0.155 [85] | |
| Hf2MnC2 | O | 0.238 [86] | |
| F | 1.027 [86] | ||
| Hf2VC2 | F | 0.4 [87] | 0.9 [87] |
| Mo2TiC2 | O | 0.041 [88], 0.052 [89] | 0.119 [88], 0.125 [89] |
| Mo2ZrC2 | O | 0.069 [88], 0.087 [89] | 0.125 [88], 0.147 [89] |
| Mo2HfC2 | O | 0.153 [88], 0.213 [89] | 0.238 [88], 0.301 [89] |
| W2TiC2 | O | 0.136 [88] | 0.290 [88] |
| W2ZrC2 | O | 1.170 [88] | 0.280 [88] |
| W2HfC2 | O | 0.285 [88] | 0.409 [88] |
| Cr2TiC2 | F | 1.35 [79] | |
| OH | 0.85 [79] |
| MXene | Magnetic Moments (Pristine) (μB) | Magnetic Moments (Termination Group) (μB) | ||
|---|---|---|---|---|
| -O | -F | -OH | ||
| Ti3C2 | 1.8~1.93 | — | Nonmagnetic | Nonmagnetic |
| Ti2N | 1.0~1.1 | — | — | — |
| Ti2C | 1.9~1.91 | — | — | — |
| Ti3N2 | 0.34/Ti atom | — | — | — |
| V2C | 0.16 | — | — | — |
| V2N | Nonmagnetic | — | — | — |
| Fe2C | 3.95 | — | — | — |
| Zr2C | 1.90 | — | — | — |
| Zr3C2 | 1.73 | — | — | — |
| Mn2N | — | 7.0 | 9.0 | 8.8 |
| Cr2C | 0.54/Cr atom | Nonmagnetic | 2.71/Cr atom | 2.24 |
| Cr2N | — | 5.6/Cr atom | 3.23/Cr atom | 3.01/Cr atom |
| Sc2N | — | 1.00 | — | — |
| Mn2N | — | 7.0 | 9.0 | 8.8 |
| (Ti2Mn)C2 | — | 2.97 | 4.24 | 3.90 |
| (Hf2Mn)C2 | — | 3.00 | 5.00 | 4.84 |
| (Hf2V)C2 | — | 1.00 | 1.27 | 1.33 |
| Ti4N3 | 7.00 | 0.37 | 0.88 | Nonmagnetic |
| (TiMn2)C2 | 16.3 | — | 4.0 | — |
| (TiCr2)C2 | 3.4 | 1.8 | 3.3 | 3.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tang, M.; Li, J.; Wang, Y.; Han, W.; Xu, S.; Lu, M.; Zhang, W.; Li, H. Surface Terminations of MXene: Synthesis, Characterization, and Properties. Symmetry 2022, 14, 2232. https://doi.org/10.3390/sym14112232
Tang M, Li J, Wang Y, Han W, Xu S, Lu M, Zhang W, Li H. Surface Terminations of MXene: Synthesis, Characterization, and Properties. Symmetry. 2022; 14(11):2232. https://doi.org/10.3390/sym14112232
Chicago/Turabian StyleTang, Mengrao, Jiaming Li, Yu Wang, Wenjuan Han, Shichong Xu, Ming Lu, Wei Zhang, and Haibo Li. 2022. "Surface Terminations of MXene: Synthesis, Characterization, and Properties" Symmetry 14, no. 11: 2232. https://doi.org/10.3390/sym14112232
APA StyleTang, M., Li, J., Wang, Y., Han, W., Xu, S., Lu, M., Zhang, W., & Li, H. (2022). Surface Terminations of MXene: Synthesis, Characterization, and Properties. Symmetry, 14(11), 2232. https://doi.org/10.3390/sym14112232







