Cerebral Arterial Asymmetries in the Neonate: Insight into the Pathogenesis of Stroke
Abstract
1. Introduction
2. Materials and Methods
2.1. Search Strategy and Selection Criteria
2.2. Neonatal Exclusion Criteria
2.3. Maternal Exclusion Criteria
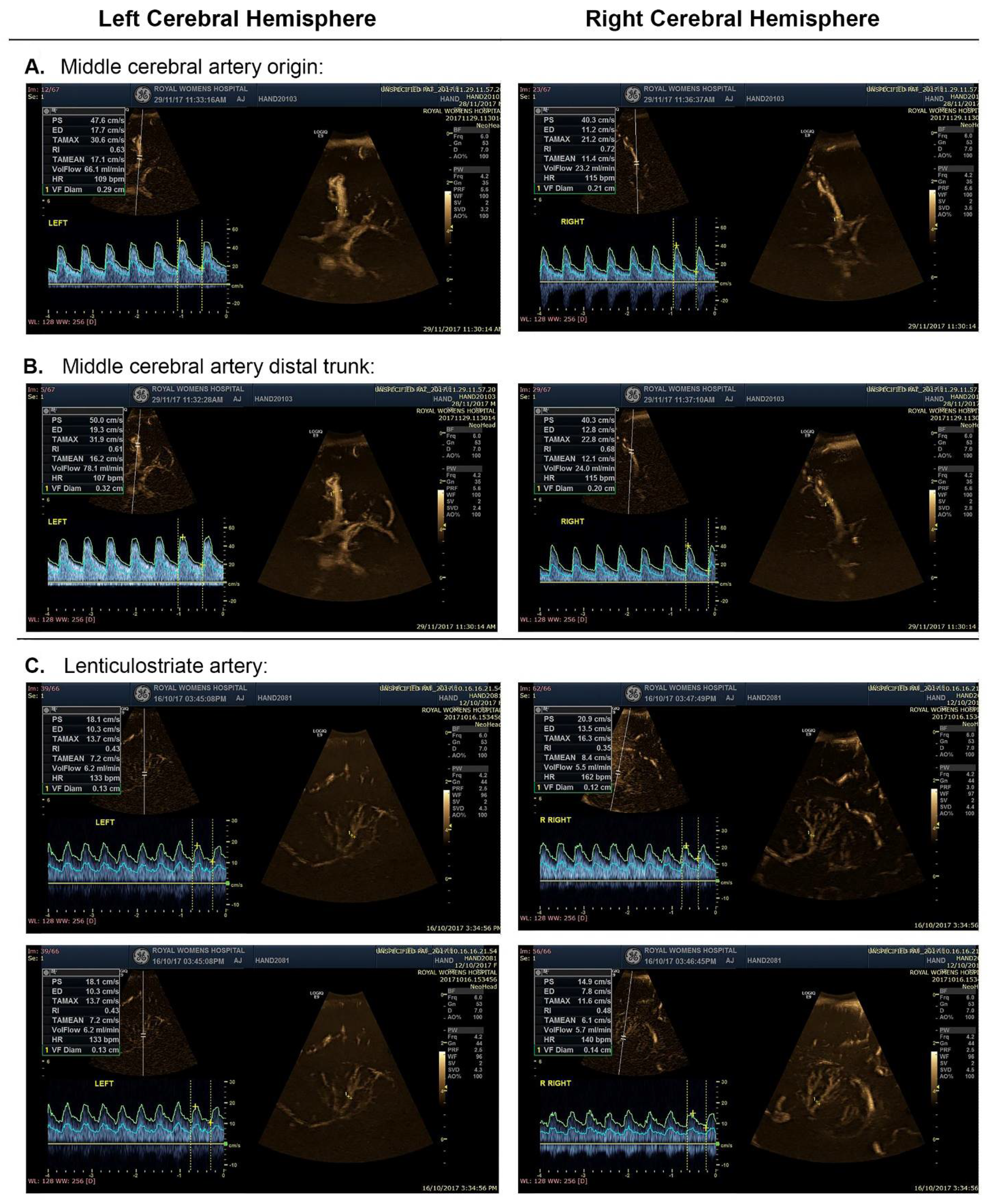
2.4. Procedure
2.5. Statistical Analyses
3. Results
3.1. Sex Differences
3.2. Structural Differences
3.3. Structurofunctional Differences
3.4. Shearing Stress Differences
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Foerch, C.; Misselwitz, B.; Sitzer, M.; Berger, K.; Steinmetz, H.; Neumann-Haefelin, T. Difference in recognition of right and left hemispheric stroke. Lancet 2005, 366, 392–393. [Google Scholar] [CrossRef]
- Hedna, V.S.; Bodhit, A.N.; Ansari, S.; Falchook, A.D.; Stead, L.; Heilman, K.M.; Waters, M.F. Hemispheric Differences in Ischemic Stroke: Is Left-Hemisphere Stroke More Common? J. Clin. Neurol. 2013, 9, 97–102. [Google Scholar] [CrossRef] [PubMed]
- Selwaness, M.; van den Bouwhuijsen, Q.; van Onkelen, R.S.; Hofman, A.; Franco, O.; van der Lugt, A.; Wentzel, J.J.; Vernooij, M. Atherosclerotic plaque in the left carotid artery is more vulnerable than in the right. Stroke 2014, 45, 3226–3230. [Google Scholar] [CrossRef] [PubMed]
- Denarié, N.; Gariepy, J.; Chironi, G.; Massonneau, M.; Laskri, F.; Salomon, J.; Levenson, J.; Simon, A. Distribution of ultrasonographically-assessed dimensions of common carotid arteries in healthy adults of both sexes. Atherosclerosis 2000, 148, 297–302. [Google Scholar] [CrossRef]
- Cagnie, B.; Petrovic, M.; Voet, D.; Barbaix, E.; Cambier, D. Vertebral artery dominance and hand preference: Is there a correlation? Man. Ther. 2006, 11, 153–156. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.; Yang, Y.; Cao, T.; Li, Z. Differences in left and right carotid intima–media thickness and the associated risk factors. Clin. Radiol. 2011, 66, 393–398. [Google Scholar] [CrossRef]
- Lin, P.-Y.; Roche-Labarbe, N.; Dehaes, M.; Fenoglio, A.; Grant, P.E.; Franceschini, M.A. Regional and Hemispheric Asymmetries of Cerebral Hemodynamic and Oxygen Metabolism in Newborns. Cereb. Cortex 2012, 23, 339–348. [Google Scholar] [CrossRef]
- Zhao, M.; Amin-Hanjani, S.; Ruland, S.; Curcio, A.; Ostergren, L.; Charbel, F. Regional Cerebral Blood Flow Using Quantitative MR Angiography. Am. J. Neuroradiol. 2007, 28, 1470–1473. [Google Scholar] [CrossRef]
- Kamath, S. Observations on the length and diameter of vessels forming the circle of Willis. J. Anat. 1981, 133, 419–423. [Google Scholar]
- Baldassarre, D.; Amato, M.; Bondioli, A.; Sirtori, C.R.; Tremoli, E. Carotid artery intima-media thickness measured by ultrasonography in normal clinical practice correlates well with atherosclerosis risk factors. Stroke 2000, 31, 2426–2430. [Google Scholar] [CrossRef]
- Herisson, F.; Heymann, M.-F.; Chétiveaux, M.; Charrier, C.; Battaglia, S.; Pilet, P.; Rouillon, T.; Krempf, M.; Lemarchand, P.; Heymann, D.; et al. Carotid and femoral atherosclerotic plaques show different morphology. Atherosclerosis 2011, 216, 348–354. [Google Scholar] [CrossRef] [PubMed]
- Oxenham, H.; Sharpe, N. Cardiovascular aging and heart failure. Eur. J. Hear. Fail. 2003, 5, 427–434. [Google Scholar] [CrossRef]
- Guzzetta, F.; Shackelford, G.D.; Volpe, S.; Perlman, J.M.; Volpe, J.J. Periventricular intraparenchymal endodensities in the premature newborn: Critical determinant of neurological outcome. Pediatrics 1986, 78, 995–1006. [Google Scholar] [CrossRef] [PubMed]
- Perlman, J.M.; Rollins, N.K.; Evans, D. Neonatal stroke: Clinical characteristics and cerebral blood flow velocity measurements. Pediatr. Neurol. 1994, 11, 281–284. [Google Scholar] [CrossRef]
- Uvebrant, P. Hemiplegic cerebral palsy. Aetiology and outcome. Acta Paediatr. Scand. Suppl. 1988, 345, 1–100. [Google Scholar] [CrossRef]
- Miller, V. Neonatal cerebral infarction. Semin. Pediatr. Neurol. 2000, 7, 278–288. [Google Scholar] [CrossRef]
- Coker, S.B.; Beltran, R.S.; Myers, T.F.; Hmura, L. Neonatal stroke: Description of patients and investigation into pathogenesis. Pediatr. Neurol. 1988, 4, 219–223. [Google Scholar] [CrossRef]
- Dahl, A.; Russell, D.; Nyberg-Hansen, R.; Rootwelt, K. A Comparison of Regional Cerebral Blood Flow and Middle Cerebral Artery Blood Flow Velocities: Simultaneous Measurements in Healthy Subjects. J. Cereb. Blood Flow Metab. 1992, 12, 1049–1054. [Google Scholar] [CrossRef]
- Oktar, S.; Yücel, C.; Karaosmanoglu, D.; Akkan, K.; Ozdemir, H.; Tokgoz, N.; Tali, T. Blood-Flow Volume Quantification in Internal Carotid and Vertebral Arteries: Comparison of 3 Different Ultrasound Techniques with Phase-Contrast MR Imaging. Am. J. Neuroradiol. 2006, 27, 363–369. [Google Scholar]
- Hayashi, T.; Ichiyama, T.; Uchida, M.; Tashiro, N.; Tanaka, H. Evaluation by color Doppler and pulsed Doppler sonography of blood-flow velocities in intracranial-arteries during the early neonatal-period. Eur. J. Pediatr. 1922, 151, 461–465. [Google Scholar] [CrossRef]
- Anwarm, M.A.; Bignall, W.; River, R.P.A. The variation with gestational age of the rheological properties of the blood of the new-born. Brit. J. Haematol. 1994, 86, 163–168. [Google Scholar] [CrossRef]
- Wu, Y.-C.; Hsieh, W.-S.; Hsu, C.-H.; Chiu, N.-C.; Chou, H.-C.; Chen, C.-Y.; Peng, S.-F.; Hung, H.-Y.; Chang, J.-H.; Chen, W.J.; et al. Relationship of Neonatal Cerebral Blood Flow Velocity Asymmetry with Early Motor, Cognitive and Language Development in Term Infants. Ultrasound Med. Biol. 2013, 39, 797–803. [Google Scholar] [CrossRef] [PubMed]
- Jahromi, A.S.; Cinà, C.S.; Liu, Y.; Clase, C.M. Sensitivity and specificity of color duplex ultrasound measurement in the estimation of internal carotid artery stenosis: A systematic review and meta-analysis. J. Vasc. Surg. 2005, 41, 962–972. [Google Scholar] [CrossRef] [PubMed]
- Bishop, C.C.; Powell, S.; Rutt, D.; Browse, N.L. Transcranial Doppler measurement of middle cerebral artery blood flow velocity: A validation study. Stroke 1986, 17, 913–915. [Google Scholar] [CrossRef]
- Kamouchi, M.; Kishikawa, K.; Okada, Y.; Inoue, T.; Ibayashi, S.; Iida, M. Reappraisal of Flow Velocity Ratio in Common Carotid Artery to Predict Hemodynamic Change in Carotid Stenosis. Am. J. Neuroradiol. 2005, 26, 957–962. [Google Scholar] [PubMed]
- Leutin, V.P.; Pystina, E.A.; Yarosh, S.V. Linear Blood Velocity in Arteries of the Brain Hemispheres in Left-Handers and Right-Handers during Hypoxia. Hum. Physiol. 2004, 30, 290–292. [Google Scholar] [CrossRef]
- Willis, M.W.; Ketter, T.A.; Kimbrell, T.A.; George, M.S.; Herscovitch, P.; Danielson, A.L.; Benson, B.E.; Post, R.M. Age, sex and laterality effects on cerebral glucose metabolism in healthy adults. Psychiatry Res. Neuroimaging 2002, 114, 23–37. [Google Scholar] [CrossRef]
- Gielecki, J.; Zurada, A.; Kozlowska, H.; Nowak, D.; Loukas, M. Morphometric and volumetric analysis of middle cerebral artery in human fetus. Acta Neurobiol. Exp. 2009, 69, 129–137. [Google Scholar]
- Seydel, H.G. The diameters of cerebral arteries of the human fetus. Anat. Rec. 1964, 150, 79–88. [Google Scholar] [CrossRef]
- Ebbing, C.; Rasmussen, S.; Kiserud, T. Middle cerebral artery blood flow velocities and pulsatility index and the cerebroplacental pulsatility ratio: Longitudinal reference ranges and terms for serial measurements. Ultrasound Obstet. Gynecol. 2007, 30, 287–296. [Google Scholar] [CrossRef]
- Kehrer, M.; Krägeloh-Mann, I.; Goelz, R.; Schöning, M. The Development of Cerebral Perfusion in Healthy Preterm and Term Neonates. Neuropediatrics 2003, 34, 281–286. [Google Scholar] [CrossRef]
- Pezzati, M.; Dani, C.; Biadaioli, R.; Filippi, L.; Biagiotti, R.; Giani, T.; Rubaltelli, F.F. Early postnatal Doppler assessment of cerebral blood flow velocity in healthy preterm and term infants. Dev. Med. Child Neurol. 2002, 44, 745–752. [Google Scholar] [CrossRef]
- Seffah, J.D.; Swarray-Deen, A. Fetal middle cerebral artery Doppler indices and clinical application at Korle Bu Teaching Hospital, Accra, Ghana. Int. J. Gynecol. Obstet. 2016, 134, 135–139. [Google Scholar] [CrossRef]
- Sinha, A.K.; Cane, C.; Kempley, S.T. Blood flow in the common carotid artery in term and preterm infants: Reproducibility and relation to cardiac output. Arch. Dis. Child.-Fetal Neonatal Ed. 2005, 91, F31–F35. [Google Scholar] [CrossRef][Green Version]
- Yoshida, H.; Yasuhara, A.; Kobayashi, Y. Transcranial Doppler sonographic studies of cerebral blood flow velocity in neonates. Pediatr. Neurol. 1991, 7, 105–110. [Google Scholar] [CrossRef]
- Bude, R.O.; Rubin, J.M. Relationship between the Resistive Index and Vascular Compliance and Resistance. Radiology 1999, 211, 411–417. [Google Scholar] [CrossRef]
- de Riva, N.; Budohoski, K.P.; Smielewski, P.; Kasprowicz, M.; Zweifel, C.; Steiner, L.A.; Reinhard, M.; Fábregas, N.; Pickard, J.D.; Czosnyka, M. Transcranial Doppler pulsatility index: What it is and what it isn’t. Neurocrit. Care 2012, 17, 58–66. [Google Scholar] [CrossRef]
- Ecury-Goossen, G.M.; Raets, M.M.A.; Camfferman, F.A.; Vos, R.H.J.; Van Rosmalen, J.; Reiss, I.K.M.; Govaert, P.; Dudink, J. Resistive indices of cerebral arteries in very preterm infants: Values throughout stay in the neonatal intensive care unit and impact of patent ductus arteriosus. Pediatr. Radiol. 2016, 46, 1291–1300. [Google Scholar] [CrossRef]
- Davies, P.F. Hemodynamic shear stress and the endothelium in cardiovascular pathophysiology. Nat. Clin. Pr. Cardiovasc. Med. 2009, 6, 16–26. [Google Scholar] [CrossRef]
- Slager, C.J.; Wentzel, J.J.; Gijsen, F.J.H.; Schuurbiers, J.C.H.; van der Wal, A.; Van Der Steen, A.F.W.; Serruys, P.W. The role of shear stress in the generation of rupture-prone vulnerable plaques. Nat. Clin. Pr. Cardiovasc. Med. 2005, 2, 401–407. [Google Scholar] [CrossRef]
- Naess, H.; Waje-Andreassen, U.; Thomassen, L.; Myhr, K.M. High incidence of left cerebral infarction among young adults. J. Stroke Cerebrovasc Dis. 2006, 15, 241–244. [Google Scholar] [CrossRef]
- Rodríguez Hernández, S.A.; Kroon, A.A.; van Boxtel, M.P.; Mess, W.H.; Lodder, J.; Jolles, J.; de Leeuw, P.W. Is there a side predilection for cerebrovascular disease? Hypertension 2003, 42, 56–60. [Google Scholar] [CrossRef]
- Lee, J.; Croen, L.A.; Backstrand, K.H.; Yoshida, C.K.; Henning, L.H.; Lindan, C.; Ferriero, D.M.; Fullerton, H.J.; Barkovich, A.J.; Wu, Y.W. Maternal and infant characteristics associated with perinatal arterial stroke in the infant. JAMA 2005, 293, 723–729. [Google Scholar] [CrossRef]
- Dammers, R.; Stifft, F.; Tordoir, J.H.M.; Hameleers, J.M.M.; Hoeks, A.P.G.; Kitslaar, P.J.E.H.M. Shear stress depends on vascular territory: Comparison between common carotid and brachial artery. J. Appl. Physiol. 2003, 94, 485–489. [Google Scholar] [CrossRef]
- Murray, C.D. The Physiological Principle of Minimum Work: I. The Vascular System and the Cost of Blood Volume. Proc. Natl. Acad. Sci. USA 1926, 12, 207–214. [Google Scholar] [CrossRef]
- Hong, Y.M. Atherosclerotic Cardiovascular Disease Beginning in Childhood. Korean Circ. J. 2010, 40, 1–9. [Google Scholar] [CrossRef]
- Menshawy, K.; Mohr, J.P.; Gutierrez, J.G.A. A Functional Perspective on the Embryology and Anatomy of the Cerebral Blood Supply. J. Stroke 2015, 17, 144–158. [Google Scholar] [CrossRef]
- Lemme, C.; Jogestrand, T.; de Faire, U. Carotid intima-media thickness and plaque in borderline hypertension. Stroke 1995, 26, 34–39. [Google Scholar]
- Önbaş, O.; Dane, Ş.; Kantarci, M.; Koplay, M.; Alper, F.; Okur, A. Clinical importance of asymmetry and handedness differences in common carotid artery intima-media thickness. Int. J. Neurosci. 2007, 117, 433–441. [Google Scholar] [CrossRef]
- Algra, A.; Gates, P.C.; Fox, A.J.; Hachinski, V.; Barnett, H.J. North American Symptomatic Carotid Endarterectomy Trial Group: Side of brain infarction and long-term risk of sudden death in patients with symptomatic carotid disease. Stroke 2003, 34, 2871–2875. [Google Scholar] [CrossRef]
- Jansen van Vuuren, A.; Saling, M.M.; Ameen, O.; Naidoo, N.; Solms, M. Hand preference is selectively related to common and internal carotid arterial asymmetry. Laterality 2016, 4, 377–398. [Google Scholar] [CrossRef]

| Left-Dominant | Right-Dominant | No Dominance | Total | |
|---|---|---|---|---|
| M (SD) | M (SD) | M (SD) | M (SD) | |
| Na | 57 | 34 | 6 | 97 |
| Sex (%) | ||||
| Male | 61.4 | 64.7 | 66.7 | 62.9 |
| Female | 39.6 | 35.3 | 33.3 | 37.1 |
| Gestational age at birth (wk) | 39.07 (1.45) | 38.76 (1.46) | 39.00 (1.67) | 38.99 (1.45) |
| Age at scan (hrs) | 48.44 (32.17) | 48.88 (23.94) | 38.00 (11.22) | 47.71 (28.58) |
| Birth weight (g) | 3418.51 (539.94) | 3525.15 (621.10) | 3428.00 (470.34) | 3460.76 (560.37) |
| AS1min | 8.40 (1.31) | 8.18 (1.49) | 8.5 (1.22) | 8.30 (1.38) |
| AS5min | 8.91 (0.39) | 8.97 (0.17) | 9 (0.00) | 8.92 (0.32) |
| Heart rate (bt/min) | 113.62 (14.64) | 111.82 (13.79) | 114.58 (17.07) | 113.87 (13.89) |
| Left-Dominant | Right-Dominant | No Dominance | Averaged a | ||
|---|---|---|---|---|---|
| Artery | Side | M (SD) | M (SD) | M (SD) | M (SD) |
| MCAO (mm) | n | 52 | 37 | 8 | 97 |
| L | 2.32 (0.35) | 1.95 (0.25) | 2.01 (0.17) | 2.13 (0.35) | |
| R | 1.91 (0.23) | 2.39 (0.43) | 2.01 (0.17) | 2.11 (0.39) | |
| p | 0.000 | 0.000 | - | 0.412 | |
| MCADT (mm) | n | 60 | 33 | 4 | 97 |
| L | 2.14 (0.35) | 1.87 (0.27) | 2.03 (0.20) | 2.04 (0.34) | |
| R | 1.81 (0.22) | 2.22 (0.34) | 2.03 (0.20) | 1.96 (0.33) | |
| p | 0.000 | 0.000 | - | 0.050 * | |
| MCAMEAN (mm) | n | 57 | 34 | 6 | 97 |
| L | 2.14 (0.35) | 1.86 (0.27) | 2.03 (0.16) | 2.09 (0.33) | |
| R | 1.81 (0.22) | 2.22 (0.35) | 2.03 (0.16) | 2.03 (0.34) | |
| p | 0.000 | 0.000 | - | 0.158 |
| Left Hemisphere | Right Hemisphere | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Artery | Dominance | Parameter | M | SD | M | SD | t | df | p | d |
| MCAO | Left | PSV (cm/s) | 54.00 | 10.23 | 51.24 | 11.28 | 2.307 | 51 | 0.013 * | 0.331 |
| EDV (cm/s) | 18.91 | 4.42 | 18.25 | 5.45 | 1.140 | 51 | 0.130 | 0.154 | ||
| VMEAN (cm/s) | 30.58 | 5.77 | 29.25 | 6.92 | 1.866 | 51 | 0.033 * | 0.257 | ||
| RI | 0.65 | 0.07 | 0.64 | 0.07 | 0.250 | 51 | 0.402 | 0.163 | ||
| PI | 0.96 | 0.14 | 0.96 | 0.15 | 0.195 | 51 | 0.423 | 0.000 | ||
| Q (mL/min) | 232.52 | 83.44 | 149.98 | 41.15 | 7.509 | 51 | 0.000 * | 1.048 | ||
| WSSSYS (dyne/cm2) | 80.36 | 18.77 | 91.32 | 22.39 | −5.118 | 51 | 0.000 * | −0.727 | ||
| WSSDIAS (dyne/cm2) | 2.80 | 0.68 | 3.24 | 1.00 | −2.022 | 51 | 0.000 * | −0.683 | ||
| Right | PSV (cm/s) | 56.04 | 14.85 | 57.67 | 14.62 | −0.945 | 36 | 0.176 | 0.155 | |
| EDV (cm/s) | 18.63 | 6.97 | 19.53 | 6.28 | −1.253 | 36 | 0.109 | 0.230 | ||
| VMEAN (cm/s) | 31.10 | 9.17 | 32.24 | 8.73 | −1.167 | 36 | 0.125 | 0.191 | ||
| RI | 0.67 | 0.07 | 0.66 | 0.06 | 0.857 | 36 | 0.199 | 0.187 | ||
| PI | 1.01 | 0.15 | 0.99 | 0.13 | 0.937 | 36 | 0.178 | 0.173 | ||
| Q (mL/min) | 167.15 | 52.67 | 266.11 | 123.28 | −5.630 | 36 | 0.000 * | 0.926 | ||
| WSSSYS (dyne/cm2) | 99.51 | 32.68 | 84.14 | 27.73 | 20.270 | 36 | 0.001 * | 0.572 | ||
| WSSDIAS (dyne/cm2) | 3.32 | 1.46 | 2.87 | 1.19 | 0.664 | 36 | 0.007 * | 0.432 | ||
| MCADT | Left | PSV (cm/s) | 53.26 | 12.72 | 51.03 | 14.23 | 1.947 | 59 | 0.028 * | 0.251 |
| EDV (cm/s) | 18.28 | 5.53 | 18.14 | 6.45 | 0.245 | 59 | 0.407 | 0.032 | ||
| VMEAN (cm/s) | 29.94 | 7.52 | 29.10 | 8.65 | 1.173 | 59 | 0.123 | 0.152 | ||
| RI | 0.66 | 0.06 | 0.65 | 0.07 | 1.773 | 59 | 0.041 * | 0.193 | ||
| PI | 0.99 | 0.14 | 0.96 | 0.15 | 1.726 | 59 | 0.045 * | 0.260 | ||
| Q (mL/min) | 193.68 | 71.06 | 130.20 | 37.92 | 8.093 | 59 | 0.000 * | 1.045 | ||
| WSSSYS (dyne/cm2) | 86.69 | 26.81 | 97.56 | 33.08 | −4.597 | 59 | 0.000 * | −0.628 | ||
| WSSDIAS (dyne/cm2) | 2.97 | 1.06 | 3.48 | 1.36 | −4.574 | 59 | 0.000 * | −0.625 | ||
| Right | PSV (cm/s) | 53.90 | 12.42 | 54.73 | 11.86 | −0.628 | 32 | 0.267 | 0.109 | |
| EDV (cm/s) | 17.06 | 5.52 | 17.56 | 4.98 | −0.735 | 32 | 0.233 | 0.127 | ||
| VMEAN (cm/s) | 29.58 | 7.71 | 29.95 | 6.70 | −0.422 | 32 | 0.338 | 0.073 | ||
| RI | 0.68 | 0.06 | 0.68 | 0.06 | 0.629 | 32 | 0.267 | 0.000 | ||
| PI | 1.03 | 0.15 | 1.03 | 0.15 | 0.588 | 32 | 0.267 | 0.000 | ||
| Q (mL/min) | 148.46 | 50.63 | 217.59 | 88.24 | −6.771 | 32 | 0.000 * | 1.178 | ||
| WSSSYS (dyne/cm2) | 100.26 | 30.39 | 84.80 | 20.63 | 4.705 | 32 | 0.000 * | 0.940 | ||
| WSSDIAS (dyne/cm2) | 3.16 | 1.22 | 2.71 | 0.80 | 3.081 | 32 | 0.002 * | 0.602 | ||
| Middle Cerebral Origin | Middle Cerebral Distal Trunk | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Source | df | F | p | ηp2 | df | F | p | ηp2 | |
| PSV | PSV | 1 | 0.266 | 0.607 | 0.003 | 1 | 0.584 | 0.447 | 0.006 |
| Geometric dominance | 1 | 2.902 | 0.092 | 0.032 | 1 | 0.663 | 0.418 | 0.007 | |
| PSV* Geometric dominance | 1 | 4.621 | 0.034 * | 0.050 | 1 | 2.795 | 0.098 | 0.030 | |
| EDV | EDV | 1 | 0.055 | 0.815 | 0.001 | 1 | 0.154 | 0.696 | 0.002 |
| Geometric dominance | 1 | 0.184 | 0.669 | 0.002 | 1 | 0.613 | 0.436 | 0.007 | |
| EDV* Geometric dominance | 1 | 2.877 | 0.093 | 0.032 | 1 | 0.484 | 0.488 | 0.005 | |
| VMEAN | VMEAN | 1 | 0.028 | 0.868 | 0.000 | 1 | 0.162 | 0.689 | 0.002 |
| Geometric dominance | 1 | 1.345 | 0.249 | 0.015 | 1 | 0.023 | 0.879 | 0.000 | |
| VMEAN* Geometric dominance | 1 | 4.382 | 0.039 * | 0.048 | 1 | 1.079 | 0.302 | 0.012 | |
| RI | RI | 1 | 0.580 | 0.448 | 0.007 | 1 | 2.441 | 0.122 | 0.026 |
| Geometric dominance | 1 | 2.390 | 0.126 | 0.027 | 1 | 4.779 | 0.031 * | 0.050 | |
| RI* Geometric dominance | 1 | 0.175 | 0.667 | 0.002 | 1 | 0.331 | 0.566 | 0.004 | |
| PI | PI | 1 | 0.658 | 0.419 | 0.008 | 1 | 2.242 | 0.138 | 0.024 |
| Geometric dominance | 1 | 2.312 | 0.132 | 0.026 | 1 | 4.818 | 0.031 * | 0.050 | |
| PI* Geometric dominance | 1 | 0.304 | 0.583 | 0.003 | 1 | 0.273 | 0.603 | 0.003 | |
| Q | Q | 1 | 0.693 | 0.408 | 0.008 | 1 | 0.188 | 0.665 | 0.002 |
| Geometric dominance | 1 | 3.294 | 0.073 | 0.036 | 1 | 3.131 | 0.080 | 0.033 | |
| Q* Geometric dominance | 1 | 84.636 | 0.000 * | 0.493 | 1 | 103.905 | 0.000 * | 0.533 | |
| WSSSYS | WSSSYS | 1 | 0.939 | 0.335 | 0.011 | 1 | 1.303 | 0.257 | 0.014 |
| Geometric dominance | 1 | 1.492 | 0.225 | 0.017 | 1 | 0.005 | 0.945 | 0.000 | |
| WSSSYS* Geometric dominance | 1 | 33.575 | 0.000 * | 0.278 | 1 | 43.048 | 0.000 * | 0.321 | |
| WSSDIAS | WSSDIAS | 1 | 0.001 | 0.976 | 0.000 | 1 | 0.046 | 0.861 | 0.001 |
| Geometric dominance | 1 | 0.109 | 0.742 | 0.001 | 1 | 1.367 | 0.245 | 0.015 | |
| WSSDIAS* Geometric dominance | 1 | 22.661 | 0.000 * | 0.207 | 1 | 27.122 | 0.000 * | 0.230 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
van Vuuren, A.J.; Saling, M.; Rogerson, S.; Anderson, P.; Cheong, J.; Solms, M. Cerebral Arterial Asymmetries in the Neonate: Insight into the Pathogenesis of Stroke. Symmetry 2022, 14, 456. https://doi.org/10.3390/sym14030456
van Vuuren AJ, Saling M, Rogerson S, Anderson P, Cheong J, Solms M. Cerebral Arterial Asymmetries in the Neonate: Insight into the Pathogenesis of Stroke. Symmetry. 2022; 14(3):456. https://doi.org/10.3390/sym14030456
Chicago/Turabian Stylevan Vuuren, Anica Jansen, Michael Saling, Sheryle Rogerson, Peter Anderson, Jeanie Cheong, and Mark Solms. 2022. "Cerebral Arterial Asymmetries in the Neonate: Insight into the Pathogenesis of Stroke" Symmetry 14, no. 3: 456. https://doi.org/10.3390/sym14030456
APA Stylevan Vuuren, A. J., Saling, M., Rogerson, S., Anderson, P., Cheong, J., & Solms, M. (2022). Cerebral Arterial Asymmetries in the Neonate: Insight into the Pathogenesis of Stroke. Symmetry, 14(3), 456. https://doi.org/10.3390/sym14030456






