Asymmetry Index Evaluation of Cerebral Volume and Cerebral Blood Flow in Neonatal Hypoxic–Ischemic Encephalopathy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Animal Models
2.2. MRI Acquisition
2.3. Image analysis
2.4. Statistical Analysis
3. Results
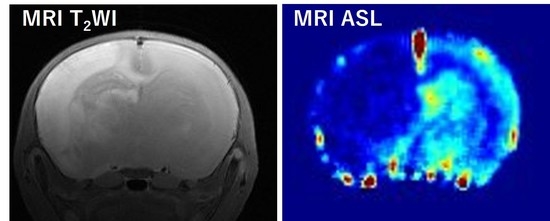
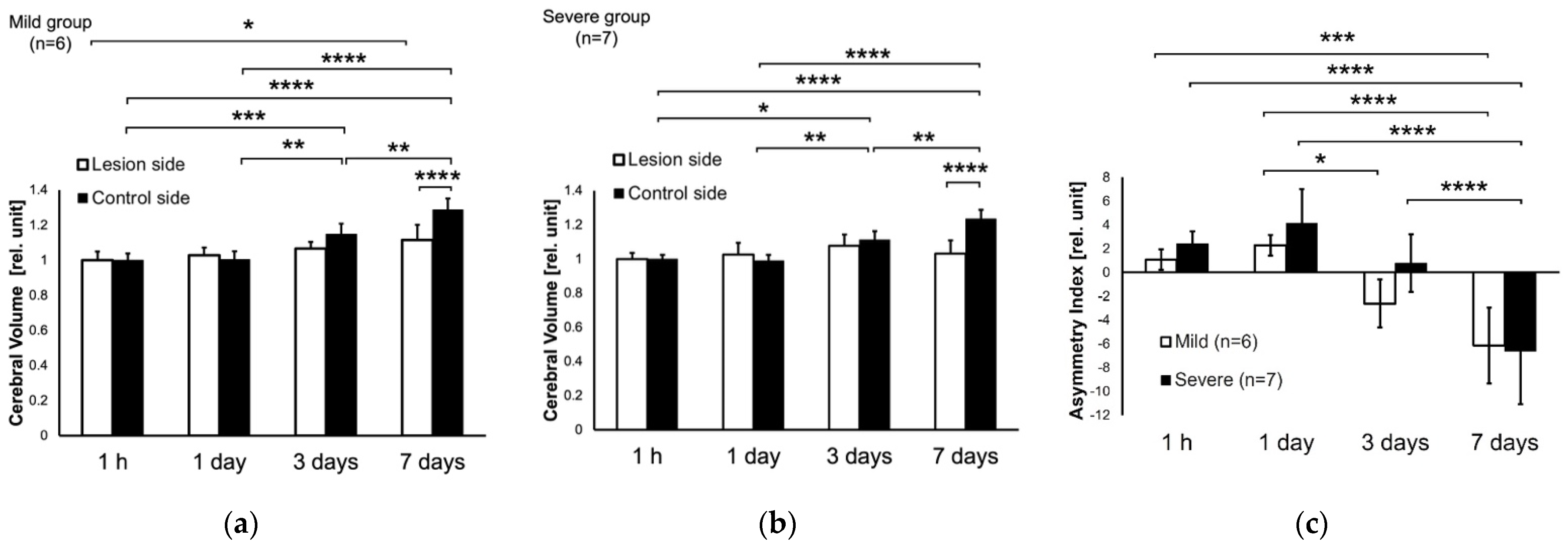
3.1. T2W Images at 1 h, and 1, 3, 7 Days after HIE
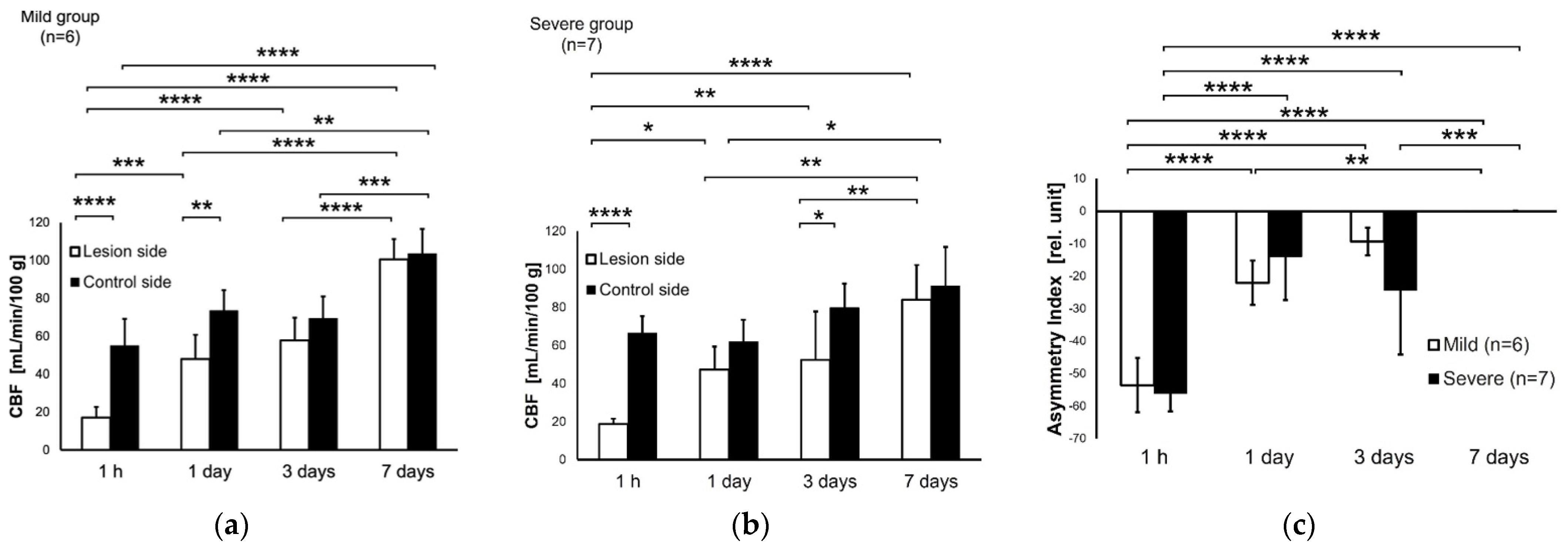
3.2. CBF at 1 h and 1, 3, 7 Days after HIE
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Van Handel, M.; Swaab, H.; de Vries, L.S.; Jongmans, M.J. Long-term cognitive and behavioral consequences of neonatal encephalopathy following perinatal asphyxia: A review. Eur. J. Pediatr. 2007, 166, 645–654. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lai, M.C.; Yang, S.N. Perinatal hypoxic-ischemic encephalopathy. J. Biomed. Biotechnol. 2011, 2011, 609813. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wassink, G.; Gunn, E.R.; Drury, P.P.; Bennet, L.; Gunn, A.J. The mechanisms and treatment of asphyxial encephalopathy. Front. Neurosci. 2014, 8, 40. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vannucci, R.C.; Vannucci, S.J. Perinatal hypoxic-ischemic brain damage: Evolution of an animal model. Dev. Neurosci. 2005, 27, 81–86. [Google Scholar] [CrossRef] [PubMed]
- Millar, L.J.; Shi, L.; Hoerder-Suabedissen, A.; Molnar, Z. Neonatal Hypoxia Ischaemia: Mechanisms, Models, and Therapeutic Challenges. Front. Cell Neurosci. 2017, 11, 78. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hamdy, N.; Eide, S.; Sun, H.S.; Feng, Z.P. Animal models for neonatal brain injury induced by hypoxic ischemic conditions in rodents. Exp. Neurol. 2020, 334, 113457. [Google Scholar] [CrossRef] [PubMed]
- Rice, J.E.; Vannucci, R.C.; Brierley, J.B. The influence of immaturity on hypoxic-ischemic brain damage in the rat. Ann. Neurol. 1981, 9, 131–141. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Silverstein, F.S.; Skoff, R.; Barks, J.D. Hypoxic-ischemic oligodendroglial injury in neonatal rat brain. Pediatr. Res. 2002, 51, 25–33. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, S.; Wu, E.X.; Tam, C.N.; Lau, H.F.; Cheung, P.T.; Khong, P.L. Characterization of white matter injury in a hypoxic-ischemic neonatal rat model by diffusion tensor MRI. Stroke 2008, 39, 2348–2353. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ten, V.S.; Wu, E.X.; Tang, H.; Bradley-Moore, M.; Fedarau, M.V.; Ratner, V.I.; Stark, R.I.; Gingrich, J.A.; Pinsky, D.J. Late measures of brain injury after neonatal hypoxia-ischemia in mice. Stroke 2004, 35, 2183–2188. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ohki, A.; Saito, S.; Hata, J.; Okano, H.J.; Higuchi, T.; Fukuchi, K. Neurite orientation dispersion and density imaging for evaluating the severity of neonatal hypoxic-ischemic encephalopathy in rats. Magn. Reson. Imaging 2019, 62, 214–219. [Google Scholar] [CrossRef]
- Doman, S.E.; Girish, A.; Nemeth, C.L.; Drummond, G.T.; Carr, P.; Garcia, M.S.; Johnston, M.V.; Kannan, S.; Fatemi, A.; Zhang, J.; et al. Early Detection of Hypothermic Neuroprotection Using T2-Weighted Magnetic Resonance Imaging in a Mouse Model of Hypoxic Ischemic Encephalopathy. Front. Neurol. 2018, 9, 304. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aden, U.; Dahlberg, V.; Fredholm, B.B.; Lai, L.J.; Chen, Z.; Bjelke, B. MRI evaluation and functional assessment of brain injury after hypoxic ischemia in neonatal mice. Stroke 2002, 33, 1405–1410. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.; Schneider, T.; Wheeler-Kingshott, C.A.; Alexander, D.C. NODDI: Practical in vivo neurite orientation dispersion and density imaging of the human brain. Neuroimage 2012, 61, 1000–1016. [Google Scholar] [CrossRef]
- Lodygensky, G.A.; Inder, T.E.; Neil, J.J. Application of magnetic resonance imaging in animal models of perinatal hypoxic-ischemic cerebral injury. Int. J. Dev. Neurosci. 2008, 26, 13–25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Silverstein, F.S.; Buchanan, K.; Hudson, C.; Johnston, M.V. Flunarizine limits hypoxia-ischemia induced morphologic injury in immature rat brain. Stroke 1986, 17, 477–482. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saito, S.; Takahashi, Y.; Ohki, A.; Shintani, Y.; Higuchi, T. Early detection of elevated lactate levels in a mitochondrial disease model using chemical exchange saturation transfer (CEST) and magnetic resonance spectroscopy (MRS) at 7T-MRI. Radiol. Phys. Technol. 2019, 12, 46–54. [Google Scholar] [CrossRef]
- Johnston, M.V.; Ishida, A.; Ishida, W.N.; Matsushita, H.B.; Nishimura, A.; Tsuji, M. Plasticity and injury in the developing brain. Brain Dev. 2009, 31, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Allen, K.A.; Brandon, D.H. Hypoxic Ischemic Encephalopathy: Pathophysiology and Experimental Treatments. Newborn Infant Nurs. Rev. 2011, 11, 125–133. [Google Scholar] [CrossRef] [Green Version]
- Rumpel, H.; Nedelcu, J.; Aguzzi, A.; Martin, E. Late glial swelling after acute cerebral hypoxia-ischemia in the neonatal rat: A combined magnetic resonance and histochemical study. Pediatr. Res. 1997, 42, 54–59. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rumpel, H.; Buchli, R.; Gehrmann, J.; Aguzzi, A.; Illi, O.; Martin, E. Magnetic resonance imaging of brain edema in the neonatal rat: A comparison of short and long term hypoxia-ischemia. Pediatr. Res. 1995, 38, 113–118. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sekhon, M.S.; Ainslie, P.N.; Griesdale, D.E. Clinical pathophysiology of hypoxic ischemic brain injury after cardiac arrest: A “two-hit” model. Crit. Care 2017, 21, 90. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, H.; Burris, M.; Fajilan, A.; Spagnoli, F.; Tang, J.; Zhang, J.H. Prolonged exposure to isoflurane ameliorates infarction severity in the rat pup model of neonatal hypoxia-ischemia. Transl. Stroke Res. 2011, 2, 382–390. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fleiss, B.; Gressens, P. Tertiary mechanisms of brain damage: A new hope for treatment of cerebral palsy? Lancet Neurol. 2012, 11, 556–566. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Banura, N.; Ohki, A.; Saito, S. Asymmetry Index Evaluation of Cerebral Volume and Cerebral Blood Flow in Neonatal Hypoxic–Ischemic Encephalopathy. Symmetry 2022, 14, 596. https://doi.org/10.3390/sym14030596
Banura N, Ohki A, Saito S. Asymmetry Index Evaluation of Cerebral Volume and Cerebral Blood Flow in Neonatal Hypoxic–Ischemic Encephalopathy. Symmetry. 2022; 14(3):596. https://doi.org/10.3390/sym14030596
Chicago/Turabian StyleBanura, Natsuo, Akiko Ohki, and Shigeyoshi Saito. 2022. "Asymmetry Index Evaluation of Cerebral Volume and Cerebral Blood Flow in Neonatal Hypoxic–Ischemic Encephalopathy" Symmetry 14, no. 3: 596. https://doi.org/10.3390/sym14030596