Abstract
Two minor phospholipids, i.e., mono- and/or dimethylphosphatidylethanolamines, are widespread in many organisms, from bacteria to higher plants and animals. A molecular mixture of methyl-PE and dimethyl-PE was obtained from total lipids by liquid chromatography and further identified by mass spectrometry. Total methyl-PE and dimethyl-PE were cleaved by phospholipase C, and the resulting diacylglycerols, in the form of acetyl derivatives, were separated into alkyl-acyl, alkenyl-acyl, and diacylglycerols. Reversed-phase LC/MS allowed dozens of molecular species to be identified and further analyzed. This was performed on a chiral column, and identification by tandem positive ESI revealed that diacyl derivatives from all four bacteria were mixtures of both R and S enantiomers. The same applied to alkenyl-acyl derivatives of anaerobic bacteria. Analysis thus confirmed that some bacteria biosynthesize phospholipids having both sn-glycerol-3-phosphate and sn-glycerol-1-phosphate as precursors. These findings were further supported by data already published in GenBank. The use of chiral chromatography made it possible to prove that both enantiomers of glycerol phosphate of some molecular species of mono- and dimethylphosphatidylethanolamines are present. The result of the analysis can be interpreted that the cultured bacteria do not have homochiral membranes but, on the contrary, have an asymmetric, i.e., heterochiral membranes.
1. Introduction
Phospholipids are a part of cells from the simplest microorganisms, i.e., from bacteria, to plants and animals. In addition to standard and dominant phospholipids several minor phospholipids, e.g., mono- and/or dimethylphosphatidylethanolamines (methyl-PE and/or dimethyl-PE) are also present [1]. Several pathways are known for their biosynthesis, and one pathway involves the sequential methylation of PE by S-adenosylmethionine [2]. Methyl-PE and dimethyl-PE have been identified as intermediates in PC biosynthesis. These reactions are catalyzed by phosphatidylethanolamine N-methyltransferase [3,4,5]. It is clear from Table S1 that the enzyme phosphatidylethanolamine N-methyltransferase, sometimes also called phospholipid N-methyltransferase, is present in all organisms studied, or at least present in a taxonomically close organism.
Furthermore, phospholipids with interesting distributions in nature are ether lipids, To date, these lipids have been identified in anaerobic bacteria and also in animals but not in fungi or plants [6]. Table S1 lists the relevant references in GenBank.
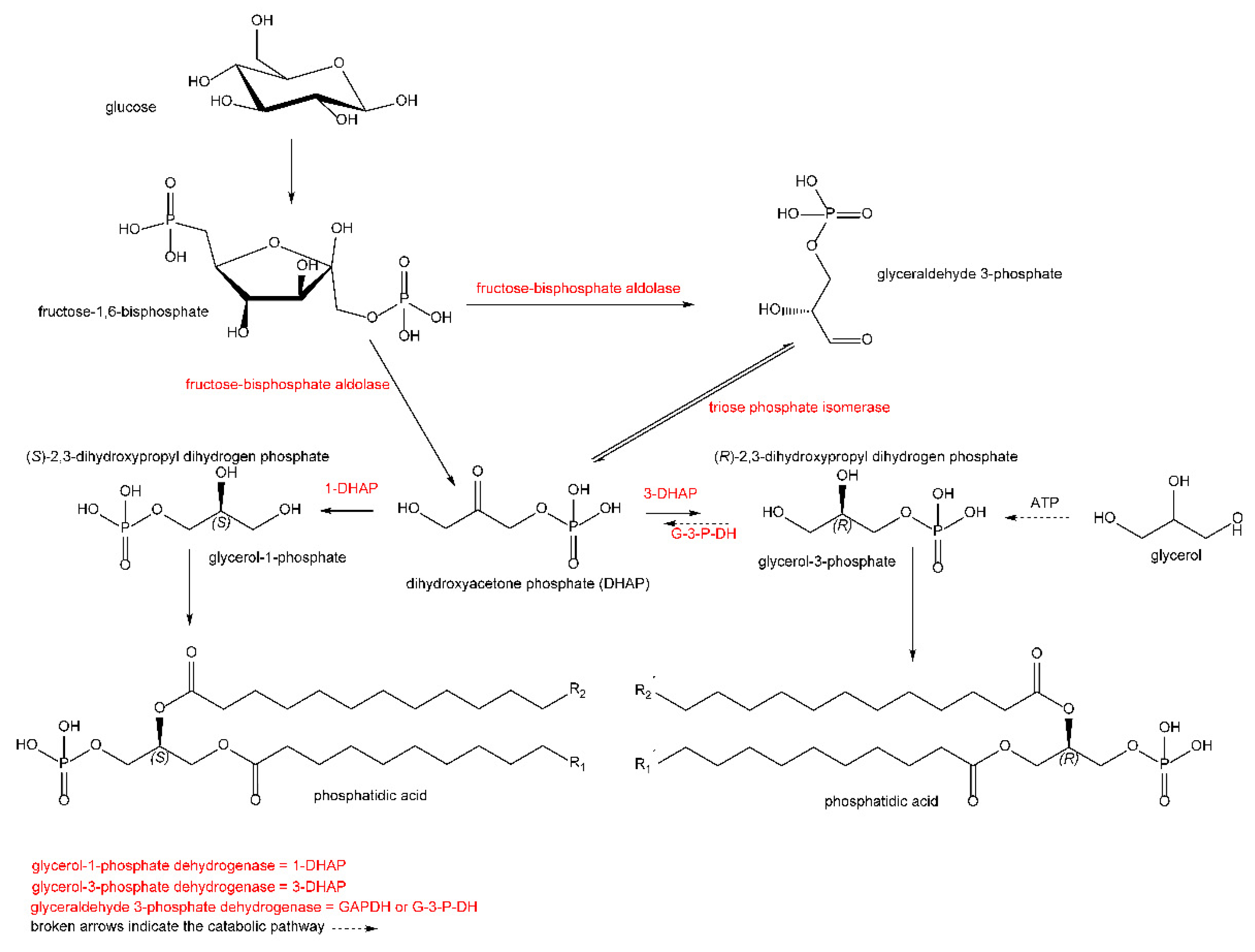
Another group of unusual lipids are glycerol derivatives, primarily phospholipids that have a unique chirality of the glycerol molecule. A common biosynthetic precursor-sn-glycerol-3-phosphate ((R)-2,3-dihydroxypropyl dihydrogen phosphate) (Figure 1) has been found in all bacteria and eukaryotes [7,8]. On the contrary, in archaeal lipids [9], its enantiomer is expected, i.e., sn-glycerol-1-phosphate ((S)-2,3-dihydroxypropyl dihydrogen phosphate). Some organisms may biosynthesize both enantiomers because both enzymes are present, e.g., in the bacterium, Arthrobacter are both glycerol-3-phosphate dehydrogenase (GenBank KUM38942.1) and glycerol-1-phosphate dehydrogenase (GenBank SDP79711.1). A recent article [10] states that bacteria from the Candidatus Cloacimonetes strain can contain mixed lipids based on glycerol-3-phosphate and glycerol-1-phosphate.
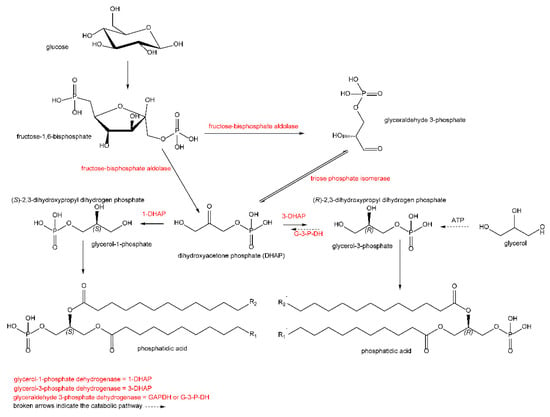
Figure 1.
Presumed biosynthesis of both enantiomers of phosphatidic acid. Both enantiomers and their sn-glycerols are designated according to the Cahn–Ingold–Prelog nomenclature.
One of the most effective methods of complex lipid analysis is based on liquid chromatography–mass spectrometry (LC–MS). In HILIC, lipids are separated based on the structure of polar head groups, while separation by chain length, number, and configuration of double bonds, etc., and is only partial. In contrast, by RP-HPLC, lipids are separated mainly according to the length and unsaturation of hydrocarbon chains [11,12].
Analysis of PE, methyl-PE, dimethyl-PE, and PC and other phospholipids was performed, for example, using precursor ion scanning (PIS) and neutral loss scanning (NLS) [13]. Another possibility is nitrogen derivatization using deuterated methyl iodide (CD3I) to give a PC containing 3, 6, or 9 deuterium atoms [14].
From various organelles of the yeast Saccharomyces cerevisiae, in addition to common phospholipids, methyl-PE were also identified [15]. Analysis of several yeast species by LC-MS, including S. cerevisiae, showed that the concentrations of methyl-PE and dimethyl-PE were approximately an order of magnitude lower than that of conventional phosphatidylethanolamine and phosphatidylcholine (PE, PC) [16]. Lipid extracts from four phyla of Planctomycetes bacteria [17] were analyzed using LC-HR/MS, and PE, PC, methyl-PE, and dimethyl-PE were identified in the genus Gemmata. In addition, shotgun lipidomics has made it possible to identify eight classes of lipids in the soil bacterium Sinorhizobium meliloti, including methyl-PE and dimethyl-PE [18].
Phospholipase C hydrolyzes the polar head group of phospholipids to alkyl-acyl, alkenyl-acyl, and diacyl-glycerols, which can be identified by HPLC [19,20]. A method using LC–MS/MS has been developed to separate individual plasmalogens and identify 1-O-alk-1′-enyl and 1-O-alkyl ether lipids in wild and genetically modified mice [21]. During the separation of plasmalogens and diacyl phospholipids, plasmenyl PE is usually eluted before diacyl PE [22,23]. The use of 2D chromatography has been used for a detailed analysis of plasmalogens, for example, in pig organs [24], mouse brain [25], blood plasma and induced sputum samples [26], and also in soil microbes [27]. Almost half a century ago, one of the first analyzes of alkenyl-acyl, alkyl-acyl, and diacylglycerols was performed as isolated acetates of total bovine brain phospholipids [28].
Based on these articles and our previous reports [29,30] that describe the identification of regioisomers and enantiomers of different lipids, lipid classes from fourteen organisms or their organs were analyzed. Using several analytical techniques (see below), all animal sources were shown to contain methyl-PE and dimethyl-PE, and anaerobic bacteria also contained plasmalogens; in all four bacteria, both enantiomers of glycerol and alkenyl-acyl or diacyl phospholipids were identified.
2. Materials and Methods
2.1. Chemicals and Standards
For a full description of all chemical compounds, see the Supplementary Materials. Two triacylglycerols (TAG) enantiomers (1-palmitoyl-2-oleoyl-3-acetyl-sn-glycerol, i.e., ((S)-1-acetoxy-3-(palmitoyloxy)propan-2-yl oleate) and its enantiomer 3-palmitoyl-2-oleoyl-1-acetyl-sn-glycerol, i.e., ((R)-1-acetoxy-3-(palmitoyloxy)propan-2-yl oleate)), see Figure S1, were prepared according to previously published papers [31,32].
2.2. Sample Preparation of Organisms
Four bacteria were cultured under standard conditions (see the Supplementary Materials). Briefly, the cultivation of Gram-positive Kocuria rhizophila CCM 552 has been described previously [30]. In addition, a description of the cultivation of the Gram-negative bacterium of the genus Raoultella sp. KDF8 has been published [33]. The culture conditions of two anaerobic bacteria, i.e., Pectinatus frisingensis (DSM 20465) and Megasphaera cerevisiae (DSM 20461) are described in detail in Supplementary Materials. Furthermore, a description of the cultivation of two algae, i.e., the extremophilic unicellular red alga Galdieria sulphuraria (ACUF 002) [34] and the green alga Desmodesmus quadricauda (CCALA 463) [35], are described in the Supplementary Materials. The mold Aspergillus niger was cultivated under conditions described in the Supplementary Materials. Mushroom Laetiporus sulphureus was collected on an oak stump near Prague. Saccharomyces pastorianus, a bottom fermenting brewer’s yeast, was obtained from a large brewery in the Czech Republic (yearly beer production of 130,000,000 L) [36]. The freshwater sponge Spongilla lacustris was collected in the Opatovický pond near Třeboň (South Bohemia). Carp (Cyprinus carpio) was purchased from the company Rybářství Třeboň a.s. (Fishing Třeboň). Beef heart and chicken breast were purchased from a local butcher, while spinach was from a gardener, respectively. Cells of all microorganisms were separated by centrifugation and lyophilized.
2.3. Extraction of Lipids
The extraction procedure was based on the method described by Bligh and Dyer [37] with modifications as previously described [38]. Briefly, lyophilized samples were suspended in a dichloromethane-methanol mixture (2:1, v/v) for 30 min with stirring, after which dichloromethane and water were added. The dichloromethane phase was evaporated to dryness under reduced pressure. Total lipids were dissolved in the mobile phase (acetonitrile: 2-propanol (99:1, v/v)) for further analysis.
2.4. Semipreparative HILIC-ESI-MS
Semipreparative HPLC equipment consisted of a 1090 Win system, a PV5 ternary pump (400 bar pressure (5801 psi)), an automatic injector (HP 1090 series, Hewlett Packard, Palo Alto, CA, USA) and a HILIC HPLC ZIC®-HILIC 250 × 10 mm, 5 μm, 200 Å column. Elution with a flow rate of 4.5 mL/min was performed and a linear gradient was carried out from the mobile phase containing methanol/acetonitrile/aqueous 1 mM ammonium acetate (50:30:20, v/v/v) to methanol/acetonitrile/aqueous 1 mM ammonium acetate (10:70:20, v/v/v) for 60 min. The column temperature was 35 °C and the re-equilibration period between runs was 30 min. The efficiency of the column was approximately 11,000 plates/25 cm. 1-Palmitoyl-2-oleoyl-glycero-3-phosphocholine was used as an external standard. The eluent from the HPLC column was split so that 2% of the flow was introduced to ESI-MS and 98% of the flow containing fractions of lipid classes were collected manually. Intervals of collection for fraction of dimethyl-PE and methyl-PE were 22.25–23.15 min and 24.50–25.45 min, respectively. The LTQ Orbitrap Velos (Thermo Fisher Scientific, Bremen, Germany) high-resolution hybrid mass spectrometer was used for ESI-MS analysis under conditions described below. Fractions of lipid classes were further evaporated and redissolved in diethyl ether for hydrolysis (chemical and enzymatic, respectively).
2.5. Analysis of Aldehydes and Fatty Acids from Phospolipids
To analyze plasmalogens, the eluted methyl-PE and/or dimethyl-PE from semipreparative HILIC were derivatized [39,40]. Briefly, fatty acid methyl esters (FAME) and aldehyde dimethylacetals (DMA) were prepared using lipids and BF3 (14% in methanol, 15 min at 70 °C) and extracted with hexane. The extracts were analyzed by GC-MS. A detailed description of additional preparations of derivatives (free alcohols, nicotinates [41], and 3-pyridylcarbonyl esters [42]) and their GC-MS analysis is provided in the Supplementary Materials. Mass spectra in the electron ionization (EI) mode were analyzed as previously described [43,44,45].
2.6. Labeling with Deuterated CD3I
Two separated lipid fractions (methyl-PE and dimethyl-PE) were dissolved in 75 μL CD3I, heated for 3 h at 90 °C, and the reaction was terminated by evaporation. Methylated lipids were extracted in a mixture of 0.1 mL of CHCl3-MeOH (1:1, v/v) and used for shotgun ESI-MS.
2.7. Shotgun Lipidomics
An LTQ-Orbitrap Velos mass spectrometer (Thermo Fisher Scientific, San Jose, CA, USA), a high-resolution hybrid mass spectrometer equipped with a heated electrospray interface (H-ESI), was operated in positive and negative ionization modes. Flow injection analysis (FIA) was used for sample introduction into the H-ESI ion source. Acetonitrile/water (50:50, v/v) was used at a flow rate of 150 μL/min. The MS scan range was performed in the Fourier transform mode and recorded within a window between 200–1000 m/z. Mass spectra were acquired with a target mass resolution of R = 110,000 at m/z 800. The ion spray voltage was set at 3.5 kV (in the positive ionization mode) and −2.5 kV (in the negative ionization mode). Both ionization modes used the following parameters: sheath gas flow, 18 arbitrary units (AU); auxiliary gas flow, 7 AU; ion source temperature, 250 °C; capillary temperature, 230 °C; capillary voltage, 50 V (in-source dissociation potential); and tube lens voltage, 170 V. Helium was used as a collision gas for collision-induced dissociation (CID) experiments. The CID normalization energy of 35% was used for the fragmentation of parental ions. The MS/MS product ions were detected in high-resolution FT mode. Calibration of the MS spectrometer was conducted using a Pierce LTQ Orbitrap positive and/or negative ion calibration solution (Thermo Fisher Scientific, San Jose, CA, USA). The internal lock mass was used in the acquisition of mass spectra, i.e., 255.2330 m/z [M − H]− palmitic acid in negative ESI. The mass accuracy was better than 0.9 ppm. The chemical structures of the compounds were confirmed with the help of the LIPID MAPS® Lipidomics Gateway spectral database (http://www.lipidmaps.org/; accessed on 11 February 2022).
2.8. Phospholipase C Hydrolysis
Phospholipid (~1 mg) and commercially available Bacillus cereus phospholipase C (2.5 units) were dispersed in 1 mL of Tris buffer (17.5 mM; pH 7.3) with 1% calcium chloride (0.1 mL) and diethyl ether (0.3 mL) [46]. The mixture was shaken vigorously at 37 °C for approximately 45 min. The degree of hydrolysis was determined by TLC (silica gel with fluorescent indicator on 20 cm × 20 cm TLC plates; Merck, Prague, Czech Republic), mobile phase dichloromethane-methanol-acetic acid (65:25:8, v/v/v). The resulting glycerol derivatives were acetylated to monoacetyldiradylglycerols [47] by standing overnight in a mixture of acetic anhydride/pyridine (3:2, v/v) at 20 °C. These derivatives of glycerol were further separated and identified.
2.9. Separation of Subclasses—Alkyl-Acyl, Alkenyl-Acyl, and Diacylglycerols
Acetyl diradylglycerols were analyzed by LC–MS on a Zorbax Rx-SIL Semi-Prep HPLC Column L × I.D. 250 × 9.4, particle size 5 µm, Agilent Technologies (Munich, Germany) using as mobile phase, n-hexane–isopropanol (99:1, v/v). The column was maintained at 30 °C and the flow rate was 3.1 mL/min.
2.10. Separation and Identification of Acetyldiradylglycerols by RP-HPLC/MS2
The LC equipment consisted of a 1090 Win system, PV5 ternary pump and automatic injector (HP 1090 series, Agilent, Santa Clara, CA, USA), and three Luna Omega 1.6 µm, C18, 100 Å, LC Columns L × I.D. 150 × 2.1 mm, connected in series. Although the manufacturer’s efficiency was not achieved, i.e., 350,000 theoretical plates/meter (see the manufacturer’s literature), we achieved an efficiency of ~311,000 theoretical plates/meter. 1-Palmitoyl-2-oleoyl-3-acetylglycerol with tR 63.76 min was used as an external standard, with a flow rate of 0.95 mL/min, an injection volume of 10 μL, and column temperature of 25 °C. The gradient program with the solvent mixture from 50% A and 50% B to 100% B was used. Solvent A contained acetonitrile-2-propanol (1:1, v/v) and solvent B had acetonitrile-2-propanol (2:3, v/v), both containing 2.5 mmol/L of ammonium acetate from 5 min to 105 min, and held for 10 min. The composition was returned to the initial conditions for 10 min. The 10 % HPLC flow was introduced into the ESI source, and 90% of the flow containing molecular species of acetyl-diradylglycerols was collected manually. For ESI-MS analysis, the LTQ Orbitrap Velos was used under conditions as described above. The two enantiomeric diacylglycerols obtained by hydrolysis of commercially available standards (1-palmitoyl-2-oleoyl-sn-glycerol and 3-palmitoyl-2-oleoyl-sn-glycerol) were also acetylated and the resulting triacylglycerols (1-palmitoyl-2-oleoyl-3-acetyl-sn-glycerol and 3-palmitoyl-2-oleoyl-1-acetyl-sn-glycerol) were used as external standards. Previously published information [48,49,50] was used in this analysis.
2.11. Chiral Analysis of Acetyldiradylacetyl Glycerols by HPLC-ESI-MS2
The LC system used for separation in the chiral mode was the same as the system used in the reversed-phase mode. Acetyldiradylacetyl glycerols (~1 mg/mL in mobile phase) were chromatographically separated on two Astec cyclobond TM I 2000 DMP (3,5-dimethyl-phenyl-carbamate modified β-cyclodextrin) chiral LC columns (5 μm, 25 cm × 4.6 mm) (Sigma-Aldrich, Prague, Czech Republic) connected in series. The mobile phase was a gradient from 95% A and 5% B at 0 min to 45% A and 55% B over 180 min, and then isocratically maintained for 30 min, where A was hexane and B was a hexane-2-propanol (97:3, v/v) mixture [29,51]. The flow rate, column temperature, and injection volume were 0.45 mL/min, 24 °C, and 10 μL, respectively. Other conditions were the same as those used for RP-HPLC/MS2, as described above. The elution order of enantiomers from the chiral column was determined based on the tR of both synthetically prepared isomers (see Chemical and standards).
2.12. Lipid Nomenclature
Lipid nomenclature was applied according to Liebisch et al. [52].
3. Results and Discussion
3.1. Analysis of Fatty Acids and Aldehydes
Individual samples from all fourteen organisms were lyophilized and extracted according to Bligh–Dyer [37] using a modification by inactivating phospholipases [38]. Hydrolytic products of total lipids were analyzed and identified by GC–MS. Analysis of fatty acid methyl esters (FAME) and dimethylacetals of aldehydes (DMA) confirmed the presence of fatty acids and aldehydes derived from 1-O-1′-(Z)-chains (vinyl ethers).
Based on the GC–MS of DMA, it was assumed that in addition to straight chains, the branched chains plasmalogens were also present in bacteria of the genus Kocuria. Therefore, free aldehydes were reduced with lithium aluminum hydride, and the resulting alcohols were derivatized with nicotinic acid (see Supplementary Materials). The resulting derivatives can be identified by GC–MS as nicotinate esters [53,54]. These are structural analogs of 3-pyridylcarbinol esters (formerly picolinyl esters), and their fragmentation is similar. In the mass spectrum of all nicotinate esters with long-chain fatty alcohols, the two most abundant ions are at m/z 107.0 and 124.1.
The last methyl (15 Da) was lost to form an ion at m/z 332.3, and ions differing by 14 Da were also present. Nicotinate of branched fatty alcohols had a gap of 28 Da between m/z 304.3 and 332.3, i.e., 14-methylhexadecanol (anteisoisomer) and/or a gap of 28 Da between m/z 318.3 and 346.3, i.e., 15-methylhexadecanol (iso-isomer) (Figures S2 and S3). This proved the presence of branched alkenyl ethers. In addition, the tR of the branched and unbranched derivatives were found to differ, as previously published [53]. The elution order was iso-, anteiso-, and straight-chain nicotinates.
In the case of the freshwater sponge, the structure of less common acids, i.e., called demospongic acids (long chain fatty acids with a Δ5,9 unsaturation system), was confirmed using GC-MS of 3-pyridylcarbinol esters. In the mass spectrum of 3-pyridylcarbinyl 5,9,19-pentacosatrienoate (5,9,19–25:3), key ions determining the Δ5,9 double bond structure were identified; these are ions at m/z 219 and 272, which indicate cleavage between carbons 7 and 8 and between 11 and 12, respectively. The ions at m/z 178, 204, 232, 258, 370, and 396 also located the double bonds, with the last pair confirming the position of the double bond Δ19 (see Figure S4).
3.2. Hydrophilic Interaction Liquid Chromatography-HILIC
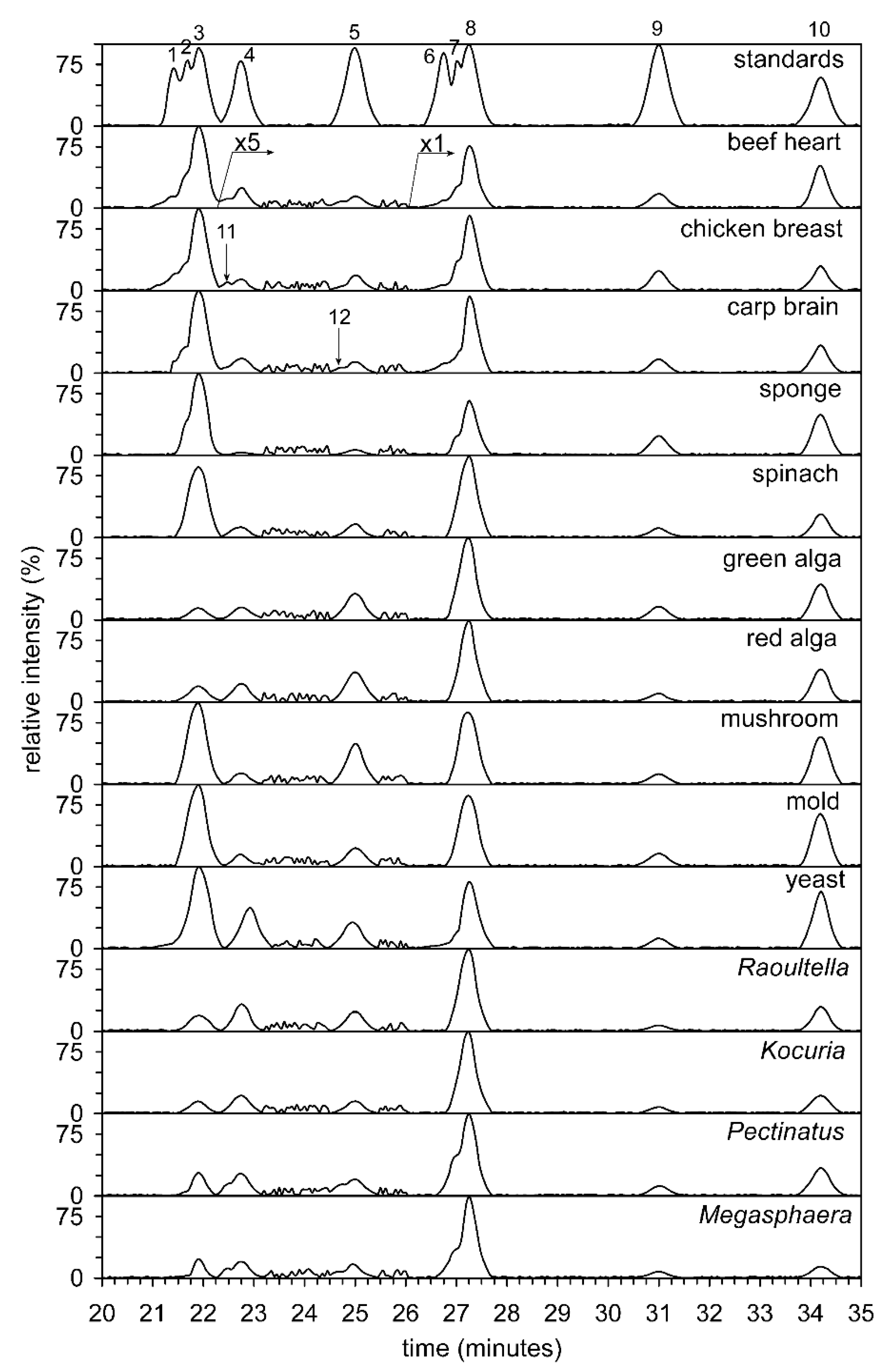
The total lipids of all 14 organisms or their organs were analyzed by HILIC (see Figure 2). Unfortunately, the separation of commercial alkyl-acyl, alkenyl-acyl, and diacyl standards of PC and PE was unsuccessful. In contrast, the separation of diacyl standards of PC, dimethyl-PE, methyl-PE, and PE was not a problem, and the classes of phospholipids were separated on a baseline. The analysis time as a result of using a semipreparative column, diameter 10 mm, was from 21 to 28 min. The abundance of different classes of phospholipids is shown in Figure 3 (heat maps). The preliminary structure of individual classes of phospholipids was determined based on retention times (tR) compared to standards. Two fractions of phospholipids, i.e., methyl- and dimethyl-PE i.e., including alkyl-acyl, alkenyl-acyl, and diacyl forms, were collected manually and further analyzed by direct-inlet high-resolution negative ESI (see below).
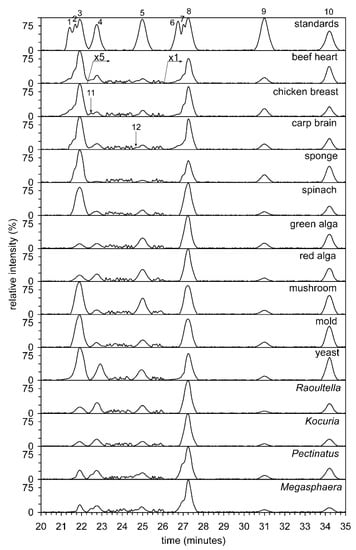
Figure 2.
Hydrophilic interaction liquid chromatography-HILIC/ESI-MS separation of the major lipid classes of the 14 different biological sources (beef heart, chicken breast, carp brain, sponge, spinach, green alga, red alga, mushroom, mold, yeast, Gram-positive bacterium Kocuria, G- bacterium Raoultella, beer spoilage bacteria Pectinatus and Megasphaera). The TIC (total ion current) was filtered and exhibits ions in the interval from 200 to 1500 Da. Abbreviations: alkyl-acyl-phosphatidylcholines (1), alk-1-enyl-acyl-phosphatidylcholines (2), phosphatidylcholines (3), dimethylphosphatidylethanolamines (4), monophosphatidylethanolamines (5), alkyl-acyl-phosphatidylethanolamines (6), alk-1-enyl-acyl-phosphatidylethanolamines (7), phosphatidylethanolamines (8), lysophosphatidylcholines (9), phosphatidylinositols (10), plasmenyl-dimethylphosphatidylethanolamines (11), plasmenyl-methylphosphatidylethanolamines (12). The TIC was filtered and indicates ions in the interval from 200 to 800 Da.
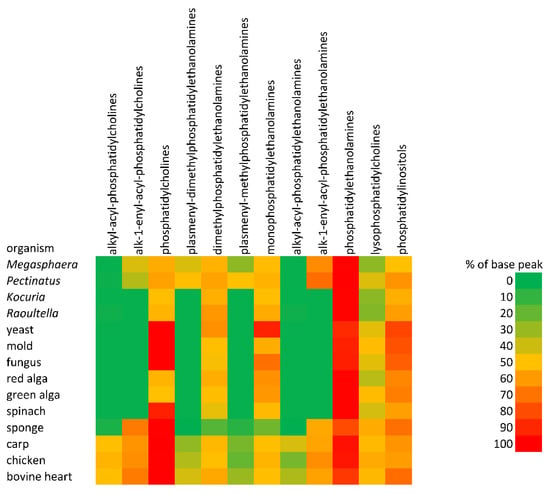
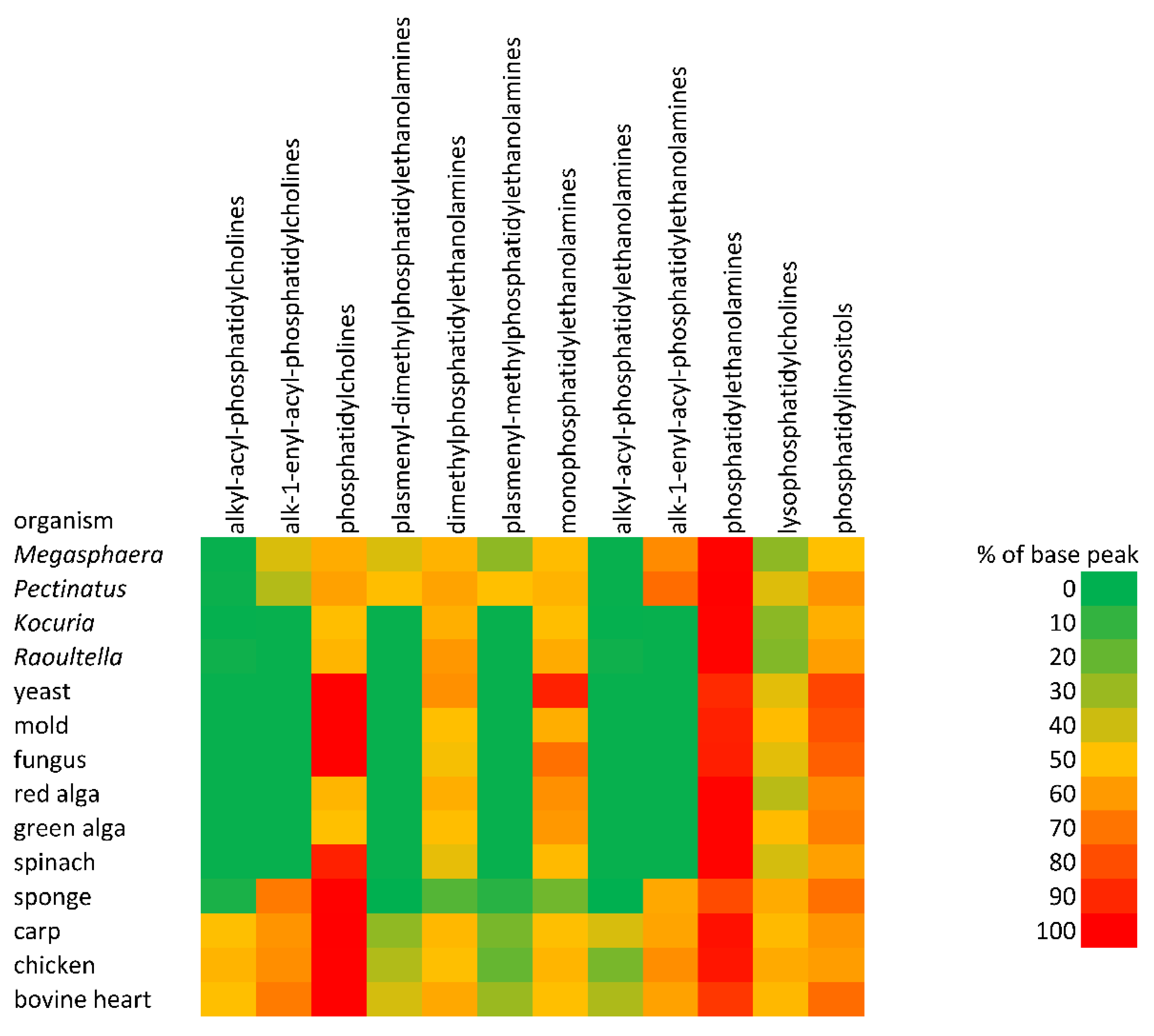
Figure 3.
The abundance of different classes of phospholipids-heat maps.
Investigated minor phospholipids are not commonly analyzed; see, e.g., the paper of Peterson and Cummings [55], which summarized published data up to 2006. They were found, for example, in mouse livers [56] or in unpasteurized milk from ewes and cows, and were analyzed by 31P-NMR, where signals for methyl-PE and dimethyl-PE were obtained [57]. Yeast was one of the first organisms in which was detected two biosynthetic pathways that led from PE to PC [58]. The direct presence of methyl-PE and dimethyl-PE was provided by a global analysis of the yeast lipidome using quantitative shotgun mass spectrometry [59]. Quantitative profiling of PE, methyl-PE, dimethyl-PE, and PC lipid species by multiple precursor ion scanning was used as a tool for monitoring PE metabolism in S. cerevisiae [14]. Hein and Hayen [16] identified both minor phospholipids in S. cerevisiae and in four hemiascomycetous yeasts, and their content was orders of magnitude lower than that of usually found phospholipids, i.e., PE, PC, or PI.
Photosynthetic organisms have also been investigated. Nakamura [4] described the biosynthesis of the phosphatidylcholine and phosphatidylethanolamine head group in seed plants. The presence of phosphoethanolamine N-methyltransferase was detected in spinach [60]. The paper of Hirashima et al. [61] describes the possibility that both red and green algae can biosynthesize methyl-PE and dimethyl-PE. These authors identified phospholipid-N-methyltransferase in green algae, e.g., in the genus Monoraphidium, which belongs to the same order (Sphaeropleales), including the alga Desmodesmus analyzed in this paper. In the red alga Galdieria sulphuraria, both methyl-PE and dimethyl-PE were identified by MS and the presence of N-methyltransferase has also been demonstrated [61]. Methyl-PE has also been found in the lipidome of the marine phytoplankton Emiliania huxleyi [62].
The situation in bacteria is similar, where about 15% of the bacteria contained phosphatidylcholine, which can be biosynthesized by the phospholipid N-methylation pathway [5]. Direct evidence for the presence of methyl-PE and dimethyl-PE was provided, for example, by shotgun lipidomic analysis in the soil bacterium Sinorhizobium meliloti [18] or acidophilic mixed microbial communities [63]. The proportion of methyl-PE and dimethyl-PE in Mesorhizobium ciceri was approximately an order of magnitude lower than that of PE and/or PC [64]. Analysis of the bacterial phylum Planctomycetes revealed that the Gemmata-like strain contained methyl-PE and dimethyl-PE [17].
Based on the above references, as well as from the results of the analysis presented in this paper, it is clear that both phospholipids are present as a minority in all four kingdoms, i.e., Eubacteria, Fungi, Plants, and Animals.
3.3. Identification of Methyl-PE and Dimethyl-PE
Preliminary analysis of the fractions from HILIC was performed using negative HR-ESI. To confirm the structure of at least one molecular species from each fraction, two significant peaks were selected at m/z 714.5445 ([C40H77NO7P]−, i.e., P-16:0/18:1-methyl-PE) and at m/z 728.5602 ([C41H79NO7P]−, i.e., P-16:0/18:1-dimethyl-PE) having structures determined by tandem MS (Tables S2 and S3). In tandem mass spectra, abundant ions result from the loss of the sn-2 acyl chain as a ketene (RCH = C = O) from [M − H]− at m/z 450.2993 (methyl-PE) and at m/z 464.3148 (dimethyl-PE). Other ions, i.e., precursor ion [M − H]−, sn-2 RCOO− ion and methyl-(dimethyl-)ethanolamine phosphate ion with loss of H2O, were also identified (see Figure S5).
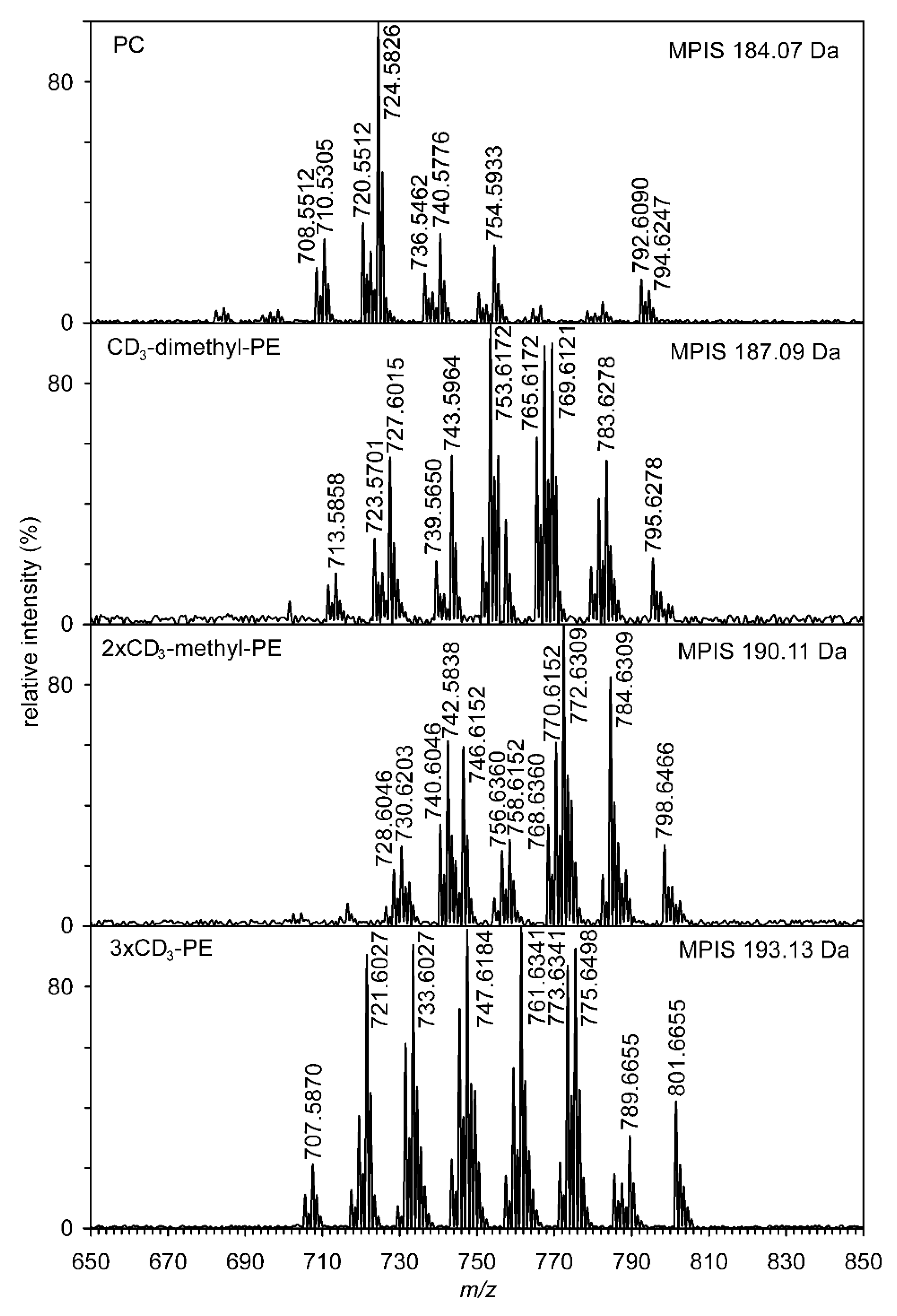
Further confirmation of the structure of methyl-PE and dimethyl-PE and, in particular, the quantification of PE, methyl-PE, dimethyl-PE, and PC was accomplished by the methylation of the nitrogen group. The reaction of NH2, NHMe, or NMe2 groups with CD3I yielded a mixture of phosphatidylcholines containing 1–3 CD3 groups. Phosphatidylcholines, because they already contain a quaternary nitrogen atom, do not react with CD3I. Additional identification of the molecular species of methyl-PE and dimethyl-PE was performed using multiple precursor ion scanning (MPIS) (see Figure 4). Scanning of ions having a value of 184.07, 187.09, 190.11, and 193.13 Da clearly showed the presence of dozens of molecular species that contained from zero to three CD3 groups. Tandem mass spectra (positive ESI) of lithium adducts of deuterium-labeled natural phospholipids 1-alkenyl-2-acyl-PC (P-16:0/18:1-dimethyl-PE with 1 × CD3 and P-16:0/18:1-methyl-PE with 2 × CD3) are shown in Figure S6. The structures of important ions used for identification are demonstrated for molecular species P-16:0/18:1-methyl-PE with 2 × CD3 in Figure S7.
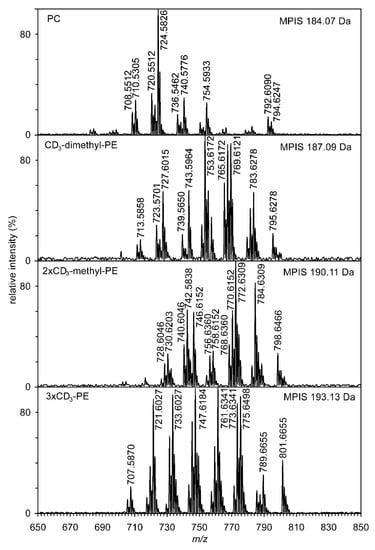
Figure 4.
High-resolution multiple precursor ion scanning of four ions having values 184.07, 187.09, 190.11, and 193.13 Da (lithium adducts in ESI positive ionization mode) acquired by shotgun lipidomic analysis. Numbers correspond to the accurate mass values.
In this way, the structure of the individual classes of phospholipids (PE, methyl-PE, dimethyl-PE, and PC) was demonstrated. However, the four classes of phospholipids have the same polar head group but differ substantially in molecular weight (always ~3 Da), and MS has similar responses, so they can be quantified. On the basis of this positive tandem MS of both lithiated and deuterium-labeled PC, the ratio of PE:methyl-PE:dimethyl-PE:PC for the molecular species of P. frisingensis containing sn-1 P-16:0 and sn-2 18:1 was found to be 100:4:3:55. This confirmed the already published results [16,64] that the content of methyl-PE and dimethyl-PE is approximately one order of magnitude lower than PC or PE.
3.4. Separation of Subclasses–Alkyl-Acyl, Alkenyl-Acyl and Diacylglycerols
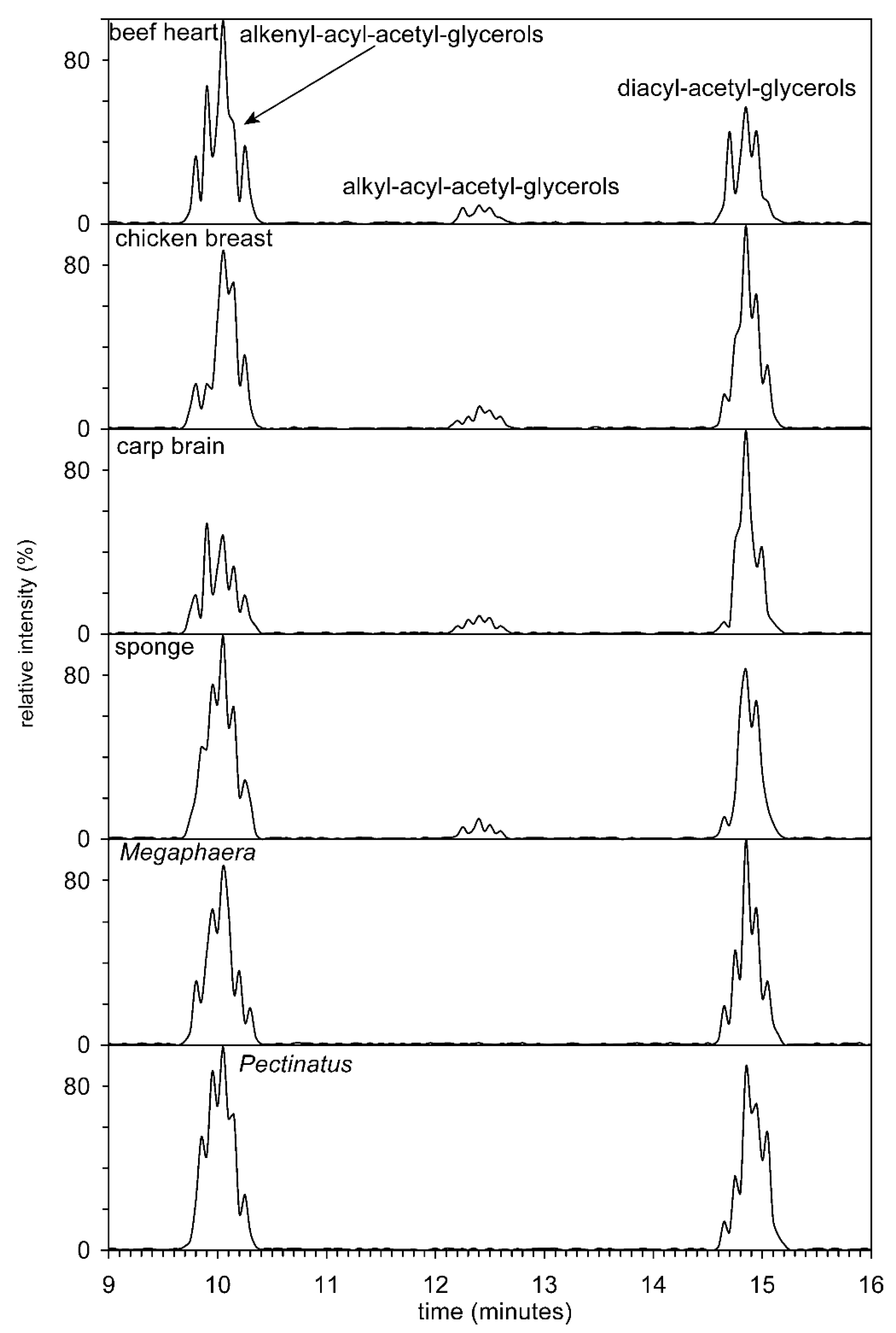
In the next step of the HILIC analysis, the fractions (methyl- and dimethyl-PE from animals and anaerobic bacteria, i.e., those organisms in which plasmenyl lipids could be expected, were further treated by phospholipase C) from HILIC were hydrolyzed with phospholipase, C and the resulting diradyl-glycerols were acetylated. All three types of acetyl diradylglycerols, that is, alkenylacyl, alkylacyl, and diacyl subclasses, were easily separated on a semipreparative Zorbax Rx-SIL column (Figure 5). However, the efficiency of the column was not sufficient to separate molecular species with different chain lengths and number of double bonds.
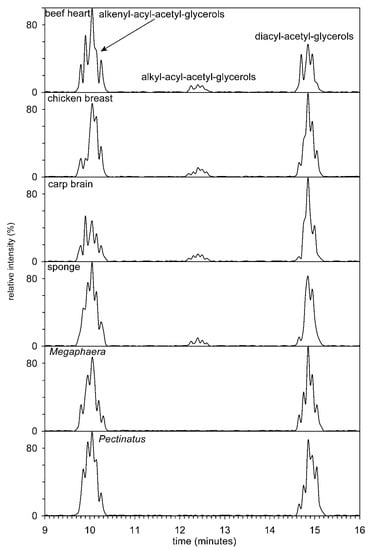
Figure 5.
Separation of acetyl diradylglycerols (alkenylacyls, alkylacyls, and diacyls) obtained after hydrolysis by phospholipase C from the beef heart, chicken breast, carp brain, sponge, and beer spoilage bacteria Pectinatus and Megasphaera. The total ion current was filtered and exhibits ions in the interval from 200 to 1500 Da.
Nakagawa and Horrocks [28] used HPLC to separate alkenylacyl, alkylacyl, and diacyl acetylglycerols obtained by PE. Total lipids of the bovine brain were divided into individual classes of phospholipids, and PC were hydrolyzed with phospholipase C and acetylated with acetic anhydride. The resulting diradyl-acetyl-glycerols were separated on a µPorasil silica column, and the molecular species of each of the three classes were analyzed by RP-HPLC. Furthermore, the compositions of several tens of molecular species were determined using gas chromatography.
A significantly improved method has been published by Guan et al. [19], where the authors esterified the free primary OH group formed after hydrolysis of phospholipids and separated the resulting mixture of alkylacyl-, alkenyl acyl, and diacyl glycerols on a Zorbax SIL column. All three classes were divided on a baseline and also quantified. Human, mouse and rat brains, mouse heart, and rat liver were used as sources. Confirmation of the structure was performed by GC–MS of fatty acid methyl esters and dimethyl acetals. Yamashita et al. [65] used HPLC with evaporative light-scattering detection for separation and detection of plasmalogen in marine invertebrates. The content of plasmalogens in the class Cephalopoda and Crustacea reached more than 60% of the total PE.
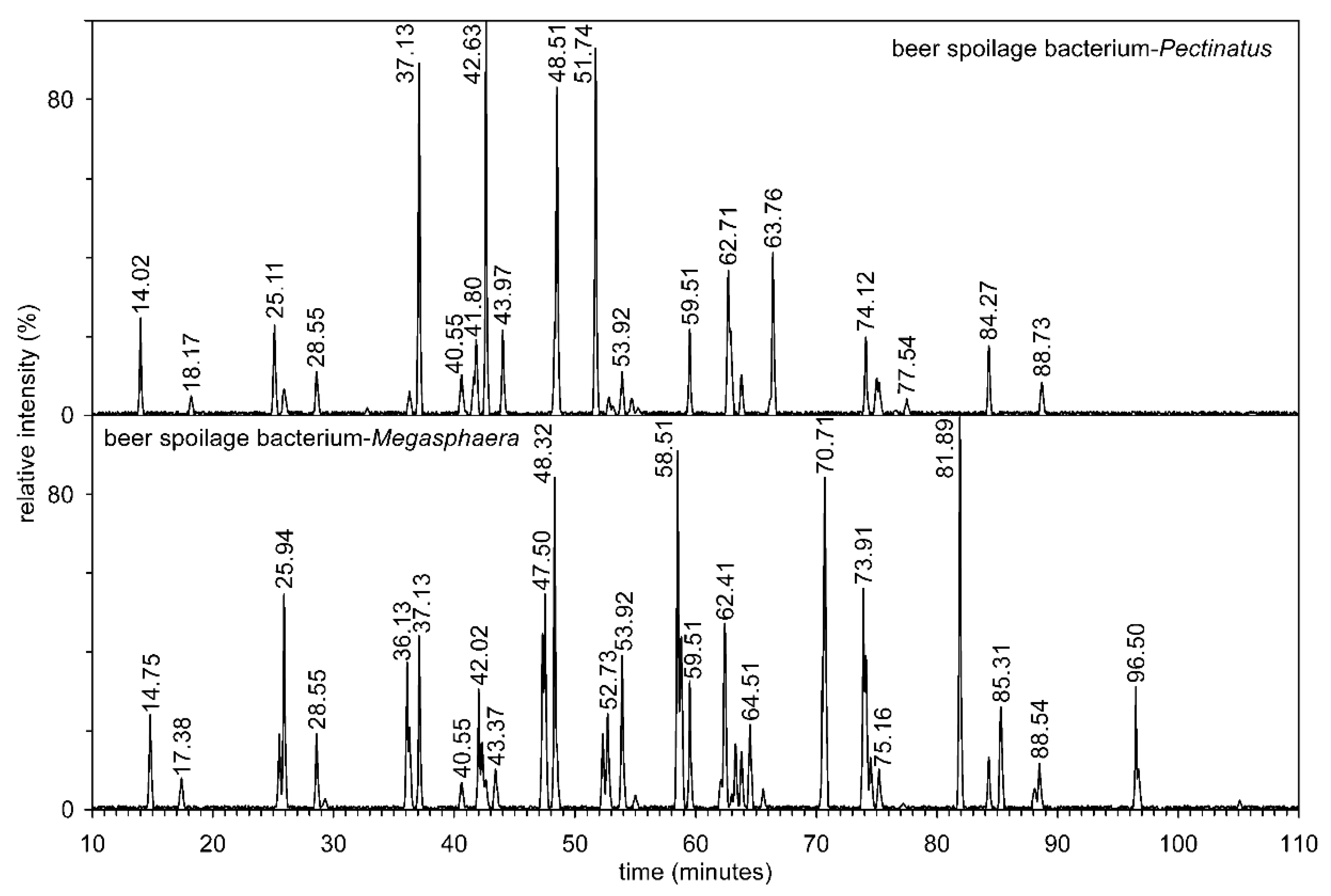
3.5. Analysis of Ether Lipids by RP-HPLC
The resulting mixture of diradyl-3-acetylglycerols was separated into three reverse phase columns connected in series (Figure 6). Tables S4 and S5 show the abundance of alkenyl-acyl-acetyl glycerol molecular species of both anaerobic bacteria, i.e., including the structures of regioisomers (e.g., P-15:1/17:0/2:0 versus P-17:1/15:0/2:0), retention times, and [M + NH4]+ values. From both tables, it is clear that due to the connection of three columns in series, about 311,000 theoretical plates per 1 m of column length were achieved. This made it possible to separate molecular species with different lengths of hydrocarbon chains, such as P-16:1/18:1/2:0 and P-17:1/17:1/2:0, and the already mentioned regioisomers (P-15:1/17:0/2:0 versus P-17:1/15:0/2:0).
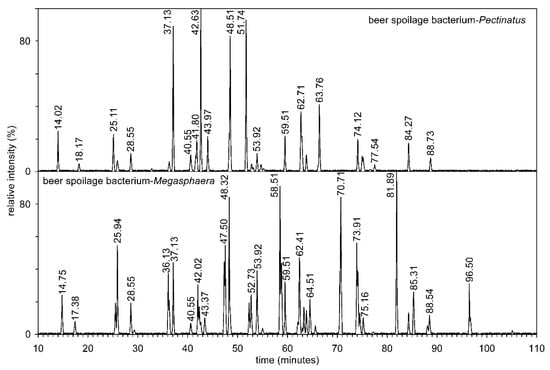
Figure 6.
The reverse phase separation of mixture of alkenyl-acyl-acetyl glycerols on three reversed phase columns connected in series from two beer spoilage bacteria Pectinatus and Megasphaera.
Tandem MS alkenyl-acyl-acetyl glycerols are characterized by abundant ions of the type: precursor ion [M + NH4]+, neutral loss of sn-2 and sn-3 RCOOH + NH3 from [M + NH4]+, and acyl chain sn-2 ([RC = O + 74]+). The other ions, whose structure is given in Table S6 and the values of m/z are shown in Figure S8 only confirm the accuracy of the individual molecular species.
Yang et al. [66] used multidimensional-mass-spectrometry-based shotgun lipidomics to analyze the molecular species alkenyl-acyl-glycerols, obtained from mouse brain tissues. These molecular species were obtained by hydrolysis with phospholipase C and identified by neutral loss scanning and precursor ion scanning and were distinguished from isomeric alkyl-acyl-glycerol species and isobaric odd chain length diacylglycerol species. From the brains of winter and summer carp, diradyl-dinitrobenzoyl derivatives were prepared using the methods mentioned above, which, after separation by RP-HPLC, were identified by retention times with standards [67]. Direct analysis of intact phospholipids, specifically PE, has been used more recently, for example, by Yamashita et al. [68], who analyzed marine and land foodstuffs by LC–MS with ESI in negative and positive ion modes. PIS identified several dozen molecular species isolated from the viscera of the scallop, cuttlefish muscle, and salmon muscle. Tandem MS demonstrated plasmalogens in marine invertebrates, i.e., blue mussel and ascidian, but not in brown algae.
As assumed based on published papers (see Introduction), ether lipids (alkyl-acyl and alkenyl-acyl) were identified only in animals as vertebrates (cow, chicken, carp) or in an invertebrate (sponge). In anaerobic bacteria, only plasmalogens (alkenyl-acyl) and diacyl-glycerols were identified using data already published.
3.6. Analysis by Chiral HPLC
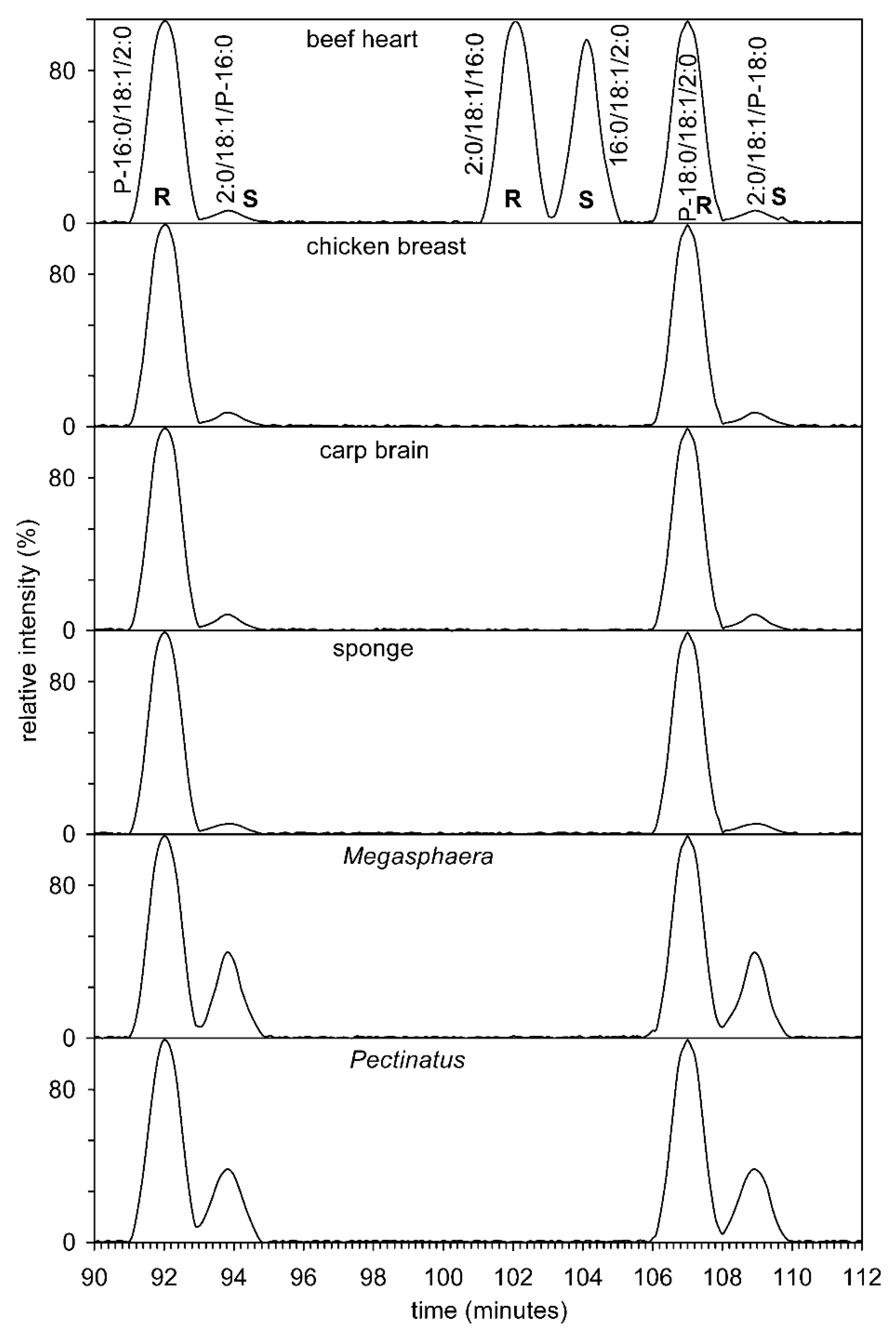
Two fractions obtained from RP-HPLC were collected at intervals of 63.57 to 64.06 min and 84.10 to 84.55 min. The molecular species from six different sources mentioned below were separated on a chiral column.
Figure 7 shows the chiral chromatography of both standards and sn-1-alkenyl-2-acyl-3-acetyl glycerols from four animals and two anaerobic bacteria. The first problem that needed to be addressed was the preparation of standards. Commercially available are 1-O-1′-(Z)-octadecenyl-2-oleoyl-sn-glycero-3-phosphocholine, 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphatidylcholine, and 3-palmitoyl-2-oleoyl-sn-glycero-1-phosphocholine. All three phospholipids were hydrolyzed with phospholipase C and further acetylated to give (S)-1-acetoxy-3-(palmitoyloxy)propan-2-yl oleate, (R)-1-acetoxy-3-(palmitoyloxy)propan-2-yl oleate, and (S)-1-acetoxy-3-((Z)-hexadec-1-enyloxy)propan-2-yl oleate. Figure 7 clearly shows that natural 1,2-diacyl-3-acetyl glycerols have the R configuration. On the contrary, 1-alkenyl-2-acyl-3-acetyl glycerols have the S configuration; this apparent difference is based on the interpretation of the Cahn–Ingold–Prelog rule [69], but the spherical arrangement is the same. A small amount of the “unnatural” S enantiomer was formed during hydrolysis and acetylation, as described previously [31]. Acyl migration can only take place from position 2 to position 3 in plasmenyl lipids. Diradyl glycerol, in this case 1-alkene-2-acyl-glycerol, has a group in position 1 which is stable in a basic medium (Tris buffer (pH 7.3)) and is therefore neither enzymatically nor chemically hydrolysed. After acetylation, 1-alkenyl-2-acetyl-3-acyl glycerol, i.e., a possible hydrolysis product, was not detected. The only possible product from the 1-O-1′-(Z)-octadecenyl-2-oleoyl-sn-glycero-3-phosphocholine standard would be 1-O-1′-(Z)-octadecenyl-2-acetyl-3-oleoyl-sn-glycerol. We performed the experiment again and because it is a regioisomer to the two detected enantiomers, it was sufficient to use a reverse phase column that had several times the number of theoretical plates (311,000 theoretical plates per 1 m of column length (this manuscript) versus 55,000 [70] to prove the presence or absence of this isomer. No peak with selected ion monitoring at m/z 367.321 (neutral loss of sn-2 RCOOH + NH3 from [M+NH4]+) was detected on this column. To be sure, a similar experiment was performed on the chiral column and no peak at m/z 367.321 was detected. Therefore, we believe that migration of the acyl group from position 2 to position 3 does not occur, or if it does occur, the amount of isomer is at most about 1% of the total mixture of isomers. The amount of the “unnatural” enantiomer reached percent levels in animals. The situation was completely different for two anaerobic bacteria, where the content of the “unnatural” enantiomer reached tens of percent. This fact was supported by a search in GenBank, in which it was found that both bacteria contained glycerol-1-phosphate dehydrogenase and glycerol-3-phosphate dehydrogenase (WP_048513017.1 and WP_122628282.1, vs. WP_132549284.1 and WP_132548237.1). Lipids whose chirality on the secondary carbon atom is opposite can thus be biosynthesized starting from sn-glycerol-3-phosphate or its enantiomer sn-glycerol-1-phosphate.
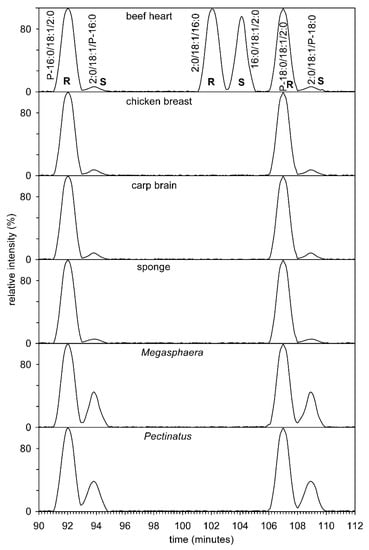
Figure 7.
Chiral separation of natural AcTAG, that is, enantiomers and regioisomers from beef heart, chicken breast, carp brain, sponge, and beer spoilage bacteria Pectinatus and Megasphaera. Total ion current was filtered and indicates the type of ions [DAG]+, i.e., ions resulting from loss of neutral RCOOH + NH3 from [M + NH4]+ in the interval from 450 to 700 Da. The [DAG]+ type ion was always the base peak.
Two enantiomers, i.e., two molecular species P-16:0/18:1/2:0 and 2:0/18:1/P-16:0, i.e., (€-1-acetoxy-3-((E)-hexadec-1-enyloxy)propan-2-yl oleate and (S)-1-acetoxy-3-((E)-hexadec-1-enyloxy)propan-2-yl oleate) were separated and their tandem MS are shown in Figure S9. From these spectra, it is clear that the majority of ions were, again, precursor ions [M + NH4]+, neutral loss of sn-2 RCOOH + NH3 from [M + NH4]+, and sn-2 acyl chain ([RC = O + 74]+) (Table S7). Furthermore, as expected from the tandem MS of the two enantiomers, their spectra were nearly identical, differing only in the insignificant abundance of ions and can only be distinguished based on tR on a chiral phase column. Figure S9 and Table S7 give an example of the tandem MS of the synthetic derivative (P-18:0/18:1/2:0), which shows a similar structure and abundance of all ions. It follows from the above mass spectra that two classes of plasmalogens (MMPA and DMPA) from the bacteria analyzed have both enantiomers of glycerol phosphate as biosynthetic precursors.
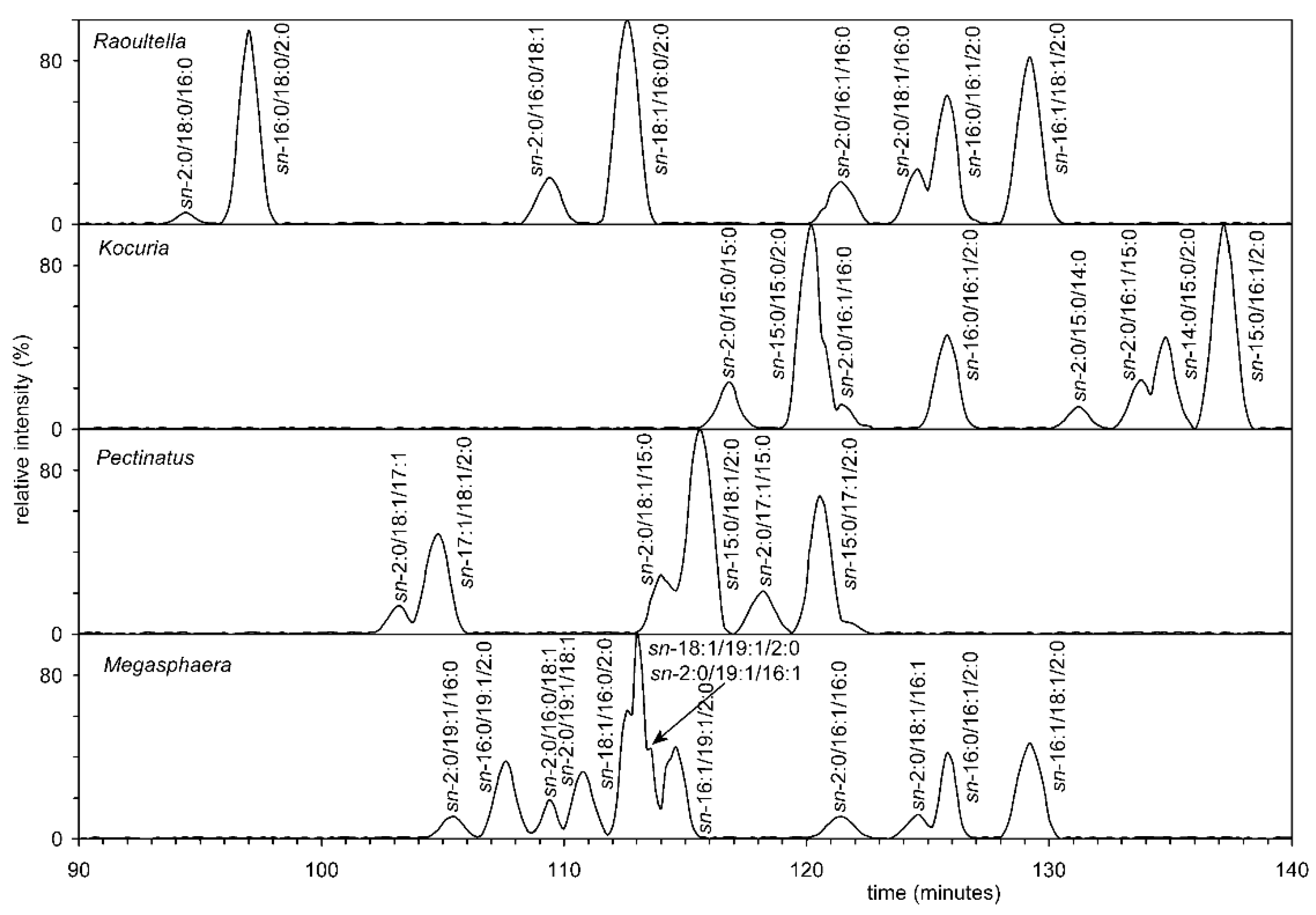
3.7. Analysis of all Four Bacteria by Chiral HPLC
Furthermore, chiral HPLC was used to analyze diacyl-acetylglycerols (AcTAG), obtained in a similar way to that previously published [29]. Briefly, individual AcTAG obtained by selective hydrolysis of the appropriate molecular species methyl-PE and dimethyl-PE, by phospholipase C (see experimental methods), and subsequent derivatization, were separated into molecular species by RP-HPLC and further chiral chromatography. Analysis showed that the AcTAG of all four bacteria were mixtures of R and S enantiomers. Based on the chiral HPLC of these standards, it was possible to determine that the S enantiomer of AcTAG always predominated, meaning that the R enantiomer was mainly present in the phospholipid (Figure 8). In the case of all eukaryotic organisms, the proportion of R enantiomers was many times lower. The hypothesis that bacteria of four different genera use two pathways, i.e., with both starting units ((S)-2,3-dihydroxypropyl dihydrogen phosphate and (R)-2,3-dihydroxypropyldihydrogen phosphate), is likely because Table S1 shows the results of the search in GenBank. This search demonstrates that both enzymes-glycerol-3-phosphate dehydrogenase and glycerol-1-phosphate dehydrogenase, are present. It can be assumed that some bacteria can form mixed membranes as described by Fiore and Buchet [71].
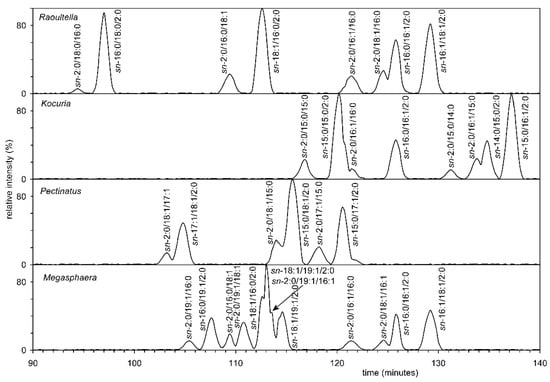
Figure 8.
The chiral HPLC of the AcTAG from all four bacteria showed that they are the mixtures of R and S enantiomers.
The most commonly found phospholipids in bacterial membranes are PE, PG, and cardiolipin (CL). However, 15% of all bacteria are predicted to encode enzymes for the biosynthesis of PC [5]. Methyl-PE and dimethyl-PE are synthesized as intermediates of the mechanisms of phosphatidylcholine biosynthesis by sequential methylation of phosphatidylethanolamine using the enzyme phosphatidylethanolamine N-methyltransferase [72]. This pathway is generally minor in animals, although it is significant in their livers, especially when there is a lack of choline in the diet. In contrast, it is the main biosynthetic pathway in yeast and bacteria [5]. They are never found in animal or plant tissues in amounts greater than trace amounts, which is not the case for yeasts and bacteria. The enzyme phosphatidylethanolamine-N-methyltransferase has been identified in some green algae of the genus Chlamydomonas [61]. However, not all bacteria were found to have complete biosynthesis, i.e., the PE-methyl-PE-dimethyl-PE-PC pathway [73].
4. Conclusions
Methyl-PE and dimethyl-PE were successfully separated by HILIC from total lipids derived from fourteen organisms, and their structures were confirmed by shotgun negative ESI and MS/MS (MPIS) of deuterated derivatives. In the next step, the polar head-group was removed by phospholipase C, and the resulting diradylglycerols after acetylation were separated by normal phase HPLC. Molecular species were obtained and identified by RP-HPLC. The column with a chiral phase allowed the separation of the R and S enantiomers of diacyl- and alkenyl-acyl-glycerols and was identified by positive ESI. Chiral HPLC revealed that both enantiomers were present in all four bacteria. Therefore, it can be assumed that both sn-glycerol-1-phosphate and sn-glycerol-3-phosphate are precursors for the biosynthesis of methyl-PE and dimethyl-PE. This finding strongly suggests that the bacteria contain the biosynthetic pathway described so far only in Archaea. This conclusion is supported by the search results shown in Table S1, which lists possible enzymes obtained by the GenBank search.
As early as 2003, the theory was expressed that phospholipid membranes of last universal common ancestor (LUCA) were heterochiral. This theory was based on the assumption that lipids of different chirality are incompatible and that two lipid enantiomers are unstable. This theory, known as the “lipid divide” theory [74], has been widely accepted but has been challenged in several papers, such as that heterochiral membranes are known to be as stable or even more stable than homochiral membranes [75]. Their increased resistance to environmental stress has been also demonstrated [76], where growth was unchanged in E. coli cells but the robustness of cells with a hybrid heterochiral membrane was slightly increased. Furthermore, bacteria discovered in deep of the Black Sea may be the first group of organisms to be thought to have the ability to biosynthesize the heterochiral membrane [10]. Based on these published papers and the results reported above, it can be assumed that at least some bacteria biosynthesize both glycerol-3-phosphate and glycerol-1-phosphate and therefore their membranes are asymmetric, i.e., heterochiral.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/sym14030616/s1, Figure S1: Structure of two enantiomeric TAG; Figure S2: Mass spectrum of nicotinate ester of 14-methylhexadecanol; Figure S3: Mass spectrum of nicotinate ester of 15-methylhexadecanol; Figure S4: Mass spectrum of 3-pyridylcarbinyl 5,9,19-pentacosatrienoate; Figure S5: Tandem MS of ions at m/z 728.5601 and 714.5443 ([M − H]−). Based on the m/z values for the ions, see Tables S2 and S3, P-16:0/18:1-DMPE and 714.5443 P-16:0/18:1-MMPE, the probable structures have been proposed; Figure S6: Tandem mass spectra (positive ESI) of lithium adducts of deuterium-labeled natural phospholipids; 1-alkenyl-2-acyl-PC (P-16:0/18:1-DMPE with 1 × CD3 and P-16:0/18:1-MMPE with 2 × CD3); Figure S7: Structures of deuterated methyl-PE key ions (P-16:0/18:1-methyl-PE with 2 × CD3) obtained by tandem MS; Figure S8: Tandem mass spectra of four AcTAG (P-16:1/18:1/2:0, P-17:1/17:1/2:0, P-17:1/15:0/2:0, P-15:1/17:0/2:0,) obtained from two beer spoilage bacteria Pectinatus and Megasphaera; Figure S9: Tandem mass spectra of two enantiomers, i.e., two molecular species P-16:0/18:1/2:0 and 2:0/18:1/P-16:0, and the synthetic derivative (P-18:0/18:1/2:0); Figure S10: Tandem mass spectra of two molecular species, i.e., molecular species P-16:0/22:6-methyl-PE (carp) and P-16:0/20:4-dimethyl-PE (chicken).; Table S1: Examples of organisms and their proteins involvement in lipid biosynthesis; Table S2: Negative tandem MS of P-16:0/18:1/MMPE; Table S3: Negative tandem MS of P-16:0/18:1/DMPE; Table S4: RP-HPLC of alkenyl-acyl-acetyl glycerols from Megasphaera cerevisiae; Table S5: RP-HPLC alkenyl-acyl-acetyl glycerols from Pectinatus frisingensis; Table S6: Tandem MS alkenyl-acyl-acetyl glycerols; Table S7: Tandem MS of both enantiomers P-16:0/18:1/2:0. References [77,78,79] are cited in the supplementary materials.
Author Contributions
Conceptualization, T.Ř. and A.P.; methodology, T.Ř., A.P., and M.V.; validation, T.B.; investigation, T.Ř., M.V., T.B., and A.P.; resources, T.Ř.; data curation, T.Ř.; writing—original draft preparation, T.Ř.; writing—review and editing, J.K.I. and M.K.; supervision, T.Ř. and T.B.; project administration, T.Ř.; funding acquisition, T.Ř. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by European fund for regional development, the program Interreg V-A Austria–Czech Republic, grant number ATCZ172 REEgain; by Institutional Research Concept (Institute of Microbiology, Prague, Czech Republic), grant number RVO61388971; by the Ministry of Agriculture of the Czech Republic, grant number MZE-RO1918.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
We acknowledge D. Brooker for critical reading and language editing of the text and also Natálie Vítová for collection of the sponge.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- Wang, M.; Wang, C.; Han, R.H.; Han, X. Novel advances in shotgun lipidomics for biology and medicine. Prog. Lipid Res. 2016, 61, 83–108. [Google Scholar] [CrossRef] [PubMed]
- Cole, L.K.; Vance, J.E.; Vance, D.E. Phosphatidylcholine biosynthesis and lipoprotein metabolism. Biochim. Biophys. Acta (BBA)-Mol. Cell Biol. Lipids 2012, 1821, 754–761. [Google Scholar] [CrossRef]
- McMaster, C.R. From yeast to humans-roles of the Kennedy pathway for phosphatidylcholine synthesis. FEBS Lett. 2018, 592, 1256–1272. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, Y. Headgroup biosynthesis of phosphatidylcholine and phosphatidylethanolamine in seed plants. Prog. Lipid Res. 2021, 82, 101091. [Google Scholar] [CrossRef] [PubMed]
- Geiger, O.; Lopez-Lara, I.M.; Sohlenkamp, C. Phosphatidylcholine biosynthesis and function in bacteria. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2013, 1831, 503–513. [Google Scholar] [CrossRef]
- Vitova, M.; Palyzova, A.; Rezanka, T. Plasmalogens-ubiquitous molecules occurring widely, from anaerobic bacteria to humans. Prog. Lipid Res. 2021, 83, 101111. [Google Scholar] [CrossRef]
- Yao, J.W.; Rock, C.O. Phosphatidic acid synthesis in bacteria. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2013, 1831, 495–502. [Google Scholar] [CrossRef]
- Athenstaedt, K.; Daum, G. Biosynthesis of phosphatidic acid in lipid particles and endoplasmic reticulum of Saccharomyces cerevisiae. J. Bacteriol. 1997, 179, 7611–7616. [Google Scholar] [CrossRef]
- Koga, Y.; Morii, H. Biosynthesis of ether-type polar lipids in archaea and evolutionary considerations. Microbiol. Mol. Biol. Rev. 2007, 71, 97–120. [Google Scholar] [CrossRef]
- Villanueva, L.; von Meijenfeldt, F.A.B.; Westbye, A.B.; Yadav, S.; Hopmans, E.C.; Dutilh, B.E.; Damste, J.S.S. Bridging the membrane lipid divide: Bacteria of the FCB group superphylum have the potential to synthesize archaeal ether lipids. ISME J. 2021, 15, 168–182. [Google Scholar] [CrossRef]
- Tang, D.Q.; Zou, L.; Yin, X.X.; Ong, C.N. HILIC-MS for metabolomics: An attractive and complementary approach to RPLC-MS. Mass Spectrom. Rev. 2016, 35, 574–600. [Google Scholar] [CrossRef] [PubMed]
- Granafei, S.; Azzone, P.; Spinelli, V.A.; Losito, I.; Palmisano, F.; Cataldi, T.R. Hydrophilic interaction and reversed phase mixed-mode liquid chromatography coupled to high resolution tandem mass spectrometry for polar lipids analysis. J. Chromatogr. A 2016, 1477, 47–55. [Google Scholar] [CrossRef]
- Schwudke, D.; Oegema, J.; Burton, L.; Entchev, E.; Hannich, J.T.; Ejsing, C.S.; Kurzchalia, T.; Shevchenko, A. Lipid profiling by multiple precursor and neutral loss scanning driven by the data-dependent acquisition. Anal. Chem. 2006, 78, 585–595. [Google Scholar] [CrossRef] [PubMed]
- Bilgin, M.; Markgraf, D.F.; Duchoslav, E.; Knudsen, J.; Jensen, O.N.; de Kroon, A.I.P.M.; Ejsing, C.S. Quantitative profiling of PE, MMPE, DMPE, and PC lipid species by multiple precursor ion scanning: A tool for monitoring PE metabolism. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2011, 1811, 1081–1089. [Google Scholar] [CrossRef] [PubMed]
- Schneiter, R.; Brugger, B.; Sandhoff, R.; Zellnig, G.; Leber, A.; Lampl, M.; Athenstaedt, K.; Hrastnik, C.; Eder, S.; Daum, G.; et al. Electrospray ionization tandem mass spectrometry (ESI-MS/MS) analysis of the lipid molecular species composition of yeast subcellular membranes reveals acyl chain-based sorting/remodeling of distinct molecular species en route to the plasma membrane. J. Cell Biol. 1999, 146, 741–754. [Google Scholar] [CrossRef]
- Hein, E.M.; Hayen, H. Comparative lipidomic profiling of S. cerevisiae and four other Hemiascomycetous yeasts. Metabolites 2012, 2, 254–267. [Google Scholar] [CrossRef]
- Moore, E.K.; Hopmans, E.C.; Rijpstra, W.I.C.; Villanueva, L.; Dedysh, S.N.; Kulichevskaya, I.S.; Wienk, H.; Schoutsen, F.; Damste, J.S.S. Novel mono-, di-, and trimethylornithine membrane lipids in Northern Wetland Planctomycetes. Appl. Environ. Microbiol. 2013, 79, 6874–6884. [Google Scholar] [CrossRef]
- Basconcillo, L.S.; Zaheer, R.; Finan, T.M.; McCarry, B.E. A shotgun lipidomics approach in Sinorhizobium meliloti as a tool in functional genomics. J. Lipid Res. 2009, 50, 1120–1132. [Google Scholar] [CrossRef]
- Guan, Z.Z.; Grunler, J.; Piao, S.F.; Sindelar, P.J. Separation and quantitation of phospholipids and their ether analogues by high-performance liquid chromatography. Anal. Biochem. 2001, 297, 137–143. [Google Scholar] [CrossRef]
- Qin, D.H.; Byun, H.S.; Bittman, R. Synthesis of plasmalogen via 2,3-bis-O-(4 ’-methoxybenzyl)-sn-glycerol. J. Am. Chem. Soc. 1999, 121, 662–668. [Google Scholar] [CrossRef]
- Koch, J.; Lackner, K.; Wohlfarter, Y.; Sailer, S.; Zschocke, J.; Werner, E.R.; Watschinger, K.; Keller, M.A. Unequivocal mapping of molecular ether lipid species by LC-MS/MS in plasmalogen-deficient mice. Anal. Chem. 2020, 92, 11268–11276. [Google Scholar] [CrossRef] [PubMed]
- Calvano, C.D.; Ventura, G.; Sardanelli, A.M.M.; Savino, L.; Losito, I.; De Michele, G.; Palmisano, F.; Cataldi, T.R.I. Searching for potential lipid biomarkers of Parkinson’s disease in Parkin-mutant human skin fibroblasts by HILIC-ESI-MS/MS: Preliminary findings. Int. J. Mol. Sci. 2019, 20, 3341. [Google Scholar] [CrossRef] [PubMed]
- Rezanka, T.; Siristova, L.; Matoulkova, D.; Sigler, K. Hydrophilic interaction liquid chromatography: ESI-MS/MS of plasmalogen phospholipids from Pectinatus bacterium. Lipids 2011, 46, 765–780. [Google Scholar] [CrossRef] [PubMed]
- Cifkova, E.; Holcapek, M.; Lisa, M. Nontargeted lipidomic characterization of porcine organs using hydrophilic interaction liquid chromatography and off-line two-dimensional liquid chromatography-electrospray ionization mass spectrometry. Lipids 2013, 48, 915–928. [Google Scholar] [CrossRef] [PubMed]
- Berkecz, R.; Tomosi, F.; Kormoczi, T.; Szegedi, V.; Horvath, J.; Janaky, T. Comprehensive phospholipid and sphingomyelin profiling of different brain regions in mouse model of anxiety disorder using online two-dimensional (HILIC/RP)-LC/MS method. J. Pharm. Biomed. Anal. 2018, 149, 308–317. [Google Scholar] [CrossRef] [PubMed]
- Vanhoenacker, D.; Ruben t’Kindt, F.D.; Pat Sandra, K.S. Unraveling the complexity of lipidomes by multiple heart-cutting Q-TOF LC/MS with the Agilent 1290 Infinity 2D-LC solution. Agil. Technol. 2019, 1–14. [Google Scholar]
- Pham, T.H.; Zaeem, M.; Fillier, T.A.; Nadeem, M.; Vidal, N.P.; Manful, C.; Cheema, S.; Cheema, M.; Thomas, R.H. Targeting modified lipids during routine lipidomics analysis using HILIC and C30 reverse phase liquid chromatography coupled to mass spectrometry. Sci. Rep. 2019, 9, 5048. [Google Scholar] [CrossRef] [PubMed]
- Nakagawa, Y.; Horrocks, L.A. Separation of alkenylacyl, alkylacyl, and diacyl analogs and their molecular species by high-performance liquid-chromatography. J. Lipid Res. 1983, 24, 1268–1275. [Google Scholar] [CrossRef]
- Vitova, M.; Stranska, M.; Palyzova, A.; Rezanka, T. Detailed structural characterization of cardiolipins from various biological sources using a complex analytical strategy comprising fractionation, hydrolysis and chiral chromatography. J. Chromatogr. A 2021, 1648, 462185. [Google Scholar] [CrossRef] [PubMed]
- Palyzova, A.; Rezanka, T. Separation and identification of diacylglycerols containing branched chain fatty acids by liquid chromatography-mass spectrometry. J. Chromatogr. A 2021, 1635, 461708. [Google Scholar] [CrossRef]
- Palyzova, A.; Rezanka, T. Enantiomeric separation of triacylglycerols containing fatty acids with a ring (cyclofatty acids). J. Chromatogr. A 2020, 1622, 461103. [Google Scholar] [CrossRef] [PubMed]
- Palyzova, A.; Rezanka, T. Separation of triacylglycerols containing allenic and acetylenic fatty acids by enantiomeric liquid chromatography-mass spectrometry. J. Chromatogr. A 2020, 1623, 461161. [Google Scholar] [CrossRef] [PubMed]
- Palyzova, A.; Maresova, H.; Novak, J.; Zahradnik, J.; Rezanka, T. Effect of the anti-inflammatory drug diclofenac on lipid composition of bacterial strain Raoultella sp. KDF8. Folia Microbiol. 2020, 65, 763–773. [Google Scholar] [CrossRef]
- Vitova, M.; Goecke, F.; Sigler, K.; Rezanka, T. Lipidomic analysis of the extremophilic red alga Galdieria sulphuraria in response to changes in pH. Algal Res. Biomass Biofuels Bioprod. 2016, 13, 218–226. [Google Scholar] [CrossRef]
- Vitova, M.; Bisova, K.; Hlavova, M.; Zachleder, V.; Rucki, M.; Cizkova, M. Glutathione peroxidase activity in the selenium-treated alga Scenedesmus quadricauda. Aquat. Toxicol. 2011, 102, 87–94. [Google Scholar] [CrossRef] [PubMed]
- Rezanka, T.; Kolouchova, I.; Gharwalova, L.; Palyzova, A.; Sigler, K. Identification and characterization of phospholipids with very long chain fatty acids in brewer’s yeast. Lipids 2017, 52, 1007–1017. [Google Scholar] [CrossRef]
- Bligh, E.G.; Dyer, W.J. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 1959, 37, 911–917. [Google Scholar]
- Kiene, R.P.; Visscher, P.T.; Keller, M.D.; Kirst, G.O. Biosynthetic pathways for phosphatidylsulfocholine, the sulfonium analogue of phosphatidylcholine, in Diatoms. In Biological and Environmental Chemistry of DMSP and Related Sulfonium Compounds; Springer: Boston, MA, USA, 1996; pp. 109–119. [Google Scholar]
- Alves, S.P.; Santos-Silva, J.; Cabrita, A.R.J.; Fonseca, A.J.M.; Bessa, R.J.B. Detailed dimethylacetal and fatty acid composition of rumen content from lambs fed lucerne or concentrate supplemented with soybean oil. PLoS ONE 2013, 8, e58386. [Google Scholar] [CrossRef]
- Morrison, W.R.; Smith, L.M. Preparation of fatty acid methyl esters and dimethylacetals from lipids with boron fluoride-methanol. J. Lipid Res. 1964, 5, 600–608. [Google Scholar] [CrossRef]
- Dobson, G.; Itabashi, Y.; Christie, W.W.; Robertson, G.W. Liquid chromatography with particle-beam electron-impact mass spectrometry of diacylglycerol nicotinates. Chem. Phys. Lipids 1998, 97, 27–39. [Google Scholar] [CrossRef]
- Destaillats, F.; Angers, P. One-step methodology for the synthesis of FA picolinyl esters from intact lipids. J. Am. Oil Chem. Soc. 2002, 79, 253–256. [Google Scholar] [CrossRef]
- Rezanka, T.; Vitova, M.; Lukavsky, J.; Sigler, K. Lipidomic study of precursors of Endocannabinoids in freshwater Bryozoan Pectinatella magnifica. Lipids 2018, 53, 413–427. [Google Scholar] [CrossRef] [PubMed]
- Dembitsky, V.M.; Rezanka, T.; Rozentsvet, O. A Lipid composition of 3 macrophytes from the caspian sea. Phytochem 1993, 33, 1015–1019. [Google Scholar] [CrossRef]
- Rezanka, T.; Podojil, M. Identification of wax esters of the fresh water green alga Chlorella kessleri by gas-chromatography mass spectrometry. J. Chromatogr. 1986, 362, 399–406. [Google Scholar] [CrossRef]
- Yokota, K.; Kanamoto, R.; Kito, M. Composition of cardiolipin molecular species in Escherichia coli. J. Bacteriol. 1980, 141, 1047–1051. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.N.; Siegenthaler, P.A. Phosphatidylglycerol molecular species of photosynthetic membranes analyzed by high-performance liquid chromatography: Theoretical considerations. Lipids 1996, 31, 223–229. [Google Scholar] [CrossRef] [PubMed]
- Itabashi, Y.; Myher, J.J.; Kuksis, A. Determination of positional distribution of short-chain fatty acids in bovine milk fat on chiral columns. J. Am. Oil Chem. Soc. 1993, 70, 1177–1181. [Google Scholar] [CrossRef]
- Limb, J.K.; Kim, Y.H.; Han, S.Y.; Jhon, G.J. Isolation and characterization of monoacetyldiglycerides from bovine udder. J. Lipid Res. 1999, 40, 2169–2176. [Google Scholar] [CrossRef]
- Kalo, P.; Kemppinen, A.; Ollilainen, V.; Kuksis, A. Regiospecific determination of short-chain triacylglycerols in butterfat by normal-phase HPLC with on-line electrospray-tandem mass spectrometry. Lipids 2004, 39, 915–928. [Google Scholar] [CrossRef]
- Palyzová, A.; Cajthaml, T.; Řezanka, T. Separation of regioisomers and enantiomers of triacylglycerols containing branched fatty acids (iso and/or anteiso). Electrophoresis 2021, 42, 1832–1843. [Google Scholar] [CrossRef] [PubMed]
- Liebisch, G.; Vizcaino, J.A.; Kofeler, H.; Trotzmuller, M.; Griffiths, W.J.; Schmitz, G.; Spener, F.; Wakelam, M.J.O. Shorthand notation for lipid structures derived from mass spectrometry. J. Lipid Res. 2013, 54, 1523–1530. [Google Scholar] [CrossRef] [PubMed]
- Harvey, D.J.; Tiffany, J.M. Comparison of derivatives for the characterization of branched long-chain alcohols and 1,2-diols by mass spectrometry. Biomed. Mass Spectrom. 1984, 11, 353–359. [Google Scholar] [CrossRef]
- Vetter, W.; Meister, W. Nicotinates as derivatives for the mass spectrometry investigation of long chain alcohols. Org. Mass Spectrom. 1981, 16, 118–122. [Google Scholar] [CrossRef]
- Peterson, B.L.; Cummings, B.S. A review of chromatographic methods for the assessment of phospholipids in biological samples. Biomed. Chromatogr. 2006, 20, 227–243. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Kim, G.H.; Wei, F.; Chen, H.; Altarejos, J.; Han, X. Improved method for quantitative analysis of methylated phosphatidylethanolamine species and its application for analysis of diabetic-mouse liver samples. Anal. Bioanal. Chem. 2015, 407, 5021–5032. [Google Scholar] [CrossRef] [PubMed]
- Murgia, S.; Mele, S.; Monduzzi, M. Quantitative characterization of phospholipids in milk fat via 31P NMR using a monophasic solvent mixture. Lipids 2003, 38, 585–591. [Google Scholar] [CrossRef] [PubMed]
- Carman, G.M.; Zeimetz, G.M. Regulation of phospholipid biosynthesis in the yeast Saccharomyces cerevisiae. J. Biol. Chem. 1996, 271, 13293–13296. [Google Scholar] [CrossRef] [PubMed]
- Ejsing, C.S.; Sampaio, J.L.; Surendranath, V.; Duchoslav, E.; Ekroos, K.; Klemm, R.W.; Simons, K.; Shevchenko, A. Global analysis of the yeast lipidome by quantitative shotgun mass spectrometry. Proc. Natl. Acad. Sci. USA 2009, 106, 2136–2141. [Google Scholar] [CrossRef] [PubMed]
- Smith, D.D.; Summers, P.S.; Weretilnyk, E.A. Phosphocholine synthesis in spinach: Characterization of phosphoethanolamine N-methyltransferase. Physiol. Plant. 2000, 108, 286–294. [Google Scholar] [CrossRef]
- Hirashima, T.; Toyoshima, M.; Moriyama, T.; Sato, N. Evolution of the phosphatidylcholine biosynthesis pathways in green algae: Combinatorial diversity of methyltransferases. J. Mol. Evol. 2018, 86, 68–76. [Google Scholar] [CrossRef]
- Aveiro, S.S.; Melo, T.; Figueiredo, A.; Domingues, P.; Pereira, H.; Maia, I.B.; Silva, J.; Domingues, M.R.; Nunes, C.; Moreira, A.S.P. The polar lipidome of cultured Emiliania huxleyi: A source of bioactive lipids with relevance for biotechnological applications. Biomolecules 2020, 10, 1434. [Google Scholar] [CrossRef] [PubMed]
- Fischer, C.R.; Wilmes, P.; Bowen, B.P.; Northen, T.R.; Banfield, J.F. Deuterium-exchange metabolomics identifies N-methyl lyso phosphatidylethanolamines as abundant lipids in acidophilic mixed microbial communities. Metabolomics 2012, 8, 566–578. [Google Scholar] [CrossRef][Green Version]
- Choma, A.; Komaniecka, I. The polar lipid composition of Mesorhizobium ciceri. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2003, 1631, 188–196. [Google Scholar] [CrossRef]
- Yamashita, S.; Abe, A.; Nakagawa, K.; Kinoshita, M.; Miyazawa, T. Separation and detection of plasmalogen in marine invertebrates by high-performance liquid chromatography with evaporative light-scattering detection. Lipids 2014, 49, 1261–1273. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.; Jenkins, C.M.; Dilthey, B.; Gross, R.W. Multidimensional mass spectrometry-based shotgun lipidomics analysis of vinyl ether diglycerides. Anal. Bioanal. Chem. 2015, 407, 5199–5210. [Google Scholar] [CrossRef] [PubMed]
- Yeo, Y.K.; Park, E.J.; Lee, C.W.; Joo, H.T.; Farkas, T. Ether lipid composition and molecular species alterations in carp brain (Cyprinus carpio L.) during normoxic temperature acclimation. Neurochem. Res. 1997, 22, 1257–1264. [Google Scholar] [CrossRef]
- Yamashita, S.; Kanno, S.; Honjo, A.; Otoki, Y.; Nakagawa, K.; Kinoshita, M.; Miyazawa, T. Analysis of plasmalogen species in foodstuffs. Lipids 2016, 51, 199–210. [Google Scholar] [CrossRef] [PubMed]
- Cahn, R.S.; Ingold, C.; Prelog, V. Specification of molecular chirality. Angew. Chem. Int. Ed. 1966, 5, 385–415. [Google Scholar] [CrossRef]
- Rezanka, T.; Kolouchova, I.; Nedbalova, L.; Sigler, K. Enantiomeric separation of triacylglycerols containing very long chain fatty acids. J. Chromatogr. A 2018, 1557, 9–19. [Google Scholar] [CrossRef] [PubMed]
- Fiore, M.; Buchet, R. Symmetry breaking of phospholipids. Symmetry 2020, 12, 1488. [Google Scholar] [CrossRef]
- Vance, D.; Walkey, C.; Agellon, L. Why has phosphatidylethanolamine N-methyltransferase survived in evolution? Biochem. Soc. Trans. 1998, 26, 337–340. [Google Scholar] [CrossRef]
- Kleetz, J.; Vasilopoulos, G.; Czolkoss, S.; Aktas, M.; Narberhaus, F. Recombinant and endogenous ways to produce methylated phospholipids in Escherichia coli. Appl. Microb. Cell Physiol. 2021, 105, 8837–8851. [Google Scholar] [CrossRef]
- Wachterhauser, G. From pre-cells to Eukarya-a tale of two lipids. Mol. Microbiol. 2003, 47, 13–22. [Google Scholar] [CrossRef]
- Shimada, H.; Yamagishi, A. Stability of heterochiral hybrid membrane made of bacterial sn-G3P lipids and archaeal sn-G1P lipids. Biochemistry 2011, 50, 4114–4120. [Google Scholar] [CrossRef]
- Caforio, A.; Siliakus, M.F.; Exterkate, M.; Jain, S.; Jumde, V.R.; Andringa, R.L.H.; Kengen, S.W.M.; Minnaard, A.J.; Driessen, A.J.M.; van der Oost, J. Converting Escherichia coli into an archaebacterium with a hybrid heterochiral membrane. Proc. Natl. Acad. Sci. USA 2018, 115, 3704–3709. [Google Scholar] [CrossRef] [PubMed]
- Rezanka, T. Polyunsaturated and unusual fatty acids from slime moulds. Phytochemistry 1993, 33, 1441–1444. [Google Scholar] [CrossRef]
- Dembitsky, V.M.; Rezanka, T.; Bychek, I.A. Fatty acids and phospholipids from lichens of the order Lecanorales. Phytochemistry 1992, 31, 851–853. [Google Scholar] [CrossRef]
- Farquhar, J.W. Identification and gas-liquid chromatographic behavior of plasmalogen aldehydes and their acetal, alcohol, and acetylated alcohol derivatives. J. Lipid Res. 1962, 3, 21–30. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).