Structural Brain Asymmetries for Language: A Comparative Approach across Primates
Abstract
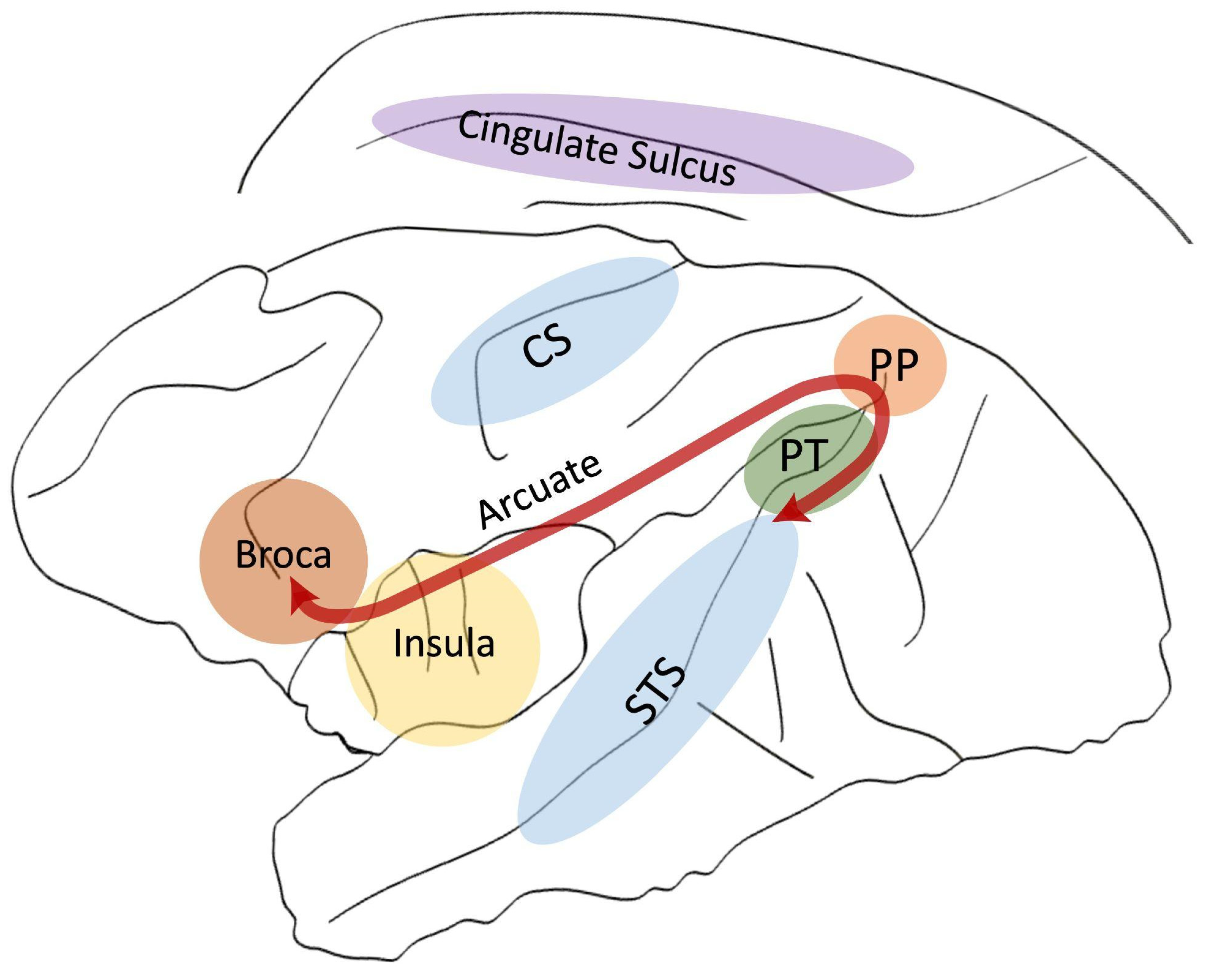
:1. Introduction
2. Planum Temporale
3. Broca’s Area
4. Arcuate Fasciculus
5. Insula
6. Cingulate Cortex/Sulcus
7. Superior Temporal Sulcus (STS)
8. Inferior Parietal Lobe
9. The Central Sulcus (CS)
10. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Berwick, R.C.; Chomsky, N. Why Only Us: Language and Evolution; MIT Press: Cambridge, MA, USA, 2016. [Google Scholar]
- Fedorenko, E. The role of domain-general cognitive control in language comprehension. Front. Psychol. 2014, 5, 335. [Google Scholar] [CrossRef] [PubMed]
- Fitch, W.T. The Evolution of Language; Cambridge University Press: Cambridge, UK, 2010. [Google Scholar]
- Liebal, K.; Waller, B.M.; Slocombe, K.E.; Burrows, A.M. Primate Communication: A Multimodal Approach; Cambridge University Press: Cambridge, UK, 2014. [Google Scholar]
- Fagot, J.; Boë, L.-J.; Berthomier, F.; Claidière, N.; Malassis, R.; Meguerditchian, A.; Rey, A.; Montant, M. The baboon: A model for the study of language evolution. J. Hum. Evol. 2019, 126, 39–50. [Google Scholar] [CrossRef]
- Geschwind, N. The Organization of Language and the Brain. Science 1970, 170, 940–944. [Google Scholar] [CrossRef]
- Toga, A.W.; Thompson, P.M. Mapping brain asymmetry. Nat. Rev. Neurosci. 2003, 4, 37–48. [Google Scholar] [CrossRef] [PubMed]
- Biduła, S.P.; Kroliczak, G. Structural asymmetry of the insula is linked to the lateralization of gesture and language. Eur. J. Neurosci. 2015, 41, 1438–1447. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Becker, Y.; Loh, K.K.; Coulon, O.; Meguerditchian, A. The Arcuate Fasciculus and language origins: Disentangling existing conceptions that influence evolutionary accounts. Neurosci. Biobehav. Rev. 2022, 134, 104490. [Google Scholar] [CrossRef] [PubMed]
- Catani, M.; Mesulam, M. The arcuate fasciculus and the disconnection theme in language and aphasia: History and current state. Cortex 2008, 44, 953–961. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Duffau, H. The error of Broca: From the traditional localizationist concept to a connectomal anatomy of human brain. J. Chem. Neuroanat. 2018, 89, 73–81. [Google Scholar] [CrossRef] [PubMed]
- Catani, M.; Dawson, M. Language Processing, Development and Evolution. Conn’s Transl. Neurosci. 2017, 679–692. [Google Scholar] [CrossRef]
- Keller, S.S.; Roberts, N.; García-Fiñana, M.; Mohammadi, S.; Ringelstein, E.B.; Knecht, S.; Deppe, M. Can the Language-dominant Hemisphere Be Predicted by Brain Anatomy? J. Cogn. Neurosci. 2010, 23, 2013–2029. [Google Scholar] [CrossRef] [PubMed]
- Amiez, C.; Sallet, J.; Hopkins, W.D.; Meguerditchian, A.; Hadj-Bouziane, F.; Ben Hamed, S.; Wilson, C.R.E.; Procyk, E.; Petrides, M. Sulcal organization in the medial frontal cortex provides insights into primate brain evolution. Nat. Commun. 2019, 10, 3437. [Google Scholar] [CrossRef] [Green Version]
- Gerrits, R.; Verhelst, H.; Dhollander, T.; Xiang, L.; Vingerhoets, G. Structural perisylvian asymmetry in naturally occurring atypical language dominance. Anat. Embryol. 2021, 227, 573–586. [Google Scholar] [CrossRef] [PubMed]
- Tzourio-Mazoyer, N.; Crivello, F.; Mazoyer, B. Is the planum temporale surface area a marker of hemispheric or regional language lateralization? Anat. Embryol. 2018, 223, 1217–1228. [Google Scholar] [CrossRef]
- Dehaene-Lambertz, G.; Dehaene, S.; Hertz-Pannier, L. Functional Neuroimaging of Speech Perception in Infants. Science 2002, 298, 2013–2015. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Catani, M.; Allin, M.P.G.; Husain, M.; Pugliese, L.; Mesulam, M.M.; Murray, R.M.; Jones, D.K. Symmetries in human brain language pathways correlate with verbal recall. Proc. Natl. Acad. Sci. USA 2007, 104, 17163–17168. [Google Scholar] [CrossRef] [Green Version]
- Darwin, C. The Descent of Man and Selection in Relation to Sex; Princeton University Press: Princeton, NJ, USA, 1981; Volume 1, [Originally Work Published 1871]. [Google Scholar]
- Hopkins, W.D.; Misiura, M.; Pope, S.M.; Latash, E.M. Behavioral and brain asymmetries in primates: A preliminary evaluation of two evolutionary hypotheses. Ann. N. Y. Acad. Sci. 2015, 1359, 65–83. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Poirier, C.; Ben Hamed, S.; Garcia-Saldivar, P.; Kwok, S.C.; Meguerditchian, A.; Merchant, H.; Rogers, J.; Wells, S.; Fox, A.S. Beyond MRI: On the scientific value of combining non-human primate neuroimaging with metadata. NeuroImage 2021, 228, 117679. [Google Scholar] [CrossRef] [PubMed]
- Milham, M.; Petkov, C.I.; Margulies, D.S.; Schroeder, C.E.; Basso, M.A.; Belin, P.; Fair, D.A.; Fox, A.; Kastner, S.; Mars, R.; et al. Accelerating the Evolution of Nonhuman Primate Neuroimaging. Neuron 2020, 105, 600–603. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hopkins, W.D.; Taglialatela, J.P.; Meguerditchian, A.; Nir, T.; Schenker, N.M.; Sherwood, C.C. Gray matter asymmetries in chimpanzees as revealed by voxel-based morphometry. NeuroImage 2008, 42, 491–497. [Google Scholar] [CrossRef] [Green Version]
- Marie, D.; Roth, M.; Lacoste, R.; Nazarian, B.; Bertello, A.; Anton, J.-L.; Hopkins, W.D.; Margiotoudi, K.; Love, S.A.; Meguerditchian, A. Left Brain Asymmetry of the Planum Temporale in a Nonhominid Primate: Redefining the Origin of Brain Specialization for Language. Cereb. Cortex 2018, 28, 1808–1815. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Knecht, S.; Dräger, B.; Deppe, M.; Bobe, L.; Lohmann, H.; Flöel, A.; Ringelstein, E.-B.; Henningsen, H. Handedness and hemispheric language dominance in healthy humans. Brain 2000, 123, 2512–2518. [Google Scholar] [CrossRef] [Green Version]
- Amunts, K.; Jäncke, L.; Mohlberg, H.; Steinmetz, H.; Zilles, K. Interhemispheric asymmetry of the human motor cortex related to handedness and gender. Neuropsychologia 2000, 38, 304–312. [Google Scholar] [CrossRef]
- Groen, M.A.; Whitehouse, A.J.O.; Badcock, N.A.; Bishop, D.V.M. Associations between Handedness and Cerebral Lateralisation for Language: A Comparison of Three Measures in Children. PLoS ONE 2013, 8, e64876. [Google Scholar] [CrossRef] [Green Version]
- Mazoyer, B.; Zago, L.; Jobard, G.; Crivello, F.; Joliot, M.; Perchey, G.; Mellet, E.; Petit, L.; Tzourio-Mazoyer, N. Gaussian Mixture Modeling of Hemispheric Lateralization for Language in a Large Sample of Healthy Individuals Balanced for Handedness. PLoS ONE 2014, 9, e101165. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ocklenburg, S.; Beste, C.; Arning, L.; Peterburs, J.; Güntürkün, O. The ontogenesis of language lateralization and its relation to handedness. Neurosci. Biobehav. Rev. 2014, 43, 191–198. [Google Scholar] [CrossRef]
- Meguerditchian, A.; Vauclair, J.; Hopkins, W.D. On the origins of human handedness and language: A comparative review of hand preferences for bimanual coordinated actions and gestural communication in nonhuman primates. Dev. Psychobiol. 2013, 55, 637–650. [Google Scholar] [CrossRef] [PubMed]
- Hewes, G.W.; Andrew, R.J.; Carini, L.; Choe, H.; Gardner, R.A.; Kortlandt, A.; Krantz, G.S.; McBride, G.; Nottebohm, F.; Pfeiffer, J.; et al. Primate Communication and the Gestural Origin of Language [and Comments and Reply]. Curr. Anthr. 1973, 14, 5–24. [Google Scholar] [CrossRef]
- Tomasello, M. Origins of Human Communication; The MIT Press: Cambridge, UK, 2008. [Google Scholar]
- Meguerditchian, A.; Vauclair, J. Communicative Signaling, Lateralization and Brain Substrate in Nonhuman Primates: Toward a Gestural or a Multimodal Origin of Language? Hum. Mente J. Philos. Stud. 2014, 7, 135–160. [Google Scholar]
- Molesti, S.; Meguerditchian, A.; Bourjade, M. Gestural communication in olive baboons [Papio anubis]: Repertoire and intentionality. Anim. Cogn. 2019, 23, 19–40. [Google Scholar] [CrossRef]
- Greenfield, P.M. Language, tools and brain: The ontogeny and phylogeny of hierarchically organized sequential behavior. Behav. Brain Sci. 1991, 14, 531–551. [Google Scholar] [CrossRef] [Green Version]
- Corballis, M.C. From mouth to hand: Gesture, speech and the evolution of right- handedness. Brain Sci. 2004, 26, 199–260. [Google Scholar] [CrossRef] [PubMed]
- Stout, D.; Toth, N.; Schick, K.; Chaminade, T. Neural correlates of Early Stone Age toolmaking: Technology, language and cognition in human evolution. Philos. Trans. R. Soc. B Biol. Sci. 2008, 363, 1939–1949. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Forrester, G.S.; Quaresmini, C.; Leavens, D.; Mareschal, D.; Thomas, M.S. Human handedness: An inherited evolutionary trait. Behav. Brain Res. 2013, 237, 200–206. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Higuchi, S.; Chaminade, T.; Imamizu, H.; Kawato, M. Shared neural correlates for language and tool use in Broca’s area. NeuroReport 2009, 20, 1376–1381. [Google Scholar] [CrossRef]
- Thibault, S.; Py, R.; Gervasi, A.M.; Salemme, R.; Koun, E.; Lövden, M.; Boulenger, V.; Roy, A.C.; Brozzoli, C. Tool use and language share syntactic processes and neural patterns in the basal ganglia. Science 2021, 374, eabe0874. [Google Scholar] [CrossRef]
- Galaburda, A.M.; LeMay, M.; Kemper, T.L.; Geschwind, N. Right-Left Asymmetries in the Brain. Science 1978, 199, 852–856. [Google Scholar] [CrossRef]
- Mesulam, M.M. From sensation to cognition. Brain 1998, 121, 1013–1052. [Google Scholar] [CrossRef]
- Wernicke, C. Der Aphasische Symptomencomplex: Eine Psychologische Studie auf Anatomischer Basis; Cohn: Breslau, Poland, 1874. [Google Scholar]
- Dronkers, N.F.; Wilkins, D.P.; Van Valin, R.D.; Redfern, B.B.; Jaeger, J.J. Lesion analysis of the brain areas involved in language comprehension. Cognition 2004, 92, 145–177. [Google Scholar] [CrossRef] [Green Version]
- Borovsky, A.; Saygin, A.P.; Bates, E.; Dronkers, N. Lesion correlates of conversational speech production deficits. Neuropsychologia 2007, 45, 2525–2533. [Google Scholar] [CrossRef] [Green Version]
- Shapleske, J.; Rossell, S.; Woodruff, P.; David, A. The planum temporale: A systematic, quantitative review of its structural, functional and clinical significance. Brain Res. Rev. 1999, 29, 26–49. [Google Scholar] [CrossRef]
- Vigneau, M.; Beaucousin, V.; Hervé, P.; Duffau, H.; Crivello, F.; Houdé, O.; Mazoyer, B.; Tzourio-Mazoyer, N. Meta-analyzing left hemisphere language areas: Phonology, semantics, and sentence processing. NeuroImage 2006, 30, 1414–1432. [Google Scholar] [CrossRef] [PubMed]
- Dehaene-Lambertz, G.; Montavont, A.; Jobert, A.; Allirol, L.; Dubois, J.; Hertz-Pannier, L.; Dehaene, S. Language or music, mother or Mozart? Structural and environmental influences on infants’ language networks. Brain Lang. 2010, 114, 53–65. [Google Scholar] [CrossRef]
- Mahmoudzadeh, M.; Dehaene-Lambertz, G.; Fournier, M.; Kongolo, G.; Goudjil, S.; Dubois, J.; Grebe, R.; Wallois, F. Syllabic discrimination in premature human infants prior to complete formation of cortical layers. Proc. Natl. Acad. Sci. USA 2013, 110, 4846–4851. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Geschwind, N.; Levitsky, W. Human Brain: Left-Right Asymmetries in Temporal Speech Region. Science 1968, 161, 186–187. [Google Scholar] [CrossRef]
- Wada, J.A.; Clarke, R.; Hamm, A. Cerebral Hemispheric Asymmetry in Humans. Arch. Neurol. 1975, 32, 239–246. [Google Scholar] [CrossRef] [PubMed]
- Witelson, S.F.; Pallie, W. Left hemisphere Spezialisation for Language in the Newborn. Brain 1973, 96, 641–646. [Google Scholar] [CrossRef]
- Glasel, H.; Leroy, F.; Dubois, J.; Hertz-Pannier, L.; Mangin, J.; Dehaene-Lambertz, G. A robust cerebral asymmetry in the infant brain: The rightward superior temporal sulcus. NeuroImage 2011, 58, 716–723. [Google Scholar] [CrossRef]
- Hill, J.; Inder, T.; Neil, J.; Dierker, D.; Harwell, J.; Van Essen, D. Similar patterns of cortical expansion during human development and evolution. Proc. Natl. Acad. Sci. USA 2010, 107, 13135–13140. [Google Scholar] [CrossRef] [Green Version]
- Dubois, J.; Benders, M.; Cachia, A.; Lazeyras, F.; Leuchter, R.H.-V.; Sizonenko, S.V.; Borradori-Tolsa, C.; Mangin, J.F.; Hüppi, P.S. Mapping the Early Cortical Folding Process in the Preterm Newborn Brain. Cereb. Cortex 2008, 18, 1444–1454. [Google Scholar] [CrossRef] [Green Version]
- Dubois, J.; Benders, M.; Lazeyras, F.; Borradori-Tolsa, C.; Leuchter, R.H.-V.; Mangin, J.; Hüppi, P. Structural asymmetries of perisylvian regions in the preterm newborn. NeuroImage 2010, 52, 32–42. [Google Scholar] [CrossRef]
- Chi, J.G.; Dooling, E.C.; Gilles, F.H. Gyral development of the human brain. Ann. Neurol. 1977, 1, 86–93. [Google Scholar] [CrossRef] [PubMed]
- Chi, J.G.; Dooling, E.C.; Gilles, F.H. Left-Right Asymmetries of the Temporal Speech Areas of the Human Fetus. Arch. Neurol. 1977, 34, 346–348. [Google Scholar] [CrossRef] [PubMed]
- Josse, G.; Hervé, P.-Y.; Crivello, F.; Mazoyer, B.; Tzourio-Mazoyer, N. Hemispheric specialization for language: Brain volume matters. Brain Res. 2006, 1068, 184–193. [Google Scholar] [CrossRef]
- Gauger, L.M.; Lombardino, L.J.; Leonard, C.M. Brain Morphology in Children with Specific Language Impairment. J. Speech Lang. Hear. Res. 1997, 40, 1272–1284. [Google Scholar] [CrossRef] [PubMed]
- Altarelli, I.; Leroy, F.; Monzalvo, K.; Fluss, J.; Billard, C.; Dehaene-Lambertz, G.; Galaburda, A.M.; Ramus, F. Planum temporale asymmetry in developmental dyslexia: Revisiting an old question. Hum. Brain Mapp. 2014, 35, 5717–5735. [Google Scholar] [CrossRef]
- Jäncke, L.; Schlaug, G.; Huang, Y.; Steinmetz, H. Asymmetry of the planum parietale. NeuroReport 1994, 5, 1161–1163. [Google Scholar] [CrossRef]
- Dorsaint-Pierre, R.; Penhune, V.B.; Watkins, K.; Neelin, P.; Lerch, J.P.; Bouffard, M.; Zatorre, R.J. Asymmetries of the planum temporale and Heschl’s gyrus: Relationship to language lateralization. Brain 2006, 129, 1164–1176. [Google Scholar] [CrossRef] [Green Version]
- Eckert, M.A.; Leonard, C.M.; Possing, E.T.; Binder, J.R. Uncoupled leftward asymmetries for planum morphology and functional language processing. Brain Lang. 2006, 98, 102–111. [Google Scholar] [CrossRef] [Green Version]
- Greve, D.N.; Van der Haegen, L.; Cai, Q.; Stufflebeam, S.; Sabuncu, M.R.; Fischl, B.; Brysbaert, M. A Surface-based Analysis of Language Lateralization and Cortical Asymmetry. J. Cogn. Neurosci. 2013, 25, 1477–1492. [Google Scholar] [CrossRef]
- Kolinsky, R.; Morais, J.; Cohen, L.; Dehaene-Lambertz, G.; Dehaene, S. The impact of literacy on the language brain areas. Rev. Neuropsychol. 2014, 6, 173–181. [Google Scholar] [CrossRef]
- Tzourio-Mazoyer, N.; Mazoyer, B. Variations of planum temporale asymmetries with Heschl’s Gyri duplications and association with cognitive abilities: MRI investigation of 428 healthy volunteers. Anat. Embryol. 2017, 222, 2711–2726. [Google Scholar] [CrossRef] [PubMed]
- Ocklenburg, S.; Friedrich, P.; Fraenz, C.; Schlüter, C.; Beste, C.; Güntürkün, O.; Genç, E. Neurite architecture of the planum temporale predicts neurophysiological processing of auditory speech. Sci. Adv. 2018, 4, eaar6830. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gannon, P.J.; Holloway, R.L.; Broadfield, D.C.; Braun, A.R. Asymmetry of Chimpanzee Planum Temporale: Humanlike Pattern of Wernicke’s Brain Language Area Homolog. Science 1998, 279, 220–222. [Google Scholar] [CrossRef] [PubMed]
- Hopkins, W.D.; Marino, L.; Rilling, J.K.; MacGregor, L.A. Planum temporale asymmetries in great apes as revealed by magnetic resonance imaging [MRI]. NeuroReport 1998, 9, 2913–2918. [Google Scholar] [CrossRef] [PubMed]
- Hopkins, W.D.; Nir, T.M. Planum temporale surface area and grey matter asymmetries in chimpanzees [Pan troglodytes]: The effect of handedness and comparison with findings in humans. Behav. Brain Res. 2010, 208, 436–443. [Google Scholar] [CrossRef] [Green Version]
- Cantalupo, C.; Pilcher, D.L.; Hopkins, W.D. Are planum temporale and sylvian fissure asymmetries directly related? A MRI study in great apes. Neuropsychologia 2003, 41, 1975–1981. [Google Scholar] [CrossRef]
- Becker, Y.; Sein, J.; Velly, L.; Giacomino, L.; Renaud, L.; Lacoste, R.; Anton, J.-L.; Nazarian, B.; Berne, C.; Meguerditchian, A. Early Left-Planum Temporale Asymmetry in newborn monkeys [Papio anubis]: A longitudinal structural MRI study at two stages of development. NeuroImage 2021, 227, e117575. [Google Scholar] [CrossRef]
- Becker, Y.; Phelipon, R.; Sein, J.; Velly, L.; Renaud, L.; Meguerditchian, A. Planum temporale grey matter volume asymmetries in newborn monkeys [Papio anubis]. Brain Struct. Funct. 2022, 227, 463–468. [Google Scholar] [CrossRef]
- Meguerditchian, A.; Gardner, M.J.; Schapiro, S.J.; Hopkins, W.D. The sound of one-hand clapping: Handedness and perisylvian neural correlates of a communicative gesture in chimpanzees. Proc. R. Soc. B Boil. Sci. 2012, 279, 1959–1966. [Google Scholar] [CrossRef] [Green Version]
- Gilissen, E.P.; Hopkins, W.D. Asymmetries of the Parietal Operculum in Chimpanzees [Pan troglodytes] in Relation to Handedness for Tool Use. Cereb. Cortex 2013, 23, 411–422. [Google Scholar] [CrossRef] [Green Version]
- Broca, P. Remarques sur le siège de la Faculté du Langage Articulé, Suivies d’une Observation d’aphémie [perte de la parole]; Bulletin et Memoires de la Societe Anatomique de Paris: Paris, France, 1861; Volume 6, pp. 330–357. [Google Scholar]
- Hickok, G.; Poeppel, D. The cortical organization of speech processing. Nat. Rev. Neurosci. 2007, 8, 393–402. [Google Scholar] [CrossRef] [PubMed]
- Friederici, A.D. Language in Our Brain: The Origins of a Uniquely Human Capacity; The MIT Press: Cambridge, MA, USA, 2017. [Google Scholar]
- Gentilucci, M.; Volta, R.D. Spoken Language and arm Gestures are Controlled by the same Motor Control System. Q. J. Exp. Psychol. 2008, 61, 944–957. [Google Scholar] [CrossRef] [PubMed]
- Koechlin, E.; Jubault, T. Broca’s Area and the Hierarchical Organization of Human Behavior. Neuron 2006, 50, 963–974. [Google Scholar] [CrossRef] [Green Version]
- Stout, D.; Hecht, E.E. Evolutionary neuroscience of cumulative culture. Proc. Natl. Acad. Sci. USA 2017, 114, 7861–7868. [Google Scholar] [CrossRef] [Green Version]
- Emmorey, K.; Grabowski, T.; McCullough, S.; Damasio, H.; Ponto, L.; Hichwa, R.; Bellugi, U. Motor-iconicity of sign language does not alter the neural systems underlying tool and action naming. Brain Lang. 2004, 89, 27–37. [Google Scholar] [CrossRef]
- Campbell, R.; Macsweeney, M.; Waters, D. Sign Language and the Brain: A Review. J. Deaf Stud. Deaf Educ. 2007, 13, 3–20. [Google Scholar] [CrossRef] [PubMed]
- Imada, T.; Zhang, Y.; Cheour, M.; Taulu, S.; Ahonen, A.; Kuhl, P.K. Infant speech perception activates Broca’s area: A developmental magnetoencephalography study. NeuroReport 2006, 17, 957–962. [Google Scholar] [CrossRef]
- Dehaene-Lambertz, G.; Hertz-Pannier, L.; Dubois, J.; Mériaux, S.; Roche, A.; Sigman, M.; Dehaene, S. Functional organization of perisylvian activation during presentation of sentences in preverbal infants. Proc. Natl. Acad. Sci. USA 2006, 103, 14240–14245. [Google Scholar] [CrossRef] [Green Version]
- Keller, S.S.; Crow, T.; Foundas, A.; Amunts, K.; Roberts, N. Broca’s area: Nomenclature, anatomy, typology and asymmetry. Brain Lang. 2009, 109, 29–48. [Google Scholar] [CrossRef]
- Sprung-Much, T.; Eichert, N.; Nolan, E.; Petrides, M. Broca’s area and the search for anatomical asymmetry: Commentary and perspectives. Anat. Embryol. 2022, 227, 441–449. [Google Scholar] [CrossRef]
- Keller, S.S.; Highley, J.R.; Garcia-Finana, M.; Sluming, V.; Rezaie, R.; Roberts, N. Sulcal variability, stereological measurement and asymmetry of Broca’s area on MR images. J. Anat. 2007, 211, 534–555. [Google Scholar] [CrossRef] [PubMed]
- Scheibel, A.B. A dendritic correlate of human speech. In Cerebral Dominance: The Biological Foundations; Harvard University Press: Cambridge, MA, USA, 1984; pp. 43–52. [Google Scholar]
- Amunts, K.; Schleicher, A.; Mohlberg, H.; Uylings, H.B.; Zilles, K. Broca’s region revisited: Cytoarchitecture and intersubject variability. J. Comp. Neurol. 1999, 412, 319–341. [Google Scholar] [CrossRef]
- Amunts, K.; Schleicher, A.; Ditterich, A.; Zilles, K. Broca’s region: Cytoarchitectonic asymmetry and developmental changes. J. Comp. Neurol. 2003, 465, 72–89. [Google Scholar] [CrossRef]
- Kurth, F.; Cherbuin, N.; Luders, E. Speaking of aging: Changes in gray matter asymmetry in Broca’s area in later adulthood. Cortex 2020, 129, 133–140. [Google Scholar] [CrossRef] [PubMed]
- Simonds, R.J.; Scheibel, A.B. The postnatal development of the motor speech area: A preliminary study. Brain Lang. 1989, 37, 42–58. [Google Scholar] [CrossRef]
- Cantalupo, C.; Hopkins, W.D. Asymmetric Broca’s area in great apes. Nature 2001, 414, 505. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Keller, S.S.; Deppe, M.; Herbin, M.; Gilissen, E. Variability and asymmetry of the sulcal contours defining Broca’s area homologue in the chimpanzee brain. J. Comp. Neurol. 2012, 520, 1165–1180. [Google Scholar] [CrossRef]
- Hopkins, W.D. Motor and Communicative Correlates of the Inferior Frontal Gyrus [Broca’s Area] in Chimpanzees. In Origins of Human Language: Continuities and Splits with Nonhuman Primates; Boë, L.-J., Fagot, J., Perrier, P., Schwartz, J.-L., Eds.; Peter Lang: Oxford, UK, 2017; pp. 153–186. [Google Scholar]
- Schenker-Ahmed, N.; Hopkins, W.D.; Spocter, M.; Garrison, A.R.; Stimpson, C.D.; Erwin, J.M.; Hof, P.R.; Sherwood, C.C. Broca’s Area Homologue in Chimpanzees [Pan troglodytes]: Probabilistic Mapping, Asymmetry, and Comparison to Humans. Cereb. Cortex 2010, 20, 730–742. [Google Scholar] [CrossRef] [Green Version]
- Graïc, J.-M.; Peruffo, A.; Corain, L.; Centelleghe, C.; Granato, A.; Zanellato, E.; Cozzi, B. Asymmetry in the Cytoarchitecture of the Area 44 Homolog of the Brain of the Chimpanzee Pan troglodytes. Front. Neuroanat. 2020, 14. [Google Scholar] [CrossRef]
- Petrides, M. Lateral prefrontal cortex: Architectonic and functional organization. Philos. Trans. R. Soc. B Biol. Sci. 2005, 360, 781–795. [Google Scholar] [CrossRef] [Green Version]
- Petrides, M.; Cadoret, G.; Mackey, S. Orofacial somatomotor responses in the macaque monkey homologue of Broca’s area. Nature 2005, 435, 1235–1238. [Google Scholar] [CrossRef] [PubMed]
- Belmalih, A.; Borra, E.; Contini, M.; Gerbella, M.; Rozzi, S.; Luppino, G. Multimodal architectonic subdivision of the rostral part [area F5] of the macaque ventral premotor cortex. J. Comp. Neurol. 2009, 512, 183–217. [Google Scholar] [CrossRef] [PubMed]
- Hage, S.R.; Nieder, A. Single neurons in monkey prefrontal cortex encode volitional initiation of vocalizations. Nat. Commun. 2013, 4, 2409. [Google Scholar] [CrossRef] [PubMed]
- Taglialatela, J.P.; Russell, J.L.; Schaeffer, J.A.; Hopkins, W.D. Communicative Signaling Activates ‘Broca’s’ Homolog in Chimpanzees. Curr. Biol. 2008, 18, 343–348. [Google Scholar] [CrossRef] [Green Version]
- Hopkins, W.D.; Meguerditchian, A.; Coulon, O.; Misiura, M.; Pope, S.; Mareno, M.C.; Schapiro, S.J. Motor skill for tool-use is associated with asymmetries in Broca’s area and the motor hand area of the precentral gyrus in chimpanzees [Pan troglodytes]. Behav. Brain Res. 2017, 318, 71–81. [Google Scholar] [CrossRef] [Green Version]
- Becker, Y.; Claidière, N.; Margiotoudi, K.; Marie, D.; Roth, M.; Nazarian, B.; Anton, J.-L.; Coulon, O.; Meguerditchian, A. Broca’s cerebral asymmetry reflects gestural communication’s lateralisation in monkeys [Papio anubis]. eLife 2022, 11, e70521. [Google Scholar] [CrossRef]
- Catani, M.; Schotten, M.T. Atlas of Human Brain Connections; OUP Oxford: Oxford, UK, 2012. [Google Scholar]
- Metellus, P.; Boussen, S.; Guye, M.; Trebuchon, A. Successful Insular Glioma Removal in a Deaf Signer Patient during an Awake Craniotomy Procedure. World Neurosurg. 2017, 98, 883-e1. [Google Scholar] [CrossRef]
- Dubois, J.; Poupon, C.; Thirion, B.; Simonnet, H.; Kulikova, S.; Leroy, F.; Hertz-Pannier, L.; Dehaene-Lambertz, G. Exploring the Early Organization and Maturation of Linguistic Pathways in the Human Infant Brain. Cereb. Cortex 2016, 26, 2283–2298. [Google Scholar] [CrossRef]
- Brauer, J.; Anwander, A.; Perani, D.; Friederici, A.D. Dorsal and ventral pathways in language development. Brain Lang. 2013, 127, 289–295. [Google Scholar] [CrossRef] [Green Version]
- Brauer, J.; Anwander, A.; Friederici, A.D. Neuroanatomical Prerequisites for Language Functions in the Maturing Brain. Cereb. Cortex 2011, 21, 459–466. [Google Scholar] [CrossRef]
- Dubois, J.; Hertz-Pannier, L.; Dehaene-Lambertz, G.; Cointepas, Y.; Le Bihan, D. Assessment of the early organization and maturation of infants’ cerebral white matter fiber bundles: A feasibility study using quantitative diffusion tensor imaging and tractography. NeuroImage 2006, 30, 1121–1132. [Google Scholar] [CrossRef] [PubMed]
- Dubois, J.; Hertz-Pannier, L.; Cachia, A.; Mangin, J.F.; Le Bihan, D.; Dehaene-Lambertz, G. Structural Asymmetries in the Infant Language and Sensori-Motor Networks. Cereb. Cortex 2009, 19, 414–423. [Google Scholar] [CrossRef] [PubMed]
- Perani, D.; Saccuman, M.C.; Scifo, P.; Anwander, A.; Spada, D.; Baldoli, C.; Poloniato, A.; Lohmann, G.; Friederici, A.D. Neural language networks at birth. Proc. Natl. Acad. Sci. USA 2011, 108, 16056–16061. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Friederici, A.D. Language Development and the Ontogeny of the Dorsal Pathway. Front. Evol. Neurosci. 2012, 4, 3. [Google Scholar] [CrossRef] [Green Version]
- Büchel, C.; Raedler, T.; Sommer, M.; Sach, M.; Weiller, C.; Koch, M. White Matter Asymmetry in the Human Brain: A Diffusion Tensor MRI Study. Cereb. Cortex 2004, 14, 945–951. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nucifora, P.G.P.; Verma, R.; Melhem, E.R.; Gur, R.E.; Gur, R.C. Leftward asymmetry in relative fiber density of the arcuate fasciculus. NeuroReport 2005, 16, 791–794. [Google Scholar] [CrossRef] [Green Version]
- Powell, H.R.; Parker, G.J.; Alexander, D.C.; Symms, M.R.; Boulby, P.A.; Wheeler-Kingshott, C.A.; Barker, G.J.; Noppeney, U.; Koepp, M.J.; Duncan, J.S. Hemispheric asymmetries in language-related pathways: A combined functional MRI and tractography study. NeuroImage 2006, 32, 388–399. [Google Scholar] [CrossRef] [PubMed]
- Takaya, S.; Kuperberg, G.R.; Liu, H.; Greve, D.N.; Makris, N.; Stufflebeam, S.M. Asymmetric projections of the arcuate fasciculus to the temporal cortex underlie lateralized language function in the human brain. Front. Neuroanat. 2015, 9, 119. [Google Scholar] [CrossRef] [Green Version]
- Thiebaut de Schotten, M.; Ffytche, D.H.; Bizzi, A.; Dell’Acqua, F.; Allin, M.; Walshe, M.; Murray, R.; Williams, S.C.; Murphy, D.G.M.; Catani, M. Atlasing location, asymmetry and inter-subject variability of white matter tracts in the human brain with MR diffusion tractography. NeuroImage 2011, 54, 49–59. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Balériaux, D.; Kavec, M.; Metens, T.; Absil, J.; Denolin, V.; Pardou, A.; Avni, F.; Van Bogaert, P.; Aeby, A. Structural asymmetries in motor and language networks in a population of healthy preterm neonates at term equivalent age: A diffusion tensor imaging and probabilistic tractography study. NeuroImage 2010, 51, 783–788. [Google Scholar] [CrossRef]
- Song, J.W.; Mitchell, P.D.; Kolasinski, J.; Ellen Grant, P.; Galaburda, A.M.; Takahashi, E. Asymmetry of White Matter Pathways in Developing Human Brains. Cereb. Cortex 2015, 25, 2883–2893. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lebel, C.; Beaulieu, C. Lateralization of the arcuate fasciculus from childhood to adulthood and its relation to cognitive abilities in children. Hum. Brain Mapp. 2009, 30, 3563–3573. [Google Scholar] [CrossRef] [PubMed]
- López-Barroso, D.; Catani, M.; Ripollés, P.; Dell’Acqua, F.; Rodríguez-Fornells, A.; de Diego-Balaguer, R. Word learning is mediated by the left arcuate fasciculus. Proc. Natl. Acad. Sci. USA 2013, 110, 13168–13173. [Google Scholar] [CrossRef] [Green Version]
- Salvan, P.; Tournier, J.D.; Batalle, D.; Falconer, S.; Chew, A.; Kennea, N.; Aljabar, P.; Dehaene-Lambertz, G.; Arichi, T.; Edwards, A.D.; et al. Language ability in preterm children is associated with arcuate fasciculi microstructure at term. Hum. Brain Mapp. 2017, 38, 3836–3847. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Propper, R.E.; O’Donnell, L.J.; Whalen, S.; Tie, Y.; Norton, I.H.; Suarez, R.O.; Zollei, L.; Radmanesh, A.; Golby, A.J. A combined fMRI and DTI examination of functional language lateralization and arcuate fasciculus structure: Effects of degree versus direction of hand preference. Brain Cogn. 2010, 73, 85–92. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Fan, Y.; Zou, Q.; Wang, J.; Gao, J.-H.; Niu, Z. Temporal Reliability and Lateralization of the Resting-State Language Network. PLoS ONE 2014, 9, e85880. [Google Scholar] [CrossRef] [PubMed]
- Silva, G.; Citterio, A. Hemispheric asymmetries in dorsal language pathway white-matter tracts: A magnetic resonance imaging tractography and functional magnetic resonance imaging study. Neuroradiol. J. 2017, 30, 470–476. [Google Scholar] [CrossRef]
- Verhelst, H.; Dhollander, T.; Gerrits, R.; Vingerhoets, G. Fibre-specific laterality of white matter in left and right language dominant people. NeuroImage 2021, 230, 117812. [Google Scholar] [CrossRef]
- Schmahmann, J.D.; Pandya, D.N. Fiber Pathways of the Brain; Oxford University Press: Oxford, UK, 2006. [Google Scholar]
- Petrides, M.; Pandya, D.N. Distinct Parietal and Temporal Pathways to the Homologues of Broca’s Area in the Monkey. PLoS Biol. 2009, 7, e1000170. [Google Scholar] [CrossRef] [Green Version]
- Petrides, M. Neuroanatomy of Language Regions of the Human Brain, 1st ed.; Elsevier/AP, Academic Press is an imprint of Elsevier: Amsterdam, The Netherlands, 2014. [Google Scholar]
- Rilling, J.K.; Glasser, M.F.; Preuss, T.M.; Ma, X.; Zhao, T.; Hu, X.; Behrens, T.E.J. The evolution of the arcuate fasciculus revealed with comparative DTI. Nat. Neurosci. 2008, 11, 426–428. [Google Scholar] [CrossRef]
- Rilling, J.K.; Glasser, M.F.; Jbabdi, S.; Andersson, J.; Preuss, T.M. Continuity, Divergence, and the Evolution of Brain Language Pathways. Front. Evol. Neurosci. 2012, 3, 11. [Google Scholar] [CrossRef] [Green Version]
- Frey, S.; Mackey, S.; Petrides, M. Cortico-cortical connections of areas 44 and 45B in the macaque monkey. Brain Lang. 2014, 131, 36–55. [Google Scholar] [CrossRef]
- Eichert, N.; Verhagen, L.; Folloni, D.; Jbabdi, S.; Khrapitchev, A.A.; Sibson, N.R.; Mantini, D.; Sallet, J.; Mars, R. What is special about the human arcuate fasciculus? Lateralization, projections, and expansion. Cortex 2019, 118, 107–115. [Google Scholar] [CrossRef] [PubMed]
- Barrett, R.L.C.; Dawson, M.; Dyrby, T.B.; Krug, K.; Ptito, M.; D’Arceuil, H.; Croxson, P.L.; Johnson, P.J.; Howells, H.; Forkel, S.; et al. Differences in Frontal Network Anatomy Across Primate Species. J. Neurosci. 2020, 40, 2094–2107. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Balezeau, F.; Wilson, B.; Gallardo, G.; Dick, F.; Hopkins, W.; Anwander, A.; Friederici, A.D.; Griffiths, T.D.; Petkov, C.I. Primate auditory prototype in the evolution of the arcuate fasciculus. Nat. Neurosci. 2020, 23, 611–614. [Google Scholar] [CrossRef] [PubMed]
- Rocchi, F.; Oya, H.; Balezeau, F.; Billig, A.; Kocsis, Z.; Jenison, R.L.; Nourski, K.; Kovach, C.; Steinschneider, M.; Kikuchi, Y.; et al. Common fronto-temporal effective connectivity in humans and monkeys. Neuron 2021, 109, 852–868.e8. [Google Scholar] [CrossRef]
- Dronkers, N.F. A new brain region for coordinating speech articulation. Nature 1996, 384, 159–161. [Google Scholar] [CrossRef]
- Oh, A.; Duerden, E.G.; Pang, E.W. The role of the insula in speech and language processing. Brain Lang. 2014, 135, 96–103. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bamiou, D.-E.; Musiek, F.E.; Luxon, L.M. The insula [Island of Reil] and its role in auditory processing: Literature review. Brain Res. Rev. 2003, 42, 143–154. [Google Scholar] [CrossRef]
- Ackermann, H.; Riecker, A. The contribution of the insula to motor aspects of speech production: A review and a hypothesis. Brain Lang. 2004, 89, 320–328. [Google Scholar] [CrossRef]
- Friederici, A.D. The neural basis for human syntax: Broca’s area and beyond. Curr. Opin. Behav. Sci. 2018, 21, 88–92. [Google Scholar] [CrossRef]
- Zaccarella, E.; Friederici, A.D. Merge in the Human Brain: A Sub-Region Based Functional Investigation in the Left Pars Opercularis. Front. Psychol. 2015, 6, 1818. [Google Scholar] [CrossRef] [Green Version]
- Lœvenbruck, H.; Baciu, M.; Segebarth, C.; Abry, C. The left inferior frontal gyrus under focus: An fMRI study of the production of deixis via syntactic extraction and prosodic focus. J. Neurolinguistics 2005, 18, 237–258. [Google Scholar] [CrossRef] [Green Version]
- Lœvenbruck, H.; Vilain, C.; Dohen, M. From gestural pointing to vocal pointing in the brain. Rev. Fr. Linguist. Appl. 2008, 13, 23–33. [Google Scholar] [CrossRef]
- Chiarello, C.; Vazquez, D.; Felton, A.; Leonard, C.M. Structural asymmetry of anterior insula: Behavioral correlates and individual differences. Brain Lang. 2013, 126, 109–122. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Economo, C.V. Eine neue art spezialzellen des lobus cinguli und lobus insulae. Z. Die Gesamte Neurol. Und Psychiatr. 1926, 100, 706–712. [Google Scholar] [CrossRef]
- Evrard, H.C. Von Economo and fork neurons in the monkey insula, implications for evolution of cognition. Curr. Opin. Behav. Sci. 2018, 21, 182–190. [Google Scholar] [CrossRef]
- Bauernfeind, A.L.; de Sousa, A.A.; Avasthi, T.; Dobson, S.D.; Raghanti, M.A.; Lewandowski, A.H.; Zilles, K.; Semendeferi, K.; Allman, J.M.; Craig, A.D.; et al. A volumetric comparison of the insular cortex and its subregions in primates. J. Hum. Evol. 2013, 64, 263–279. [Google Scholar] [CrossRef] [Green Version]
- Nimchinsky, E.A.; Gilissen, E.; Allman, J.M.; Perl, D.P.; Erwin, J.M.; Hof, P.R. A neuronal morphologic type unique to humans and great apes. Proc. Natl. Acad. Sci. USA 1999, 96, 5268–5273. [Google Scholar] [CrossRef] [Green Version]
- Toga, A.W.; Thompson, P.M. Temporal Dynamics of Brain Anatomy. Annu. Rev. Biomed. Eng. 2003, 5, 119–145. [Google Scholar] [CrossRef] [PubMed]
- Amodio, D.M.; Frith, C.D. Meeting of minds: The medial frontal cortex and social cognition. Nat. Rev. Neurosci. 2006, 7, 268–277. [Google Scholar] [CrossRef] [PubMed]
- Loh, K.K.; Petrides, M.; Hopkins, W.D.; Procyk, E.; Amiez, C. Cognitive control of vocalizations in the primate ventrolateral-dorsomedial frontal [VLF-DMF] brain network. Neurosci. Biobehav. Rev. 2017, 82, 32–44. [Google Scholar] [CrossRef] [PubMed]
- Loh, K.K.; Procyk, E.; Neveu, R.; Lamberton, F.; Hopkins, W.D.; Petrides, M.; Amiez, C. Cognitive control of orofacial motor and vocal responses in the ventrolateral and dorsomedial human frontal cortex. Proc. Natl. Acad. Sci. USA 2020, 117, 4994–5005. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Talairach, J.; Bancaud, J.; Geier, S.; Bordas-Ferrer, M.; Bonis, A.; Szikla, G.; Rusu, M. The cingulate gyrus and human behaviour. Electroencephalogr. Clin. Neurophysiol. 1973, 34, 45–52. [Google Scholar] [CrossRef]
- Ackermann, H.; Ziegler, W. Akinetischer Mutismus-eine Literaturübersicht. Fortschr. Neurol. Psychiatr. 1995, 63, 59–67. [Google Scholar] [CrossRef]
- Benga, O. Intentional communication and the anterior cingulate cortex. Interact. Stud. 2005, 6, 201–221. [Google Scholar] [CrossRef]
- Mundy, P. A review of joint attention and social-cognitive brain systems in typical development and autism spectrum disorder. Eur. J. Neurosci. 2018, 47, 497–514. [Google Scholar] [CrossRef]
- Amiez, C.; Petrides, M. Neuroimaging Evidence of the Anatomo-Functional Organization of the Human Cingulate Motor Areas. Cereb. Cortex 2014, 24, 563–578. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ide, A.; Dolezal, C.; Fernández, M.; Labbé, E.; Mandujano, R.; Montes, S.; Segura, P.; Verschae, G.; Yarmuch, P.; Aboitiz, F. Hemispheric differences in variability of fissural patterns in parasylvian and cingulate regions of human brains. J. Comp. Neurol. 1999, 410, 235–242. [Google Scholar] [CrossRef]
- Amiez, C.; Wilson, C.R.E.; Procyk, E. Variations of cingulate sulcal organization and link with cognitive performance. Sci. Rep. 2018, 8, 13988. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paus, T.; Tomaiuolo, F.; Otaky, N.; Macdonald, D.; Petrides, M.; Atlas, J.; Morris, R.; Evans, A.C. Human Cingulate and Paracingulate Sulci: Pattern, Variability, Asymmetry, and Probabilistic Map. Cereb. Cortex 1996, 6, 207–214. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jürgens, U.; Pratt, R. The cingular vocalization pathway in the squirrel monkey. Exp. Brain Res. 1979, 34, 499–510. [Google Scholar] [CrossRef] [PubMed]
- Jürgens, U.; Ploog, D. Cerebral representation of vocalization in the squirrel monkey. Exp. Brain Res. 1970, 10, 532–554. [Google Scholar] [CrossRef] [PubMed]
- Aitken, P.G. Cortical control of conditioned and spontaneous vocal behavior in rhesus monkeys. Brain Lang. 1981, 13, 171–184. [Google Scholar] [CrossRef]
- Allman, J.M.; Tetreault, N.A.; Hakeem, A.Y.; Manaye, K.F.; Semendeferi, K.; Erwin, J.M.; Park, S.; Goubert, V.; Hof, P.R. The von Economo neurons in frontoinsular and anterior cingulate cortex in great apes and humans. Anat. Embryol. 2010, 214, 495–517. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hakeem, A.Y.; Sherwood, C.C.; Bonar, C.J.; Butti, C.; Hof, P.R.; Allman, J.M. Von Economo Neurons in the Elephant Brain. Anat. Rec. 2009, 292, 242–248. [Google Scholar] [CrossRef] [PubMed]
- Hopkins, W.D.; Procyk, E.; Petrides, M.; Schapiro, S.J.; Mareno, M.C.; Amiez, C. Sulcal Morphology in Cingulate Cortex is Associated with Voluntary Oro-Facial Motor Control and Gestural Communication in Chimpanzees [Pan troglodytes]. Cereb. Cortex 2021, 31, 2845–2854. [Google Scholar] [CrossRef]
- Deen, B.; Koldewyn, K.; Kanwisher, N.; Saxe, R. Functional Organization of Social Perception and Cognition in the Superior Temporal Sulcus. Cereb. Cortex 2015, 25, 4596–4609. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Belin, P.; Zatorre, R.J.; Lafaille, P.; Ahad, P.A.; Pike, B. Voice-selective areas in human auditory cortex. Nature 2000, 403, 309–312. [Google Scholar] [CrossRef] [PubMed]
- Pitcher, D.; Dilks, D.D.; Saxe, R.R.; Triantafyllou, C.; Kanwisher, N. Differential selectivity for dynamic versus static information in face-selective cortical regions. NeuroImage 2011, 56, 2356–2363. [Google Scholar] [CrossRef]
- Pelphrey, K.A.; Morris, J.P.; Michelich, C.R.; Allison, T.; McCarthy, G. Functional Anatomy of Biological Motion Perception in Posterior Temporal Cortex: An fMRI Study of Eye, Mouth and Hand Movements. Cereb. Cortex 2005, 15, 1866–1876. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Taylor, K.I.; Moss, H.E.; Stamatakis, E.A.; Tyler, L.K. Binding crossmodal object features in perirhinal cortex. Proc. Natl. Acad. Sci. USA 2006, 103, 8239–8244. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ciaramidaro, A.; Adenzato, M.; Enrici, I.; Erk, S.; Pia, L.; Bara, B.; Walter, H. The intentional network: How the brain reads varieties of intentions. Neuropsychologia 2007, 45, 3105–3113. [Google Scholar] [CrossRef]
- Wyk, B.C.V.; Hudac, C.M.; Carter, E.J.; Sobel, D.M.; Pelphrey, K.A. Action Understanding in the Superior Temporal Sulcus Region. Psychol. Sci. 2009, 20, 771–777. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bodin, C.; Takerkart, S.; Belin, P.; Coulon, O. Anatomo-functional correspondence in the superior temporal sulcus. Anat. Embryol. 2018, 223, 221–232. [Google Scholar] [CrossRef]
- Moreno, A.; Limousin, F.; Dehaene, S.; Pallier, C. Brain correlates of constituent structure in sign language comprehension. NeuroImage 2018, 167, 151–161. [Google Scholar] [CrossRef]
- Bonte, M.; Frost, M.A.; Rutten, S.; Ley, A.; Formisano, E.; Goebel, R. Development from childhood to adulthood increases morphological and functional inter-individual variability in the right superior temporal cortex. NeuroImage 2013, 83, 739–750. [Google Scholar] [CrossRef]
- Brauer, J.; Neumann, J.; Friederici, A.D. Temporal dynamics of perisylvian activation during language processing in children and adults. NeuroImage 2008, 41, 1484–1492. [Google Scholar] [CrossRef] [Green Version]
- Skeide, M.A.; Brauer, J.; Friederici, A.D. Syntax gradually segregates from semantics in the developing brain. NeuroImage 2014, 100, 106–111. [Google Scholar] [CrossRef]
- Leroy, F.; Cai, Q.; Bogart, S.L.; Dubois, J.; Coulon, O.; Monzalvo, K.; Fischer, C.; Glasel, H.; Van der Haegen, L.; Bénézit, A.; et al. New human-specific brain landmark: The depth asymmetry of superior temporal sulcus. Proc. Natl. Acad. Sci. USA 2015, 112, 1208–1213. [Google Scholar] [CrossRef] [Green Version]
- Leroy, F.; Glasel, H.; Dubois, J.; Hertz-Pannier, L.; Thirion, B.; Mangin, J.-F.; Dehaene-Lambertz, G. Early Maturation of the Linguistic Dorsal Pathway in Human Infants. J. Neurosci. 2011, 31, 1500–1506. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Le Guen, Y.; Leroy, F.; Auzias, G.; Riviere, D.; Grigis, A.; Mangin, J.-F.; Coulon, O.; Dehaene-Lambertz, G.; Frouin, V. The chaotic morphology of the left superior temporal sulcus is genetically constrained. NeuroImage 2018, 174, 297–307. [Google Scholar] [CrossRef] [PubMed]
- Sliwa, J.; Freiwald, W.A. A dedicated network for social interaction processing in the primate brain. Science 2017, 356, 745–749. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marciniak, K.; Atabaki, A.; Dicke, P.W.; Thier, P. Disparate substrates for head gaze following and face perception in the monkey superior temporal sulcus. eLife 2014, 3, e03222. [Google Scholar] [CrossRef] [PubMed]
- Fisher, C.; Freiwald, W.A. Contrasting Specializations for Facial Motion within the Macaque Face-Processing System. Curr. Biol. 2015, 25, 261–266. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roumazeilles, L.; Eichert, N.; Bryant, K.L.; Folloni, D.; Sallet, J.; Vijayakumar, S.; Foxley, S.; Tendler, B.C.; Jbabdi, S.; Reveley, C.; et al. Longitudinal connections and the organization of the temporal cortex in macaques, great apes, and humans. PLoS Biol. 2020, 18, e3000810. [Google Scholar] [CrossRef] [PubMed]
- Sallet, J.; Mars, R.B.; Noonan, M.P.; Andersson, J.L.; O’Reilly, J.X.; Jbabdi, S.; Croxson, P.L.; Jenkinson, M.; Miller, K.L.; Rushworth, M.F.S. Social Network Size Affects Neural Circuits in Macaques. Science 2011, 334, 697–700. [Google Scholar] [CrossRef] [Green Version]
- Ghazanfar, A.A.; Chandrasekaran, C.; Logothetis, N.K. Interactions between the Superior Temporal Sulcus and Auditory Cortex Mediate Dynamic Face/Voice Integration in Rhesus Monkeys. J. Neurosci. 2008, 28, 4457–4469. [Google Scholar] [CrossRef]
- Petkov, C.I.; Kayser, C.; Steudel, T.; Whittingstall, K.; Augath, M.; Logothetis, N.K. A voice region in the monkey brain. Nat. Neurosci. 2008, 11, 367–374. [Google Scholar] [CrossRef]
- Belin, P.; Bodin, C.; Aglieri, V. A “voice patch” system in the primate brain for processing vocal information? Hear. Res. 2018, 366, 65–74. [Google Scholar] [CrossRef]
- Bodin, C.; Trapeau, R.; Nazarian, B.; Sein, J.; Degiovanni, X.; Baurberg, J.; Rapha, E.; Renaud, L.; Giordano, B.L.; Belin, P. Functionally homologous representation of vocalizations in the auditory cortex of humans and macaques. Curr. Biol. 2021, 31, 4839–4844.e4. [Google Scholar] [CrossRef] [PubMed]
- Khandhadia, A.P.; Murphy, A.P.; Romanski, L.M.; Bizley, J.K.; Leopold, D.A. Audiovisual integration in macaque face patch neurons. Curr. Biol. 2021, 31, 1826–1835.e3. [Google Scholar] [CrossRef] [PubMed]
- Meguerditchian, A. “Human-specific” brain lateralization landmarks found in monkeys and their socio-cognitive correlates in both adults and infants [Papio anubis]. In Proceedings of the Communication at NeuroFrance, Strasbourg, France, 19–21 May 2021. [Google Scholar]
- Meguerditchian, A.; Marie, D.; Love, S.A.; Margiotoudi, K.; Bertello, A.; Lacoste, R.; Roth, M.; Nazarian, B.; Anton, J.-L.; Coulon, O. Human-Like Brain Specialization in Baboons: An in vivo Anatomical Mri Study of Language Areas Homologs in 96 Subjects. In The Evolution of Language, Proceedings of the 11th International Conference [EVOLANG11], New Oleans, LA, USA, 20–24 March 2016; Roberts, S.G., Cuskley, C., McCrohon, L., Barceló-Coblijn, L., Feher, O., Verhoef, T., Eds.; EvoLang Scientific Committee: New Orleans, Tulane, 2016. [Google Scholar] [CrossRef]
- Vilberg, K.L.; Rugg, M.D. Memory retrieval and the parietal cortex: A review of evidence from a dual-process perspective. Neuropsychologia 2008, 46, 1787–1799. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jacquemot, C.; Scott, S.K. What is the relationship between phonological short-term memory and speech processing? Trends Cogn. Sci. 2006, 10, 480–486. [Google Scholar] [CrossRef] [PubMed]
- Stout, D.; Chaminade, T. Stone tools, language and the brain in human evolution. Philos. Trans. R. Soc. B Biol. Sci. 2012, 367, 75–87. [Google Scholar] [CrossRef] [PubMed]
- Hecht, E.E.; Gutman, D.A.; Khreisheh, N.; Taylor, S.V.; Kilner, J.; Faisal, A.A.; Bradley, B.A.; Chaminade, T.; Stout, D. Acquisition of Paleolithic toolmaking abilities involves structural remodeling to inferior frontoparietal regions. Anat. Embryol. 2015, 220, 2315–2331. [Google Scholar] [CrossRef]
- Flechsig, P. Developmental [myelogenetic] localisation of the cerebral cortex in the human subject. Lancet 1901, 2, 1027–1029. [Google Scholar] [CrossRef] [Green Version]
- Geschwind, N. Disconnexion syndromes in animals and man. Brain 1965, 88, 237. [Google Scholar] [CrossRef] [Green Version]
- Catani, M.; Bambini, V. A model for Social Communication and Language Evolution and Development [SCALED]. Curr. Opin. Neurobiol. 2014, 28, 165–171. [Google Scholar] [CrossRef] [PubMed]
- Lattner, S.; Meyer, M.E.; Friederici, A.D. Voice perception: Sex, pitch, and the right hemisphere. Hum. Brain Mapp. 2005, 24, 11–20. [Google Scholar] [CrossRef]
- Vanduffel, W.; Fize, D.; Peuskens, H.; Denys, K.; Sunaert, S.; Todd, J.T.; Orban, G.A. Extracting 3D from Motion: Differences in Human and Monkey Intraparietal Cortex. Science 2002, 298, 413–415. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grefkes, C.; Fink, G.R. REVIEW: The functional organization of the intraparietal sulcus in humans and monkeys. J. Anat. 2005, 207, 3–17. [Google Scholar] [CrossRef]
- Orban, G.A.; Claeys, K.; Nelissen, K.; Smans, R.; Sunaert, S.; Todd, J.T.; Wardak, C.; Durand, J.-B.; Vanduffel, W. Mapping the parietal cortex of human and non-human primates. Neuropsychologia 2006, 44, 2647–2667. [Google Scholar] [CrossRef]
- Budisavljevic, S.; Castiello, U.; Begliomini, C. Handedness and White Matter Networks. Neuroscientist 2021, 27, 88–103. [Google Scholar] [CrossRef] [PubMed]
- Arbib, M.A. From monkey-like action recognition to human language: An evolutionary framework for neurolinguistics. Behav. Brain Sci. 2005, 28, 105–124. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rizzolatti, G.; Sinigaglia, C. The functional role of the parieto-frontal mirror circuit: Interpretations and misinterpretations. Nat. Rev. Neurosci. 2010, 11, 264–274. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eickhoff, S.B.; Schleicher, A.; Zilles, K.; Amunts, K. The Human Parietal Operculum. I. Cytoarchitectonic Mapping of Subdivisions. Cereb. Cortex 2006, 16, 254–267. [Google Scholar] [CrossRef]
- Habib, M.; Robichon, F.; Levrier, O.; Khalil, R.; Salamon, G. Diverging Asymmetries of Temporo-parietal Cortical Areas: A Reappraisal of Geschwind/Galaburda Theory. Brain Lang. 1995, 48, 238–258. [Google Scholar] [CrossRef] [PubMed]
- Johnson-Frey, S.H.; Newman-Norlund, R.; Grafton, S.T. A Distributed Left Hemisphere Network Active during Planning of Everyday Tool Use Skills. Cereb. Cortex 2005, 15, 681–695. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lewis, J.W. Cortical Networks Related to Human Use of Tools. Neurosci 2006, 12, 211–231. [Google Scholar] [CrossRef]
- Cheng, L.; Zhang, Y.; Li, G.; Wang, J.; Hopkins, W.D.; Sherwood, C.C.; Gong, G.; Fan, L.; Jiang, T. Divergent Connectional Asymmetries of the Inferior Parietal Lobule Shape Hemispheric Specialization in Humans, Chimpanzees, and Macaque Monkeys. Neuroscience 2021. [Google Scholar] [CrossRef]
- Anderson, J.R.; Gallup, G.G. Mirror self-recognition: A review and critique of attempts to promote and engineer self-recognition in primates. Primates 2015, 56, 317–326. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hecht, E.; Mahovetz, L.M.; Preuss, T.M.; Hopkins, W.D. A neuroanatomical predictor of mirror self-recognition in chimpanzees. Soc. Cogn. Affect. Neurosci. 2017, 12, 37–48. [Google Scholar] [CrossRef] [Green Version]
- Stout, D.; Hecht, E.; Khreisheh, N.; Bradley, B.; Chaminade, T. Cognitive Demands of Lower Paleolithic Toolmaking. PLoS ONE 2015, 10, e0121804. [Google Scholar] [CrossRef] [Green Version]
- Gannon, P.J.; Kheck, N.M.; Braun, A.R.; Holloway, R.L. Planum parietale of chimpanzees and orangutans: A comparative resonance of human-like planum temporale asymmetry. Anat. Rec. Part A 2005, 287, 1128–1141. [Google Scholar] [CrossRef] [PubMed]
- Taglialatela, J.P.; Dadda, M.; Hopkins, W.D. Sex differences in asymmetry of the planum parietale in chimpanzees [Pan troglodytes]. Behav. Brain Res. 2007, 184, 185–191. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Penfield, W.; Boldrey, E. somatic motor and sensory representation in the cerebral cortex of man as studied by electrical stimulation. Brain 1937, 60, 389–443. [Google Scholar] [CrossRef]
- Yousry, T. Localization of the motor hand area to a knob on the precentral gyrus. A New Landmark. Brain 1997, 120, 141–157. [Google Scholar] [CrossRef] [PubMed]
- Amunts, K.; Schlaugb, G.; Schleichera, A.; Steinmetzb, H.; Dabringhausa, A.; Roland, P.E.; Zilles, K. Asymmetry in the Human Motor Cortex and Handedness. NeuroImage 1996, 4, 216–222. [Google Scholar] [CrossRef]
- Cykowski, M.D.; Coulon, O.; Kochunov, P.V.; Amunts, K.; Lancaster, J.L.; Laird, A.R.; Glahn, D.C.; Fox, P.T. The Central Sulcus: An Observer-Independent Characterization of Sulcal Landmarks and Depth Asymmetry. Cereb. Cortex 2008, 18, 1999–2009. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, Z.Y.; Klöppel, S.; Rivière, D.; Perrot, M.; Frackowiak, R.; Siebner, H.; Mangin, J.-F. The effect of handedness on the shape of the central sulcus. NeuroImage 2012, 60, 332–339. [Google Scholar] [CrossRef] [PubMed]
- Häberling, I.S.; Corballis, P.M.; Corballis, M.C. Language, gesture, and handedness: Evidence for independent lateralized networks. Cortex 2016, 82, 72–85. [Google Scholar] [CrossRef]
- Crow, T. Directional asymmetry is the key to the origin of modern Homo sapiens [the Broca-Annett axiom]: A reply to Rogers’ review of The Speciation of Modern Homo Sapiens. Laterality Asymmetries Body Brain Cogn. 2004, 9, 233–242. [Google Scholar] [CrossRef]
- Warren, J.M. Handedness and laterality in humans and other animals. Physiol. Psychol. 1980, 8, 351–359. [Google Scholar] [CrossRef] [Green Version]
- Hobaiter, C.; Byrne, R.W. Laterality in the gestural communication of wild chimpanzees. Ann. N. Y. Acad. Sci. 2013, 1288, 9–16. [Google Scholar] [CrossRef] [Green Version]
- Hopkins, W.D.; Cantalupo, C. Handedness in Chimpanzees [Pan troglodytes] Is Associated with Asymmetries of the Primary Motor Cortex but Not With Homologous Language Areas. Behav. Neurosci. 2004, 118, 1176–1183. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dadda, M.; Cantalupo, C.; Hopkins, W.D. Further evidence of an association between handedness and neuroanatomical asymmetries in the primary motor cortex of chimpanzees [Pan troglodytes]. Neuropsychologia 2006, 44, 2582–2586. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Margiotoudi, K.; Marie, D.; Claidière, N.; Coulon, O.; Roth, M.; Nazarian, B.; Lacoste, R.; Hopkins, W.D.; Molesti, S.; Fresnais, P.; et al. Handedness in monkeys reflects hemispheric specialization within the central sulcus. An in vivo MRI study in right- and left-handed olive baboons. Cortex 2019, 118, 203–211. [Google Scholar] [CrossRef] [PubMed]
- Phillips, K.A.; Sherwood, C.C. Primary motor cortex asymmetry is correlated with handedness in capuchin monkeys [cebus apella]. Behav. Neurosci. 2005, 119, 1701–1704. [Google Scholar] [CrossRef]
- Nudo, R.; Jenkins, W.; Merzenich, M.; Prejean, T.; Grenda, R. Neurophysiological correlates of hand preference in primary motor cortex of adult squirrel monkeys. J. Neurosci. 1992, 12, 2918–2947. [Google Scholar] [CrossRef] [Green Version]
- Bouziane, S.; Loh, K.K.; Becker, Y.; Brunschvig, S.; Picchiottino, A.; Sein, J.; Coulon, O.; Velly, L.; Renaud, L.; Meguerditchian, A. Early structural asymmetry in the central sulcus is associated with handedness in infant baboons. In Proceedings of the OHBM 2021: 27th Annual Meeting of the Organization for Human Brain Mapping, Monday, Virtual, 21–25 June 2021. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Becker, Y.; Meguerditchian, A. Structural Brain Asymmetries for Language: A Comparative Approach across Primates. Symmetry 2022, 14, 876. https://doi.org/10.3390/sym14050876
Becker Y, Meguerditchian A. Structural Brain Asymmetries for Language: A Comparative Approach across Primates. Symmetry. 2022; 14(5):876. https://doi.org/10.3390/sym14050876
Chicago/Turabian StyleBecker, Yannick, and Adrien Meguerditchian. 2022. "Structural Brain Asymmetries for Language: A Comparative Approach across Primates" Symmetry 14, no. 5: 876. https://doi.org/10.3390/sym14050876
APA StyleBecker, Y., & Meguerditchian, A. (2022). Structural Brain Asymmetries for Language: A Comparative Approach across Primates. Symmetry, 14(5), 876. https://doi.org/10.3390/sym14050876





