3. Small-Molecule-Based Fluorescent Probes
Small molecular probes are always advantageous compared to labelled methods, where biomolecules such as peptides and nucleic acids are generally covalently attached to the fluorophores [
63,
64,
65,
66,
67]. In this labelling approach, where biomacromolecules are site specifically modified with fluorescent labels, usually suffers from various drawbacks—viz., susceptibility to cellular ions, proteases and nucleases; and always demanding transfecting agents to enter the cells. In contrast, small molecules can enter the cells through passive modes without getting degraded by proteases or nucleases [
68,
69,
70]. Furthermore, small molecules are usually not accompanied by quenching units (such as BHQ) and can have a ability to trigger dual emission (AIE-based materials monomer ↔ excimer) depending on the molecular confinements. Considering these facts, various small molecule based blue/green/red/NIR emissive fluorescent materials were designed for the recognition of ALP.
Zhang et al. reported phosphorylated tetraphenylethylene-based, AIE-based fluorescent probes
ALP-1,
ALP-2 and
ALP-3 [
71] for monitoring of ALPs during osteogenic differentiation of stem cells (
Figure 2). In these probes, hydrophilicities, were tuned by incorporating mono-, di-, and tetra-phosphate units at the periphery, resulting in a very low quantum yield in aqueous conditions. Probes showed characteristics λ
max at ~338 (±4) nm and λ
em at 460 (±10) nm in UV–Vis and fluorescence spectra, respectively. In contrast, upon incubation with ALP, the probes showed a turn response within 5 min, at the bluish green region centered at λ
em = ~470 nm, which was blue shifted to λ
em = ~450 nm after 60 min.
This substantial blue shift, attributed to complete hydrolysis of phosphorylated probes into insoluble hydroxylated tetraphenylethylene units, resulted in highly ordered aggregates. An ALP-regulated switch on response was found to be highest in the case of ALP-2, followed by ALP-4 and ALP-1, under physiological conditions. In contrast, mono-phosphorylated probe ALP-1 was found to be very sensitive compared to di- and tetra-phosphorylated probes in similar experimental conditions. The authors speculate that the presence of multiple phosphorylated units in ALP-3 induced a complex mechanism of dephosphorylation with ALP, resulting in low sensitivity. Due to these reasons, even though di-phosphorylated probe showed a higher enhancement ratio upon interaction with ALP, the lowest detection limit was found for the mono-phosphorylated probe. Stoichiometrically, at the specified concentration, the mono-phosphorylated probe has quantitatively half of the di-phosphorylated one’s units. Only mono- and di-phosphorylated probes were analyzed in biological samples because of their higher sensitivity and stability.
Cytotoxicity studies analyzed through propidium iodide revealed that both
ALP-1 and
ALP-2 can be used effectively at 2 to 20 μM. Upon incubation of 20 μM of probe with
ALP-1/2 during osteogenic differentiation in a bone marrow mesenchymal stem cell (BMSC) culture (0 to 7 days), fluorescence intensities were increased in the green channel, and can be visualized through fluorescence confocal laser scanning microscopic images (
Figure 3). Overexpression of ALP during osteogenic differentiation of stem cells was further supported through the flow cytometric Western blotting and rt-PCR analysis. It was found that, in cellular conditions, the di-phosphorylated probe
ALP-2 showed significant changes compared to that of the mono-phosphorylated counterpart.
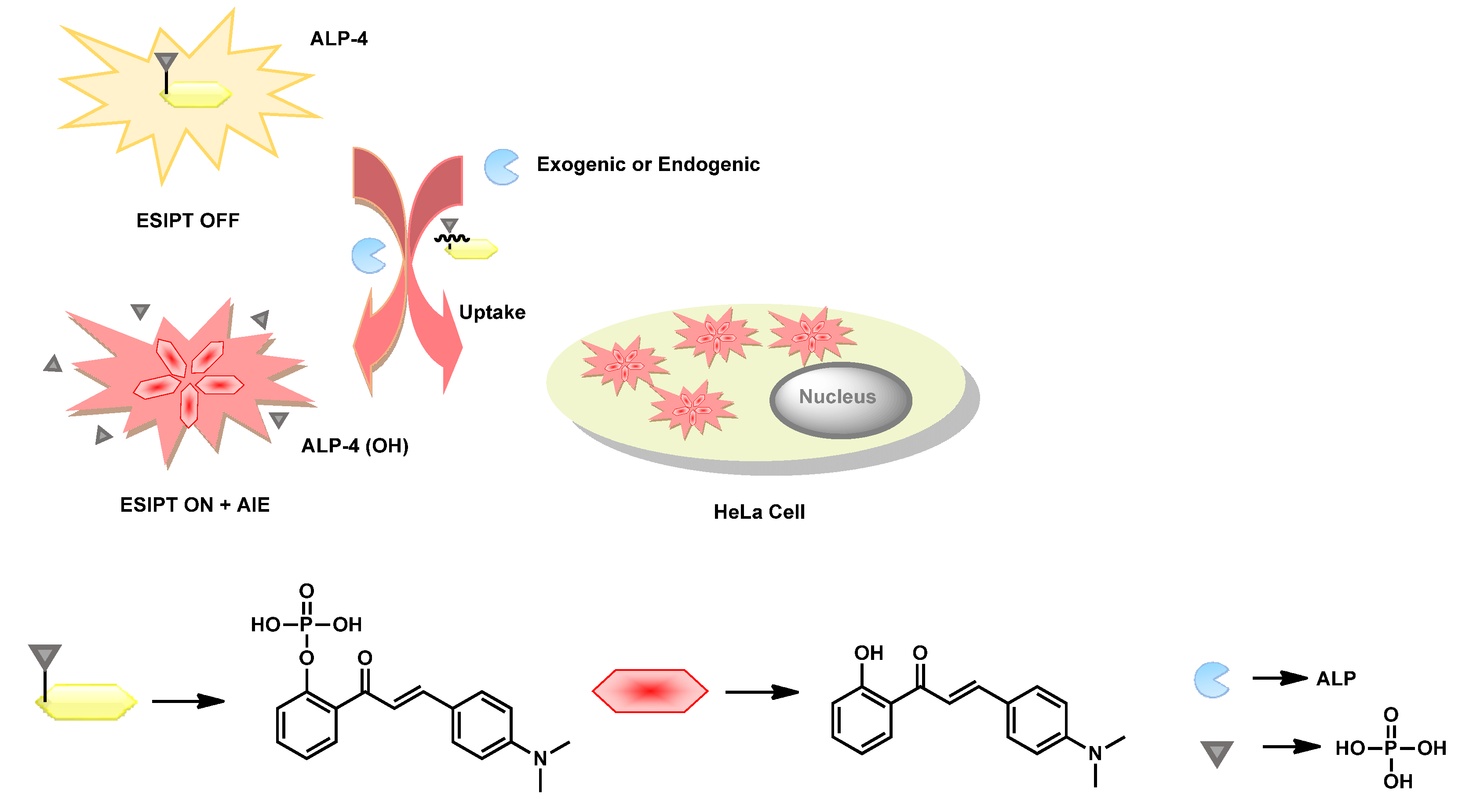
Upon exploiting the AIE properties in combination with ESIPT behavior, Tang et al. reported a 2′-hydroxychalcone-based phosphorylated probe
ALP-4 (
Figure 4) for ALP recognition in in vitro and in cellulo [
72]. The rationally designed probe triggered far-red emission centered at 640 nm upon ALP-catalyzed dephosphorylation under physiological conditions.
ALP-4 showed absorption maxima at λ
max 430 and 416 nm in UV–Vis spectra and weak emission (greenish-yellow λ
em 538 nm). This weakly green emissive behavior in the phosphorylated state was attributed to the presence of
N,N,dimethylaniline and vinyl ketone units, thereby creating an ICT process in the chalcone unit in the twisted conformation. However, upon dephosphorylation, the presence of the ketone unit at the
ortho position induced strong intramolecular hydrogen bonding upon photochemical excitation, leading to the highly stable 6-membered ring, resulting in keto-enol tautomerism. Based on pH-dependent study, the authors speculated that fluorescence properties in the probe should be attributed to the combination of synergistic ESIPT and ICT processes. Single crystal analysis of
ALP-4 (
OH) revealed head-to-head and edge–face stacking resulted in strong intermolecular-coupling-aided AIE behavior. Exploiting such dual photophysical properties in phosphorylated and dephosphorylated 2-hydroxychalcone resulted in ratiometric fluorescence switching properties in green and red channels. The probe detected ALP as scarce as 0.15 mU/mL in a ratiometric manner. The authors did not specify the kinetic parameters in their work.
ALP-4 showed excellent biocompatibility towards HeLa cell lines. Upon incubation of
ALP-4 in endogenously overexpressed HeLa cells, orange fluorescence was seen in the cytoplasm (
Figure 5). The authors speculated on the existence of unusual blue-shifted emission in the orange region in cellular conditions, as compared to that of in vitro conditions (red emissive), could have been due to the twisted molecular confinements of
ALP-4 (
OH) upon interactions with bio-macromolecules in biological fluids. To validate the intracellular phosphate hydrolysis of
ALP-4, fluorescence confocal imaging studies (FCIS) were performed in dual channels in the absence and presence of the ALP inhibitor levamisole. According to the results, the authors postulated that intercellular ALPs readily cleaved the phosphorylated probe in extracellular fluid, and thereafter the fluorescent
ALP-4 (
OH) was transfected passively, resulting in bright fluorescence in the cytosol (
Figure 6).
Sun et al. and co-workers reported the naphthalene-based green emissive two-photon florescent probe
ALP-5 (
Figure 7) for in vitro and in cellulo recognition of ALPs [
73]. A PO
43− group was incorporated into the self-cleavable benzyl-ester unit at the ortho position in such a way that, upon dephosphorylation, it could trigger the release of an amino-naphthalene moiety. Under physiological conditions, the probe showed absorption maxima at λ
max 300 nm in UV–Vis spectrum and emission maxima centered at λ
em 450 nm in the fluorescence spectrum. Upon dephosphorylation in the presence of ALP, the absorption and emission maxima shifted to 365 and 500 nm respectively. Such selective bathochromicity in the absorption spectrum, along with a
switch on response in the green channel, made the authors interested in performing ALP recognitions in biological systems.
The phospho-ester hydrolysis kinetics of ALP-5 was analyzed through the well-established Lineweaver–Burk equations, revealing appreciably good Km (8.56 µM) and Vmax (0.073 µM) values. Under the specified experimental conditions, the probe showed excellent sensitivity of up to 0.3 U/L towards ALP, with a good linearity range from 0.1 to 10 U/L. The authors speculated that the ortho-substituted phospho-phenolic ester hydrolysis by phosphatase thermodynamically favors cascade cleavage of the para-substituted amidic linkage in 2-acetyl-naphthalene. This process restoring the strong ICT process between the amino and acetyl substituents across the naphthalene’s core resulted in the bright green fluorescence. Two-photon cross-section studies of ALP-assisted dephosphorylated product showed 90 GM at 740 nm. In contrast ALP-5’s two-photon cross-section in similar experimental conditions was found to be too small. Due to these reasons, one-photon and two-photon excitations showed similar emission spectra under physiological conditions.
Upon detailed in vitro analysis, two-photon
ALP-5 probe cytotoxicity was evaluated in HeLa cell models, based on MTT assays. Upon incubation of the probe in HeLa cells, it displayed strong intracellular green fluorescence, due to two-photon excitations (λ
ex 720 nm). However, Na
3VO
4-pre-treated HeLa cells also revealed fluorescence in the green channel upon two-photon excitations (
Figure 8a–d). Interestingly
ALP-5 did not show any fluorescence in HEK cell lines, even at higher concentrations, revealing that the two-photon probe was able to recognize ALP in a cell-dependent manner (
Figure 8e,f).
Two-photon imaging studies of
ALP-5 were performed in various live tissues, such as the hippocampi, livers and kidneys of rats at 30 to 230 μm depth levels.
ALP-5-incubated tissues showed bright green fluorescence within 120 min in all tissues, which was not observed in the presence of the inhibitor Na
3VO
4 (
Figure 9). This validated successful tracking of endogenous ALP in tissues and organs. Due to the green emission range, the in vivo recognition capability of the probe was not demonstrated.
Nie et al. developed a new near-infrared, emissive, hemi-cyanin-based fluorescent probe,
ALP-6 (
Figure 10), for in vitro and in vivo recognition of endogenous ALP activity [
74].
ALP-6 involves phosphorylated chromene units conjugated with benz[e]indolium salts. In buffered conditions, the probe showed an absorption maximum at λ
max at 604 nm and was very weakly emissive (λ
em 738 nm). Upon incubation with ALP at roughly physiological pH, 8.0, there was a 10-fold enhancement (at 738 nm) in the emission intensity, along with a substantial redshift from 604 to 738 nm in the UV–Vis spectra within 20 min. The designed hemicyanine-based probe showed a highly selective and robust
switch on response in the NIR region, towards phosphatase, without allowing any interference by biologically relevant ions or macromolecules. The ALP-induced dephosphorylation of
ALP-6 retained a strong ICT process, resulting in high fluorescence with redshifts in the UV–Vis spectrum, which were significantly inhibited by the phosphorylated probe due to the electron pulling effects from the electronegative phosphorous atom in the PO
43− unit.
Probe
ALP-6’s dephosphorylation kinetic parameters,
Vmax and
Km, were 0.693 μM/min and 9.32 μM, respectively, under similar experimental conditions. The probe’s LOD towards ALP was found to be 0.003 U/mL (linearity range from 0.01 to 2.0 U/mL). In detailed in vitro studies,
ALP-6 was tested in HeLa cells. The MTT assay revealed the probe was negligibly toxic until 30 μM. Upon incubation of
APL-6 in ALP-overexpressing HeLa cells, bright fluorescence in red channel was exhibited, but it was greatly diminished in the presence of the inhibitor Na
3VO
4, as can be seen in the fluorescence confocal images (FCIMs) (
Figure 11a–c). Fluorescence signals in rat liver tissues collected through Z-scan mode revealed that
ALP-6 has excellent tissue penetration capabilities (
Figure 11d). Due to its excellent tissue penetration capabilities, the probe’s in vivo ALP recognition capabilities were demonstrated in Kunming mice. Upon intraperitoneal injection of
ALP-6, mice produced deep red NIR emissions over time. Such emissive behavior was not observed in the Na
3VO
4-pretreated mice, which revealing that the probe can be effectively used to track endogenous ALP in in vitro and in vivo models.
Liang’s group reported fluorescent peptide
ALP-7 (
Figure 12) for the in vitro recognition of ALP. The designed FITC-conjugated, phosphorylated peptide undergoes dephosphorylation in the presence of ALP, leading to the three-dimensional, self-assembled, fibrous-type material—a “gel” [
75] at pH 8.0 (TRIS-HCl). Enzyme-assisted sol to gel transformation resulted in quenching of fluorescence in the green channel attributed to conventional aggregation caused quenching (ACQ). The authors did not specify kinetic parameters such as
Vmax and
Km.
ALP-7 showed strong green emission upon illumination at 365 nm (UV light), whereas upon incubation with ALP, in physiological pH buffers, a substantial decrement in emission intensity in the green channel was observed. Concomitantly, a significant color change from light green to transparent yellow was clearly seen with the naked eye. Probe was able to recognize ALP as lowest as 0.06 U/mL in similar experimental conditions.
MTT assay against human colorectal cancer (LoVo) cells did not show any cytotoxic behavior (99% cell survival was observed) until 160 μM, after a 12 h incubation. ALP overexpressed LoVo cells showed very weak fluorescence upon incubation with
ALP-7, whereas in the presence of the inhibitor L-phenylalanine (L-Phe), they showed bright greenish fluorescence (
Figure 13a). From these studies, the authors speculate that FITC-conjugated peptides, while dispersed in biological fluids, can cause bright greenish fluorescence. However, upon dephosphorylations with the aid of ALP, they resulted in the formation of a supramolecular assembly, causing significant ACQ. Such drastic variations in emission intensity in the green channel helped with the qualitative and quantitative recognition of ALP in in vitro and in cellulo models.
Tan et al. reported a hemicyanine-based NIR based fluorescent probe,
ALP-8 (
Figure 14), for in vitro and in vivo recognition of ALP [
76]. In buffered conditions, the probe showed absorption maxima at λ
max 600 and 650 nm in the UV–Vis spectrum, and did not induce any fluorescence band in the NIR region. Upon dephosphorylation, absorption maxima λ
max were red-shifted to 650 and 680 nm, elicited with bright NIR fluorescence centered at 700 nm. They observed blue shifts in the absorption and emission wavelengths compared to
ALP-6 (benz[e]-indolium core), revealing that indolyl core’s conjugation extension can significantly affect the ICT process in hemicyanine-based fluorophore units.
ALP-8 showed a detection limit of 0.07 U/mL for phosphatase, without inducing interference from structurally similar biomolecules and cell metabolites (cations, anions and other molecules). The observed dephosphorylation kinetic parameters, such as Km and Kcat, were found to be 21.2 μM and 2.14 S−1 respectively. The catalytic efficiency (Kcat/Km) value of 1.01 × 105 M−1 S−1 is much higher than that of commercially available 4-methylumbelliferyl phosphate and enzyme labelled fluorescence substance 97 (ELF-97).
The intracellular ALP-recognizing capabilities of
ALP-8 were evaluated in ALP-positive (HeLa cells) and negative (HEK 293) cell models. The ALP-positive HeLa cells showed a strong fluorescence pattern, whereas the ALP-negative HEK 293 cells exhibited negligible fluorescence (
Figure 15A) upon
ALP-8 incubation. When
ALP-8 was incubated in Na
3VO
4 (a well-known ALP inhibitor)-pretreated cells, low emission was exhibited in both cell lines in the red channel, supporting the probe’s capability of successfully tracking endogenously produced ALP. Intraperitoneal injection of
ALP-8 in Kunming mice in the absence and presence of Na
3VO
4 inhibitor revealed bright and low emissive behavior, respectively (
Figure 15B,C). From these results, the authors speculated that bright NIR emission from cell and mouse models can be attributed to endogenously produced ALP-regulated dephosphorylation of probe.
Wei et al. reported a highly efficient, NIR-regulated far-red probe,
ALP-9 (
Figure 16) based on the dicyanomethylene-4H-chromene unit conjugated with dichrolorophenyl phosphate ester [
77]. To achieve best possible tumor microenvironment (TME) localization and controlled time-resolved intracellular ALP imaging,
ALP-9 was loaded into am NIR-responsive nano-container.
The rationale behind this design is that incorporation of dichlorination at the ortho position via phenolic OH enhances the biocompatibility, through the high degree of dissociation (pKa 6.97), which is much lower in the case of its mono-chlorinated (pKa ~ 8.43)/non-substituted (pKa ~ 9.85) counterpart. Additionally, nano-encapsulated-ALP-9 showed a peculiar absorption maximum at λab ca. ~380 nm, and was weakly emissive (λem ca. 683 nm); thus, the debilitated ICT process (dicyanomethylene as the acceptor and the phosphophenolate unit as the donor) operated between π-bridged acceptor–donor couples. ALP-regulated biocatalytic dephosphorylation of ALP-9 → ALP-9 (OH) exhibited a new peak at ca. ~510 nm and strong emission at ca. 683 nm (~15 fold) in UV–Vis and fluorescence spectra, respectively. Such dramatic changes in the optical behavior of the dephosphorylated probe were attributed to the presence of a strong ICT process operating between the dechlorinated phenolate unit and dicyanomethylene across the styryl unit. Dephosphorylation kinetic parameters Km and Vmax were found to be 13.58 µM and 0.083 µM/s, respectively. The lowest fluorogenic response towards ALP was 0.072 U/L under physiological conditions. In order to demonstrate the time-resolved ALP recognition, ALP-9 was loaded into NIR-responsive nano-containers consisting of a lanthanide core (NaYF4:Yb/Tm@NaYF4) and a mesoporous silica layer decorated with an azo compound. In this strategy, ALP-9 molecules loaded in the nano-container were selectively released, upon irradiating by NIR light, by up-converting it to UV–Visible light. During the up-conversion, the generated UV–Visible light induced trans to cis transition in the azo compound, thereby regulating controlled release of the phosphorylated probe from the nano-container.
HeLa cells and L02 cell lines were chosen for this study due to their up- and down- regulation of ALPs levels. When the cells were initially incubated with the nano-
ALP-9, no obvious fluorescence was seen, but upon irradiation with NIR, the pre-treated HeLa cells exhibited very strong fluorescence, indicating that
ALP-9 was efficiently released, followed by subsequent recognition of endogenously produced ALP. However, in the presence of the ALP inhibitor Na
3VO
4, fluorescence in the red channel enormously decreased, even upon NIR irradiation (
Figure 17). Similarly, ALP downregulated L02 cells also exhibited extremely weak emission in the red channel, even in the presence of NIR irradiation. Based on the observed results, the authors speculated that generation of red mission in the cell lines solely resulted from phosphatase-catalyzed dephosphorylation of the probe.
Since the in vitro studies showed remarkable results, the efficiency of the nano-encapsulated probe was further studied in Kunming mice, as an in vivo model. Results revealed that the nano-
ALP-9 impregnated mice showed red fluorescence only upon irradiation by NIR light. In contrast, mice treated with the ALP inhibitor (Na
3VO
4) with or without NIR irradiation did not exhibit any fluorescence (
Figure 18). Further, HepG2/xenograft tumor-bearing nude mice were chosen to recognize the ALP activity in acidic tumor environments in in vivo models. Interestingly, intratumoral injection of the nano form of the
ALP-9 probe showed excellent ALP recognition, especially in the tumor microenvironment upon irradiation by NIR radiation.
Ding et al. reported a novel D–π–A-tuned mitochondria-targeting fluorescence probe (
ALP-10) whose emission properties are regulated through the ESIPT phenomenon [
78]. Molecular engineering was performed by the combination of 2-(2′-hydroxyphenyl)-benzothiazole (ESIPT core) with a 4-styryl-pyridinium (robust mitochondrial targeting unit) core (
Figure 19).
ALP-10 showed a characteristic broad absorption peak with maximal absorptivity at ca. ~358 nm and an emission maximum at 514 nm (ϕ = 0.21) in UV–Vis and fluorescence spectra, respectively. Upon ALP induced dephosphorylation, the free phenolate unit induced a strong ESIPT process in
ALP-10 (
OH), resulting in the generation of a new absorption band (λ
max ca. ~510 nm) and emission band (λ
em ca. ~650 nm). The dephosphorylated product with free phenolic OH emitted a strong red fluorescent signal in a ratiometric manner. The ALP recognition capability of the probe was found to be appreciable in the physiological pH range (5.0 to 8.0). However, at an alkaline pH (pH > 8.0) the ratiometric ALP recognition capability was significantly diminished, which was attributed to inhibition of the ESIPT process pertaining to phenolate ion formation.
The dephosphorylation kinetic parameters Km and Vmax were 1.48 µM and 0.63 µM/s, respectively, under in vitro diagnostic conditions. In physiological conditions, ALP-10 did not show any fluorescence instability due to biologically relevant ions or molecules, and showed a minimum detection limit of 0.072 mU/mL towards phosphatase.
The endogenous ALP activity of the probe was monitored in ALP positive cells, such as HeLa, A549 and ALP-negative HUVEC cell lines.
ALP-10-treated HeLa and A549 cell lines exhibited bright red fluorescence with complete disappearance of emission intensity in the green channel after 30 to 60 min (
Figure 20). In the HUVEC cell lines, only weak green fluorescence was observed, due to the downregulation of endogenous ALP. Colocalization experiments with mito-tracker green showed a Pearson’s correlation coefficient of >0.96, further supporting the idea that
ALP-10 can recognize mitochondrial ALP.
ALP inhibition was monitored in HeLa cells with Na
3VO
4 and NaH
2PO
4. Disregarding the control experiments (without inhibitor), the remaining HeLa cell lines (with inhibitor) showed dose-dependent red and green fluorescence upon incubation with
ALP-10 (
Figure 21). Additionally, at a low concentration of inhibitor, the probe incubated cells’ green emission did not completely disappear. From these experiments, the authors speculated that the red emission in cells should be attributed to phosphatase-regulated dephosphorylation of
ALP-10.
The in vivo ALP recognition capabilities of the probe were tested in tumor-bearing BALB/C nude mice. Upon intratumoral injection of
ALP-10 into the mouse model, a time dependent enhancement in the red signal, along with a substantial intensity decrement in the green channel, was observed. In contrast, when Na
3VO
4-pretreated tissue was injected with
ALP-10, mice exhibited their lowest red emission intensity in the tumor (
Figure 22) and these results are consistent with in vitro (cell imaging) experiments.
Han et al. reported the 2′-(2′-hydroxyphenyl)-benzothiazole (HBT) fluorescent conjugates (
Figure 23) were linked to the 5′ position of the ribonucleoside (adenosine/guanosine) residues for recognition of ALP in cellular conditions [
79]. These 5′-ribonucleotide-fluorophore analogues showed weak emission, due to the phosphorylated phenolic OH in HBT unit. P–O bond cleavage (dephosphorylation) generates free HBT and phenolic OH, which exists in a keto–enol tautomerization equilibration. Upon photochemical excitation, the ESIPT core equilibrium shifted toward the enol tautomer (more predominant isomer), resulting in red shifts in the emission spectrum.
ALP-11/12 are non-fluorescent in aqueous buffer, but otherwise have three emission maxima, 370, 375 and 380 nm. Similarly, these probes exhibited a bright fluorescent signal centered at 512 nm in the presence of ALP.
Due to the presence of two phosphoester linkages in ALP-11/12 probes, they showed two hyperbolic peaks. The apparent Km values were 1.89 and 238.6 μM for adenosine conjugates (ALP-11) and 0.1 and 65.26 μM for guanosine conjugates (ALP-12). Vmax values for ALP-11 were 9.8 × 105 and 1.64 × 107 pmol min−1 nmol−1. Similarly, for ALP-12, Vmax values were 3.48 × 105 and 5.33 × 106 pmol min−1 nmol−1, revealing that guanosine conjugates have higher affinities towards ALP in similar experimental conditions.
The cytotoxicity of
ALP-11/
ALP-12 probes assessed by sulforhodamine B (SRB) assays showed a small cytotoxic effect in both HeLa cells and HT29 cells. It was also observed that
ALP-12 has less toxicity than
ALP-11. Due to the upregulated placental ALP in the HeLa cells upon
ALP-11 incubation, green fluorescence intensity in the intracellular region was gradually increased until 30 min had gone by. (
Figure 24). By contrast, no fluorescence was seen in ALP-downregulated HT-29 cell lines. In cellular conditions, the adenosine analogue (
ALP-11) showed better fluorescence in response to endogenous ALP than the guanosine conjugate (
ALP-12).
Kim et al. reported a new iminocoumarin-benzothiazole-based fluorophore,
ALP-13 (
Figure 25) to recognize the phosphatase at the single-cell level [
80]. In an aqueous buffer solution, probe showed an absorption maximum of 472 nm and emission maximum (λ
em) of 542 nm, in the UV–Vis and fluorescence spectra, respectively.
ALP-13 was very weakly emissive (ϕ = 0.002) in aqueous conditions, which was attributed to free rotation across the vinyl unit. Upon phosphor-ester hydrolysis, the ortho-substituted OH group undergoes rapid cyclisation by the nucleophilic attack on the C atom of the nitrile unit, which thereby inhibits free rotation of the vinyl units. Such planar arrangements induce hypsochromic changes (ϕ = 0.10) in the emission maxima and bathochromic shifts in the absorption maxima. The ALP-13 enzymatic dephosphorylation biocatalytic kinetic parameters were found to be KM = 19.2 µM and kcat = 0.27 s−1. The enzymatic efficiency of ALP-13 was measured as kcat/KM = 1.4 × 104 M−1 s−1, which is moderate compared to that of commercially available phosphatase probes under similar experimental conditions.
Intracellular the ALP recognition capability of
ALP-13 was demonstrated in HeLa cells (human cervical carcinoma) as a positive control and HT29 cell lines as a negative control due their up and downregulation of ALP levels, respectively.
ALP-13-incubated HeLa cells showed bright green emission within 2 min; however, such intensified green emission was not observed in the case of inhibitor levamisole-pretreated HeLa cells. Similarly, when
ALP-13 was incubated with HT29 cells, no such substantial increment in the green channel was observed (
Figure 26). Based on these results, the authors speculate that the imino-coumarine–benzothiazole conjugate exhibits bright green emission only upon selective dephosphorylation by the endogenously produced ALP.
Li et al. reported the self-immolative resorufin-based probe
ALP-14 (
Figure 27) for an ALP assay in cellular conditions [
81]. Molecular engineering was performed based on the conjugation of an ALP-recognizing substrate (phosphoester unit) to a red emissive resorufin unit through the reactive
p-hydroxybenzyl unit, which acts as self-immolative linker. This design is such that ALP can easily access the phosphor-ester terminus of the probe without steric hindrance, for biocatalytic dephosphorylation. When
ALP-14 is dephosphorylated, it intermediately undergoes 1,6-eliminations to produce a
p-quinone methide ether derivative and a free
Resorufin unit. This transformation is associated with changing of the solution’s color from orange to purple and generating intense fluorescence emission centered at the 585 nm region.
In physiological conditions, ALP-14 showed a UV–Vis absorption maximum at 484 nm with a shoulder peak at 400 nm, and was very weakly emissive (λem 585 nm and ϕ = 0.0023). This weakly emissive behavior was attributed to photoinduced electron transfer (PET) from 7-hydroxy substitution (ohydroxy-benzyl) in the resorufin unit. This phenomenon was not observed upon dephosphorylation, due to the formation of the free resorufin unit.
The dephosphorylation kinetic parameters of probe, KM and kcat, were found to be 15.38 µM and 0.26 s−1, respectively. The enzymatic efficiency kcat/KM = 1.7 × 104 M−1 s−1 was greatly reduced in the absence of a self-immolative linker between resorufin (signaling unit) and the phosphate (receptor) group. ALP-14 has high affinity, as compared to commercially available probes such as 4-MUP, and was very sensitive, being able detect as little as 1.09 U/L of ALP in physiological conditions. The inhibition assay revealed IC50 values for Na3VO4 and levamisole of 7.58 and 79.4 µM, respectively.
The
ALP-14 probe’s intracellular phosphatase recognition capabilities were demonstrated in HeLa cells and HEK 293T cells as ALP positive and negative controls, respectively.
ALP-14-incubated HeLa cells showed intense red fluorescence within 5 min, whereas under the same conditions, no visible fluorescence signals were observed inside HEK 293T cells (
Figure 28). The validated emission signals in the red channel were attributed to phosphatase-catalyzed dephosphorylation of
ALP-14.
Sun et al. reported a novel hydrazone-based fluorescent probe,
ALP-15 (
Figure 29), for the in cellulo recognition of ALP. The opto-analytical behavior of the probe is regulated through the dual photophysical phenomena, such as aggregation-induced emission (AIE) and excited state intramolecular proton transfer (ESIPT) [
82]. The design strategy targeted a simple and small molecular architecture, which showed excellent selectivity and sensitivity, by showing a large Stokes shift upon interacting with phosphatase in physiological conditions.
In ALP-15, ESIPT and AIE are regulated through the ortho-hydroxy unit. In the phosphorylated state, the probe exhibited good water solubility and prevents the ESIPT and AIE phenomena. Selective blocking of the ortho-OH group in the probe did not elicit significant fluorescence in the green channel. In buffer, ALP-15 exhibited very weak emission (λem 536 nm) in the green channel. In contrast, the dephosphorylated probe with free hydroxyl groups showed bright green emissive behavior with a large Stokes shift (Δλ = 180 nm), which was attributed to synergistic ESIPT and AIE properties. Intensity-based emission profiling revealed that the dephosphorylation rate was high at pH 9. Dephosphorylation kinetic parameters Km and Vmax, calculated through Lineweaver–Burk analysis, were 7.66 µM and 0.408 µM min−1, respectively. The probe showed a limit of detection of 0.012 UL−1 towards ALP without inducing interference from biologically relevant ions or molecules.
Intracellular ALP activity was measured in various cell lines, such as MG-63, WI-38, B6F10, RAW264.7 and HEK293, using confocal fluorescence imaging. Amongst them, MG3 showed bright green emission, owing to overexpression of ALP during differentiation of osteosarcoma cells (
Figure 30). Other cells, such as WI-38, B16F10 and RAW 264.7 exhibited a mild fluorescence signal due to moderate expression of ALP. However, HEK 293 cells did not show any significant fluorescence signal, due to the complete downregulation of ALP. Hence, the authors believed that depending on the amount of ALP in cells, the intensity profiles in the green channel were different in the various cell lines.
Wu et al. developed a 1,8-naphthalimide derivative,
ALP-16, for real time ratiometric recognition of (
Figure 31) the organ damage biomarker ALP in cells and in vivo models [
83]. A phosphor-ester group was incorporated at the 4th position of the 1,8-naphthalimide core to induce dual emission (blue–green region) and incorporation of a peripheral amine-
N-oxide group facilitated water solubility and biocompatibility. Upon phosphor-ester hydrolysis, the 4th position of the naphthalimide unit is transformed into free OH. This chemical transformation enormously changes the photophysical properties of the probe, causing a dramatic color change and a ratiometric signal in the emission spectra. In the biological buffer,
ALP-16 exhibited an absorption maximum λ
max at ca. ~375 nm, and an emission maximum at λ
em 468 nm in the UV–Vis and fluorescence spectra, respectively. Upon ALP-regulated biocatalytic dephosphorylation, electron deficient substituents (phosphoester) were changed to electron donating substituents (phenolic OH) at the 4th position of the 1,8-naphthalimide unit of
ALP-15 (OH). Due to these reasons, significant bathochromic behavior (λ
max 375 → 450 nm) was observed in UV–Vis spectra. Similarly, significant ratiometric changes were observed through the concomitant decrement at 468 nm and a significant enhancement at 554 nm (redshift) in the emission signal.
Dephosphorylation kinetics parameters calculated through the Michaelis–Menten equation revealed appreciably good values: Vmax = 1.506 µM min−1 and Km = 22.514 µM in physiological conditions. This naphthalimide-based probe showed a detection limit of 0.38 UL−1 towards ALP without inducing interference from biologically relevant ions and molecules.
ALP-16’s cytotoxicity assay was evaluated in L929 and HeLa cell lines via MTT assay. Even at high concentrations of probe, no substantial reduction in cell viability was observed in cells. HeLa cells incubated with
ALP-16 exhibited the green fluorescence within 30 min (
Figure 32(I)). On the other hand, due to the downregulation of ALP in L929 cells, only blue fluorescence was observed. In support to the above studies, fluorescence images collected from the levamisole-preincubated cell lines exhibited only blue emission (
Figure 32(II)). Based on these results, the authors speculated that the bright green signal originated in the cells due to the biocatalytic dephosphorylation of
ALP-16 by endogenously produced phosphatase.
N-acetyl-p-aminophenol (NAPA) is a drug generally used to damage zebrafish larvae organs by the upregulation of ALP. Accordingly, NAPA dose-dependent studies in zebrafish larvae exhibited bright green emission in damaged liver, stomach and intestine due to the presence of elevated ALP (
Figure 32(III)). Based on these studies, the authors speculated that
ALP-16 can be used to detect organ damage in in vivo models, such as zebrafish larvae.
Zhou et al. reported a naphthalene-based two-photon fluorescent probe
ALP-17 [
84] for the recognition of ALP (
Figure 33) in a ratiometric manner. The designed probe is associated with a typical donor–π–acceptor (D–π–A) structure and is able to emit very low fluorescence in its phosphorylated state, which is attributed to inhibition of the ICT process from the naphthalene core to the benzo-thiazole unit due to the electron withdrawing properties of the phosphoester unit. However, upon dephosphorylation, strong ICT is restored, due to the resonance effects of phenolic OH (strong electron donor) in the naphthalene core. As a result, the emission band undergoes a bathochromic shift, relative to the phosphorylated structure, along with a peculiar ratiometric response.
The probe’s fluorescence intensity ratio showed a linear relationship with ALP concentration in the range of 20 to 180 U/L, and had a detection limit of 2.3 UL
−1 in physiological conditions. The authors did not specify the dephosphorylation kinetic parameters in their work. In a detailed in vitro investigation,
ALP-17 was used to recognize the intracellular ALP activity using one-photon (405 nm) or two-photon (720 nm) excitation techniques. The probe’s quantum yield was found to be 0.41. When excited at 720 nm, the probe has a 65 GM two-photon action absorption cross-section at 428 nm. The probe’s cytotoxicity was evaluated based on MTT assay in HeLa cells, revealing that
ALP-17 did not have a toxic effect until 20 µM. During incubation with
ALP-17, HeLa cells exhibited bright green fluorescence after 30 min. In contrast, probe incubated in levamisole-hydrochloride-pre-treated HeLa cells resulted in only blue emission (
Figure 34), which supports that
ALP-17 is able to detect intracellular ALP. The probe-incubated HeLa cells resulted in bright fluorescence images upon excitation at λ
ex 720 nm, supporting the idea that
ALP-17 is suitable for two-photon imaging as well (
Figure 35).
Podder et al. developed the rhodol-based green fluorescent probe
ALP-18 (
Figure 36), which was used to distinguish cancer cells from normal cells based on lysosomal phosphatase expression [
85]. In vitro studies revealed that the probe causes a ~9-fold increment in UV-absorption at λ
abs of 490 nm and a ~33-fold emission enhancement at λ
em 532 nm in the presence of 1.72 U mL
−1 of ALP. In this probe, fluorescence emission behavior was tuned through the closed and open structure of the conventional spiro-lactam ring in the rhodol skeleton. In the phosphorylated state, the probe exists in spirocyclic form and exhibited weak fluorescence. However, upon dephosphorylation, the spiro-lactam ring will be opened, resulting in revival of rhodol fluorescence in the green channel. Dephosphorylation kinetic parameters
KM, k
cat and k
cat/
KM were found to be 7.0 µM, 9.52 s
−1, and 13.6 × 10
5 M
−1 s
−1, respectively. The probe did not suffer from interference in the presence of biologically relevant ions and molecules in physiological conditions.
MTT-based toxicity assays in NIH-3T3 (normal) and HeLa (cancer) cells revealed that at µM concentrations,
ALP-18 did not show antiproliferative activity towards either cell line. The probe’s adhesion to HeLa (cancer) cells was relatively stronger than to normal cells. Based on a dose-dependent assay, the relative expression of ALP was examined through fluorescence imaging in both cancer and normal cells (
Figure 37). When compared to HeLa cells, the fluorescence intensity in regular NIH-3T3 cells was much lower (~2 folds).
In order to evaluate the
ALP-18’s sub cellular localization and inhibitory activity in HeLa cells, colocalization experiments were carried out in the presence and absence of levamisole (inhibitor) (
Figure 38). Lyso tracker co-incubated cells showed bright green and non-emissive behavior in the absence and presence of inhibitor, respectively, and a good co-localization coefficient (Pearson’s coefficient 0.93) with the red channel supported that
ALP-18 is primely localized in lysosomes.
Lu et al. reported ICT tuned dicyano-based fluorogenic substrate (
ALP-19) for the ratiometric recognition of ALP (
Figure 39) [
86]. The probe showed a significant bathochromic shift (550 → 650 nm) in the emission spectrum, upon biocatalytic dephosphorylation: a substantial color change from yellow-green to light orange. In its phosphorylated state, the probe was weakly emissive due to lowering of electron densities on the phenolic oxygen, which resulted in inhibition of the ICT phenomenon in D–π–A skeleton. However, upon phosphate hydrolysis, phenolic OH enhanced the ICT phenomenon in the probe, resulting in noticeable spectral shifts in the UV–Vis and emission signals. The absorption and emission bands on the probe
ALP-19 exhibited peaks at 440 nm (ε = 2.2 × 10
4 M
−1 cm
−1) and 550 nm (ϕ = 10.5%) respectively. Upon dephosphorylation, a new band appeared at 650 nm (ϕ = 7.5%). It is worth noting that due to the weak emission at 650 nm, the dephosphorylated probe exhibited a low quantum yield compared to the phosphorylated form.
The probe detected ALP very selectively with a LOD of 3.8 UL−1. The authors did not specify the kinetic parameters of the probe towards ALP.
MTT-based cell viability assays showed the probe was non-toxic to HeLa cells under the μM range. Thereafter, endogenously produced ALP activity was monitored in the presence and absence of Na
3VO
4 (inhibitor) in HeLa cells. In the absence of Na
3VO
4,
ALP-19-incubated HeLa cells showed bright fluorescence in the red channel. However, such bright emissive behavior was not observed in the presence of inhibitor (
Figure 40). From these studies, the authors speculate that emission intensity in the red channel solely came from ALP-catalyzed dephosphorylation of the probe.
Hu et al. reported a new flavone-based fluorescent probe,
ALP-20 (
Figure 41) (3-hydroxy-2-(p-tolyl)-4H-chromen-4-one), for the in vitro and in cellulo recognition of ALP [
87]. Tuning of photophysical behavior in the probe is attributed to inhibition and facilitation of the ESIPT process in phosphorylated and dephosphorylated states, respectively. Due to the presence of a phosphorylated phenolic unit, it is unable to undergo a tautomerization process; as a result, emission intensity is significantly quenched. However, ALP-regulated dephosphorylation induced the keto-enol tautomers through the phenolic OH group, allowing for intensified ESIPT-based emission.
The probe showed absorption and emission maxima at 335 and 480 nm in UV–Vis and fluorescence spectra, respectively. However, upon the addition of ALP, a decrement and an increment at 335 and 410 nm were observed in UV–Vis spectra, respectively. Similarly, a dramatic peak in the emission spectrum centered at λem 480 nm was observed. The dephosphorylation kinetic parameters Km and Vmax were found to be 1.919 µM and 1.21 × 10−3 µmol/min, respectively. Due to high affinity of the probe towards phosphatase (affinity constant of Ka 1.84 × 107 L/mol), its detection limit was good, 0.032 U/L; and it showed no interference from other proteins and biologically relevant ions. The authors also demonstrated the probe’s phosphatase recognition capabilities in serum samples.
MTT-based cytotoxicity assays performed in ALP-downregulated (L929) and upregulated (HeLa) cell lines revealed that
ALP-20 is biocompatible at µM concentrations. The probe-incubated HeLa cells exhibited bright green emission; however, no such bright emission was observed in L929 cells. Furthermore, upon incubation of
ALP-20 in levamisole-preincubated HeLa cells did not show any emission signal in green channel, supporting successful tracking of endogenously produced ALP (
Figure 42).
Liu et al. reported a hemicyanine-based NIR fluorescent probe,
ALP-21 (
Figure 43), for turn-on trapping of ALP activity in cancer cells and tumor-xenografted nude mouse models [
88]. The probe rationale was based on regulation of the intramolecular charge transfer (ICT) principle. The phosphate group was tethered directly to the fluorophore’s phenolic OH group, thereby preventing fluorescence in the hemicyanine core by repressing the ICT process. Upon biocatalytic dephosphorylation, free phenolic OH was regenerated, thereby reviving a strong ICT process which triggered a bright emission signal in the NIR region.
ALP-21 showed absorption maxima at λmax at 590 and 635 nm in the UV–Vis spectrum in buffer. However, upon addition of ALP, a substantial red-shift was observed (635 → 680 nm): a distinct color change from blue to blue–green (bluish green). Paradoxically, dramatic enhancement in the emission spectra (at λem 706 nm) was observed. It is worth noting that the phosphorylated probe exhibited very weak fluorescence at λem 706 nm, which was attributed to inhibition of the ICT process from the phosphoester unit to the indolium unit. The dephosphorylation kinetic parameters Km and Vmax (reaction rate) were found to be 52.45 μM and 4.41 μM min−1, respectively. The probe did not show any interference from biologically relevant proteins, ions or biomolecules, and showed a detection limit of 0.28 U/L towards ALP under physiological conditions.
Initially
ALP-21’s cytotoxicity was evaluated in HeLa, HepG2 and HEK293 cells with MTT assays. At µM concentrations, the probe did not show any significant cytotoxicity in the cell lines. Upon incubation of
ALP-21 in ALP-upregulated cells, such as HeLa and HepG2, it exhibited bright emission in the NIR/far-red channel. However, in the presence of Na
3VO
4, no such bright emission was observed. Additionally, ALP-downregulated HEK293 cells did not show significant emission in the NIR/far-red channel, which was attributed to dephosphorylation of the probe in the presence of endogenously produced ALP (
Figure 44).
In vivo ALP recognition capabilities were demonstrated in BALB/c nude mouse models. Intratumoral injection of
ALP-21 into tumor-bearing living mice showed concomitant increments in fluorescence intensities over time (
Figure 45a). This type of NIR emission intensity increment was not observed in Na
3VO
4-preincubated mice, even after 1 h under (
Figure 45b). However, after 2 h, a weak fluorescence signal was generated, which was not comparable with fluorescence obtained in the absence of Na
3VO
3.
Li et al. reported
ALP-22, a water-soluble hemicyanine-based NIR fluorescent probe (
Figure 46) tethered with a quinolinium ethyl iodide unit to induce water solubility [
89].
ALP-22 showed absorption maxima at λ
max 568 and 720 nm in UV–Vis spectrum, before and after addition of phosphatase, respectively. Substantial changes in absorption maxima involved a dramatic color transition from purple to blue. The phosphorylated probe was weakly emissive due to the inhibition of ICT by the phosphoester unit. Upon dephosphorylation, a strong ICT process was resurrected between the quinolinium unit and the chromene-type phenolic unit, resulting in bright emission centered at 770 nm (~7 fold).
ALP-22 showed excellent selectivity and sensitivity towards ALP in vitro, allowing it to be used to visualize ALP activity in various cells.
The dephosphorylation kinetic parameters Vmax and Michaelis constant (Km) were found to be 0.491 μM min−1 and 8.90 μM, respectively. The probe had peak activity at pH 8.0 and exhibited a LOD of 0.017 U mL−1 towards ALP.
The cytotoxicity of probe
ALP-22 was investigated in HeLa, HepG2, HCT116 and GT1 cell lines based on the MTT assay and was non-toxic at µM concentrations.
ALP-22-incubated HeLa cells showed bright NIR emission in the absence of inhibitor (
Figure 47). However, such emissive behavior was not observed when the probe was incubated in inhibitor (Na
3VO
4)-pretreated cells, validating the selective red fluorescence being attributed to successful dephosphorylation of
ALP-22 by the intracellular phosphatase.
In detailed in vitro studies,
ALP-22’s in vivo recognition capabilities were tested in mouse models (normal, diabetic and treatment). The fluorescence intensity in normal mice increased slowly over time, indicating that the probe detected ALP activity in normal mice. The fluorescence signal of diabetic (induced by intraperitoneal injection of streptozotocin, a toxin for pancreatic β-cells) mice was much greater than that of the normal mice, indicating upregulation of the ALP level in diabetic mice. Interestingly, when diabetic mice were treated with the hypoglycemic drug
metformin, fluorescence intensity was reduced significantly (
Figure 48a). From these studies, the authors believed that the generation of bright NIR fluorescence in the mouse models was attributable to ALP-catalyzed dephosphorylation of the probe. These results were found concordant with fluorescence images of normal, diabetic and treatment-given mice, in organs such as liver, heart and spleen (
Figure 48b) and blood samples (
Figure 48c).
Zhang et al. reported a far-red fluorescent probe based on malononitrile tethered chromene analogue
ALP-23 (
Figure 49) for in vitro and in vivo detection of ALP [
90]. The design rationale of
ALP-23 was based on the phosphorylation and dephosphorylation of the phenolic OH unit, accomplished through the inhibiting and restraining of the ICT process in D–π–A confinements. The ALP-regulated dephosphorylation reaction restores the ICT effect by unveiling the “turn-on” fluorescence in the
ALP-23 (
OH). The probe showed absorption spectra ranging from 420 to 580 nm with a sharp absorption maximum at 510 nm and annihilated red emission upon excitation at 600 nm. However, upon addition of ALP, the absorption band at 510 nm was decreased and saw a substantial shift to 605 nm. Additionally, a prominent emission signal was observed with maximal intensity at 640 nm. Such substantial changes in optical behavior in
ALP-23 were attributed to biocatalytic dephosphorylation, via the strong ICT process in
ALP-23 (
OH).
The dephosphorylation kinetic parameters Michaelis constant (Km) and Vmax were found to be 2.094 μM and 0.244 μM min−1, respectively, and it exhibited a LOD of 0.28 U/L towards ALP under physiological conditions.
The endogenous ALP recognition capabilities of the probe were evaluated in LO2 and HepG2 cells lines. In the MTT-based cytotoxicity assay,
ALP-23 was not toxic to either of the cell lines at below-µM concentrations. Due to the upregulated ALP expression in HepG2 cells, upon incubation with the probe, cells showed bright emission in the red channel; however, such significant emissive behavior was not observed in the case of LO2 cells due to the downregulation of phosphatase (
Figure 50). Additionally, when the probe was incubated with inhibitor Na
3VO
4-pre-treated cells, it did not show significant emission in the NIR channel. From these results, the authors speculated that bright emission in the far-red region in cells should be attributed to dephosphorylation of the probe by endogenously produced phosphatase.
ALP-23’s phosphatase recognition capabilities were successfully demonstrated in zebrafish larvae through the fluorescence confocal imaging studies (
Figure 51).
Khatun et al. reported coumarin-tethered triphenylphosphonium salt
ALP-24 for the recognition of (
Figure 52) mitochondrial ALP in cellular conditions [
91]. The rationale was based on a receptor-fluorophore-subcellular targeting group where coumarin acts as a signaling unit (blue fluorophore), in which the ALP receptor (diethyl phosphate) is attached to the 8th position, and the mitochondrial targeting moiety triphenylphosphine is connected to a coumarin unit through the ethyl spacer. In this work, the diethyl-phosphate moiety was retained in the molecule to enhance the cellular uptake, instead of the conventional negatively charged free phosphate terminus.
Biocatalytic cleaving of the diethyl-phospho-ester unit in the presence of phosphatase resulted in a significant bathochromic shift (350 → 410 nm) in the UV–Vis absorption spectrum, along with a substantial increment in the emission spectrum centered at ca. ~450 nm (~18 fold). After exposing the probe solution to a 150 W Xenon lamp for several hours, the emission intensity at 450 nm did not alter. From these results, the authors believe that the designed probe has good photostability in buffered conditions. The dephosphorylation kinetic parameters Michaelis constant (Km = 16.0 μM), catalytic efficiency constant (Kcat/Km ~ 3.12 × 10−16 M−1 s−1) and turnover number (Kcat 4.23 s−1) were found to be moderately good and favorable, even in cellular conditions.
Its ALP recognition capabilities were demonstrated in cancer (HeLa cells) and normal (NIH3T3 cells) cell lines. In neither the cell lines did
ALP-24 show negative effects on cell proliferation at µM concentrations. It was found that, due to downregulation of ALP in normal cells, emission signal intensity in the blue channel was too low. However, probe-incubated HeLa cells exhibited bright blue emission; such emissive behavior was significantly diminished in levamisole-preincubated cell lines. Furthermore, colocalization studies with mito-tracker red showed a good correlation coefficient (~0.9) in HeLa cells (
Figure 53). From these results, the authors speculated that the bright blue emissive behavior in mitochondrial regions can be attributed to dephosphorylation of the probe in the presence of endogenously produced phosphatase in subcellular organelles (mitochondria).
Park et al. reported the two dihydroxanthene-based NIR fluorescent probes
ALP-25 and “
ALP-26” (
Figure 54) for the detection of ALP in cells and tissues [
92]. To induce better water solubility, phenolic and sulfonate groups were incorporated at each terminus. The rationally designed probes were non-fluorescent in their phosphorylated states; however, upon biocatalytic dephosphorylation, the probes exhibited bright NIR fluorescence, in a dose-dependent manner. ALP-regulated
off–on fluorescence switching in the probes was attributed to inhibition and restoration of the ICT process in the hemicyanine core.
ALP-25 and
ALP-26, due to the minor difference in the hemicyanine core substitution, both showed a characteristic electronic absorption band around 600–700 nm in their UV–Vis absorption spectra. Both probes were weakly emissive (Φ
F = ~0.006) due to the effective inhibition of the ICT process. However, in the presence of ALP, a new band was generated at ~710 nm with a substantial increment in NIR emission intensity (~200 fold, Φ
F = ~0.009), upon excitation at 685 nm. The probes showed a rapid
switch on response towards ALP (0.1 U min
−1) within 1.5 min. However, the authors did not report any kinetic parameters, such as
Km and
Vmax, in their work.
Upon detailed in vitro analysis, the probes’ (ALP-25 and ALP-26) binding affinities towards hydroxyapatite (HA) were studied in the presence of various types of calcium salts, such as phosphates, carbonates, chlorides and nitrates. Intriguingly, they showed high binding affinities towards the HA surface. The authors believed that the phosphate unit in the probes being able to interact with the Ca2+ ions on the HA surface is the main reason for their superior adsorption. It was found that the probe-holding calcium phosphates scaffolds have moderately good ALP recognition capabilities in physiological conditions.
The cell viability of the respective probes was analyzed through CCK-8 assay studies. It was found that the probes did not induce any serious cytotoxicity below 50 µM towards HeLa, HepG2 or MC3T3-E1 cell lines. After incubation of (
ALP-25/
ALP-26) probes in the ALP-upregulated cells HeLa and HepG2, bright NIR emission was seen. In contrast, ALP-downregulated cells, MC3T3-E3, exhibited extremely weak emission, supporting their successful tracking of endogenous ALP in cellular conditions (
Figure 55). Their in vivo ALP recognition capabilities were demonstrated by using the probe-holding calcium phosphate scaffolds under the skin of nude mice (
Figure 56). Bright NIR emission was detected on day 0, and concomitant increments were found in the emission intensities as the days passed on. From these studies, the authors concluded that the designed probes detect ALP activity during bone formation, even in in vivo models.
Yaqian et al. reported hydrazone-based AIE-tuned fluorescent probe €-2-(((9H-fluoren-9-ylidene) hydrazono)methyl)phenyl dihydrogen phosphate,
ALP-27, for the recognition of cellular ALP [
93]. The probe’s rationale is based on the dual photophysical phenomena ESIPT and AIE.
ALP-27 exhibited a characteristic absorption maximum at 400 nm and was weakly emissive upon excitation at λ
ex 380 nm in physiological conditions. However, upon biocatalytic dephosphorylation, the probe showed bright emission centered at 586 nm by showing minor changes (blue shift 380 →350 nm) in the UV–Vis absorption spectra (
Figure 57). ALP-regulated dramatic green emissive behavior exhibited a large Stokes shift (>200 nm), which was attributed to the synergistic AIE and ESIPT mechanism.
The presence of an o-phosphorylated phenolic hydrazone in the probe makes it unable to induce an ESIPT effect with the nitrogen atoms through the hydrogen bonding mechanism. However, upon dephosphorylation, ALP-27’s (OH) free ortho-hydroxyl group (phenolic OH) interacts with nitrogen atoms (forming stable 6 membered ring) through the hydrogen bonding process, during photo-chemical excitation, which is the main reason for the ESIPT effect. The observed in vitro dephosphorylation kinetics included a Km value of 8.49 μM, revealing that the probe has a high affinity towards phosphatase in biological buffers. The probe did not suffer from optical interference from biologically relevant enzymes, ions, H2O2, bio-mercaptan or amino acids, and had a detection limit of 0.6 U/L (linear range 1 to 100 U/L) towards ALP in physiological conditions. ALP-27’s cytotoxicity assay used HeLa cells, finding that it and its dephosphorylated product have negligible cytotoxicity up to the concentration of 10 μM.
Due to the excellent biocompatible properties of
ALP-27 at under μM concentrations, the probe was employed for imaging of endogenous ALP in living cells (
Figure 58). Upon incubation of
ALP-27 in HeLa cells, bright yellow emission was observed. In contrast, L-cysteine (ALP inhibitor)-pretreated HeLa cells incubated with probe did not show any significant emission in the yellow channel. Based on the imaging results, the authors speculate that the bright yellow emission from HeLa cells can be attributed to dephosphorylation of the probe by the endogenously produced ALP.
Yin et al. reported a novel red emissive fluorescent probe,
ALP-28 (
Figure 59), based on isophorone for intracellular ALP detection [
94]. In physiological conditions, the probe showed an absorption maximum at 404 nm and weak emission centered at 570 nm in UV–Vis and fluorescence spectra, respectively. Upon phosphatase catalyzed hydrolysis, the probe exhibited substantial bathochromicity (404 → 421 nm) in the UV–Vis spectrum and an intense orange–yellow fluorescence signal centered at ca. 570 nm in the emission spectrum. The probe showed excellent sensitivity towards ALP with a detection limit of 0.088 U/L in in vitro assay conditions. Biocatalytic dephosphorylation of
ALP-28 was authentically validated through the
31P NMR spectroscopic methods. In the studies before and after dephosphorylation, the phosphorous resonance peak was at δ −4.56 ppm or δ 0.81 ppm, respectively, unambiguously supporting the ALP-regulated phosphoester hydrolysis.
A Cell Counting Kit-8 (CCK-8)-based cytotoxicity assay in HeLa and HepG2 cell lines revealed that
ALP-28 was biocompatible and did not show a toxic effect until 10 µM. Incubation of the probe in ALP-upregulated HeLa and HepG2 cell lines caused different emission intensities in the yellow channels (
Figure 60a). When the probe was incubated with levamisole and NaH
2PO
4-pre-treated cells, there was no significant difference in the yellow channel (
Figure 60b). From these results, the authors speculated that the bright emission in the yellow channel is attributable to ALP-catalyzed dephosphorylation of the probe.
Yangyang et al. reported a benzothiazole-based ratiometric probe, (4-benzamio-2-(benzo[d] thiazol-2-yl)phenyl dihydrogen phosphate (
ALP-29), for the intracellular recognition of ALP [
95]. Its molecular engineering associated it with the phosphatase-regulated ESIPT phenomenon (
Figure 61). In order to reduce the pH-related instability in ESIPT-based probes, the phenolic‘s unit para position was functionalized with a benzoamide moiety. Under physiological conditions, the probe showed characteristic excitation and fluorescence emission peaks at ca. 340 and ca. 425 nm respectively. Upon biocatalytic dephosphorylation, bright red-shifted (120 nm) fluorescence emission was observed (425 nm → 545 nm). Such dramatic changes in fluorescence intensity were attributed to revival of the ESIPT mechanism, resulting from the phenolic OH unit with a benzothiazole nitrogen forming the partial quinone structure upon photochemical excitation.
ALP-29 showed a ratiometric emission enhancement ratio (I545/I425) of ~122-fold towards phosphatase and a low detection limit of 0.004 mU/mL in physiological conditions. Based on pH-dependent spectral measurements, pH 8.0 was found to be the most suitable for the ALP activity measurements. ALP-29 showed excellent selectivity towards ALP without showing any interference from the other enzymes, such as acetylcholinesterase, trypsin or lysozyme. The MTT-based cytotoxicity assay in HeLa cell lines revealed that the probe did not show any toxicity and showed cell viability at 5 µmol/L to 20 µmol/L concentrations.
Upon detailed in vitro analysis and cytotoxicity evaluations,
ALP-29 was employed for the imaging of endogenous ALP in HeLa cells (
Figure 62). Accordingly, probe-incubated HeLa cells exhibited bright yellow emission; such bright emissive behavior was not observed in the presence of levamisole-pretreated cells. From these results, the authors speculated that the dramatic fluorescence enhancement in cells should be attributed to endogenous phosphatase-catalyzed dephosphorylation of
ALP-29.
Gao et al. reported a new blue–green emissive fluorescent probe,
ALP-30 (
Figure 63), based on a naphthalimide core, for in cellulo ratiometric imaging of ALP [
96]. In physiological conditions,
ALP-30 exhibited an absorption maximum at λ
abs ca. 355 nm and itself has an emission maximum at ca. 472 nm, due to the weak electron withdrawing effect (weak ICT) of its phosphoester group. Upon phosphatase assisted cleavage of the phosphoester unit, there was a new absorption maximum at λ
abs ca. 448 nm and a new emission peak at 556 nm, owing to the strong electron donating ability of -OH (strong ICT). Strong green emissive behavior in the dephosphorylated probe (
ALP-30 (
OH)) can be attributed to the formation of 4-hydroxyl-naphthalimide, which resulted in the generation of the ICT process with the electron donating (resonance stabilized) phenolic OH and the electron accepting imide core.
The probe showed a detection limit of 0.25 U/L towards ALP (linearity range from 0 to 200 U/L) under physiological conditions. The probe exhibited good selectivity towards ALP over other enzymes, such as lipase, peroxidase and carboxylesterase. The authors did not specify the dephosphorylation kinetic parameters, such as Km and Vm, in their work.
A cytotoxicity assay was performed with HepG2 cells based on the MTT assay. Even at high concentrations,
ALP-30 exhibited low cytotoxicity towards HepG2 cells. Upon incubation with
ALP-30, HepG2 cells showed significant green fluorescence. In contrast, when the cells were incubated with an ALP inhibitor (Na
3VO
4) beforehand, the cells showed a substantial decrement in the green emission intensity (
Figure 64). From said studies, the authors speculated that the bright green emission should be attributed to the biocatalytic dephosphorylation of
ALP-30 by endogenously produced ALP.
Wang et al. reported a novel hemicyanine-based water-soluble NIR fluorophore
ALP-31 (
OH) (
Figure 65) (3-ethyl-2-(2-(6-hydroxy-2,3-dihydro-1H-xanthen-4-yl)vinyl)benzo[d]thiazol-3-ium) for exogenous and endogenous ALP imaging in cells [
97]. The probe’s rationale revolved around a simple push–pull tuned ICT process between the phosphorylated/dephosphorylated phenolic OH and the benzothiazolium unit. The phosphorylated state’s ICT process is inhibited due to the electron withdrawing capability of the phosphoester unit; as a result, the probe was weakly emissive (ϕ = 0.15). However, dephosphorylated
ALP-31 (
OH) exhibited a strong fluorescence signal with an excitation maximum at ca. 680 nm and an emission maximum at ca. 723 nm. The strong ICT process between the phenolic OH and thiazolium units culminated in a Stokes’ shift of 43 nm.
Based on pH dependent fluorescence studies, it was found that the probe exhibited maximal dephosphorylation kinetics at pH 8. The probe showed excellent sensitivity and exhibited a detection limit of 0.042 U/L (linear range 0–8 U/L) towards phosphatase. ALP-31 did not show optical interference from other enzymes or anions under similar experimental conditions.
Upon detailed in vitro studies, the probe’s intracellular ALP recognition capabilities were monitored in ALP-downregulated HEK 293 cell lines (
Figure 66). After incubation of
ALP-31 in the HEK 293 cells, they exhibited low emission signals in the red channel. In contrast, a strong red fluorescence signal was observed after the cells were transfected by exogenous ALP. From these observations, the authors concluded that
ALP-31 can be used even in ALP-downregulated cell lines to detect exogenously transfected ALP activity.
Thereafter, the probe’s cytotoxicity was evaluated in ALP-upregulated BEL 7402 cells through the MTT assay. Until 20 µM, the probe did not show any cytotoxic effects. Encouraged by these results, the authors tested the endogenous ALP activity in a BEL 7402 cell lines (
Figure 67). As expected, untreated BEL 7402 cells did not show any fluorescence in the red channels; on the other hand, the cells treated with
ALP-31 showed strong red fluorescence. When the probe was incubated in Na
3VO
4-pre-treated cells, there was extremely low fluorescence intensity. When the probe was incubated in exogenously transfected ALP, the cells had significant emission in the red channel, supporting that
ALP-31 can be used to recognize both endogenous and exogenous ALP activity in cells.
Wu et al. reported an AIE-responsive tetraphenylenthene fluorescent probe,
ALP-32 (
Figure 68), for the recognition of ALP in living cells [
98]. The probe’s rationale is associated with the incorporation of a hydrophilic polar phosphate terminus—a specific receptor for ALP with a hydrophobic TPE core. In biological buffers, the probe showed a characteristic absorption maximum at λ
max ca. 375 nm and extremely weak emissive behavior. Upon dephosphorylation, due to the elimination and formation of polar phosphate and phenol groups, respectively,
ALP-32 (
OH) resulted in bright green emission. These biochemically catalytic transformations led to characteristic absorption at λ
max ca. 400 nm and a ~12-fold enhancement in emission intensity at λ
em ca. 545 nm. Elevated hydrophobicity in
ALP-32 (
OH) resulted in poor water solubility and strong green fluorescence upon aggregation through the AIE mechanism.
The probe’s fluorescence intensity ratio showed a linear relationship with ALP from 0 to 200 U/L and exhibited a detection limit of 14.2 U/L under physiological conditions. ALP-32 showed good selectivity towards the ALP compared to the other biologically relevant proteins, such as BSA and trypsin, under physiological conditions.
Upon detailed in vitro analysis,
ALP-32’s MTT-based cytotoxicity was evaluated in HeLa cells, L929 cells and HepG2 cells. Until 50 µM,
ALP-32 did not show any significant cytotoxicity in the cell lines. After incubation of
ALP-32 in the ALP-upregulated cell lines, such as HeLa and HepG2, there was strong emission in the green channel (
Figure 69a,b). In contrast, when the probe was incubated with levamisole-hydrochloride-pretreated cells, there was no significant emission in the green channel (negative control). Likewise, when the probe was incubated in ALP-downregulated L929 cells, there were no remarkable changes in fluorescence under similar experimental conditions (
Figure 69c). From these results, the authors speculated that the bright green emission in cells should be attributed to selective dephosphorylation of
ALP-32 by the cellular phosphatase (
Table 1).
The rationale was the conjugation of a phosphatase recognition unit (phosphoester unit) and subcellular targeting moieties (lysosome, mitochondria, cancer cell receptors, etc.) to the fluorophore in a sterically free environment. It is evident from the literature, most cancer cells substantially upregulate ALP; hence, it is highly desirable to develop novel fluorescent probes that selectively target the cancer cells rather than normal cells in tissues or in vivo models. Conventionally, it is possible to design novel small fluorescent probes that can exclusively target cancer cells in tissues by incorporating cancer-cell-targeting units such as biotin, nitroimidazole, anthocyanine and many more, in a sterically free environment. Several NIR emissive small-molecule-based fluorescent probes have been reported for the selective recognition of ALP in mouse (in vivo) models, in the tumor microenvironment (TME) and cancer tissues, but very few of them addressed the ALP association with cancer pathophysiology. As cancer is very broad area, there are several biochemical issues that need to be addressed, especially at the molecular level. Therefore, in the current scenario, it is highly essential to develop label-free fluorescent probes for ALP-like cancer biomarkers in the simplest way. In that sense, simple and robust non-classical small molecular probes that can exclusively address ALP-associated diseases such as cancers could provide a better platform for bioanalytical chemists to develop a future generation of novel cancer diagnosing tools.