A Theoretical Study of the In Situ Structural Reconstruction of Pdn (n = 6, 19, 44) Clusters for Catalytic Hydrogen Evolution
Abstract
:1. Introduction
2. Calculation Methods
3. Results
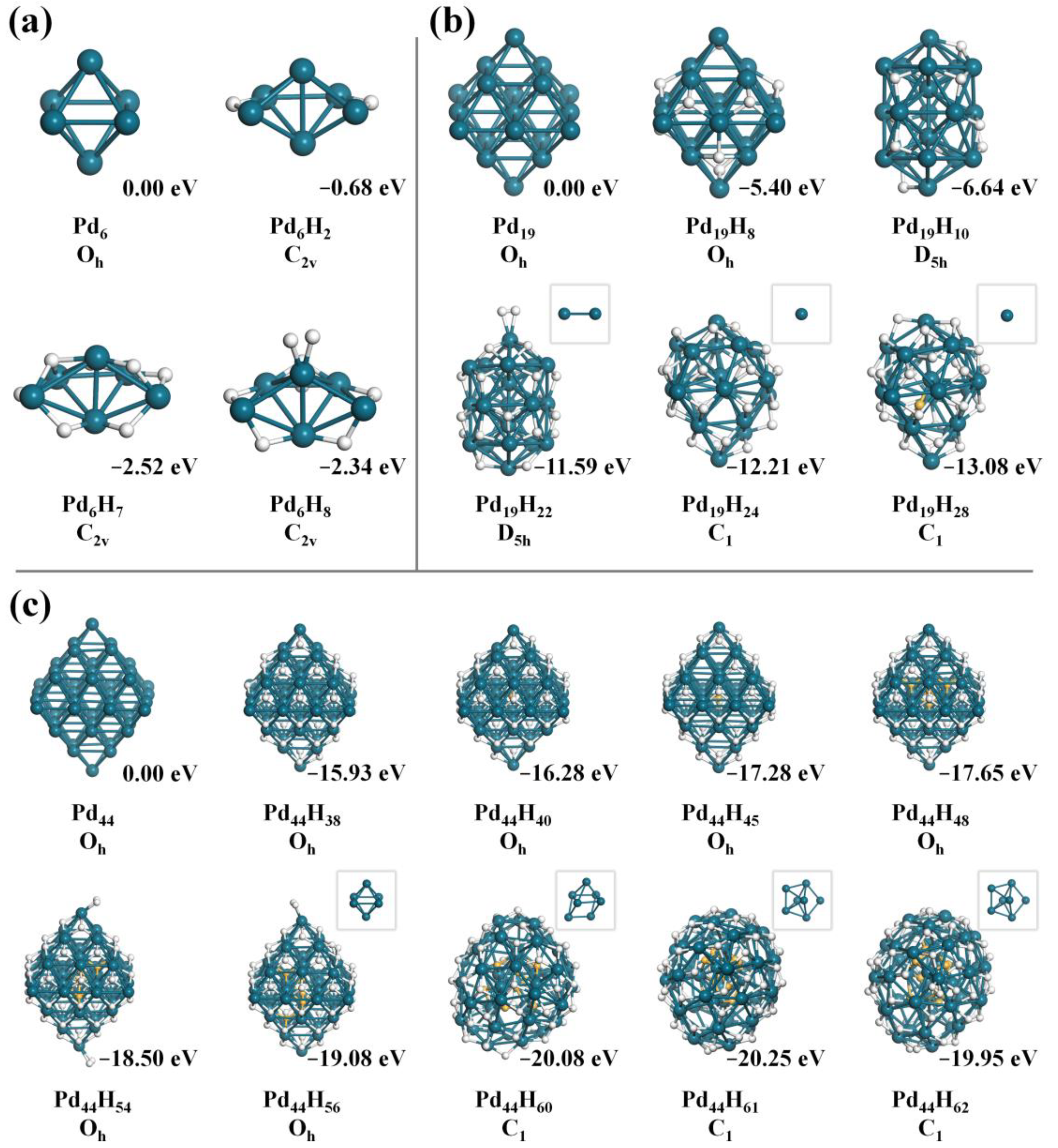
3.1. Structure Reconstruction of Pdn (n = 6, 19, 44) Clusters under HER Environment
3.1.1. Pd6
3.1.2. Pd19
3.1.3. Pd44
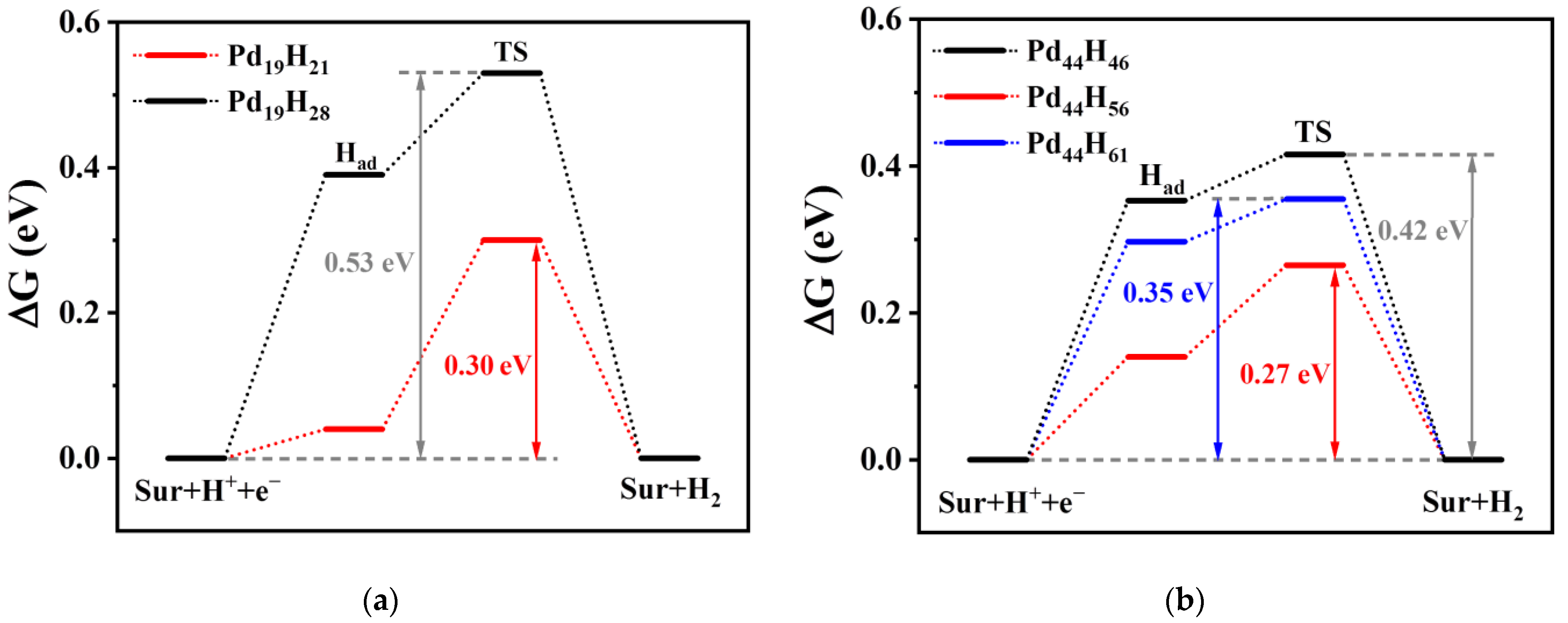
3.2. Effects of Structural Reconstruction on HER Activity
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chu, S.; Majumdar, A. Opportunities and challenges for a sustainable energy future. Nature 2012, 488, 294–303. [Google Scholar] [CrossRef] [PubMed]
- Norskov, J.K.; Christensen, C.H. Chemistry—Toward efficient hydrogen production at surfaces. Science 2006, 312, 1322–1323. [Google Scholar] [CrossRef] [PubMed]
- Turner, J.A. Sustainable hydrogen production. Science 2004, 305, 972–974. [Google Scholar] [CrossRef] [PubMed]
- Walter, M.G.; Warren, E.L.; McKone, J.R.; Boettcher, S.W.; Mi, Q.X.; Santori, E.A.; Lewis, N.S. Solar Water Splitting Cells. Chem. Rev. 2010, 110, 6446–6473. [Google Scholar] [CrossRef]
- Durst, J.; Siebel, A.; Simon, C.; Hasche, F.; Herranz, J.; Gasteiger, H.A. New insights into the electrochemical hydrogen oxidation and evolution reaction mechanism. Energy Environ. Sci. 2014, 7, 2255–2260. [Google Scholar] [CrossRef] [Green Version]
- McKone, J.R.; Warren, E.L.; Bierman, M.J.; Boettcher, S.W.; Brunschwig, B.S.; Lewis, N.S.; Gray, H.B. Evaluation of Pt, Ni, and Ni-Mo electrocatalysts for hydrogen evolution on crystalline Si electrodes. Energy Environ. Sci. 2011, 4, 3573–3583. [Google Scholar] [CrossRef] [Green Version]
- Seh, Z.W.; Kibsgaard, J.; Dickens, C.F.; Chorkendorff, I.B.; Norskov, J.K.; Jaramillo, T.F. Combining theory and experiment in electrocatalysis: Insights into materials design. Science 2017, 355, eaad4998. [Google Scholar] [CrossRef] [Green Version]
- Faber, M.S.; Jin, S. Earth-abundant inorganic electrocatalysts and their nanostructures for energy conversion applications. Energy Environ. Sci. 2014, 7, 3519–3542. [Google Scholar] [CrossRef]
- Koper, M.T. Blank voltammetry of hexagonal surfaces of Pt-group metal electrodes: Comparison to density functional theory calculations and ultra-high vacuum experiments on water dissociation. Electrochim. Acta 2011, 56, 10645–10651. [Google Scholar] [CrossRef]
- Koutauarapu, R.; Reddy, C.V.; Babu, B.; Reddy, K.R.; Cho, M.; Shim, J. Carbon cloth/transition metals-based hybrids with controllable architectures for electrocatalytic hydrogen evolution—A review. Int. J. Hydrog. Energy 2020, 45, 7716–7740. [Google Scholar] [CrossRef]
- Lai, W.H.; Zhang, L.F.; Hua, W.B.; Indris, S.; Yan, Z.C.; Hu, Z.; Zhang, B.W.; Liu, Y.N.; Wang, L.; Liu, M.; et al. General pi-Electron-Assisted Strategy for Ir, Pt, Ru, Pd, Fe, Ni Single-Atom Electrocatalysts with Bifunctional Active Sites for Highly Efficient Water Splitting. Angew. Chem. Int. Ed. 2019, 58, 11868–11873. [Google Scholar] [CrossRef] [PubMed]
- Peng, X.; Pi, C.R.; Zhang, X.M.; Li, S.; Huo, K.F.; Chu, P.K. Recent progress of transition metal nitrides for efficient electrocatalytic water splitting. Sustain. Energy Fuels 2019, 3, 366–381. [Google Scholar] [CrossRef]
- Huang, H.; Bao, S.X.; Chen, Q.L.; Yang, Y.A.; Jiang, Z.Y.; Kuang, Q.; Wu, X.Y.; Xie, Z.X.; Zheng, L.S. Novel hydrogen storage properties of palladium nanocrystals activated by a pentagonal cyclic twinned structure. Nano Res. 2015, 8, 2698–2705. [Google Scholar] [CrossRef]
- Durst, J.; Simon, C.; Hasche, F.; Gasteiger, H.A. Hydrogen Oxidation and Evolution Reaction Kinetics on Carbon Supported Pt, Ir, Rh, and Pd Electrocatalysts in Acidic Media. J. Electrochem. Soc. 2015, 162, F190–F203. [Google Scholar] [CrossRef]
- Ludwig, W.; Savara, A.; Madix, R.J.; Schauermann, S.; Freund, H.J. Subsurface Hydrogen Diffusion into Pd Nanoparticles: Role of Low-Coordinated Surface Sites and Facilitation by Carbon. J. Phys. Chem. C 2012, 116, 3539–3544. [Google Scholar] [CrossRef]
- Wang, G.X.; Liu, J.Y.; Sui, Y.M.; Wang, M.; Qiao, L.; Du, F.; Zou, B. Palladium structure engineering induced by electrochemical H intercalation boosts hydrogen evolution catalysis. J. Mater. Chem. A 2019, 7, 14876–14881. [Google Scholar] [CrossRef]
- Chen, M.J.; Lu, S.M.; Peng, Y.Y.; Ding, Z.F.; Long, Y.T. Tracking the Electrocatalytic Activity of a Single Palladium Nanoparticle for the Hydrogen Evolution Reaction. Chem. Eur. J. 2021, 27, 11799–11803. [Google Scholar] [CrossRef]
- Fan, J.X.; Du, H.X.; Zhao, Y.; Wang, Q.; Liu, Y.N.; Li, D.Q.; Feng, J.T. Recent Progress on Rational Design of Bimetallic Pd Based Catalysts and Their Advanced Catalysis. ACS Catal. 2020, 10, 13560–13583. [Google Scholar] [CrossRef]
- Newton, M.A.; Belver-Coldeira, C.; Martinez-Arias, A.; Fernandez-Garcia, M. Dynamic in situ observation of rapid size and shape change of supported Pd nanoparticles during CO/NO cycling. Nat. Mater. 2007, 6, 528–532. [Google Scholar] [CrossRef]
- Shang, C.; Liu, Z.-P. Stochastic Surface Walking Method for Structure Prediction and Pathway Searching. J. Chem. Theory Comput. 2013, 9, 1838–1845. [Google Scholar] [CrossRef]
- Zhang, X.-J.; Shang, C.; Liu, Z.-P. Double-ended surface walking method for pathway building and transition state location of complex reactions. J. Chem. Theory Comput. 2013, 9, 5745–5753. [Google Scholar] [CrossRef] [PubMed]
- Wei, G.F.; Liu, Z.P. Restructuring and Hydrogen Evolution on Pt Nanoparticle. Chem. Sci. 2015, 6, 1485–1490. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kresse, G.; Furthmuller, J. Efficiency of ab-initio total energy calculations for metals and semiconductors using a plane-wave basis set. Comput. Mater. Sci. 1996, 6, 15–50. [Google Scholar] [CrossRef]
- Blochl, P.E. Projector augmented-wave method. Phys. Rev. B 1994, 50, 17953–17979. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kresse, G.; Joubert, D. From ultrasoft pseudopotentials to the projector augmented-wave method. Phys. Rev. B 1999, 59, 1758–1775. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef] [Green Version]
- Monkhorst, H.J.; Pack, J.D. Special points for brillouin-zone integrations. Phys. Rev. B 1976, 13, 5188–5192. [Google Scholar] [CrossRef]
- Wang, H.-F.; Liu, Z.-P. Comprehensive mechanism and structure-sensitivity of ethanol oxidation on platinum: New transition-state searching method for resolving the complex reaction network. J. Am. Chem. Soc. 2008, 130, 10996–11004. [Google Scholar] [CrossRef]
- Shang, C.; Liu, Z.-P. Constrained Broyden minimization combined with the dimer method for locating transition state of complex reactions. J. Chem. Theory Comput. 2010, 6, 1136–1144. [Google Scholar] [CrossRef]
- Haynes, W.M.; Lide, D.R.; Bruno, T.J. CRC Handbook of Chemistry and Physics, 84th ed.; CRC Press: Boca Raton, FL, USA, 2003. [Google Scholar]
- Liu, Z.P.; Jenkins, S.J.; King, D.A. Car exhaust catalysis from first principles: Selective NO reduction under excess O-2 conditions on Ir. J. Am. Chem. Soc. 2004, 126, 10746–10756. [Google Scholar] [CrossRef]
- Zhang, L.N.; Qiao, L.J.; Bligaard, T.; Su, Y.J. A first-principle study of H adsorption and absorption under the influence of coverage. Appl. Surf. Sci. 2018, 457, 280–286. [Google Scholar] [CrossRef]
- Fang, Y.H.; Wei, G.F.; Liu, Z.P. Catalytic Role of Minority Species and Minority Sites for Electrochemical Hydrogen Evolution on Metals: Surface Charging, Coverage, and Tafel Kinetics. J. Phys. Chem. C 2013, 117, 7669–7680. [Google Scholar] [CrossRef]
- Wei, G.F.; Zhang, L.R.; Liu, Z.P. Group-VIII transition metal boride as promising hydrogen evolution reaction catalysts. Phys. Chem. Chem. Phys. 2018, 20, 27752–27757. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Zhang, L.R.; Yao, L.Y.; Fang, Y.H.; He, L.; Wei, G.F.; Lu, Z.P. Metal boride better than Pt: HCP Pd2B as a superactive hydrogen evolution reaction catalyst. Energy Environ. Sci. 2019, 12, 3099–3105. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, D.; Wei, G. A Theoretical Study of the In Situ Structural Reconstruction of Pdn (n = 6, 19, 44) Clusters for Catalytic Hydrogen Evolution. Symmetry 2022, 14, 1753. https://doi.org/10.3390/sym14091753
Zhang D, Wei G. A Theoretical Study of the In Situ Structural Reconstruction of Pdn (n = 6, 19, 44) Clusters for Catalytic Hydrogen Evolution. Symmetry. 2022; 14(9):1753. https://doi.org/10.3390/sym14091753
Chicago/Turabian StyleZhang, De, and Guangfeng Wei. 2022. "A Theoretical Study of the In Situ Structural Reconstruction of Pdn (n = 6, 19, 44) Clusters for Catalytic Hydrogen Evolution" Symmetry 14, no. 9: 1753. https://doi.org/10.3390/sym14091753
APA StyleZhang, D., & Wei, G. (2022). A Theoretical Study of the In Situ Structural Reconstruction of Pdn (n = 6, 19, 44) Clusters for Catalytic Hydrogen Evolution. Symmetry, 14(9), 1753. https://doi.org/10.3390/sym14091753





