Abstract
The oldest forms of living organisms on Earth are about 3.5 billion years old, and they are found in hydrothermal deposits, and it is often hypothesized that life originated there. However, hydrothermal systems with a fairly strong flow of chemical components are not the optimal place for the prebiological self-assembly of biomolecules and for the emergence of homochirality. This article examines the possibility that the self-assembly of homochiral molecules took place in an aqueous environment in the Earth’s crust. Based on the latest literature regarding the conditions in the lithosphere, there are several factors that point to the fact that the crust could be the location for the prebiological self-assembly of biomolecules, and there is nothing against it. The crust and the mantle contain a substantial amount of water, and at the time prior to the emergence of life, the crust most likely contained the necessary chemical substances for the synthesis of biomolecules and an aqueous environment where homochirality could be established.
1. Introduction
Theories about the origin of life often deal with the emergence of homochiral peptides of L-amino acids and homochiral oligomers of D-carbohydrates. However, not only are the chiral amino acids unstable and have a tendency to racemize [1], it is the peptides also [2,3]. The homochirality in the proteins is vital for the survival of an organism, and the racemization of Aspartic acid in endospores [4] and hyperthermophiles [5,6] limits the long-time survivability of microorganisms. Therefore, the fundamental question is not only how to get the homochiral peptides but also how to maintain the homochirality in the peptides over such a long time that self-assembly of the complex homochiral molecules in living systems can take place and a living organism can be created.
The racemization of amino acids ranges from a time span of thousands of years at low temperatures to days at 100 °C, and the racemization is enhanced in acidic as well as basic solutions [7]. Even if somewhere there was a pool of water with pH ≈ 7 of homochiral amino acid, a simple synthesis of homochiral peptides from an aqueous solution of homochiral amino acids can only happen if the synthesis of biomolecules with homochiral amino acids was completed within a few thousand years in cold water or within some days in a hot aqueous solution. However, since no one can imagine that it took so short a time to establish the prebiological environment with the synthesis of the complex biomolecules, one must search for environments with the right conditions where it is possible to obtain homochiral peptides from a racemic mixture of amino acids and maintain the environment for millions of years sufficient to ensure the self-assembly of the homochiral building blocks in the living organisms. This article focuses on the possibility of such an environment with the formation and stability of homochiral peptides in an aqueous solution. If it is possible to synthesize homochiral peptides from a racemic mixture of amino acids in an inorganic aqueous solution under the right conditions, this can explain the emergence of homochirality in peptides [8].
2. The Right Conditions
Most theories about the emergence of homochirality of the peptides take their starting point from the synthesis of peptides from homochiral amino acids. However, this is, as mentioned, unrealistic, and furthermore, it is not necessary. Kinetic models indicate that one can obtain homochiral peptides from the spontaneous polymerization of racemic mixtures of amino acids [9,10,11,12,13]. The conditions for obtaining homochiral peptides from racemic compositions can be specified. There are thermodynamic conditions [14], but there are also other conditions, e.g., the chemistry at Abiogenesis and the location with the right thermodynamic and chemical environment.
2.1. The Right Location
All living systems consist of cells with an aqueous solution (cytosol) of ions and suspensions of biomolecules, and this simple fact makes some demands on the environment and the location where the self-assembly of the biomolecules appears [15]. One demand on the location of the environment is that it must have been an aqueous environment and that life must have arisen in water. Today, water on Earth is present in oceans, lakes, and many other locations in our biosphere, but the main part of water on Earth is present in the crust and mantle beneath the continents and the oceans [16].
Earth’s lithosphere constitutes the hard and rigid outer layer of the Earth and includes the crust and the uppermost mantle. The crust is the outermost solid layer of the Earth below the oceans and the continents. The estimates of the thickness of the crust vary from ≈10 km below the oceans to ≈70 km below the continents. The crust is separated from the upper part of the mantle by the “Moho discontinuity” by a distinct change in density. The temperature in the lithosphere increases with the distance from the Earth’s surface and reaches a temperature of more than a thousand degrees at ≈200 km [17]. The synthesis and the stability of peptides require, however, a significantly lower temperature [18,19]. The crust, mantle, and the “Hadean ocean” were presumably formed relative shortly after the Earth was created ≈4.56 billion years ago [20], and the Moon, which was formed shortly after the formation of the Earth, was much closer than it is today, resulting in very strong tide waves in the Hadean ocean [21,22,23] (The strong tide waves have accelerated the Moon out to its present position.) Today the water on Earth is present in the oceans, lakes, and many other locations in our biosphere, but the main part of water on Earth is present in the crust and mantle beneath the continents and the oceans. Part of the water in the crust and the mantle is bound as crystal water, HO(c), but an essential part is present as water HO(aq). The evidence for water in the crust and mantle is indirect and based on extensive seismic velocity measurements, electrical and thermal conductivity measurements, geodetic data, mineral physics, geochemical data, and modeling [16,24]. However, although there are indications of water in the crust and the mantle, there are no direct determinations of whether appears as capillary or bulk water. The review [16] gives an estimate of the total amount of water of 2.6–8.3 ocean masses ((c) + (aq)). The water in the crust and mantle is released in the hydrothermal vents, and the right place for prebiological self-assembly of organic molecules could very well be the somewhere in the Earth’s crust.
Support for this hypothesis is the fact that the oldest signs of life, which are at least 3.5 billion years old, appear as fossilized microorganisms (bacteria) found in hydrothermal vent precipitates [25,26,27,28]. There are also direct measurements of a biosphere in the upper part of the wet crust, which today contains bacteria [29,30]. Here we argue that the location for prebiological self-assembly of homochiral peptides is a wet crust in the Hadean Eon.
A relationship that supports the hypothesis is the chemical composition of the cytosol. The cytosol contains sodium and potassium ions, and the concentrations of potassium and sodium in living systems differs significantly from the corresponding composition in the oceans. The ion content in the cytosol points to a location in the crust for the self-assembly of biomolecules and not to the Hadean ocean with a composition of salt, which probably was similar to the composition in today’s oceans (ref. [24] (Chapter 10); [31]). The concentration of potassium 0.10 M, and the ratio between the concentration of potassium ions and sodium ions
in the cytosol in the biocells deviate very much from the corresponding concentration of potassium 0.01022 M and the ratio
in today’s oceans. The ratio is crucial for the membrane potential and the sodium-potassium pump in the cells, and a high concentration of potassium and the right ratio between sodium and potassium could be present somewhere in the crust and in mica sheets in the upper part of the mantle [32]. Another example in this context is the appearance of phosphor esters in biological systems, in the membranes, in RNA, in DNA, in glycolysis, and in many other biomolecules. However, whereas the concentration of phosphates in the oceans, in general, is low [33], phosphates are more common in the crust [34,35].
Capillaries are locations where it opposed to the oceans it is possible to obtain and maintain a relatively high concentration of amino acids. This is an important condition for the thermodynamics and kinetics at the prebiological bio-synthesis (see later). Many scientists have suggested that life originated in the hydrodynamic vents and serpentinites in the oceans [36,37] in accordance with the earliest sign of life on Earth. The environment in the hydrothermal vents and serpentinites above the wet crust and mantle is turbulent and has a rapid exchange of matter [38]. If the prebiological synthesis of the biomolecules appeared over a long period of time, it seems more likely that a confined water system in the crust with a small but constant input of the chemical reactants in the biosynthesis and with a slow synthesis of the biomolecules over a time period of many millions of years is the right location for the prebiological synthesis of the biomolecules.
This article examines the possibility that the self-assembly of homochiral molecules originated in an aqueous environment in the Earth’s crust.
2.2. The Thermodynamic Condition
A suspension of homochiral peptides in an aqueous environment is stable if the peptides are in structures with minimal free energy. The peptides are polymers of units of amino acids combined via amide bonds. The stability of peptides increases with temperature relative to hydrolysis reactions in aqueous solutions and becomes a facile process in hydrothermal systems deep in sedimentary basins [39,40,41]. A homochiral L-peptide in an aqueous ionic suspension is stable if there is sufficient “chiral discrimination”. The chiral discrimination for a homochiral L-peptide is given by the difference in Gibbs free energies of the peptide and the peptide with one (or more) D-configuration of its chiral units. This requirement can be specified as follows:
The increase in reaction enthalpy by a change in the chirality of one L-configuration to a D-configuration in the homochiral peptide in an aqueous ionic suspension shall exceed the decrease in the reaction energy by the gain in entropy. The excess in reaction enthalpy measured in units of 2.5 kJ/mol must be several units in order to ensure the stability of the homochiral conformation [14]. As mentioned, the Aspartic acid units in the proteins are unstable and racemizate with a consequence for the survivability of biosystems [4,6].
The structure of a homochiral peptide is primarily given by its secondary structure, first determined by Pauling [42]. Perhaps the figure of the -helix structure in Pauling et al.’s article best illustrates the conditions for thermodynamic stability of a homochiral peptide in an aqueous suspension. The clockwise (positive) -helix structure (to the left) in Figure 1 with amide units of L-amino acids was derived from spectroscopic data for bond lengths and angles at the planar peptide units, and with an intramolecular stabilizing hydrogen bond between a hydrogen atom at the substituted -N-H and an oxygen atom in a -C=O group in the helix. The helix is obtained for peptides with homochiral amino acids, by which the overall orientation changes with a constant angle at each peptide bond. The angle of the peptide bond is changed if an L configuration is changed to a D configuration, and the -helix structure is destroyed. This fact is confirmed by the observation that a chiral change of an L-structure to a D-structure in one chiral unit is associated with a loss of the secondary structure of the peptide and an increased concentration of water molecules in a peptide [43].
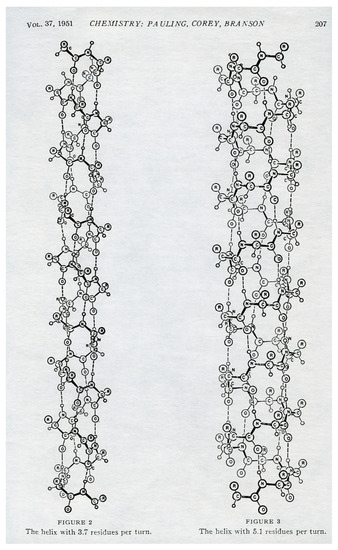
Figure 1.
The secondary -helix structure of a homochiral peptide chain [42]. The secondary structure is stabilized by (weak) hydrogen bonds. Each amide group is hydrogen bound to the third (or fifth) amide group beyond it along the helix. The -helix structure to the left of the figure is found in nature.
The effect of an ionic aqueous solution on the enthalpy of a homochiral peptide is not known, but one shall expect that the stability of the -helix structure is stabilized by increasing ionic activity a priori and thereby decrease the activity of the water molecules (Debye–Hückel theory). The energy of the -N-HO=C- hydrogen bond (≈10 kJ/mol) is only of the order of one-half of the energy of a hydrogen bond in pure water [44], and the stabilizing intramolecular hydrogen bond is in competition with hydrogen bonds to the water molecules in the solvent. The ions in the cytosol decrease the water activity and, thereby, the difference in energies between the intramolecular hydrogen bonds in the peptide and a hydrogen bond with water molecules in the cytosol. This fact is in agreement with the simulations of simple chain molecules in a solvent with different activities [8], which show that a decreasing activity of the solvent stabilizes the compact conformation of the polymer.
There is another thermodynamic requirement for a prebiological synthesis of peptides in a wet crust. The synthesis of the biomolecules in the aqueous environment requires, on the one hand, a certain temperature, and on the other hand, biomolecules that are unstable and decompose at high temperatures. However, only very little is known about the temperature in the crust and mantle in the Hadean Eon [24] (Chapter 10).
The thermodynamic requirement of the right place for maintaining peptides over long times in homochiral secondary (or higher order) structures is an aqueous solution with reduced water activity, given by the concentrations of ions in the water. A high electrolyte concentration, higher than in the cytosol, can be present in wet capillaries in the crust.
2.3. The Conditions of the Chemistry at the Abiogenesis
The conditions of the chemistry at the Abiogenesis can be specified: There are the conditions of the chemical reactions by which the homochiral peptides and carbohydrates are synthesized from racemic compositions, and there are the conditions of the chemical composition in the crust.
2.3.1. The Conditions of the Chemical Reactions: The Frank Model
The synthesis of homochiral polymers can be obtained by consecutive reactions, first described by C. F. Frank [45]. In 1953 Frank published a theoretical model for spontaneous asymmetric synthesis, and the emergence of homochirality at the polymerization of peptides is an example of such a synthesis. Since then, numerous articles with asymmetrical polymerization and chiral amplification have been published [9,10,11,12,13,46]. An important and necessary condition of the network of consecutive reactions is the inclusion of the racemization of the monomers. This is included in several of the reaction schemes in the articles [10,11,12,13], where the kinetic reactions for the racemization of the chiral monomers are associated with autocatalytic polymerization and the merging of polymers, and the reaction equations with homochiral peptides are solved for various values of reaction constants.
The asymmetric polymerization, obtained by Frank’s models, can lead to homochiral peptides, but with equal possibility for both enantiomers (Pauling’s polymers in Figure 1 are, in fact, for secondary structures of peptides with D-amino acid units [47]). The dominance of the L-conformations of peptides may be caused by the weak parity violating nuclear forces. This impact is, however, very small, and the dominance can also be obtained by a self-reinforcing dominance of homochiral domains [14,48].
The necessary chiral discrimination for a symmetry break and homochiral purification is indirectly given by the ratios of the reaction constants [48]. According to the thermodynamic condition in Section 2.2, important details on the reaction scheme for the consecutive polymerization reaction is the chiral discrimination, given by the hydrogen bonds in the secondary structure of the peptides in an ionic aqueous solution. The impact of these complicated effects of the conformation of the polymer in the ionic environment is, however, not included in the Frank models for homochirality obtained by polymerization, where the chiral discrimination from the reaction enthalpies is given by the ratios of the reaction constants.
The articles with chiral amplification are for polymerization with uniform (bulk) concentrations of the species [9,10,11,12,13,46]. An extension to more realistic reaction-diffusion Turing models with local concentrations [49] is, however, complicated, and moreover, the homochiral purification might have been obtained in capillaries in the crust. A reaction-diffusion Frank model for polymerization in a confined geometry is complicated and difficult to evaluate, but reaction-diffusion models for consecutive reactions in confined geometry have been developed and evaluated [50].
Chemical reactions in fluid phases are rather insensitive to pressure changes. The changes in the partial Gibbs free energies with respect to a pressure change are given by the partial volumes of the components in the solution, and the Gibbs free energy effects from a pressure change are generally small. The polymerization to homochiral peptides might lead to a slightly higher density in the suspension, so the increased pressure in the crust will enhance the polymerization rate, but it will not affect the symmetry break because homochiral suspensions with peptides with pure D- or L-units have the same density.
In summary: The kinetics with racemization kinetics of the amino acids and with polymerization to homochiral peptides can be described by a Frank model. The thermodynamic stability of the homochiral peptides is ensured by intra-molecular hydrogen bonds in the higher-order structures in competition with hydrogen bonds with the water molecules in the solvent, which destabilize the secondary structure. It is, however, difficult to take this effect into account in the Frank models.
2.3.2. The Chemical Composition
The chemical composition of the Earth’s crust and upper part of the mantle 4–4.5 billion years ago is not known, but today, the solid mantle consists mainly of Mg, Si, and Fe [51,52]. In determining the chemical composition of the crust and the mantle, one utilizes cosmochemical data based on the assumption that the sun, planets and meteorites all originated from the solar nebula. Chondritic meteorites are considered the most representative samples of solid nebular material [52]. Today, the crust may only contain a small amount of organic materials, such as amino acids [53] and carbohydrates [54], and 2.7 billion-year-old rocks contain acetate and formate [55].
The most interesting chondrites are the carbonaceous chondrites. Not only do they contain carbon but also organic molecules, and among these are amino acids and purine and pyrimidine nucleobases [56]. There exists many investigations into the chemical composition of the carbonaceous chondrites and the composition of amino acids [57,58]. Amino acids appear with a small excess of L-amino acids, which is to be expected since strong baryonic forces in the universe favor the L-configurations. However, these small excesses of chirality are, as mentioned, irrelevant to the origin of the homochirality of peptides in aqueous solutions because the amino acids racemizate rapidly. However, if a part of the crust or the mantle consisted of the same materials as the carbonaceous chondrites, some organic molecules might have survived the heating of the Earth at its formation, and the carbonaceous chondrite’s material might have been a source of amino acids at the polymerization of peptides in the crust. There are also investigations that make it probable that amides can be directly synthesized in thermodynamic conditions similar to the conditions in the crust [59,60,61].
Carbohydrates are found in carbonaceous chondrites [62], but they can also be synthesized from carbon dioxide and methane, two components that were also present in the nebular material at the formation of the Earth and our solar system [63]. The first step in the synthesis of carbohydrates is formaldehyde, CHO, which is synthesized from carbon dioxide and methane [64], and the succeeding spontaneous condensation of formaldehyde is the formose reaction, which was discovered in 1861 [65]. The formose reaction is well known and is believed to be the basic synthesis of bio-carbohydrates and related bio-organic molecules [66,67,68,69,70]. The condensation into carbohydrates is catalyzed by not only amino acids [71]; but also by natural aluminosilicates occurring in the mantle [51].
In summary, the early lithosphere most likely contained the basic chemical ingredients, carbon dioxide and methane and ammonia, for the synthesis of carbohydrates and peptides.
3. Conclusions
The oldest signs of cellular life on Earth are about 3.5 billion years old [25,27], and they are found in a volcanic-hydrothermal (hot springs, geysers) systems and in hydrothermal veins deposits; it is often hypothesized that life originated there. However, hydrothermal systems with a fairly strong flow of chemical components are not the optimal place for the prebiological self-assembly of biomolecules and for the emergence of homochirality. This article examines the possibility of the self-assembly of homochiral molecules that originated in an aqueous environment in the Earth’s crust.
Based on the latest literature regarding the conditions in the Earth’s crust and upper part of the mantle, there are several factors that point to the fact that the crust could be the location for the prebiological self-assembly of biomolecules, and there is nothing against it. The crust and the upper part of the mantle contain a substantial amount of water [16] and a substantial biomass and biodiversity [30], with an estimated number of (Prokaryotic) cells of the order 2 to 6 [72]. The crust and upper part of the mantle at the time prior to the emergence of life contained the necessary chemical substances for the synthesis of biomolecules, and an aqueous environment in the crust beneath hydrothermal locations is a more likely place for the prebiological synthesis of peptides and carbohydrates and for the establishment of homochirality.
A wet crust can generally be a suitable place for the biochemical evolution primordial stages. Here we have advocated for the Earth’s crust in the Hadean Eon as the most likely place, but it might as well have been on Mars. The requirement for a moderate temperature for the synthesis of stable biomolecules sets a limit to how soon after the emergence of the solar system, such a biosynthesis could take place, and this fact is in favor of Mars, which is a terrestrial planet similar to Earth. It was created at the same time as the Earth but cooled down before the Earth [73].
Funding
This work was supported by the VILLUM Foundation’s Matter project, grant No. 16515.
Acknowledgments
The anonymous but constructive referee reports are gratefully acknowledged.
Conflicts of Interest
The author declares no conflict of interest.
References
- Bada, J.L. Kinetics of razimization of amino acids as a function of pH. J. Am. Chem. Soc. 1972, 94, 1371–1373. [Google Scholar] [CrossRef]
- Aki, K.; Fujii, N.; Fujii, N. Kineticsof Isomerization and Inversion of Aspartate 58 of α A-Crystallin Peptide Mimics ubder Physilogical Conditions. PLoS ONE 2013, 8, e58515. [Google Scholar] [CrossRef]
- Fujii, N.; Takata, T.; Fujii, N.; Aki, K.; Sakaue, H. D-Amino acids in protein: The mirror of life as a molecular index of aging. Biochim. Biophys. Acta Proteins Proteom. 2018, 1866, 840–847. [Google Scholar] [CrossRef] [PubMed]
- Liang, R.; Lau, M.C.Y.; Baars, O.; Robb, F.T.; Onstott, C. Aspartic acid racemization constrains long-term viability and longevity of endospores. FEMS Microbiol. Ecol. 2019, 95, fiz132. [Google Scholar] [CrossRef]
- Onstott, T.C.; Magnabosco, C.; Aubrey, A.D.; Burton, A.S.; Dworkin, J.P.; Elsila, J.E.; Grunsfeld, S.; Cao, B.H.; Hein, J.E.; Glavin, D.P.; et al. Does aspartic acid racemization constrain the depth limit of the subsurface biosphere? Geobiology 2014, 12, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Liang, R.; Robb, F.T. Aspartic acid racemization and repair in the survival and recovery of hyperthermophiles after prolonged starvation at high temperatures. FEMS Microbiol. Ecol. 2019, 97, fiab112. [Google Scholar] [CrossRef]
- Bada, J.L. Racemization of Amino Acids. In Chemistry and Biochemistry of Amino Acids; Barrett, G.C., Ed.; Chapman and Hall: London, UK, 1985. [Google Scholar]
- Toxvaerd, S. The role of the peptides at the origin of life. J. Theor. Biol. 2017, 429, 164–169. [Google Scholar] [CrossRef] [PubMed]
- Brandenburg, A.; Andersen, A.C.; Höfner, S.; Nilsson, M. Homochiral growth through enantiomeric cross-inhibition. Orig. Life Evol. Biosph. 2005, 35, 225–241. [Google Scholar] [CrossRef] [PubMed]
- Ribó, J.M.; Crusats, J.; El-Hachemi, Z.; Moyano, A.; Hochberg, D. Spontaneous mirror symmetry breaking in heterocatalytically coupled enantioselectivve replicators. Chem. Sci. 2017, 8, 763. [Google Scholar] [CrossRef] [PubMed]
- Blanco, C.; Stich, M.; Hochberg, D. Mechanically Induced Homochirality in Nucleated Enantioselective Polymerization. J. Phys. Chem. B 2017, 121, 942–955. [Google Scholar] [CrossRef]
- Buhse, T.; Micheau, J.-C. Spontaneous Emergence of Transient Chirality in Closed, Reversible Frank-like Deterministic Models. Orig. Life Evol. Biosph. 2022, 52, 3–20. [Google Scholar] [CrossRef] [PubMed]
- Piñeros, W.D.; Tlusty, T. Spontaneous Chiral symmetry breaking in a random driven chemical system. Nat. Commun. 2022, 13, 2244. [Google Scholar] [CrossRef] [PubMed]
- Toxvaerd, S. Origin of Homochirality in Biosystems. Int. J. Mol. Sci. 2009, 10, 1290–1299. [Google Scholar] [CrossRef] [PubMed]
- Toxvaerd, S. A Prerequisity for life. Theor. Biol. 2019, 474, 48–51. [Google Scholar] [CrossRef]
- Ohtani, E. Hydration and Dehydration in Earth’s Interior. Ann. Rev. Earth Planet. Sci. 2021, 49, 253–278. [Google Scholar] [CrossRef]
- Jeanloz, R.; Morris, S. Temperature Distribution in the Crust and Mantle. Rev. Earth Planet. Sci. 1986, 14, 377–415. [Google Scholar] [CrossRef]
- Vogt, G.; Woell, S.; Argos, P. Protein Thermal Stability, Hydrogen Bonds, and Ion Pairs. J. Mol. Biol. 1997, 269, 631–643. [Google Scholar] [CrossRef]
- Shao, Q.; Gao, Y.Q. Temperature Dependence of Hydrogen-Bond Stability in β-Hairpin Structures. J. Chem. Theory Comput. 2010, 6, 3750–3760. [Google Scholar] [CrossRef]
- Miyazaki, Y. A wet heterogeneous mantle creates a habitable wold in the Hadean. Nature 2022, 625, 86. [Google Scholar] [CrossRef]
- Barboni, M.; Boehnke, P.; Keller, B.; Kohl, I.; Schoene, B.; Young, E.D.; McKeegan, K.D. Early formation of the Moon 4.51 billion years ago. Sci. Adv. 2017, 3, e1602365. [Google Scholar] [CrossRef]
- Thiemens, M.M.; Sprung, P.; Fonseca, R.O.C.; Leitzke, F.P.; Münker, C. Early Moon formation inferred from hafnium-tungsten systematics. Nat. Geosci. 2019, 12, 696. [Google Scholar] [CrossRef]
- Green, J.A.M.; Huber, M.; Waltham, D.; Buzan, J.; Wells, M. Explicitly modelled deep-time tidal dissipation and its implication for Lunar history. Earth Planet. Sci. Lett. 2017, 461, 46. [Google Scholar] [CrossRef]
- Harrison, T.M. Hadean Earth; Springer: Cham, Switzerland, 2020; ISBN 978–3-030-46687-9. [Google Scholar]
- Djokic, T.; Van Kranendonk, M.J.; Campbell, K.A.; Walter, M.R.; Ward, C.R. Eaiest signs of life on land preserved in ca. 3.5 Ga hot spring deposits. Nat. Commun. 2017, 8, 15263. [Google Scholar] [CrossRef] [PubMed]
- Schopf, J.W.; Kitajima, K.; Spicuzza, M.J.; Kudryavtsev, A.B.; Valley, J.W. SIMS analyses of the oldest known assemblage of microfossils document their taxon-correlated carbon isotope compositions. Proc. Natl. Acad. Sci. USA 2018, 115, 53–58. [Google Scholar] [CrossRef] [PubMed]
- Cavalazzi, B.; Lemelle, L.; Simionovici, A.; Cady, S.L.; Russell, M.J.; Bailo, E.; Canteri, R.; Enrico, E.; Manceau, A.; Maris, A.; et al. Cellular remains in a ≈ 3.42-billion-year-old subseafloor hydrothermal environment. Sci. Adv. 2021, 7, eabf3963. [Google Scholar] [CrossRef]
- Dodd, M.S.; Papineau, D.; Grenne, T.; Slack, J.F.; Rittner, M.; Pirajno, F.; O’Neil, J.; Little, C.T. Evidence for early life in Earth’s oldest hydrothermal vent precipitates. Nature 2017, 543, 60–74. [Google Scholar] [CrossRef]
- Li, J.; Mara, P.; Schubotz, F.; Sylvan, J.B.; Burgaud, G.; Klein, F.; Beaudoin, D.; Wee, S.Y.; Dick, H.J.; Lott, S.; et al. Recycling and metabolic flexibility dictate life in the lower oceanic crust. Nature 2020, 579, 250–260. [Google Scholar] [CrossRef]
- Takamiya, H.; Kouduka, M.; Suzuki, Y. The Deep Rocky Biosphere: New Geomicrobiological Insight and Prospects. Front. Microbiol. 2021, 12, 785743. [Google Scholar] [CrossRef]
- Harrison, T.M. The Hadean Crust: Evidence from >4 Ga Zircons. Annu. Rev. Earth Planet. Sci. 2009, 37, 479–505. [Google Scholar] [CrossRef]
- Hansma, G.H. Potassium at the Origin of Life: Did Biology Emerge from Biotite in Micaceous Clay? Life 2022, 12, 301. [Google Scholar] [CrossRef]
- Paytan, A.; McLaughlin, K. The Oceanic Phosphorus Cycle. Chem. Rev. 2007, 107, 563–576. [Google Scholar] [CrossRef]
- Walton, C.R.; Shorttle, O.; Jenner, F.E.; Williams, H.M.; Golden, J.; Morrison, S.M.; Downs, R.T.; Zerkle, R.M.; Hazen, R.M.; Pasek, M. Phosphorus mineral evolution and prebiotic chemistry: From minerals to microbes. Earth Sci. Rev. 2021, 221, 103806. [Google Scholar] [CrossRef]
- Flores, E.; Martinez, E.; Rodriguez, L.E.; Weber, J.M.; Khodayari, A.; VanderVelde, D.G.; Barge, L.M. Effects of Amino Acids on Phosphata Adsorption Onto Iron (Oxy)hydroxide Minerals under Early Earth Conditions. ACS Earth Space Chem. 2021, 5, 1048–1057. [Google Scholar] [CrossRef]
- Cleaves, H.J.; Aubrey, A.D.; Bada, J.L. An Evaluation of the Critical Parameters for Abiotic Peptide Synthesis in Submarine Hydrothermal Systems. Orig. Life Evol. Biosph. 2009, 39, 109–126. [Google Scholar] [CrossRef] [PubMed]
- Branscomb, E.; Russell, M.J. Frankenstein or a Submarine Alkaline Vent: Who is Responsible for Abiogenesis? BioEssays 2018, 40, 1700182. [Google Scholar] [CrossRef]
- Moore, W.S.; Frankle, J.D.; Benitez-Nelson, C.R.; Früh-Green, G.L.; Lang, S.Q. Activities of 223Ra and 226Ra in Fluids From the Lost city Hydrothermal Field Require Short Fluid Residence Times. JGR Oceans 2021, 126, e2021JC017886. [Google Scholar] [CrossRef]
- Shock, E.L. Stability of peptides in high-temperature aqueous solutions. Geochim. Cosmochim. Acta 1992, 56, 3481–3491. [Google Scholar] [CrossRef]
- Takahagi, W.; Seo, K.; Shibuya, T.; Takano, Y.; Fujiishima, K.; Saitoh, M.; Shimamura, S.; Matsui, Y.; Tomita, M.; Takai, K. Peptide Synthesis under the Alkaline Hydrothermal Conditions on Enceladus. ACS Earth Space Chem. 2019, 3, 2559–2568. [Google Scholar] [CrossRef]
- Pedreira-Segade, U.; Hao, J.; Montagnac, G.; Cardon, H.; Daniel, I. Spontaneous Polymerization of Glycine under Hydrothermal Conditions. ACS Earth Space Chem. 2019, 3, 1669–1677. [Google Scholar] [CrossRef]
- Pauling, L.; Corey, R.B.; Branson, H.R. The structure of proteins: Two hydrogen-bonded helical configurations of the polypeptide chain. Proc. Natl. Acad. Sci. USA 1951, 37, 205–211. [Google Scholar] [CrossRef]
- Fujii, N.; Fijii, N.; Kida, M.; Kinouchi, T. Influence of Lβ-, Dα-Asp isomers of the Asp-76 residue of the properties of αA-crystallin 70–88 peptide. Amino Acids 2010, 39, 1393–1399. [Google Scholar] [CrossRef] [PubMed]
- Wendler, K.; Thar, J.; Zahn, S.; Kirchner, B. Estimating the Hydrogen Bond Energy. J. Phys. Chem. A 2010, 114, 9529–9536. [Google Scholar] [CrossRef] [PubMed]
- Frank, F.C. On spontaneous asymmetric Synthesis. Biochin. Biophys. Acta 1953, 11, 459–463. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Wang, Y.; Hamley, I.W.; Qi, W.; Su, R.; He, Z. Chiral self-assembly of peptides: Toward the design of supramolecular polymers with enhanced chemical and biological functions. Prog. Polym. Sci. 2021, 123, 101469. [Google Scholar] [CrossRef]
- Dunitz, J.D. Pauling’s left-handed α-helix. Angew. Chem. Int. Ed. 2001, 40, 4167–4173. [Google Scholar] [CrossRef]
- Toxvaerd, S. Molecular Dynamics simulations of isomerization kinetics in condensed fluids. Phys. Rev. Lett. 2000, 85, 4747. [Google Scholar] [CrossRef]
- Turing, A.M. The Chemical Basis of Morphogenesis. Phil. Trans. R. Soc. 1952, B237, 37–72. [Google Scholar] [CrossRef]
- Hunding, A.; Kauffman, S.A.; Goodwin, B.C. Drosophila Segmentation: Supercomputer Simulation of Prepattern Hierarchy. J. Theor. Biol. 1990, 145, 369–384. [Google Scholar] [CrossRef]
- Anderson, D.L. Chemical Composition of the Mantle. J. Geophys. Res. 1983, 88, B41–B52. [Google Scholar] [CrossRef]
- Lyubetskaya, T.; Korenaga, J. Chemical composition of Earth’s primitive mantle and its variance: 1. Method and results. J. Geophys. Res. 2007, 112, B03211. [Google Scholar] [CrossRef]
- Ménez, B.; Pisapia, C.; Jamme, F.; Vanbellingen, Q.; Brunell, A.; Richard, L.; Dumas, P.; Réfreégies, M. Abiotic synthesis of amino acids in the recesses of the oceanic lithosphere. Nature 2018, 564, 59–63. [Google Scholar] [CrossRef] [PubMed]
- Sforna, M.C.; Brunelli, D.; Pisapia, C.; Pasini, V.; Malferrari, D.; Ménez, B. Abiotic formation of condensed carbonaceous matter in the hydrating oceanic crust. Nat. Commun. 2018, 9, 5049. [Google Scholar] [CrossRef] [PubMed]
- Lollar, B.; Heuer, V.B.; McDermott, J.; Tille, S.; Warr, O.; Moran, J.J.; Telling, J.; Hinrichs, K.-U. A window into the abiotic carbon cycle- Acetate and formate in fracture waters in 2.7 billion year-old rocks of the Canadian Shield. Geochim. Cosmochim. Acta 2021, 294, 295–314. [Google Scholar] [CrossRef]
- Oba, Y.; Takano, Y.; Furukawa, Y.; Koga, T.; Glavin, D.P.; Dwokin, J.P.; Naraoka, H. Identifying the wide diversity of extraterrestrial purine and pyrimidine nucleobases in carbonaceous meteorites. Nat. Commun. 2022, 13, 2008. [Google Scholar] [CrossRef] [PubMed]
- Aponte, J.C.; Elsila, J.E.; Hein, J.E.; Dworkin, J.P.; Glavin, D.P.; McLain, H.L.; Parker, E.T.; Cao, T.; Berger, E.L.; Burton, A.S. Analysis of amino acids, hydroxy acids, and amine in CR condrites. Meteor. Planet. Sci. 2020, 55, 2422–2439. [Google Scholar] [CrossRef]
- Glavin, D.P.; Burton, A.S.; Elsila, J.E.; Aponte, J.C.; Dwokin, J.P. The Search for Chiral Asymmetry as a Potential Biosignature in our Solar Systsem. Chem. Rev. 2020, 120, 4660–4689. [Google Scholar] [CrossRef]
- Robinson, K.J.; Bockisch, C.; Gould, I.R.; Liao, Y.; Yang, Z.; Glein, C.R.; Shaver, G.D.; Hartnett, H.E.; Williams, L.B.; Scock, E.L. Quantifying the extent of amide and peptide bond synthesis across conditions relevant to gologic and planetary environment. Geochim. Cosmochim. Acta 2021, 300, 318–332. [Google Scholar] [CrossRef]
- Fu, X.; Liao, Y.; Glein, C.R.; Jamison, M.; Hayes, K.; Zaporski, J.; Yang, Z. Direct Synthesis of Adides from Amines and Carboxylic Acids under Hydrothermal Conditions. ACS Earth Space Chem. 2020, 4, 722–729. [Google Scholar] [CrossRef]
- Yang, B.; Niu, K.; Haag, F.; Cao, N.; Zhang, J.; Zhang, H.; Li, Q.; Allegretti, F.; Björk, J.; Barth, J.V.; et al. Abiotic Formation of an Amide Bond via Surface-Supported Direct Carboxyl-Amine Coupling. Angew. Chem. Int. Ed. 2022, 61, e202113590. [Google Scholar]
- Furukawa, Y.; Chikaraishi, Y.; Ohkouchi, N.; Ogawa, N.O.; Glavin, D.P.; Dworkin, J.P.; Abe, C.; Nakamura, T. Extraterrestrial ribose and other sugars in primitive meteorites. Proc. Natl. Acad. Sci. USA 2019, 116, 24440–24445. [Google Scholar] [CrossRef]
- Guzmań-Marmolejo, A.; Segura, A. Methane in the Solar System. Bol. Soc. Geol. Mex. 2015, 67, 377–385. [Google Scholar] [CrossRef]
- Schlesinger, G.; Miller, S.L. Prebiotic synthesis in atmospheres containing CH4, CO, and CO2. J. Mol. Evol. 1983, 19, 383–390. [Google Scholar] [CrossRef] [PubMed]
- Butlerow, A. Formation synthetique d’une substance sucree. Compt. Rend. Acad. Sci. 1861, 53, 145–147. [Google Scholar]
- Gabel, N.W.; Ponnamperuma, C. Model for Origin of Monosaccharides. Nature 1967, 216, 453–455. [Google Scholar] [CrossRef]
- Washington, J. The possible Role of Volcanic Aquifers in Prebiologic Genesis of Organic Compounds and RNA. Orig. Life Evol. Biosph. 2000, 30, 53–79. [Google Scholar] [CrossRef]
- Kim, H.-J.; Ricardo, A.; Illangkoon, H.I.; Kim, M.J.; Carrigan, M.A.; Frye, F.; Benner, S.A. Synthesis of Carbohydrates in Mineral-Guided Prebiotic Cycles. J. Am. Chem. Soc. 2011, 133, 9457–9468. [Google Scholar] [CrossRef]
- Jalbout, A.F.; Abrell, L.; Adamowicz, L.; Polt, R.; Apponi, A.J.; Ziurys, L.M. Sugar synthesis from a gas-phase formose reaction. Astrobiology 2007, 7, 433–442. [Google Scholar] [CrossRef] [PubMed]
- Lambert, J.B.; Gurusamy-Thangavelu, S.A.; Ma, K. The silica-mediated formose reaction: Bottom-up synthesis of sugar silicates. Science 2010, 327, 984–986. [Google Scholar] [CrossRef]
- Weber, A.L. The Sugar Model: Catalysis by Amines and Amino Acid Products. Orig. Life Evol. Biosph. 2001, 31, 71–86. [Google Scholar] [CrossRef]
- Magnabosco, C.; Lin, L.-H.; Dong, H.; Bomberg, M.; Ghiorse, W.; Stan-Lotter, H.; Pedersen, K.; Kieft, T.L.; van Heeden, E.; Onstott, T.C. The biomass and biodiversity of the continental subsurface. Nat. Geosci. 2018, 11, 707–717. [Google Scholar] [CrossRef]
- Plesa, A.-C.; Padovan, S.; Tosi, N.; Breuer, D.; Grott, M.; Wieczorek, M.A.; Spohn, T.; Smrekar, S.E.; Banerdt, W.B. The Thermal State and Interior Structure of Mars. Geophys. Res. Lett. 2018, 45, 12198–12209. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).