Catalytic Pyrolysis of Plastic Waste and Molecular Symmetry Effects: A Review
Abstract
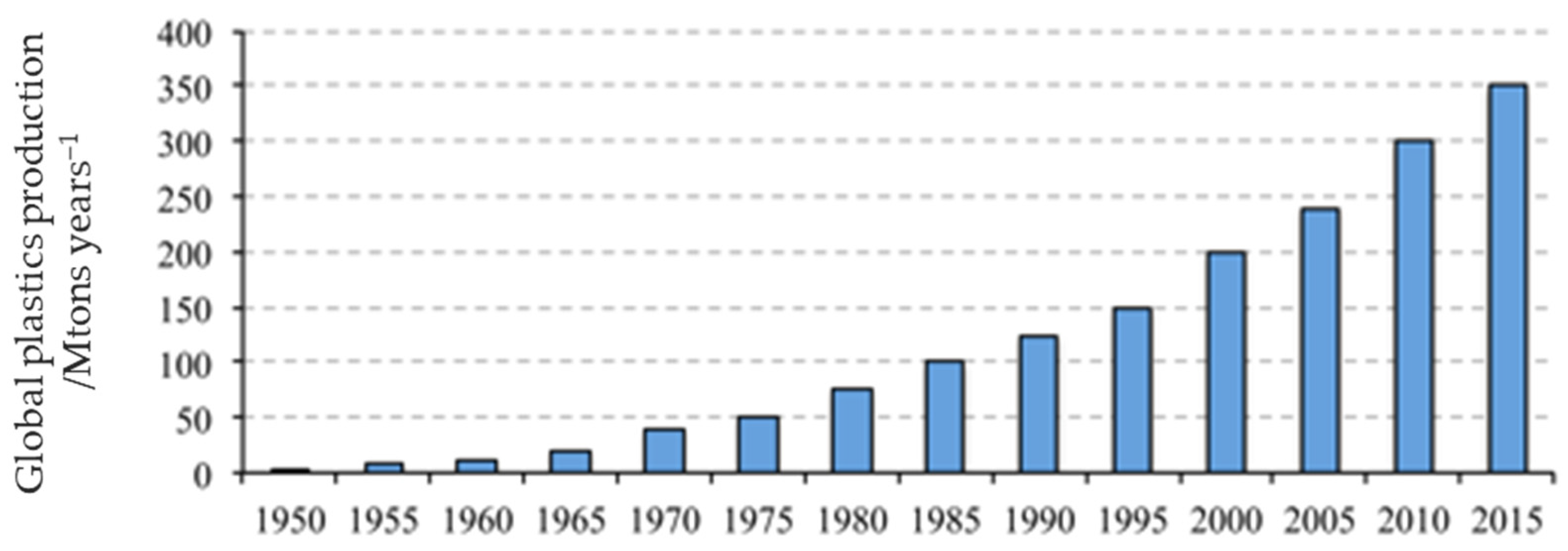
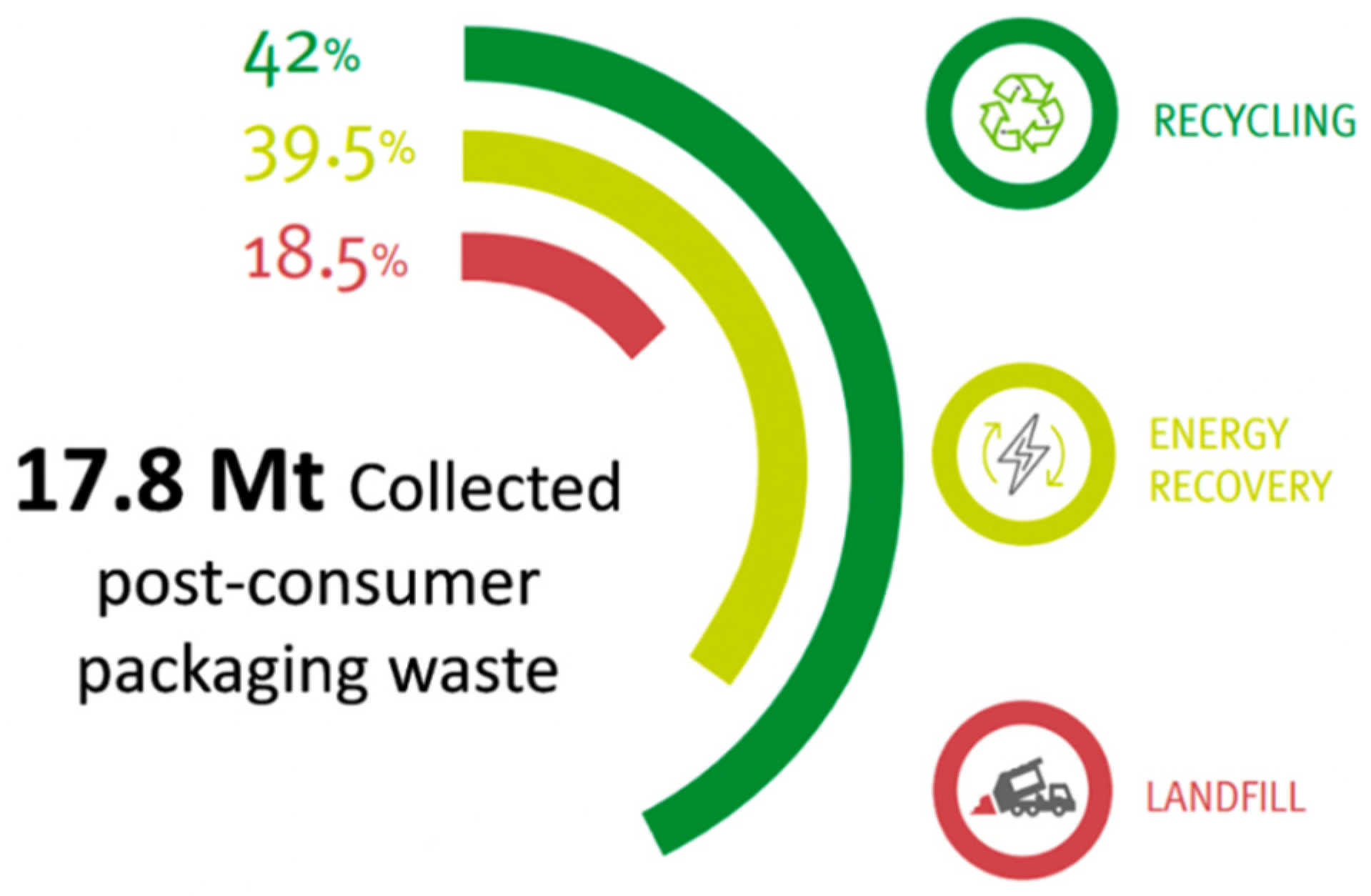
:1. Introduction
2. Thermal Pyrolysis
3. Catalytic Pyrolysis
3.1. Catalyst Structure and the Pyrolysis Mechanism
3.2. Application of Zeolites for the Catalytic Pyrolysis of Plastic
3.3. Application of Natural Catalysts for Plastic Pyrolysis
3.4. Application of Other Types of Catalysts for the Catalytic Pyrolysis of Plastic
4. Comparison between Thermal and Catalytic Pyrolysis
5. Molecular Symmetry Effects in Pyrolysis Processes
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Miandad, R.; Barakat, M.A.; Aburiazaiza, A.S.; Rehan, M.; Nizami, A.S. Catalytic pyrolysis of plastic waste: A review. Process Saf. Environ. Prot. 2016, 102, 822–838. [Google Scholar] [CrossRef]
- Ritchie, H.; Roser, M. OurWorldInData.org. Available online: https://ourworldindata.org/plastic-pollution (accessed on 18 December 2022).
- Selpiana; Aprianti, T.; Rayosa, I.; Fuspitasarie, D. Expanded polystyrene and multilayer plastic waste conversion into liquid fuel by the pyrolysis process. In AIP Conference Proceedings; AIP Publishing LLC: Surakarta, Indonesia, 2018; p. 020151. [Google Scholar] [CrossRef]
- Ma, C.; Yu, J.; Wang, B.; Song, Z.; Xiang, J.; Hu, S.; Su, S.; Sun, L. Catalytic pyrolysis of flame retarded high impact polystyrene over various solid acid catalysts. Fuel Process. Technol. 2017, 155, 32–41. [Google Scholar] [CrossRef]
- Rhodes, C.J. Plastic Pollution and Potential Solutions. Sci. Prog. 2018, 101, 207–260. [Google Scholar] [CrossRef] [PubMed]
- Paula, T.P.; Marques, M.D.F.V.; Marques, M.R.D.C.; Oliveira, M.S.; Monteiro, S.N. Thermal and Catalytic Pyrolysis of Urban Plastic Waste: Modified Mordenite and ZSM-5 Zeolites. Chemistry 2022, 4, 297–315. [Google Scholar] [CrossRef]
- Zhang, Y.; Ji, G.; Chen, C.; Wang, Y.; Wang, W.; Li, A. Liquid oils produced from pyrolysis of plastic wastes with heat carrier in rotary kiln. Fuel Process. Technol. 2020, 206, 106455. [Google Scholar] [CrossRef]
- Pinto, F.; Costa, P.; Gulyurtlu, I.; Cabrita, I. Pyrolysis of plastic wastes 2. Effect of catalyst on product yield. J. Anal. Appl. Pyrolysis 1999, 51, 57–71. [Google Scholar] [CrossRef]
- Semilu Rosesar, J.; Andari Kristanto, G. Household solid waste composition and characterization in Indonesia Urban Kampong. In IOP Conference Series: Materials Science and Engineering, Proceedings of the International Conference on Advanced Mechanical and Industrial engineering, Banten, Indonesia, 8–9 July 2020; IOP Publishing: Bristol, UK, 2020; Volume 909, p. 012077. [Google Scholar] [CrossRef]
- Li, P.; Wang, X.; Su, M.; Zou, X.; Duan, L.; Zhang, H. Characteristics of Plastic Pollution in the Environment: A Review. Bull. Environ. Contam. Toxicol. 2021, 107, 577–584. [Google Scholar] [CrossRef]
- Almeida, D.; Marques, M.D.F. Thermal and catalytic pyrolysis of plastic waste. Polímeros 2016, 26, 44–51. [Google Scholar] [CrossRef] [Green Version]
- Abbas-Abadi, M.S.; Haghighi, M.N.; Yeganeh, H. The effect of temperature, catalyst, different carrier gases and stirrer on the produced transportation hydrocarbons of LLDPE degradation in a stirred reactor. J. Anal. Appl. Pyrolysis 2012, 95, 198–204. [Google Scholar] [CrossRef]
- Kusenberg, M.; Eschenbacher, A.; Djokic, M.R.; Zayoud, A.; Ragaert, K.; De Meester, S.; Van Geem, K.M. Opportunities and challenges for the application of post-consumer plastic waste pyrolysis oils as steam cracker feedstocks: To decontaminate or not to decontaminate? Waste Manag. 2022, 138, 83–115. [Google Scholar] [CrossRef]
- Xue, Y.; Johnston, P.; Bai, X. Effect of catalyst contact mode and gas atmosphere during catalytic pyrolysis of waste plastics. Energy Convers. Manag. 2017, 142, 441–451. [Google Scholar] [CrossRef]
- Aishwarya, K.N.; Sindhu, N. Microwave Assisted Pyrolysis of Plastic Waste. Procedia Technol. 2016, 25, 990–997. [Google Scholar] [CrossRef] [Green Version]
- Marcilla, A.; Gómez-Siurana, A.; Berenguer, D. Study of the influence of the characteristics of different acid solids in the catalytic pyrolysis of different polymers. Appl. Catal. A Gen. 2006, 301, 222–231. [Google Scholar] [CrossRef]
- Oyedun, A.O.; Gebreegziabher, T.; Ng, D.K.S.; Hui, C.W. Mixed-waste pyrolysis of biomass and plastics waste—A modelling approach to reduce energy usage. Energy 2014, 75, 127–135. [Google Scholar] [CrossRef]
- Fivga, A.; Dimitriou, I. Pyrolysis of plastic waste for production of heavy fuel substitute: A techno-economic assessment. Energy 2018, 149, 865–874. [Google Scholar] [CrossRef]
- Anuar Sharuddin, S.D.; Abnisa, F.; Wan Daud, W.M.A.; Aroua, M.K. A review on pyrolysis of plastic wastes. Energy Convers. Manag. 2016, 115, 308–326. [Google Scholar] [CrossRef]
- Swamardika, I.B.A.; Winaya, I.N.S.; Hartati, R.S. Utilization plastic waste using pyrolysis fixed bed. In IOP Conference Series: Materials Science and Engineering, Proceedings of the International Conference on Design, Energy, Materials and Manufacture, Bali, Indonesia, 24–25 October 2018; IOP Publishing: Bristol, UK, 2020; Volume 539, p. 012021. [Google Scholar] [CrossRef]
- Abdy, C.; Zhang, Y.; Wang, J.; Yang, Y.; Artamendi, I.; Allen, B. Pyrolysis of polyolefin plastic waste and potential applications in asphalt road construction: A technical review. Resour. Conserv. Recycl. 2022, 180, 106213. [Google Scholar] [CrossRef]
- Oliveux, G.; Dandy, L.O.; Leeke, G.A. Current status of recycling of fibre reinforced polymers: Review of technologies, reuse and resulting properties. Prog. Mater. Sci. 2015, 72, 61–99. [Google Scholar] [CrossRef] [Green Version]
- Maqsood, T.; Dai, J.; Zhang, Y.; Guang, M.; Li, B. Pyrolysis of plastic species: A review of resources and products. J. Anal. Appl. Pyrolysis 2021, 159, 105295. [Google Scholar] [CrossRef]
- Mirkarimi, S.M.R.; Bensaid, S.; Chiaramonti, D. Conversion of mixed waste plastic into fuel for diesel engines through pyrolysis process: A review. Appl. Energy 2022, 327, 120040. [Google Scholar] [CrossRef]
- Panda, A.K.; Singh, R.K.; Mishra, D.K. Thermolysis of waste plastics to liquid fuelA suitable method for plastic waste management and manufacture of value added products—A world prospective. Renew. Sustain. Energy Rev. 2010, 14, 233–248. [Google Scholar] [CrossRef]
- Gvero, P.; Mujanić, I.; Papuga, S.; Vasković, S.; Anatunović, R. Review of Synthetic Fuels and New Materials Production Based on Pyrolysis Technologies. In Advances in Applications of Industrial Biomaterials; Pellicer, E., Nikolic, D., Sort, J., Baró, M., Zivic, F., Grujovic, N., Grujic, R., Pelemis, S., Eds.; Springer International Publishing: Cham, Switzerland, 2017; pp. 65–85. Available online: http://link.springer.com/10.1007/978-3-319-62767-0_4 (accessed on 18 December 2022).
- Kaminsky, W.; Zorriqueta, I.J.N. Catalytical and thermal pyrolysis of polyolefins. J. Anal. Appl. Pyrolysis 2007, 79, 368–374. [Google Scholar] [CrossRef]
- Buekens, A. Introduction to Feedstock Recycling of Plastics. In Feedstock Recycling and Pyrolysis of Waste Plastics; Scheirs, J., Kaminsky, W., Eds.; John Wiley & Sons, Ltd.: Chichester, UK, 2006; pp. 1–41. Available online: https://onlinelibrary.wiley.com/doi/10.1002/0470021543.ch1 (accessed on 4 October 2022).
- Gvero, P.; Papuga, S.; Mujanic, I.; Vaskovic, S. Pyrolysis as a key process in biomass combustion and thermochemical conversion. Therm. Sci. 2016, 20, 1209–1222. [Google Scholar] [CrossRef]
- Kalargaris, I.; Tian, G.; Gu, S. Combustion, performance and emission analysis of a DI diesel engine using plastic pyrolysis oil. Fuel Process. Technol. 2017, 157, 108–115. [Google Scholar] [CrossRef] [Green Version]
- Kyaw, K.T.; Hmwe, C.S.S. Effect of various catalysts on fuel oil pyrolysis process of mixed plastic wastes. Int. J. Adv. Eng. Technol. 2015, 8, 794–802. [Google Scholar]
- Dewangga, P.B.; Rochmadi; Purnomo, C.W. Pyrolysis of polystyrene plastic waste using bentonite catalyst. In IOP Conference Series: Earth and Environmental Science, Proceedings of the 1st International Seminar on Natural Resources and Environmental Management 2019, Bogor, Indonesia, 15 August 2019; IPB International Convention Center (IICC): Bogor, Indonesia, 2019; Volume 399, p. 012110. [Google Scholar] [CrossRef]
- Hoang, A.T.; Ong, H.C.; Fattah, I.M.R.; Chong, C.T.; Cheng, C.K.; Sakthivel, R.; Ok, Y.S. Progress on the lignocellulosic biomass pyrolysis for biofuel production toward environmental sustainability. Fuel Process. Technol. 2021, 223, 106997. [Google Scholar] [CrossRef]
- Gebre, S.H.; Sendeku, M.G.; Bahri, M. Recent Trends in the Pyrolysis of Non-Degradable Waste Plastics. Chemistryopen 2021, 10, 1202–1226. [Google Scholar] [CrossRef] [PubMed]
- Papuga, S.; Gvero, P.; Vukic, L. Temperature and time influence on the waste plastics pyrolysis in the fixed bed reactor. Therm. Sci. 2016, 20, 731–741. [Google Scholar] [CrossRef] [Green Version]
- Anene, A.; Fredriksen, S.; Sætre; Tokheim, L.A. Experimental Study of Thermal and Catalytic Pyrolysis of Plastic Waste Components. Sustainability 2018, 10, 3979. [Google Scholar] [CrossRef] [Green Version]
- Quesada, L.; Calero de Hoces, M.; Martín-Lara, M.A.; Luzón, G.; Blázquez, G. Performance of Different Catalysts for the In Situ Cracking of the Oil-Waxes Obtained by the Pyrolysis of Polyethylene Film Waste. Sustainability 2020, 12, 5482. [Google Scholar] [CrossRef]
- Hazrat, M.A.; Rasul, M.G.; Khan, M.M.K.; Azad, A.K.; Bhuiya, M.M.K. Utilization of Polymer Wastes as Transport Fuel Resources- a Recent Development. Energy Procedia 2014, 61, 1681–1685. [Google Scholar] [CrossRef] [Green Version]
- Panda, A.K. Thermo-catalytic degradation of different plastics to drop in liquid fuel using calcium bentonite catalyst. Int. J. Ind. Chem. 2018, 9, 167–176. [Google Scholar] [CrossRef] [Green Version]
- Fadillah, G.; Fatimah, I.; Sahroni, I.; Musawwa, M.M.; Mahlia, T.M.I.; Muraza, O. Recent Progress in Low-Cost Catalysts for Pyrolysis of Plastic Waste to Fuels. Catalysts 2021, 11, 837. [Google Scholar] [CrossRef]
- Kremer, I.; Tomić, T.; Katančić, Z.; Erceg, M.; Papuga, S.; Vuković, J.P.; Schneider, D.R. Catalytic pyrolysis of mechanically non-recyclable waste plastics mixture: Kinetics and pyrolysis in laboratory-scale reactor. J. Environ. Manag. 2021, 296, 113145. [Google Scholar] [CrossRef]
- Zhou, N.; Dai, L.; Lyu, Y.; Wang, Y.; Li, H.; Cobb, K.; Chen, P.; Lei, H.; Ruan, R. A structured catalyst of ZSM-5/SiC foam for chemical recycling of waste plastics via catalytic pyrolysis. Chem. Eng. J. 2022, 440, 135836. [Google Scholar] [CrossRef]
- Li, Q.; Faramarzi, A.; Zhang, S.; Wang, Y.; Hu, X.; Gholizadeh, M. Progress in catalytic pyrolysis of municipal solid waste. Energy Convers. Manag. 2020, 226, 113525. [Google Scholar] [CrossRef]
- Iribarren, D.; Dufour, J.; Serrano, D.P. Preliminary assessment of plastic waste valorization via sequential pyrolysis and catalytic reforming. J. Mater. Cycles Waste Manag. 2012, 14, 301–307. [Google Scholar] [CrossRef]
- Al-Salem, S.M. Feedstock and Optimal Operation for Plastics to Fuel Conversion in Pyrolysis. In Plastics to Energy; Elsevier: Amsterdam, The Netherlands, 2019; pp. 117–146. Available online: https://linkinghub.elsevier.com/retrieve/pii/B9780128131404000054 (accessed on 4 October 2022).
- Soni, V.K.; Singh, G.; Vijayan, B.K.; Chopra, A.; Kapur, G.S.; Ramakumar, S.S.V. Thermochemical Recycling of Waste Plastics by Pyrolysis: A Review. Energy Fuels 2021, 35, 12763–12808. [Google Scholar] [CrossRef]
- Yan, G.; Jing, X.; Wen, H.; Xiang, S. Thermal Cracking of Virgin and Waste Plastics of PP and LDPE in a Semibatch Reactor under Atmospheric Pressure. Energy Fuels 2015, 29, 2289–2298. [Google Scholar] [CrossRef]
- Adrados, A.; de Marco, I.; Caballero, B.M.; López, A.; Laresgoiti, M.F.; Torres, A. Pyrolysis of plastic packaging waste: A comparison of plastic residuals from material recovery facilities with simulated plastic waste. Waste Manag. 2012, 32, 826–832. [Google Scholar] [CrossRef]
- Esposito, L.; Cafiero, L.; De Angelis, D.; Tuffi, R.; Vecchio Ciprioti, S. Valorization of the plastic residue from a WEEE treatment plant by pyrolysis. Waste Manag. 2020, 112, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Demirbas, A. Pyrolysis of municipal plastic wastes for recovery of gasoline-range hydrocarbons. J. Anal. Appl. Pyrolysis 2004, 72, 97–102. [Google Scholar] [CrossRef]
- Sivagami, K.; Divyapriya, G.; Selvaraj, R.; Madhiyazhagan, P.; Sriram, N.; Nambi, I. Catalytic pyrolysis of polyolefin and multilayer packaging based waste plastics: A pilot scale study. Process Saf. Environ. Prot. 2021, 149, 497–506. [Google Scholar] [CrossRef]
- Cafiero, L.; Castoldi, E.; Tuffi, R.; Vecchio Ciprioti, S. Identification and characterization of plastics from small appliances and kinetic analysis of their thermally activated pyrolysis. Polym. Degrad. Stab. 2014, 109, 307–318. [Google Scholar] [CrossRef]
- Cafiero, L.; Fabbri, D.; Trinca, E.; Tuffi, R.; Vecchio Ciprioti, S. Thermal and spectroscopic (TG/DSC-FTIR) characterization of mixed plastics for materials and energy recovery under pyrolytic conditions. J. Therm. Anal. Calorim. 2015, 121, 1111–1119. [Google Scholar] [CrossRef]
- Budsaereechai, S.; Hunt, A.J.; Ngernyen, Y. Catalytic pyrolysis of plastic waste for the production of liquid fuels for engines. RSC Adv. 2019, 9, 5844–5857. [Google Scholar] [CrossRef] [Green Version]
- Ratnasari, D.K.; Nahil, M.A.; Williams, P.T. Catalytic pyrolysis of waste plastics using staged catalysis for production of gasoline range hydrocarbon oils. J. Anal. Appl. Pyrolysis 2017, 124, 631–637. [Google Scholar] [CrossRef]
- Kunwar, B.; Cheng, H.N.; Chandrashekaran, S.R.; Sharma, B.K. Plastics to fuel: A review. Renew. Sustain. Energy Rev. 2016, 54, 421–428. [Google Scholar] [CrossRef]
- Martín, A.J.; Mondelli, C.; Jaydev, S.D.; Pérez-Ramírez, J. Catalytic processing of plastic waste on the rise. Chem 2021, 7, 1487–1533. [Google Scholar] [CrossRef]
- Anantharaman, A.P.; Pillai, N.S. Heterogeneous Catalyst for Pyrolysis and Biodiesel Production. In Liquid Biofuels, 1st ed.; Shadangi, K.P., Ed.; Wiley: New York, NY, USA, 2021; pp. 77–118. Available online: https://onlinelibrary.wiley.com/doi/10.1002/9781119793038.ch3 (accessed on 30 September 2022).
- Yansaneh, O.Y.; Zein, S.H. Latest Advances in Waste Plastic Pyrolytic Catalysis. Processes 2022, 10, 683. [Google Scholar] [CrossRef]
- Al-Salem, S.M.; Antelava, A.; Constantinou, A.; Manos, G.; Dutta, A. A review on thermal and catalytic pyrolysis of plastic solid waste (PSW). J. Environ. Manag. 2017, 197, 177–198. [Google Scholar] [CrossRef] [PubMed]
- Armenise, S.; SyieLuing, W.; Ramírez-Velásquez, J.M.; Launay, F.; Wuebben, D.; Ngadi, N.; Rams, J.; Muñoz, M. Plastic waste recycling via pyrolysis: A bibliometric survey and literature review. J. Anal. Appl. Pyrolysis 2021, 158, 105265. [Google Scholar] [CrossRef]
- Daligaux, V.; Richard, R.; Manero, M.H. Deactivation and Regeneration of Zeolite Catalysts Used in Pyrolysis of Plastic Wastes—A Process and Analytical Review. Catalysts 2021, 11, 770. [Google Scholar] [CrossRef]
- Uthpalani, P.G.I.; Premachandra, J.K.; De Silva, D.S.M.; Weerasinghe, V.P.A. Pyrolysis as a Value Added Method for Plastic Waste Management: A Review on Converting LDPE and HDPE Waste into Fuel; Research Square: Durham, NC, USA, 2022; Available online: https://www.researchsquare.com/article/rs-1693804/v1 (accessed on 4 October 2022).
- Siddiqui, M.Z.; Park, Y.K.; Kang, Y.; Watanabe, A.; Kim, S.; Kim, Y.M. Effective use of aluminum-plastic laminate as a feedstock for catalytic pyrolysis over micro and mesoporous catalysts. J. Clean. Prod. 2019, 229, 1093–1101. [Google Scholar] [CrossRef]
- Mark, L.O.; Cendejas, M.C.; Hermans, I. The Use of Heterogeneous Catalysis in the Chemical Valorization of Plastic Waste. Chem. Sus. Chem. 2020, 13, 5808–5836. [Google Scholar] [CrossRef] [PubMed]
- Raveh-Amit, H.; Lemont, F.; Bar-Nes, G.; Klein-BenDavid, O.; Banano, N.; Gelfer, S.; Charvin, P.; Bin Rozaini, T.; Sedan, J.; Rousset, F. Catalytic Pyrolysis of High-Density Polyethylene: Decomposition Efficiency and Kinetics. Catalysts 2022, 12, 140. [Google Scholar] [CrossRef]
- Cai, N.; Li, X.; Xia, S.; Sun, L.; Hu, J.; Bartocci, P.; Fantozzi, F.; Williams, P.T.; Yang, H.; Chen, H. Pyrolysis-catalysis of different waste plastics over Fe/Al2O3 catalyst: High-value hydrogen, liquid fuels, carbon nanotubes and possible reaction mechanisms. Energy Convers. Manag. 2021, 229, 113794. [Google Scholar] [CrossRef]
- Colantonio, S.; Cafiero, L.; De Angelis, D.; Ippolito, N.M.; Tuffi, R.; Vecchio Ciprioti, S. Thermal and catalytic pyrolysis of a synthetic mixture representative of packaging plastics residue. Front. Chem. Sci. Eng. 2020, 14, 288–303. [Google Scholar] [CrossRef]
- Vouvoudi, E.C.; Achilias, D.S. Pyrolytic degradation of common polymers present in packaging materials. J. Therm. Anal. Calorim. 2019, 138, 2683–2689. [Google Scholar] [CrossRef]
- Santella, C.; Cafiero, L.; De Angelis, D.; La Marca, F.; Tuffi, R.; Vecchio Ciprioti, S. Thermal and catalytic pyrolysis of a mixture ofplastics from small waste electrical and electronic equipment (WEEE). Waste Manag. 2016, 54, 143–152. [Google Scholar] [CrossRef]
- Kremer, I.; Tomić, T.; Katančić, Z.; Erceg, M.; Papuga, S.; Vuković, J.P.; Schneider, D.R. Catalytic pyrolysis and kinetic study of real-world waste plastics: Multi-layered and mixed resin types of plastics. Clean Technol. Environ. Policy 2022, 24, 677–693. [Google Scholar] [CrossRef]
- Tuffi, R.; D’Abramo, S.; Cafiero, L.M.; Trinca, E.; Vecchio Ciprioti, S. Thermal behavior and pyrolytic degradation kinetics of polymeric mixtures from waste packaging plastics. Express Polym. Lett. 2018, 12, 82–99. [Google Scholar] [CrossRef]
- Araújo, S.A.; Araujo, A.S.; Fernandes, N.S.; Fernandes, V.J.; Ionashiro, M. Effect of the catalyst MCM-41 on the kinetic of the thermal decomposition of poly(ethylene terephthalate). J. Therm. Anal. Calorim. 2010, 99, 465–469. [Google Scholar] [CrossRef]
- Park, J.W.; Kim, J.H.; Seo, G. The effect of pore shape on the catalytic performance of zeolites in the liquid-phase degradation of HDPE. Polym. Degrad. Stab. 2002, 76, 495–501. [Google Scholar] [CrossRef]
- Borsella, E.; Aguado, R.; De Stefanis, A.; Olazar, M. Comparison of catalytic performance of an iron-alumina pillared montmorillonite and HZSM-5 zeolite on a spouted bed reactor. J. Anal. Appl. Pyrolysis 2018, 130, 320–331. [Google Scholar] [CrossRef]
- Garforth, A.A. Production of hydrocarbons by catalytic degradation of high density polyethylene in a laboratory fluidised-bed reactor. Appl. Catal. A Gen. 1998, 169, 331–342. [Google Scholar] [CrossRef]
- López, A.; de Marco, I.; Caballero, B.M.; Laresgoiti, M.F.; Adrados, A.; Aranzabal, A. Catalytic pyrolysis of plastic wastes with two different types of catalysts: ZSM-5 zeolite and Red Mud. Appl. Catal. B Environ. 2011, 104, 211–219. [Google Scholar] [CrossRef]
- Lee, S.Y.; Yoon, J.H.; Kim, J.R.; Park, D.W. Catalytic degradation of polystyrene over natural clinoptilolite zeolite. Polym. Degrad. Stabil. 2001, 74, 297–305. [Google Scholar] [CrossRef]
- Hwang, E.Y.; Choi, J.K.; Kim, D.H.; Park, D.W.; Woo, H.C. Catalytic degradation of polypropylene I. Screening of catalysts. Korean J. Chem. Eng. 1998, 15, 434–438. [Google Scholar] [CrossRef]
- Maryudi; Salamah, S.; Aktawan, A. Product distribution of pyrolysis of polystyrene foam waste using catalyst of natural zeolite and nickel/silica. In IOP Conference Series: Earth and Environmental Science, Proceedings of the International Conference on Industrial Technology for Sustainable Development (ICon-ITSD) 2017, Makassar, Indonesia, 25–26 October 2017; IOP Publishing: Bristol, UK, 2017; Volume 175, p. 012012. [Google Scholar] [CrossRef]
- Erawati, E.; Hamid, H.; Ilma, A.A. Pyrolysis Process of Mixed Polypropylene (PP) and High-Density Polyethylene (HDPE) Waste with Natural Zeolite as Catalyst. Molekul 2018, 13, 106. [Google Scholar] [CrossRef]
- Moorthy Rajendran, K.; Chintala, V.; Sharma, A.; Pal, S.; Pandey, J.K.; Ghodke, P. Review of catalyst materials in achieving the liquid hydrocarbon fuels from municipal mixed plastic waste (MMPW). Mater. Today Commun. 2020, 24, 100982. [Google Scholar] [CrossRef]
- Li, K.; Lei, J.; Yuan, G.; Weerachanchai, P.; Wang, J.Y.; Zhao, J.; Yang, Y. Fe-, Ti-, Zr- and Al-pillared clays for efficient catalytic pyrolysis of mixed plastics. Chem. Eng. J. 2017, 317, 800–809. [Google Scholar] [CrossRef]
- Auxilio, A.R.; Choo, W.L.; Kohli, I.; Chakravartula Srivatsa, S.; Bhattacharya, S. An experimental study on thermo-catalytic pyrolysis of plastic waste using a continuous pyrolyser. Waste Manag. 2017, 67, 143–154. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, L.T.T.; Poinern, G.E.J.; Le, H.T.; Nguyen, T.A.; Tran, C.M.; Jiang, Z. A LaFeO3 supported natural-clay-mineral catalyst for efficient pyrolysis of polypropylene plastic material. Asia-Pac. J. Chem. Eng. 2021, 16, e2695. [Google Scholar] [CrossRef]
- Maafa, I. Pyrolysis of Polystyrene Waste: A Review. Polymers 2021, 13, 225. [Google Scholar] [CrossRef] [PubMed]
- Miandad, R.; Rehan, M.; Barakat, M.A.; Aburiazaiza, A.S.; Khan, H.; Ismail, I.M.I.; Dhavamani, J.; Gardy, J.; Hassanpour, A.; Nizami, A.-S. Catalytic Pyrolysis of Plastic Waste: Moving Toward Pyrolysis Based Biorefineries. Front. Energy Res. 2019, 7, 27. [Google Scholar] [CrossRef]
- Cocchi, M.; Angelis, D.D.; Mazzeo, L.; Nardozi, P.; Piemonte, V.; Tuffi, R.; Ciprioti, S.V. Catalytic Pyrolysis of a Residual Plastic Waste Using Zeolites Produced by Coal Fly Ash. Catalysts 2020, 10, 1113. [Google Scholar] [CrossRef]
- Dai, L.; Zhou, N.; Lv, Y.; Cobb, K.; Cheng, Y.; Wang, Y.; Liu, Y.; Chen, P.; Zou, R.; Lei, H.; et al. Pyrolysis-catalysis for waste polyolefin conversion into low aromatic naphtha. Energy Convers. Manag. 2021, 245, 114578. [Google Scholar] [CrossRef]
- Utami, M.; Wijaya, K.; Trisunaryanti, W. Pt-promoted sulfated zirconia as catalyst for hydrocracking of LDPE plastic waste into liquid fuels. Mater. Chem. Phys. 2018, 213, 548–555. [Google Scholar] [CrossRef]
- Ahmad, I.; Khan, M.I.; Khan, H.; Ishaq, M.; Tariq, R.; Gul, K.; Ahmad, W. Pyrolysis Study of Polypropylene and Polyethylene Into Premium Oil Products. Int. J. Green Energy 2015, 12, 663–671. [Google Scholar] [CrossRef]
- Muhammad, C.; Onwudili, J.A.; Williams, P.T. Thermal Degradation of Real-World Waste Plastics and Simulated Mixed Plastics in a Two-Stage Pyrolysis–Catalysis Reactor for Fuel Production. Energy Fuels 2015, 29, 2601–2609. [Google Scholar] [CrossRef]
- Obeid, F.; Zeaiter, J.; Al-Muhtaseb, A.H.; Bouhadir, K. Thermo-catalytic pyrolysis of waste polyethylene bottles in a packed bed reactor with different bed materials and catalysts. Energy Convers. Manag. 2014, 85, 1–6. [Google Scholar] [CrossRef]
- Saeaung, K.; Phusunti, N.; Phetwarotai, W.; Assabumrungrat, S.; Cheirsilp, B. Catalytic pyrolysis of petroleum-based and biodegradable plastic waste to obtain high-value chemicals. Waste Manag. 2021, 127, 101–111. [Google Scholar] [CrossRef] [PubMed]
- Gulab, H.; Jan, M.R.; Shah, J.; Manos, G. Plastic catalytic pyrolysis to fuels as tertiary polymer recycling method: Effect of process conditions. J. Environ. Sci. Health Part A 2010, 45, 908–915. [Google Scholar] [CrossRef] [PubMed]
- Susastriawan, A.A.P.; Purnomo; Sandria, A. Experimental study the influence of zeolite size on low-temperature pyrolysis of low-density polyethylene plastic waste. Therm. Sci. Eng. Prog. 2020, 17, 100497. [Google Scholar] [CrossRef]
- Aisien, E.T.; Otuya, I.C.; Aisien, F.A. Thermal and catalytic pyrolysis of waste polypropylene plastic using spent FCC catalyst. Environ. Technol. Innov. 2021, 22, 101455. [Google Scholar] [CrossRef]
- Onwudili, J.A.; Muhammad, C.; Williams, P.T. Influence of catalyst bed temperature and properties of zeolite catalysts on pyrolysis-catalysis of a simulated mixed plastics sample for the production of upgraded fuels and chemicals. J. Energy Inst. 2019, 92, 1337–1347. [Google Scholar] [CrossRef]
- Serinyel, Z.; Dayma, G.; Glasziou, V.; Lailliau, M.; Dagaut, P. A pyrolysis study on C4–C8 symmetric ethers. Proc. Comb. Inst. 2021, 38, 329–336. [Google Scholar] [CrossRef]
- Kirss, R.U.; Brown, D.W.; Higa, K.T.; Gedridge, R.W. Pyrolysis pathways of symmetrical and unsymmetrical organotellurium(II) compounds. Organometallics 1991, 10, 3589–3596. [Google Scholar] [CrossRef]
- Erceg, M.; Krešić, I.; Vrandečić, N.S.; Jakić, M. Different approaches to the kinetic analysis of thermal degradation of poly(ethylene oxide). J. Therm. Anal. Calorim. 2018, 131, 325–334. [Google Scholar] [CrossRef]
- Eimontas, J.; Striūgas, N.; Abdelnaby, M.A.; Yousef, S. Catalytic pyrolysis kinetic behavior and TG-FTIR-GC–MS analysis of metallized food packaging plastics with different concentrations of ZSM-5 zeolite catalyst. Polymers 2021, 13, 702. [Google Scholar] [CrossRef] [PubMed]
- Tian, L.; Shen, B.; Xu, H.; Li, F.; Wang, Y.; Singh, S. Thermal behavior of waste tea pyrolysis by TG-FTIR analysis. Energy 2016, 103, 533–542. [Google Scholar] [CrossRef]
- Tsagkalias, I.S.; Vlachou, A.; Verros, G.D.; Achilias, D.S. Effect of Graphene oxide or Functionalized Graphene Oxide on the Copolymerization Kinetics of Styrene/n-butyl Methacrylate. Polymers 2019, 11, 999. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haas, C.H.; Ring, M.A. Reaction of silyl radical and silylene with acetylene and application of orbital symmetry to the pyrolysis of silane and disilane. Inorg. Chem. 1975, 14, 2253–2256. [Google Scholar] [CrossRef]
- Bagri., R.; Williams, P.T. Catalytic pyrolysis of polyethylene. J. Anal. Appl. Pyrolysis 2002, 63, 29–41. [Google Scholar] [CrossRef]
- Dwivedi, P.; Mishra, P.K.; Mondal, M.K.; Srivastava, N. Non-biodegradable polymeric waste pyrolysis for energy recovery. Heliyon 2019, 5, e02198. [Google Scholar] [CrossRef]
- Lopez, G.; Artetxe, M.; Amutio, M.; Bilbao, J.; Olazar, M. Thermochemical routes for the valorization of waste polyolefinic plastics to produce fuels and chemicals. A review. Renew. Sustain. Energy Rev. 2007, 73, 346–368. [Google Scholar] [CrossRef]
- Tomić, T.; Kremer, I.; Vecchio Ciprioti, S.; Schneider, D.R. Efficiency of municipal packaging waste recovery chain and suitability of separated residual waste fractions for use in alternative fuels production. J. Environ. Manag. 2022, 322, 116056. [Google Scholar] [CrossRef]
- Caldeira, V.P.S.; Santos, A.G.D.; Oliveira, D.S.; Lima, R.B.; Souza, L.D.; Pergher, S.B.C. Polyethylene catalytic cracking by thermogravimetric analysis: Effects of zeolitic properties and homogenization process. J. Therm. Anal. Calorim. 2017, 130, 1939–1951. [Google Scholar] [CrossRef]
- Kremer, I.; Tomić, T.; Katančić, Z.; Hrnjak-Murgić, Z.; Erceg, M.; Ciprioti, S.V.; Schneider, D.R. Effect of Zeolite Catalyst on the Pyrolysis Kinetics of Multi-Layered Plastic Food Packaging. Symmetry 2022, 14, 1362. [Google Scholar] [CrossRef]
- Zhang, Z.; Gora-Marek, K.; Watson, J.S.; Tian, J.; Ryder, M.R.; Tarach, K.A.; López-Pérez, L.; Martínez-Triguero, J.; Melián-Cabrera, I. Recovering waste plastics using shape-selective nano-scale reactors as catalysts. Nat. Sustain. 2019, 2, 39–42. [Google Scholar] [CrossRef]
- Aguado, J.; Serrano, D.P.; Escola, J.M. Fuels from Waste Plastics by Thermal and Catalytic Processes: A Review. Ind. Eng. Chem. Res. 2008, 47, 7982–7992. [Google Scholar] [CrossRef]
- Rezaei, P.S.; Oh, D.; Hong, Y.; Kim, Y.M.; Jae, J.; Jung, S.C.; Jeon, J.-K.; Park, Y.-K. In-situ catalytic co-pyrolysis of yellow poplar and high-density polyethylene over mesoporous catalysts. Energy Convers. Manag. 2017, 151, 116–122. [Google Scholar] [CrossRef]
- Santos, B.P.S.; Almeida, D.; Marques, M.D.F.V.; Henriques, C.A. Petrochemical feedstock from pyrolysis of waste polyethylene and polypropylene using different catalysts. Fuel 2018, 215, 515–521. [Google Scholar] [CrossRef]
- Constantinescu, M.; Bucura, F.; Ionete, E.I.; Ion-Ebrasu, D.; Sandru, C.; Zaharioiu, A.; Marin, F.; Miricioiu, M.; Niculescu, V.; Oancea, S.; et al. From Plastic to Fuel-New Challenges. Mater. Plast. 2019, 56, 721–729. [Google Scholar] [CrossRef]
- Aubert, M.; Nicolas, R.C.; Pawelec, W.; Wilén, C.-E.; Michael Roth, M.; Pfaendner, R. Azoalkanes—Novel flame retardants and their structure–property relationship. Polym. Adv. Technol. 2011, 22, 1529–1538. [Google Scholar] [CrossRef]
- Charitopoulou, M.A.; Stefanidis, S.D.; Lappas, A.A.; Achilias, D.S. Catalytic pyrolysis of polymers with brominated flame-retardants originating in waste electric and electronic equipment (WEEE) using various catalysts. Sustain. Chem. Pharm. 2022, 26, 100612. [Google Scholar] [CrossRef]
- Beccagutti, B.; Cafiero, L.; Pietrantonio, M.; Pucciarmati, S.; Tuffi, R.; Vecchio Ciprioti, S. Characterization of Some Real Mixed Plastics from WEEE: A Focus on Chlorine and Bromine Determination by Different Analytical Methods. Sustainability 2016, 8, 1107. [Google Scholar] [CrossRef]

| Thermal Pyrolysis | Catalytic Pyrolysis |
|---|---|
| Requires high temperatures | Lower reaction temperature |
| Resulting oils have low quality | Better conversion rate, with reduction in reaction time, temperature, and activation energy |
| Gases from the process can be used for different gas engines | Improved oil quality and better distribution of hydrocarbons |
| Production of a wide range of HC and solid residues | Smaller fractions of solid residue |
| Homogeneous or heterogeneous catalysts |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Papuga, S.; Djurdjevic, M.; Ciccioli, A.; Vecchio Ciprioti, S. Catalytic Pyrolysis of Plastic Waste and Molecular Symmetry Effects: A Review. Symmetry 2023, 15, 38. https://doi.org/10.3390/sym15010038
Papuga S, Djurdjevic M, Ciccioli A, Vecchio Ciprioti S. Catalytic Pyrolysis of Plastic Waste and Molecular Symmetry Effects: A Review. Symmetry. 2023; 15(1):38. https://doi.org/10.3390/sym15010038
Chicago/Turabian StylePapuga, Saša, Milica Djurdjevic, Andrea Ciccioli, and Stefano Vecchio Ciprioti. 2023. "Catalytic Pyrolysis of Plastic Waste and Molecular Symmetry Effects: A Review" Symmetry 15, no. 1: 38. https://doi.org/10.3390/sym15010038
APA StylePapuga, S., Djurdjevic, M., Ciccioli, A., & Vecchio Ciprioti, S. (2023). Catalytic Pyrolysis of Plastic Waste and Molecular Symmetry Effects: A Review. Symmetry, 15(1), 38. https://doi.org/10.3390/sym15010038








