Heterogeneous Catalytic and Non-Catalytic Supercritical Water Oxidation of Organic Pollutants in Industrial Wastewaters Effect of Operational Parameters
Abstract
:1. Introduction
2. Experimental
2.1. Materials
2.2. Synthesis of Catalysts
2.3. Catalyst Characterization
2.4. Catalyst’s Characteristics
2.5. SCWO Reactor
2.6. Chemical Oxygen Demand
3. Results and Discussions
3.1. Textural Characteristics of Catalysts
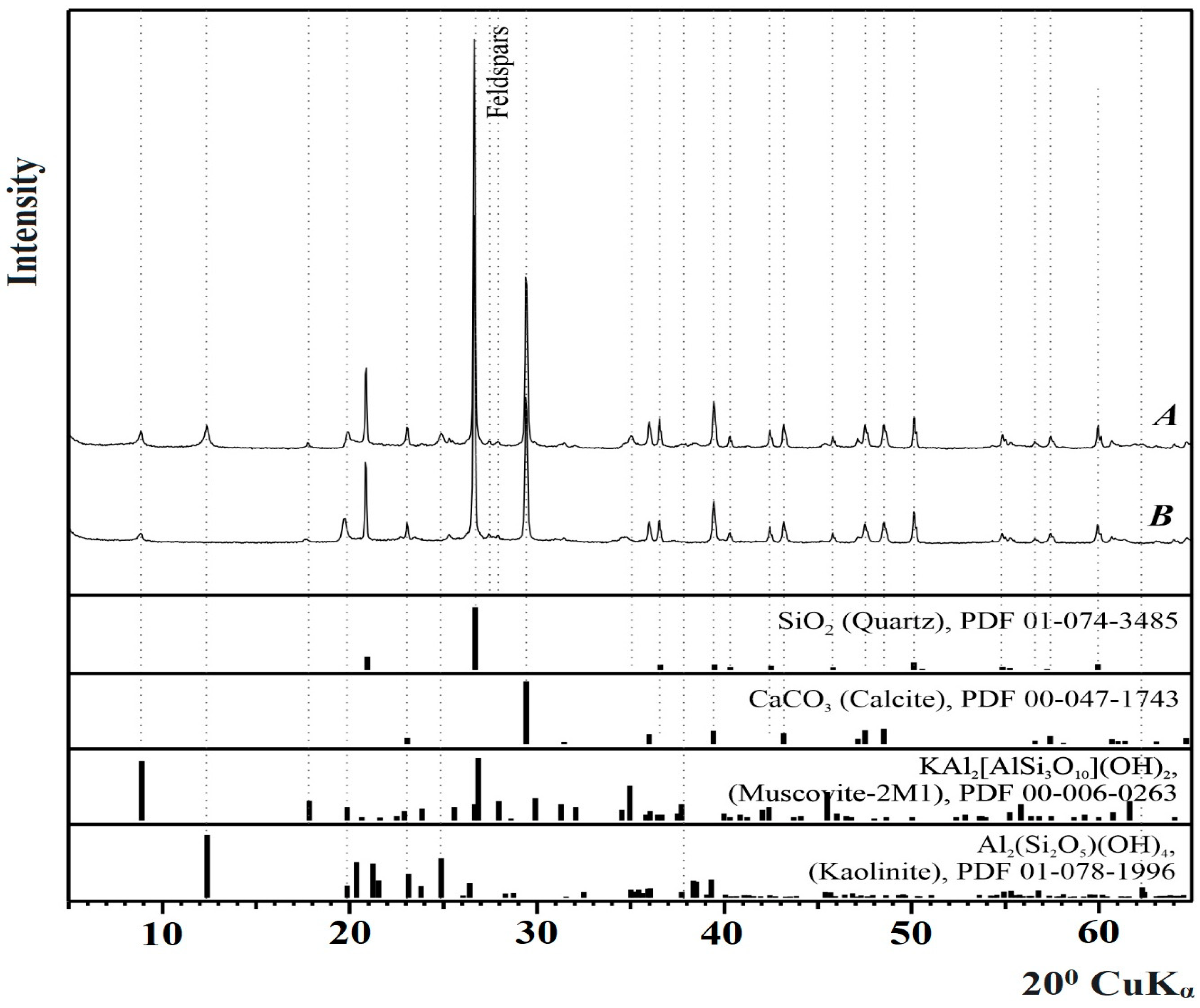
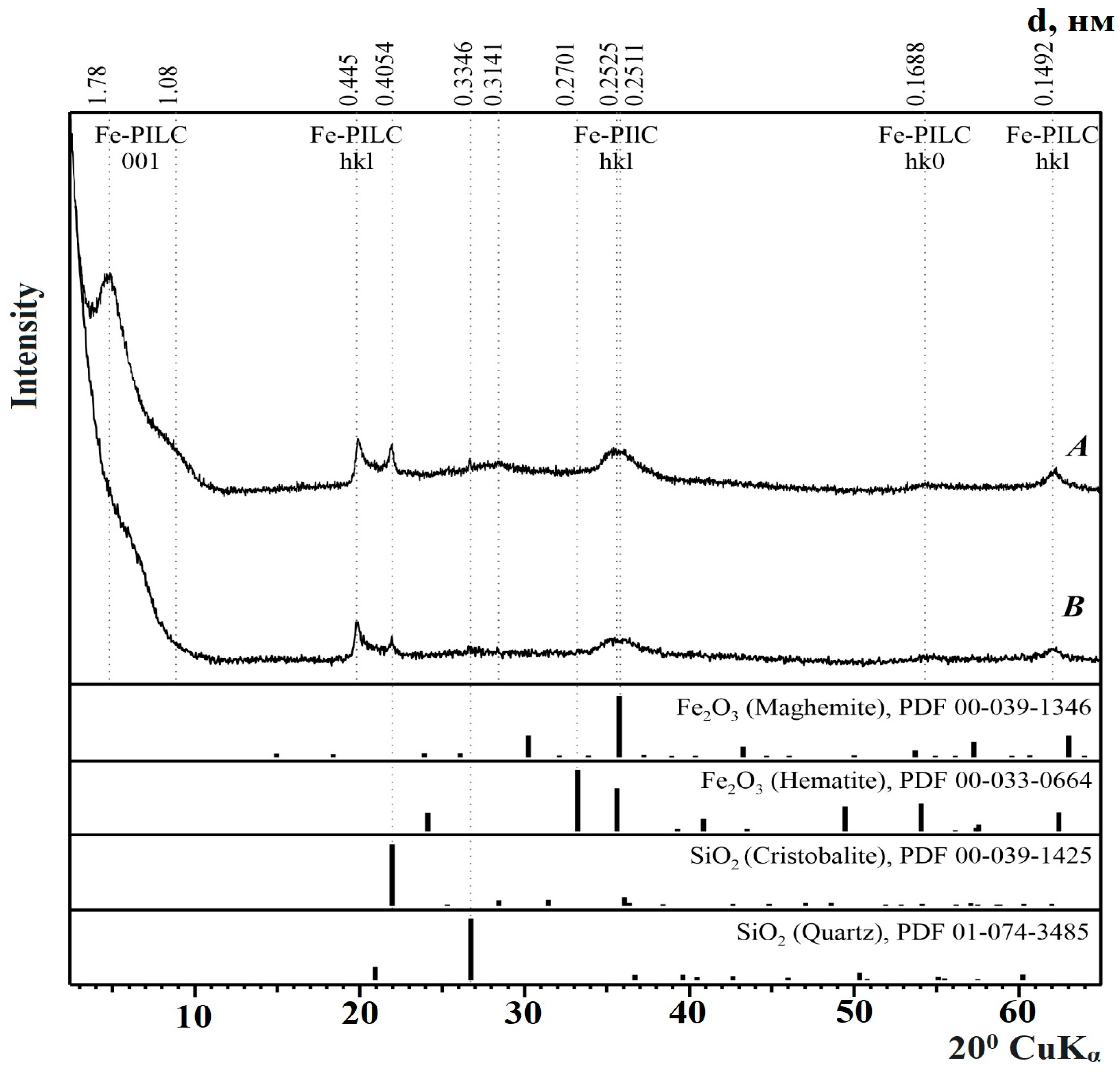
3.2. Phase Composition of Catalysts
3.3. The Results of Oxidation of the Industrial Wastewater
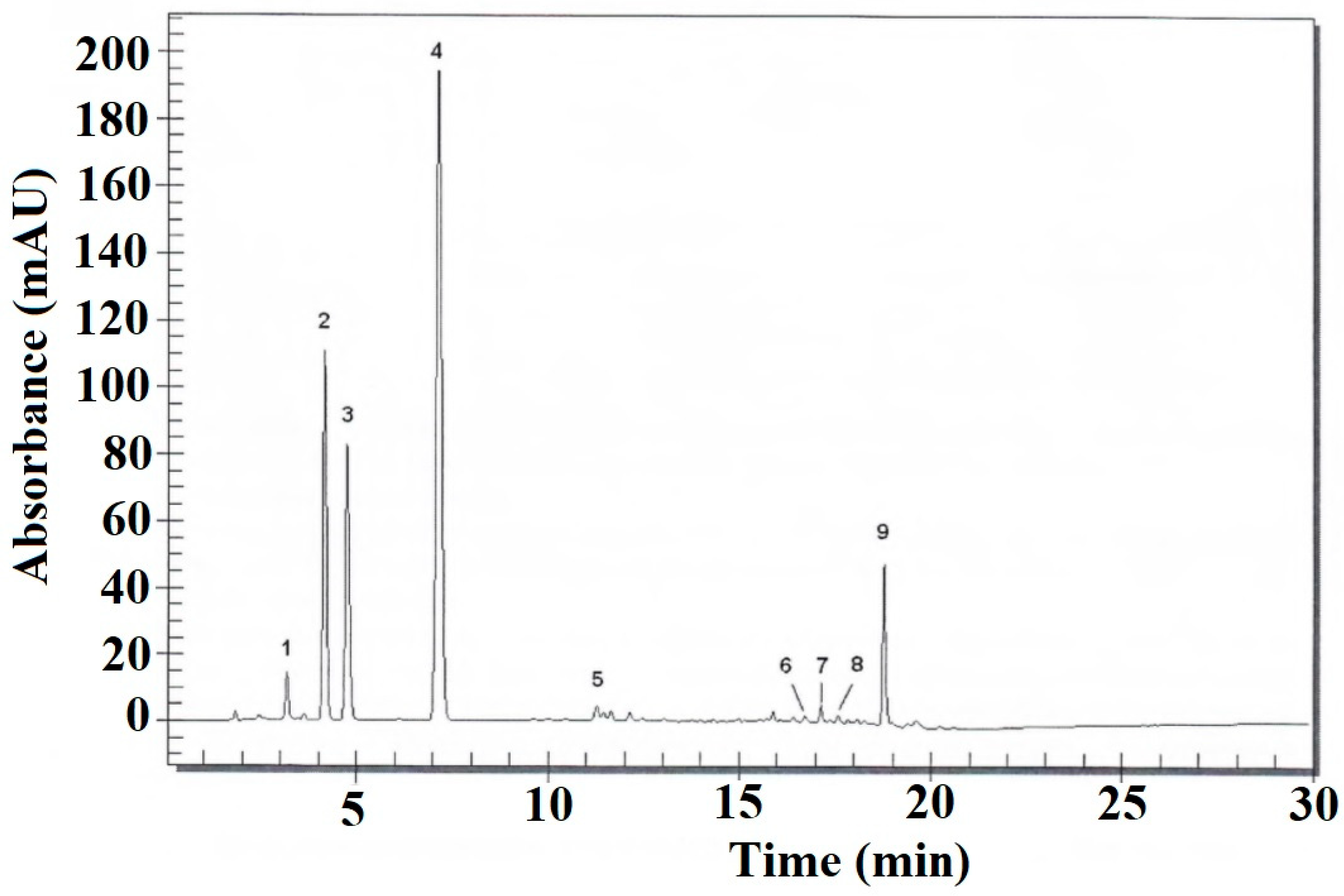
3.4. Chromatographic Analysis
3.5. Chemical Oxygen Demand and Conversion Coefficient
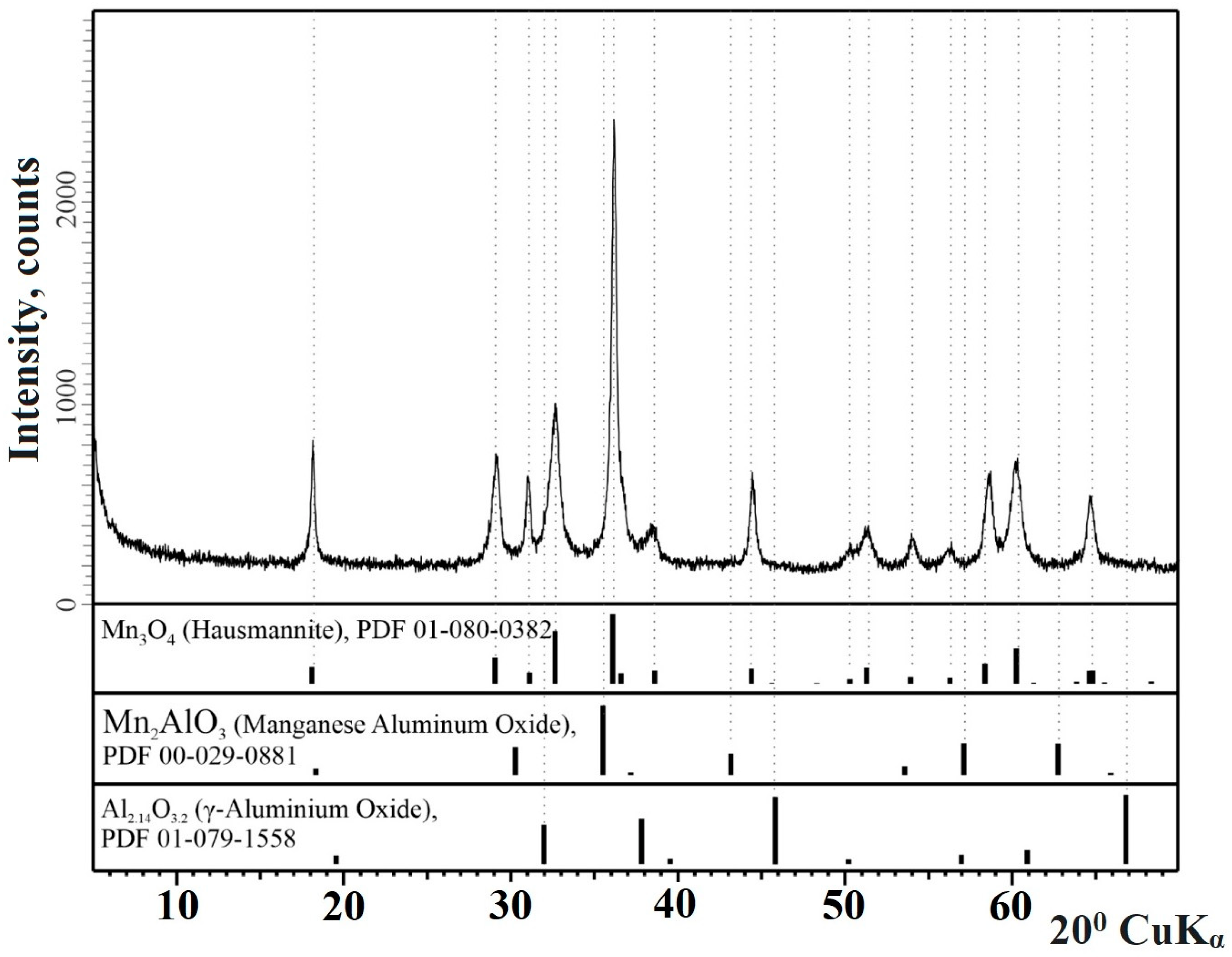
3.6. Phase Contents (Composition) of the Inorganic Residues
3.7. Kinetic of the Process of Deep Oxidation of Organic Compounds under SCF Conditions
- (1)
- The determination of the oxygen concentration in the condensed phase under experimental conditions is extremely difficult; therefore, as an assumption, we accept that this value does not depend on both the temperature and the composition of the system, despite the fact that both of these parameters affect the gas solubility in the case when the condensed phase is a liquid;
- (2)
- In the all-catalytic experiments, the mass of the loaded catalyst was the same.
4. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sun, Y.; Li, D.; Zhou, S.; Xiao, X. Research Progress of Advanced Oxidation Water Treatment Technology. Mater. Res. Forum LLC 2021, 102, 1–47. [Google Scholar] [CrossRef]
- Jiang, Z.; Li, Y.; Wang, S.; Cui, C.; Yang, C.; Li, J. Review on Mechanisms and Kinetics for Supercritical Water Oxidation Processes. Appl. Sci. 2020, 10, 4937. [Google Scholar] [CrossRef]
- Garcia-Segura, S.; Ocon, J.D.; Chong, M.N. Electrochemical oxidation remediation of real wastewater effluents—A review. Process Saf. Environ. Prot. 2018, 113, 48–67. [Google Scholar] [CrossRef]
- Miller, A.; Espanani, R.; Junker, A. Supercritical water oxidation of a model fecal sludge without the use of a co-fuel. Chemosphere 2015, 141, 189–196. [Google Scholar] [CrossRef] [Green Version]
- Wei, N.; Xu, D.; Hao, B. Chemical reactions of organic compounds in supercritical water gasification and oxidation. Water Res. 2021, 190, 116634. [Google Scholar] [CrossRef]
- Meireles de Souza, G.B.; Pereira, M.B.; Mourão, L.C.; dos Santos, M.P.; de Oliveira, J.A.; Aldaya Garde, I.A.; Alonso Ch, G.; Jegatheesan, V.; Cardozo-Filho, L. Supercritical water technology: An emerging treatment process for contaminated wastewaters and sludge. Rev. Environ. Sci. Bio/Technol. 2022, 21, 75–104. [Google Scholar] [CrossRef]
- Prasad Mylapilli, S.V.; Reddy, S.N. Sub and supercritical water oxidation of pharmaceutical wastewater. J. Environ. Chem. Eng. 2019, 7, 103165. [Google Scholar] [CrossRef]
- Javaid, R.; Qazi, U.Y.; Ikhlaq, A.; Zahid, M.; Alazmi, A. Subcritical and supercritical water oxidation for dye decomposition. J. Environ. Manag. 2021, 290, 112605. [Google Scholar] [CrossRef]
- Ren, J.; Yao, Z.; Wei, Q.; Wang, R.; Wang, L.; Liu, Y.; Ren, Z.; Guo, H.; Niu, Z.; Wang, J.; et al. Catalytic degradation of chloramphenicol by water falling film dielectric barrier discharge and FeO catalyst. Sep. Purif. Technol. 2022, 290, 120826. [Google Scholar] [CrossRef]
- Guoa, H.; Li, Z.; Zhang, Y.; Jiang, N.; Wang, H.; Li, J. Degradation of chloramphenicol by pulsed discharge plasma with heterogeneous Fenton process using Fe3O4 nanocomposites. Sep. Purif. Technol. 2020, 253, 117540. [Google Scholar] [CrossRef]
- Li, Y.; Wang, S.H. Supercritical Water Oxidation for Environmentally Friendly Treatment of Organic Wastes. In Advanced Supercritical Fluids Technologies; IntechOpen: London, UK, 2019; pp. 1–27. [Google Scholar] [CrossRef] [Green Version]
- Yang, J.; Wang Sh Li, Y.; Jiang, Z. Oxidation-sulfidation attacks on alloy 600 in supercritical water containing organic sulfides. Mater. Lett. 2020, 263, 127218. [Google Scholar] [CrossRef]
- Yao, G.; Chen Zh Chen, Q.; Li, D.; Xie Zh Zhou, Y.; Xiong, X.; Xu, Y. Behaviors of Organic and Heavy Metallic Pollutants during Supercritical Water Oxidation of Oil-Based Drill Cuttings. Water Air Soil Pollut. 2018, 229, 102. [Google Scholar] [CrossRef]
- Wang, Y. Metal-Based Nanocatalysts for Oxidative Degradation of Aqueous Organic Pollutants in Contaminated Water. Ph.D. Thesis, Curtin University, Perth, Australia, 2015. [Google Scholar]
- Mato, F.A.; Peña, M.; García-Rodríguez, Y.; Bermejo, M.-D.; Martín, Á. Analysis of the Energy Flow in a Municipal Wastewater Treatment Plant Based on a Supercritical Water Oxidation Reactor Coupled to a Gas Turbine. J. Processes 2021, 9, 1237. [Google Scholar] [CrossRef]
- Yan, Z.; Örmeci, B.; Han, Y.; Zhang, J. Supercritical water oxidation for treatment of wastewater sludge and recalcitrant organic contaminants. Environ. Technol. Innov. 2020, 18, 100728. [Google Scholar] [CrossRef]
- Al-Duri, B.; Alsoqyani, F. Supercritical water oxidation (SCWO) for the removal of N-containing heterocyclic hydrocarbon wastes. Part I: Process enhancement by addition of isopropyl alcohol. J. Supercrit. Fluids 2016, 116, 155–163. [Google Scholar] [CrossRef]
- Golmohammadi, M.; Ahmadi, S.J.; Towfighi, J. Catalytic supercritical water destructive oxidation of tributyl phosphate: Study on the effect of operational parameters. J. Supercrit. Fluids 2018, 40, 32–40. [Google Scholar] [CrossRef]
- Kayumov, R.A.; Sagdeev, A.A.; Gumerov, F.M. Utilization of the Molybdenum-Containing Wastes Using Supercritical Fluids; Publisher Brig: Nizhnekamsk, Russia, 2016. [Google Scholar]
- Kayumov, R.A.; Galimova, A.T.; Sagdeev, A.A.; Petukhov, A.A.; Gumerov, F.M. Extraction of the industrial waste components of the propylene epoxidation process using supercritical CO2. Russ. J. Supercrit. Fluids 2012, 17, 3–12. [Google Scholar]
- Gumerov, F.M.; Kayumov, R.A.; Usmanov, R.A.; Sagdeev, A.A.; Abdullin, I.S.; Sharafeev, R.F. Waste management in propylene epoxidation process with the use of supercritical fluid media. Amer. J. Anal. Chem. 2012, 3, 950–957. [Google Scholar] [CrossRef] [Green Version]
- Gumerov, F.M.; Khairutdinov, V.F.; Zaripov, Z.I. Additional Condition of Efficiency of the Supercritical Fluid Extraction Process. Theor. Found. Chem. Eng. 2021, 55, 348–358. [Google Scholar] [CrossRef]
- Usmanov, R.A.; Mazanov, S.V.; Gabitova, A.R.; Miftakhova LKh Gumerov, F.M.; Musin, R.Z.; Abdulagatov, I.M. The effect of fatty acid ethyl esters concentration on the kinematic viscosity of biodiesel fuel. J. Chem. Eng. 2015, 60, 3404–3413. [Google Scholar] [CrossRef]
- Mazanov, S.V.; Gabitova, A.R.; Usmanov, R.A.; Gumerov, F.M.; Labidi, S.; Amar, M.B.; Passarello, J.P.; Kanaev, A.; Volle, F.; Le Neindre, B. Continuous production of biodiesel from rapeseed oil by ultrasonic assist transesterification in supercritical ethanol. J. Supercrit. Fluids 2016, 118, 107–118. [Google Scholar] [CrossRef]
- Mazanov, S.V.; Aetov, A.U.; Usmanov, R.A.; Gabitov, R.R.; Zaripov, Z.I.; Gumerov, F.M. Oxidation of Fatty Acids by Hydrogen Peroxide in Aqueous Medium Under Supercritical Fluid Conditions. Russ. J. Phys. Chem. B 2017, 3, 1000116. [Google Scholar] [CrossRef] [Green Version]
- Aetov, A.U.; Usmanov, R.A.; Mazanov, S.V.; Gumerov, F.M. Treatment of molybdenum-containing wastewater in supercritical environment. Tsvetnye Met. 2020, 7, 68–73. [Google Scholar] [CrossRef]
- Dong, X.; Gan, Z.; Lu, X.; Jin, W.; Yu, Y.; Zhang, M. Study on catalytic and non-catalytic supercritical water oxidation of p-nitrophenol wastewater. Chem. Eng. J. 2015, 277, 30–39. [Google Scholar] [CrossRef]
- Kosari, M.; Golmohammadi, M.; Towfighi, J.; Ahmadi, S.J. Decomposition of tributhyl phosphate at supercritical water oxidation conditions: Non-catalytic, catalytic, and kinetic reaction studies. J. Supercrit. Fluids 2018, 133, 103–113. [Google Scholar] [CrossRef]
- Guolin, J.; Mingming, L.; Tingting, C. Heterogeneous Catalytic Supercritical Water Oxidation of Refractory Organic Pollutants in Industrial Wastewaters. J. Adv. Oxid. Technol. 2012, 15, 133–144. [Google Scholar] [CrossRef]
- Kazemi, N.; Tavakoli, O.; Seif, S.; Nahangi, M. High-strength distillery wastewater treatment using catalytic sub- and supercritical water. J. Supercrit. Fluids 2015, 97, 74–80. [Google Scholar] [CrossRef]
- Cao, W.; Wang, S.; Ma, L.; Liu, S.; Jin, H.; Wei, W.; Guo, L. Catalytic gasification of phenol in supercritical water with different metal cations. Fuel 2022, 324, 124754. [Google Scholar] [CrossRef]
- Jafarinejad, S.; Vahdat, N. Non-catalytic and Catalytic Supercritical Water Oxidation of Phenol in the Wastewaters of Petroleum and Other Industries. Adv. Nanotech. Appl. Supercrit. Fluids 2020, 239, 33–51. [Google Scholar] [CrossRef]
- Soheil, A.; Rafael, M.S. Recent advances in heterogeneous catalysis for supercritical water oxidation/gasification processes: Insight into catalyst development. Proc. Saf. Environ. Prot. 2021, 149, 169–184. [Google Scholar] [CrossRef]
- Ammar, A.A.; Farooq Sh Abu, H.; Ayesha Zafar Hafiz, M.N.I.; Karahmet, E.; Lester, E. Supercritical water oxidation of phenol and process enhancement with in situ formed Fe2O3 nano catalyst. Environ. Sci. Poll. Res. 2022, 29, 61896–61904. [Google Scholar] [CrossRef]
- Ali, M.E.; Rahman, M.M.; Sarkar, S.M.; Hamid, S.B.A. Heterogeneous Metal Catalysts for Oxidation Reactions. J. Nanomater. 2014, 2014, 209. [Google Scholar] [CrossRef] [Green Version]
- Bineesh, K.V.; Kim, D.K.; Kim, M.I.; Park, D.W. Design, synthesis and characterization of vanadia-doped iron-oxide pillared montmorillonite clay for the selective catalytic oxidation of H2S. J. Dalton Trans. 2011, 40, 3938–3945. [Google Scholar] [CrossRef] [PubMed]
- Aisawa, S.; Hirahara, H.; Uchiyama, H.; Takahashi, S.; Narita, E. Synthesis and thermal decomposition of Mn–Al layered double hydroxides. J. Solid State Chem. 2002, 167, 152–159. [Google Scholar] [CrossRef]
- Tsai, H.; Sato, S.; Takahashi, R.; Sodesawa, T.; Takenaka, S. Liquid-phase hydrogenation of ketones in the mesopores of nickel catalysts. Phys. Chem. Phys. 2002, 4, 3537–3542. [Google Scholar] [CrossRef]
- Sun, S.; Heinen, M.; Jusys, Z.; Behm, R.J. Electrooxidation of acetaldehyde on a carbon supported Pt catalyst at elevated temperature/pressure: An on-line differential electrochemical mass spectrometry study. J. Power Sources 2012, 204, 1–13. [Google Scholar] [CrossRef]
- Duplančić, M.; Kurajica, S.; Tomašić, V.; Minga, I. Catalytic Oxidation of Toluene on Hydrothermally Prepared Ceria Nanocrystals. Chem. Biochem. Eng. 2017, 31, 375–383. [Google Scholar] [CrossRef]
- Dehmani, Y.; Abouarnadasse, S. Total Oxidation of Isopropanol in the Liquid Phase, under Atmospheric Pressure and Low Temperature, on Transition Metal Oxides Catalysts Cr2O3 and Fe2O3. J. Chem. 2020, 2020, 6129526. [Google Scholar] [CrossRef]
- Dehmani, Y.; Amhoud, A.; Abouarnadasse, S. Total Oxidation of Isopropanol in Its Liquid Phase, at a Low Temperature in the Presence of Prepared and Characterized Zinc Oxide. Int. J. Environ. A 2021, 2021, 6667551. [Google Scholar] [CrossRef]
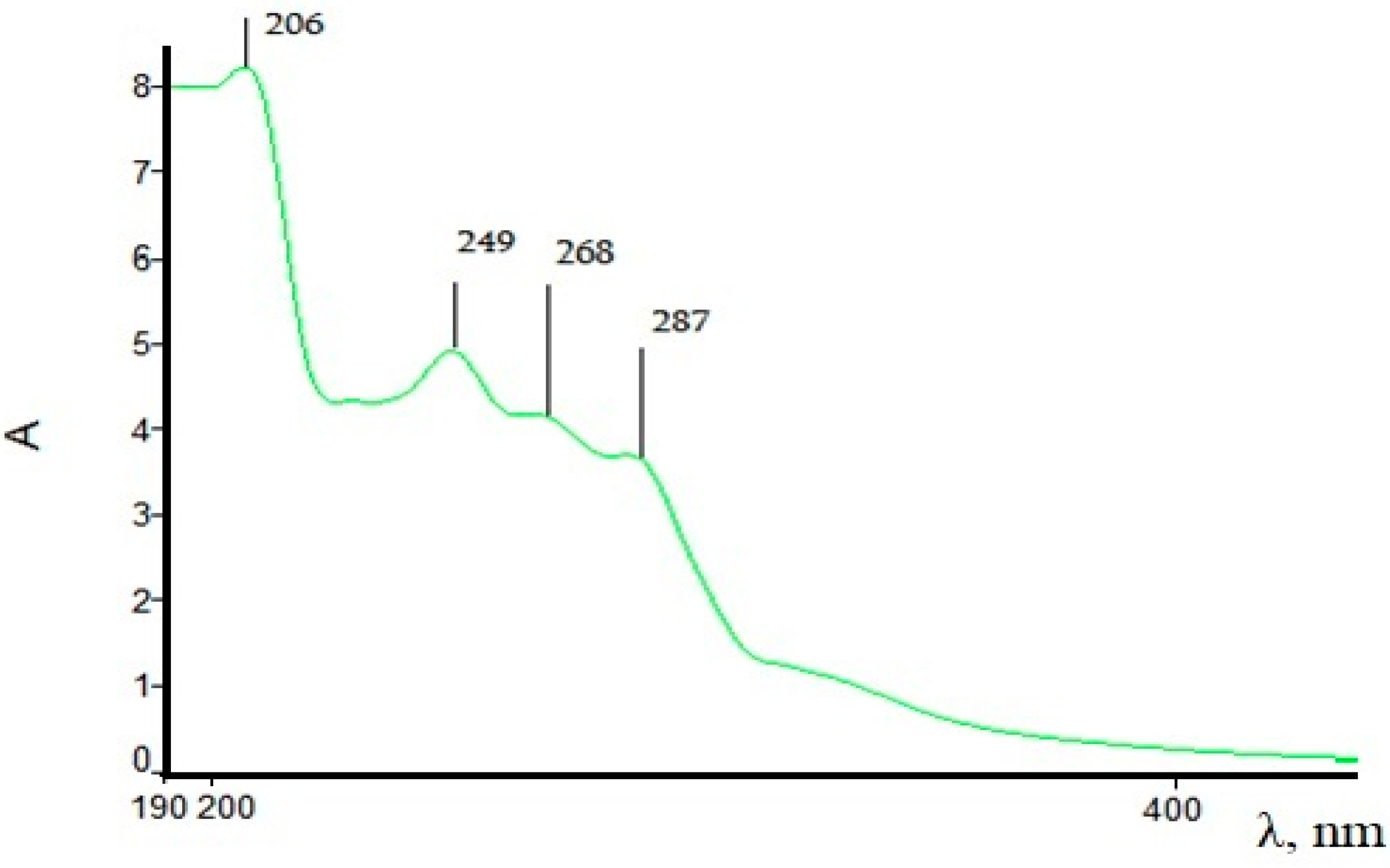
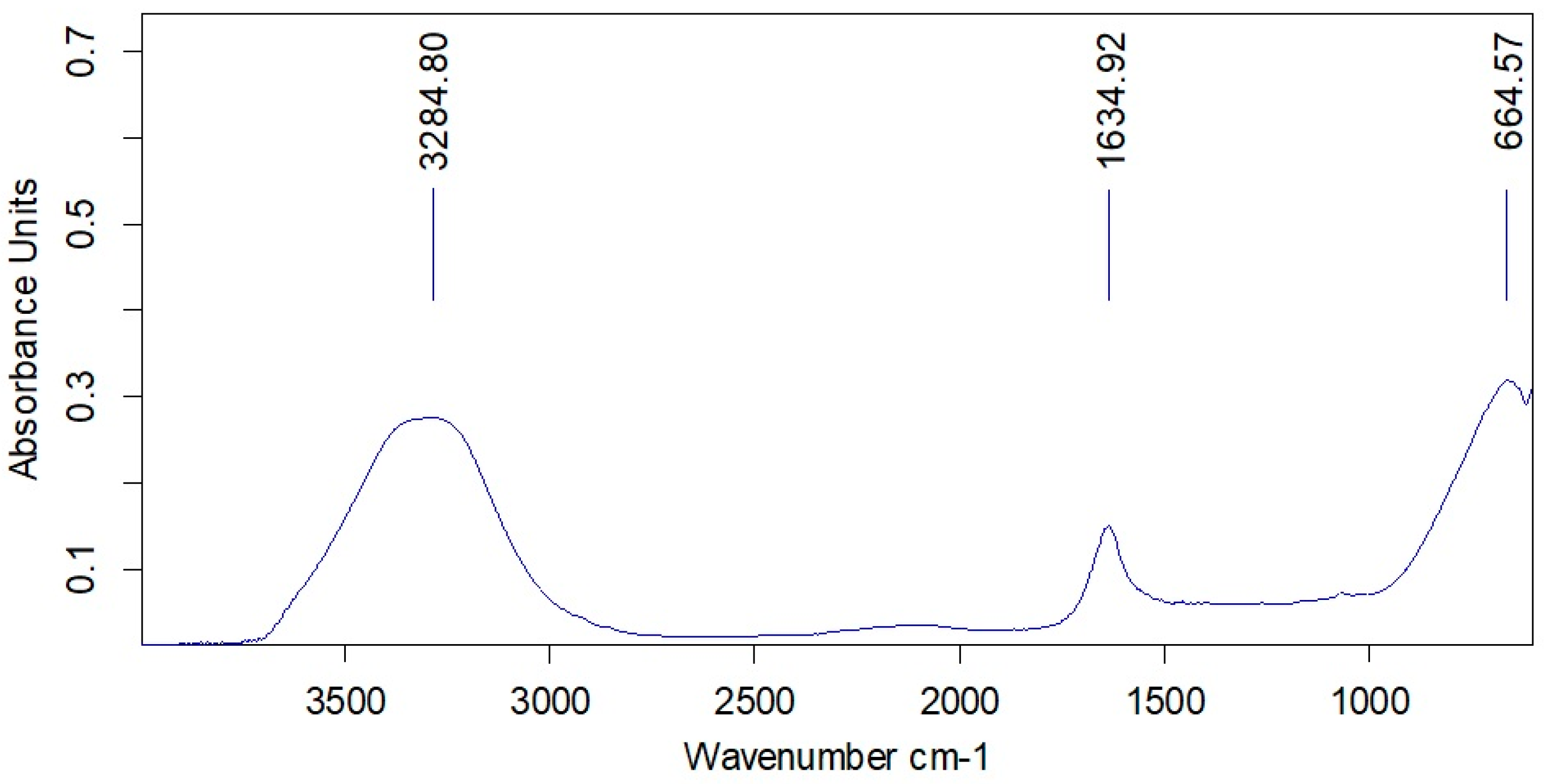
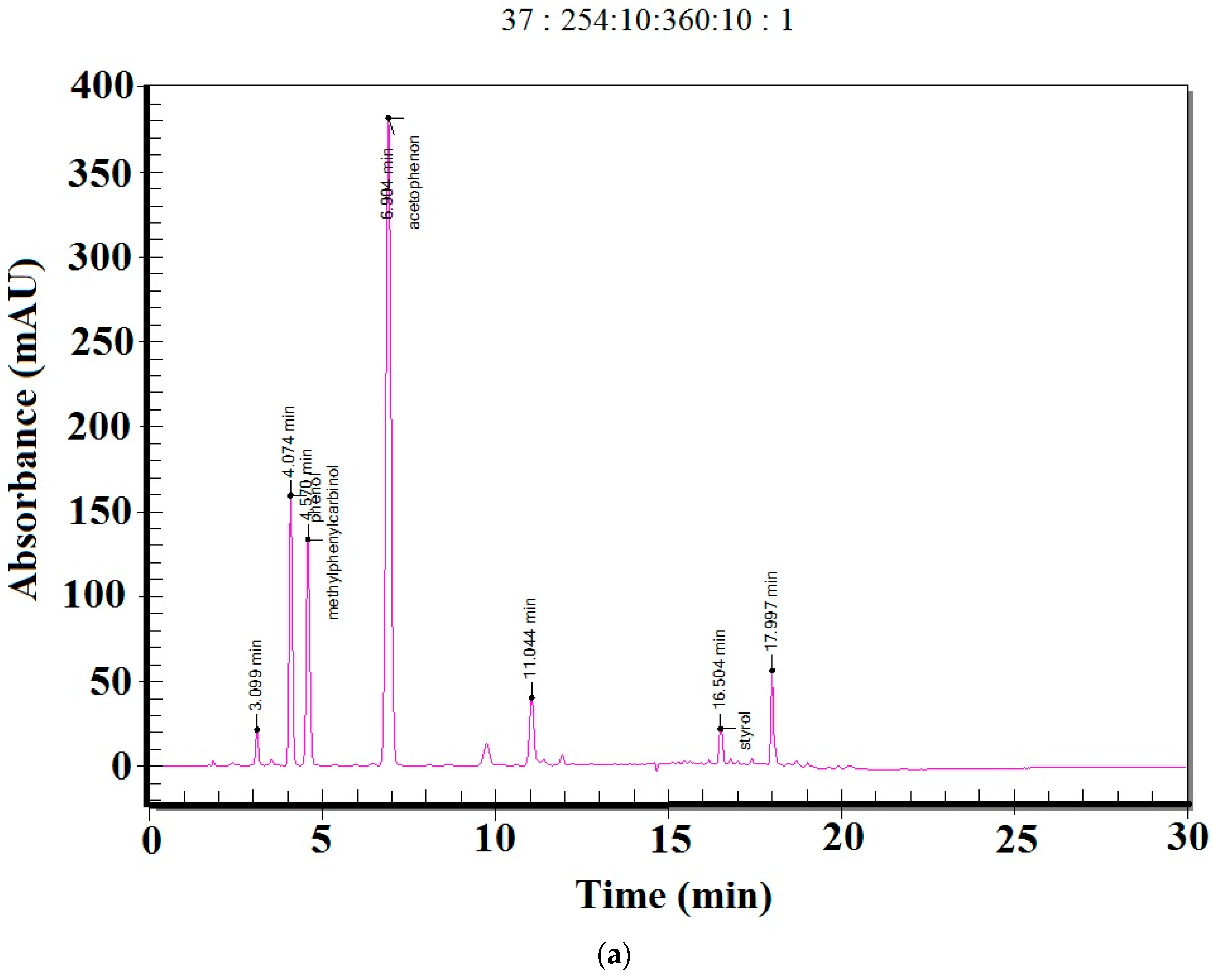


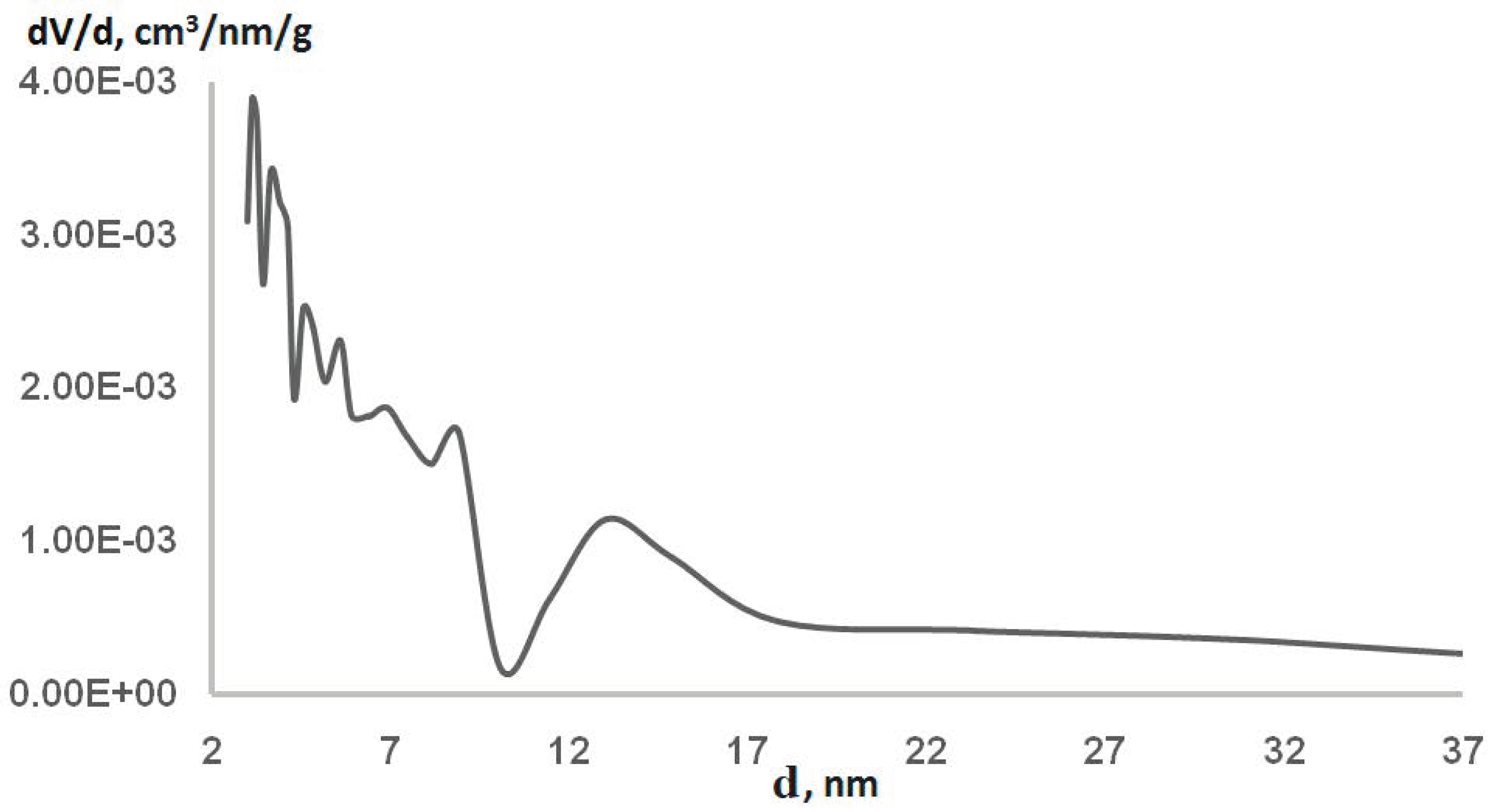


| Samples | Phenol/ mg/L | 1-Phenylethanol/mg/L | Methyl Phenyl ketone/mg/L | Toluene/ mg/L | Styrene, mg/L | Ethylbenzene, mg/L | Propylene Glycol, mg/L |
|---|---|---|---|---|---|---|---|
| Initial wastewater | 1256.64 | 1449.32 | 2598.225 | 0.03 | 92.588 | 0.046 | 1370 |
| pH | 13.25 |
|---|---|
| Ash content/% | 15.01 |
| Density/kg/m3 | 1175.0 |
| COD/mgO2/l | 335.000 |
| Sample | Elemental Composition (Mass %) | |||||
|---|---|---|---|---|---|---|
| SiO2 | Al2O3 | Fe2O3 | MgO | CaO | Na2O | |
| Fe-pillared aluminosilicate (hydrocomlex oligocations | 33.7 | 11.4 | 53.5 | 1.4 | - | - |
| Fe-pillared aluminosilicate (triaquahexacetate trioxo iron cations) | 39.1 h | 12.5 | 46.9 | 1.5 | - | - |
| SBET/m2∙g−1 | VΣ (P/P0 = 0.99)/cm3∙g−1 | Micropores (t-Plot Method) | Dave/nm | Porous Structure (in the Mesopores) | |
|---|---|---|---|---|---|
| Smp/m2 | Vmp/cm3∙g−1 | ||||
| 23.8 | 0.0410 | 4.8 | 0.0021 | 7.0 | Polymodal |
| Pore Diameter Range/nm | Mesopore Volume Fraction a/% | Specific Surface Fraction b/% |
|---|---|---|
| 2–5 | 20.3 | 50.3 |
| 5–10 | 20.7 | 27.8 |
| 10–20 | 27.0 | 16.0 |
| above 20 | 32.0 | 5.90 |
| Sample/mg∙l−1 | Initial Wastewater | Diluted Wastewater | Sample-1 | Sample-2 | Sample-3 | Sample-4 | Sample-5 |
|---|---|---|---|---|---|---|---|
| Phenol | 1256.6 | 62.8 | 10.139 | 1.462 | <1.000 | 17.8 | 10.94 |
| 1-Phenyl-ethanol | 1449.3 | 72.466 | 7.204 | 2.8004 | <1 | 7.64 | 4.9 |
| Methylphenylketone | 2598.2 | 155.8 | 20.391 | 3.019 | 1.94 | 62.8 | 22.24 |
| Toluene | 0.03 | 0.002 | <0.001 | <0.001 | <0.001 | n/a | n/a |
| Styrene | 92.6 | 4.61 | 1.506 | 0.539 | 0.55 | 1.607 | 1.24 |
| Ethylbenzene | 0.046 | 0.0019 | <0.001 | <0.001 | <0.001 | n/a | n/a |
| Propylene glycol | 1370 | 69.5 | n/a | n/a | n/a | n/a | n/a |
| Oxygen Excess (OER) 2 | Oxygen Excess (OER) 2.5 | |||||||
|---|---|---|---|---|---|---|---|---|
| Reaction Time τ Minute | Reaction Time τ Minute | |||||||
| T, K | 1.8 | 2.91 | 4.08 | 4.83 | 1.8 | 2.91 | 4.08 | 4.83 |
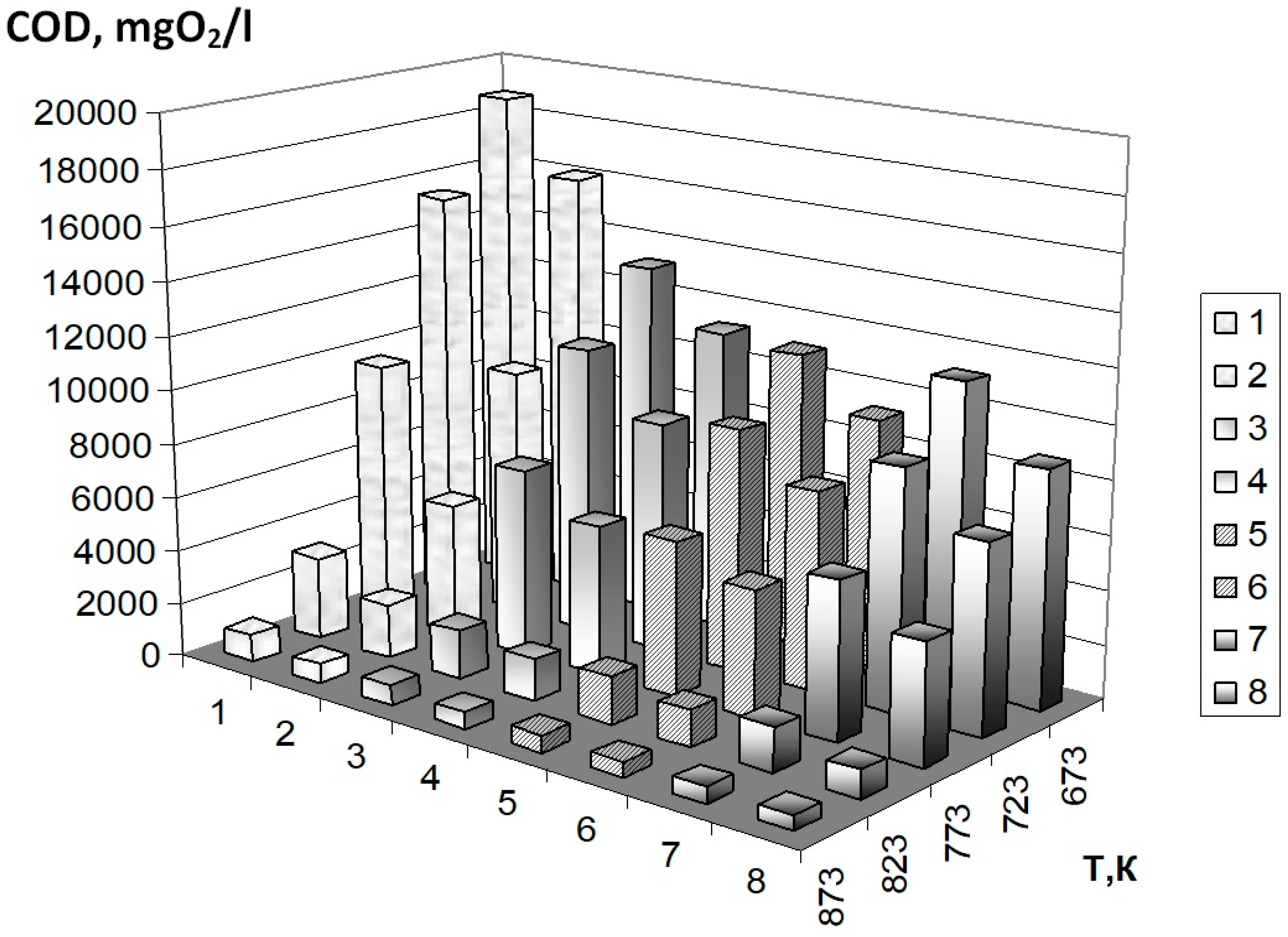
| 673.15 | 18598 | 16875 | 14763 | 12914 | 18296 | 16136 | 14320 | 12708 |
| 723.15 | 16040 | 13783 | 13056 | 12367 | 15793 | 13110 | 12855 | 12604 |
| 773.15 | 12358 | 8942 | 8125 | 7682 | 11895 | 8471 | 7993 | 7541 |
| 823.15 | 2983 | 2420 | 2403 | 2386 | 2910 | 2319 | 2305 | 2291 |
| 873.15 | 2185 | 2017 | 1802 | 1988 | 2125 | 1933 | 1800 | 1709 |
| Oxygen excess (OER) 3.0 | Oxygen excess (OER) 4.0 | |||||||
| Reaction time τ minute | Reaction time τ minute | |||||||
| T, K | 1.80 | 2.91 | 4.08 | 4.83 | 1.80 | 2.91 | 4.08 | 4.83 |
| 673.15 | 17369 | 15396 | 13222 | 11355 | 16443 | 14186 | 12125 | 10362 |
| 723.15 | 14747 | 12438 | 11829 | 11249 | 13701 | 11429 | 10803 | 10210 |
| 773.15 | 10450 | 8000 | 7804 | 7437 | 9006 | 7194 | 6882 | 6583 |
| 823.15 | 2830 | 2218 | 2105 | 1998 | 2750 | 2017 | 1905 | 1800 |
| 873.15 | 1958 | 1848 | 1754 | 1665 | 1891 | 1580 | 1488 | 1400 |
| Oxygen Excess (OER) 2 | Oxygen Excess (OER) 3 | Oxygen Excess (OER) 4 | |||||||
|---|---|---|---|---|---|---|---|---|---|
| T/K | CSCWO (Fe-OH) | CSCWO (Mn-Al) | catalyst carrier | CSCWO (Fe-OH) | CSCWO (Mn-Al) | catalyst carrier | CSCWO (Fe-OH) | CSCWO (Mn-Al) | catalyst carrier |
| 673.15 | 11250 | 11045 | 15325 | 10950 | 10080 | 14965 | 9250 | 8755 | 14535 |
| 723.15 | 9001 | 8955 | 13900 | 8255 | 8010 | 12358 | 7450 | 7020 | 12495 |
| 773.15 | 5820 | 5753 | 8733 | 5340 | 5205 | 8096 | 4735 | 4485 | 7145 |
| 823.15 | 1733 | 1635 | 2502 | 1505 | 1405 | 2015 | 1393 | 1025 | 1945 |
| 873.15 | 636 | 610 | 1915 | 615 | 595 | 1795 | 576 | 580 | 1398 |
| Oxygen Excess (OER) = 2 | Oxygen Excess (OER) = 3 | Oxygen Excess (OER) = 4 | |||||
|---|---|---|---|---|---|---|---|
| Reaction Time τ = min | Reaction Time τ = 2.91 min | Reaction Time τ = min | |||||
| T, K | 1.80 | 2.91 | 4.08 | 1.80 | 2.91 | 4.08 | |
| 723.15 | 12495 | 12124 | 10613 | 10785 | 10681 | 9731 | 8529 |
| 773.15 | 8335 | 8088 | 7080 | 6725 | 6680 | 6430 | 5640 |
| 798.15 | 4697 | 4558 | 3990 | 4060 | 3714 | 3665 | 3216 |
| 823.15 | 2130 | 2067 | 1809 | 1850 | 1729 | 1677 | 1533 |
| OER | Reactor’s Temperature, K | SCWO | CSCWO (Fe-Ac) | CSCWO (Fe-OH) | CSCWO (Mn-Al) | Catalyst Carrier |
|---|---|---|---|---|---|---|
| X | X | X | X | X | ||
| 2 | 673.15 | 0.749 | 0.809 | 0.832 | 0.835 | 0.772 |
| 723.15 | 0.795 | 0.820 | 0.866 | 0.866 | 0.793 | |
| 773.15 | 0.867 | 0.879 | 0.913 | 0.914 | 0.867 | |
| 823.15 | 0.964 | 0.969 | 0.974 | 0.975 | 0.963 | |
| 873.15 | 0.970 | 0.988 | 0.990 | 0.990 | 0.971 | |
| 3 | 673.15 | 0.741 | 0.819 | 0.837 | 0.850 | 0.777 |
| 723.15 | 0.781 | 0.839 | 0.877 | 0.880 | 0.816 | |
| 773.15 | 0.844 | 0.899 | 0.920 | 0.922 | 0.879 | |
| 823.15 | 0.957 | 0.974 | 0.977 | 0.979 | 0.970 | |
| 873.15 | 0.971 | 0.989 | 0.990 | 0.991 | 0.973 | |
| 4 | 673.15 | 0.789 | 0.832 | 0.862 | 0.869 | 0.783 |
| 723.15 | 0.830 | 0.855 | 0.889 | 0.895 | 0.814 | |
| 773.15 | 0.893 | 0.904 | 0.929 | 0.933 | 0.893 | |
| 823.15 | 0.970 | 0.975 | 0.979 | 0.984 | 0.971 | |
| 873.15 | 0.977 | 0.990 | 0.991 | 0.991 | 0.979 |
| Fraction | Mass % | Phase Composition, Mass % | |||||
|---|---|---|---|---|---|---|---|
| Fe2O3 | Fe3O4 | MoO3 | ZnO | NiO | Zn5(CO3)2(OH)6 | ||
| Magnetic | 63.4 | 8.9 | 8.0 | 60.6 | 7.6 | 10.5 | 4.5 |
| Not magnetic | 36.7 | 0.6 | - | 48.1 | 19.8 | 4.3 | 27.1 |
| Total | 100 | 5.9 | 5.1 | 56.0 | 12.1 | 8.2 | 12.7 |
| System | Temperature Range, T/K | Activation Energy /kJ·mol−1 | ln A | ·102/s−1 (at 773 K) |
|---|---|---|---|---|
| SCWO (non-catalytic) | 673–873 | 23.7 ± 4.5 | −0.694 ± 0.7 | 1.250 |
| CSCWO (catalytic Fe-Ac) | 723–873 | 22.8 ± 5.1 | −0.524 ± 0.8 | 1.705 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mazanov, S.V.; Phan, Q.M.; Aetov, A.U.; Zaripov, Z.I.; Starshinova, V.L.; Karalin, E.A.; Usmanov, R.A.; Gumerov, F.M.; Abdulagatov, I.M. Heterogeneous Catalytic and Non-Catalytic Supercritical Water Oxidation of Organic Pollutants in Industrial Wastewaters Effect of Operational Parameters. Symmetry 2023, 15, 340. https://doi.org/10.3390/sym15020340
Mazanov SV, Phan QM, Aetov AU, Zaripov ZI, Starshinova VL, Karalin EA, Usmanov RA, Gumerov FM, Abdulagatov IM. Heterogeneous Catalytic and Non-Catalytic Supercritical Water Oxidation of Organic Pollutants in Industrial Wastewaters Effect of Operational Parameters. Symmetry. 2023; 15(2):340. https://doi.org/10.3390/sym15020340
Chicago/Turabian StyleMazanov, Sergei V., Quang M. Phan, Almaz U. Aetov, Zufar I. Zaripov, Valentina L. Starshinova, Ernest A. Karalin, Rustem A. Usmanov, Farid M. Gumerov, and Ilmutdin M. Abdulagatov. 2023. "Heterogeneous Catalytic and Non-Catalytic Supercritical Water Oxidation of Organic Pollutants in Industrial Wastewaters Effect of Operational Parameters" Symmetry 15, no. 2: 340. https://doi.org/10.3390/sym15020340





